Differences in Cholesterol Metabolism, Hepato-Intestinal Aging, and Hepatic Endocrine Milieu in Rats as Affected by the Sex and Age
Abstract
1. Introduction
2. Results
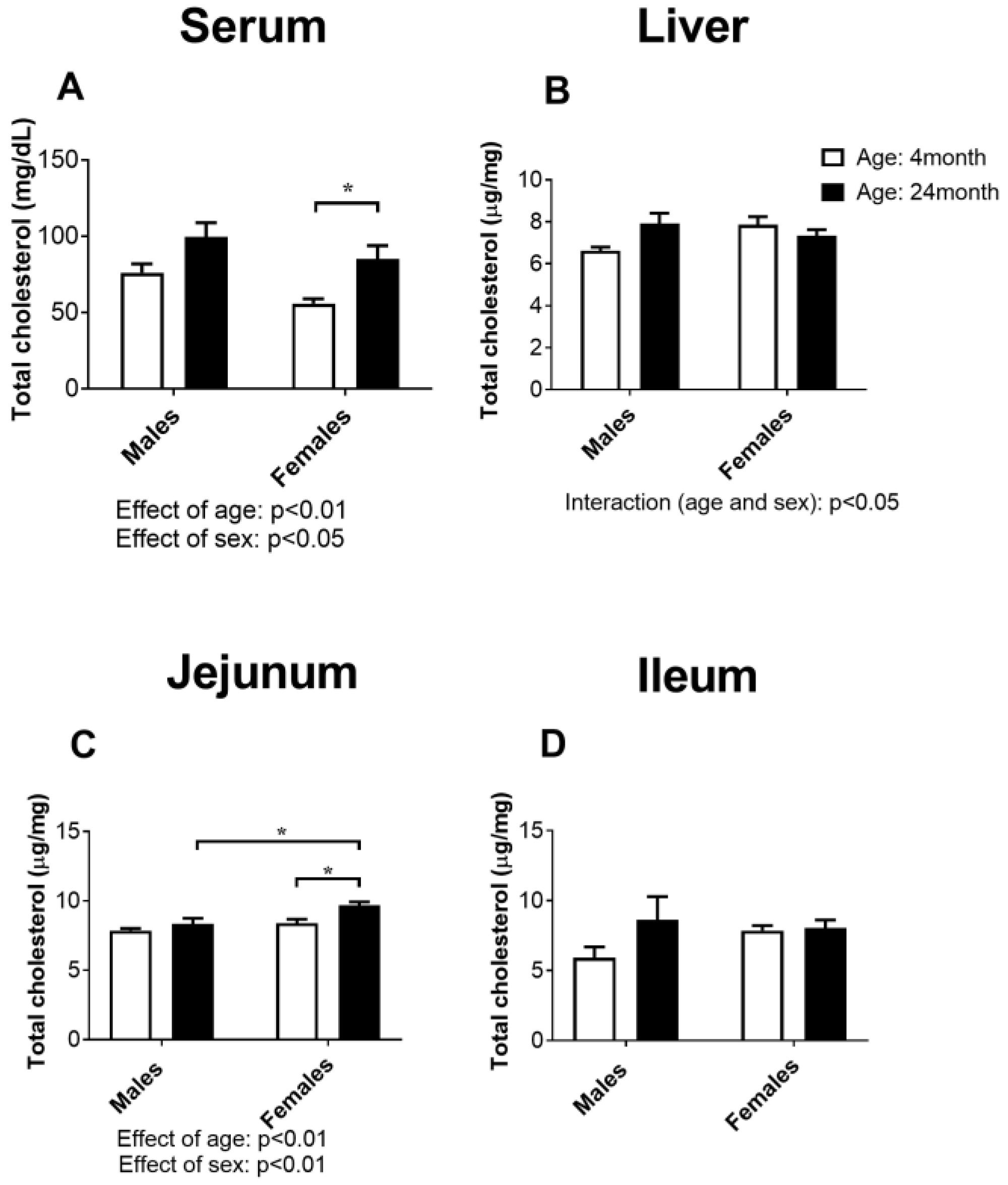
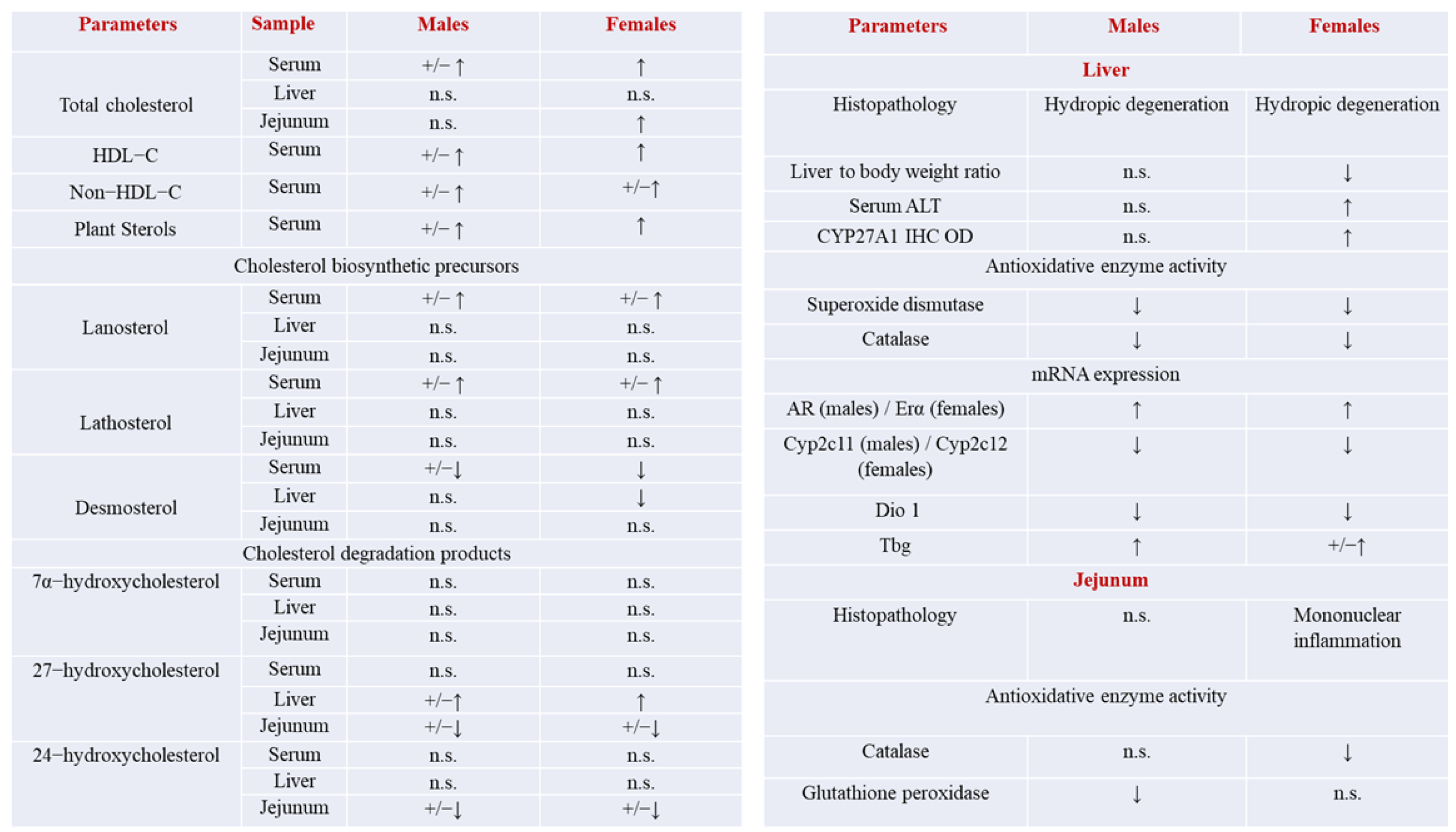
2.1. Concentration of Total Cholesterol in Serum, the Liver, Jejunum, and Ileum
2.2. Concentration of HDL-Cholesterol and Non-HDL-Cholesterol in Serum
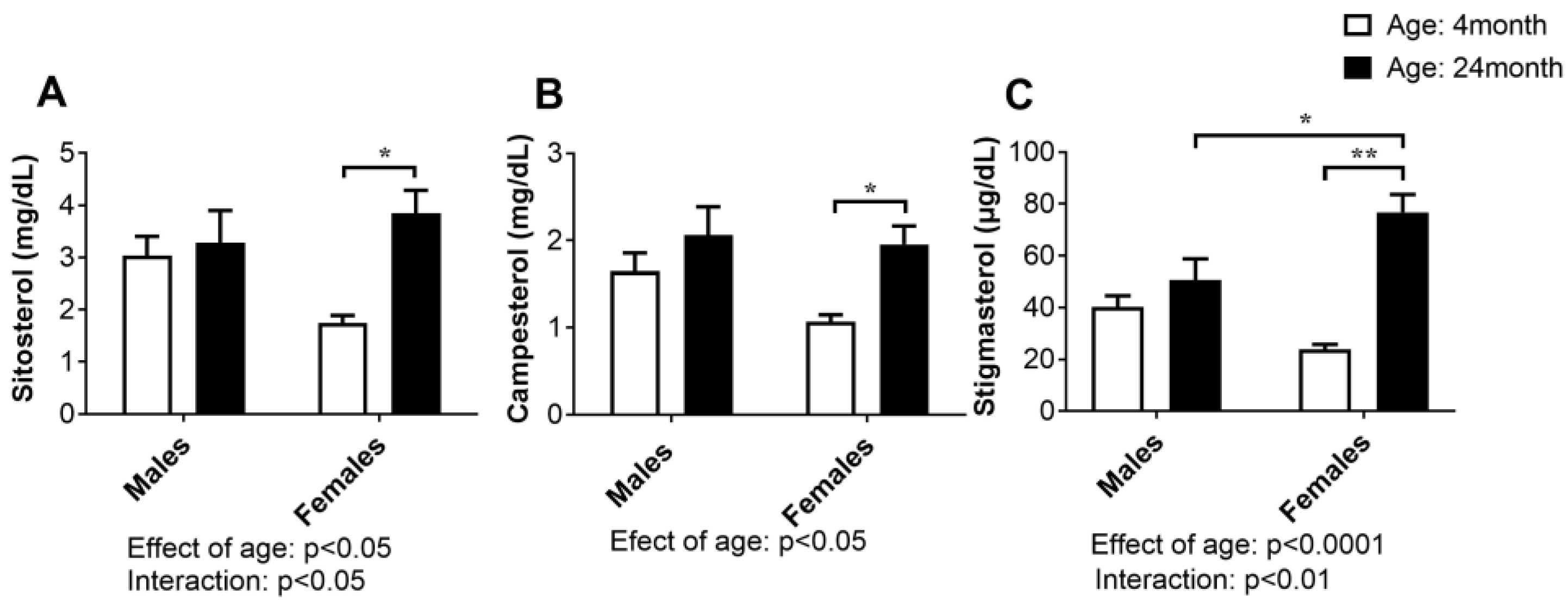
2.3. Concentration of Plant Sterols in Serum
2.4. Concentration of Cholesterol Precursors in Serum, Liver, Jejunum and Ileum
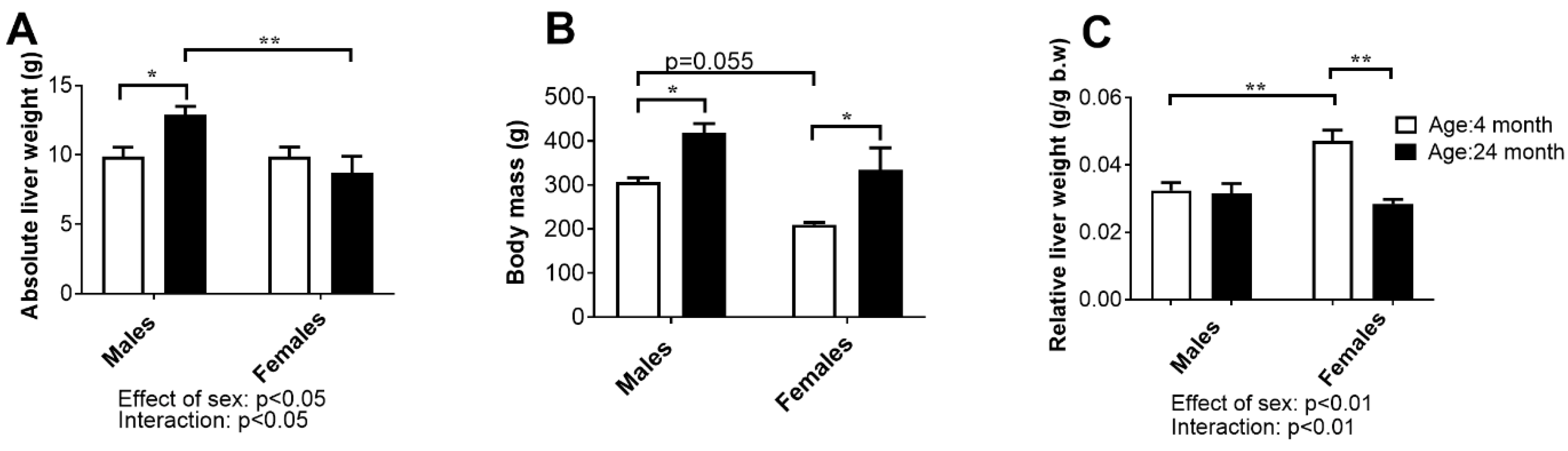
2.5. Liver Weight, Histology, and Concentration of Aminotransferases in Serum
2.6. Oxidative Stress Parameters in the Liver
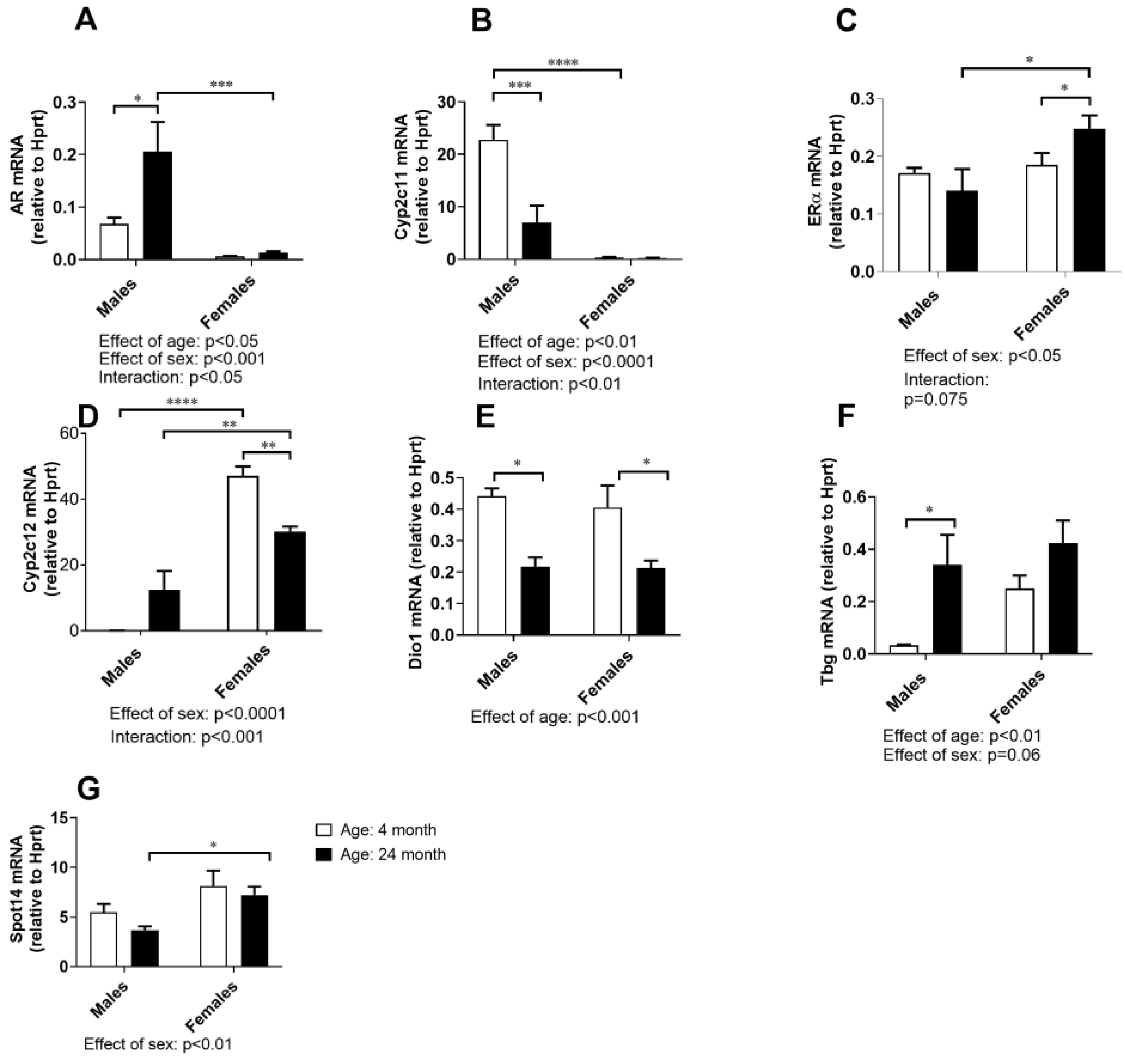
2.7. Hormone-Responsive Gene Expressions in the Liver
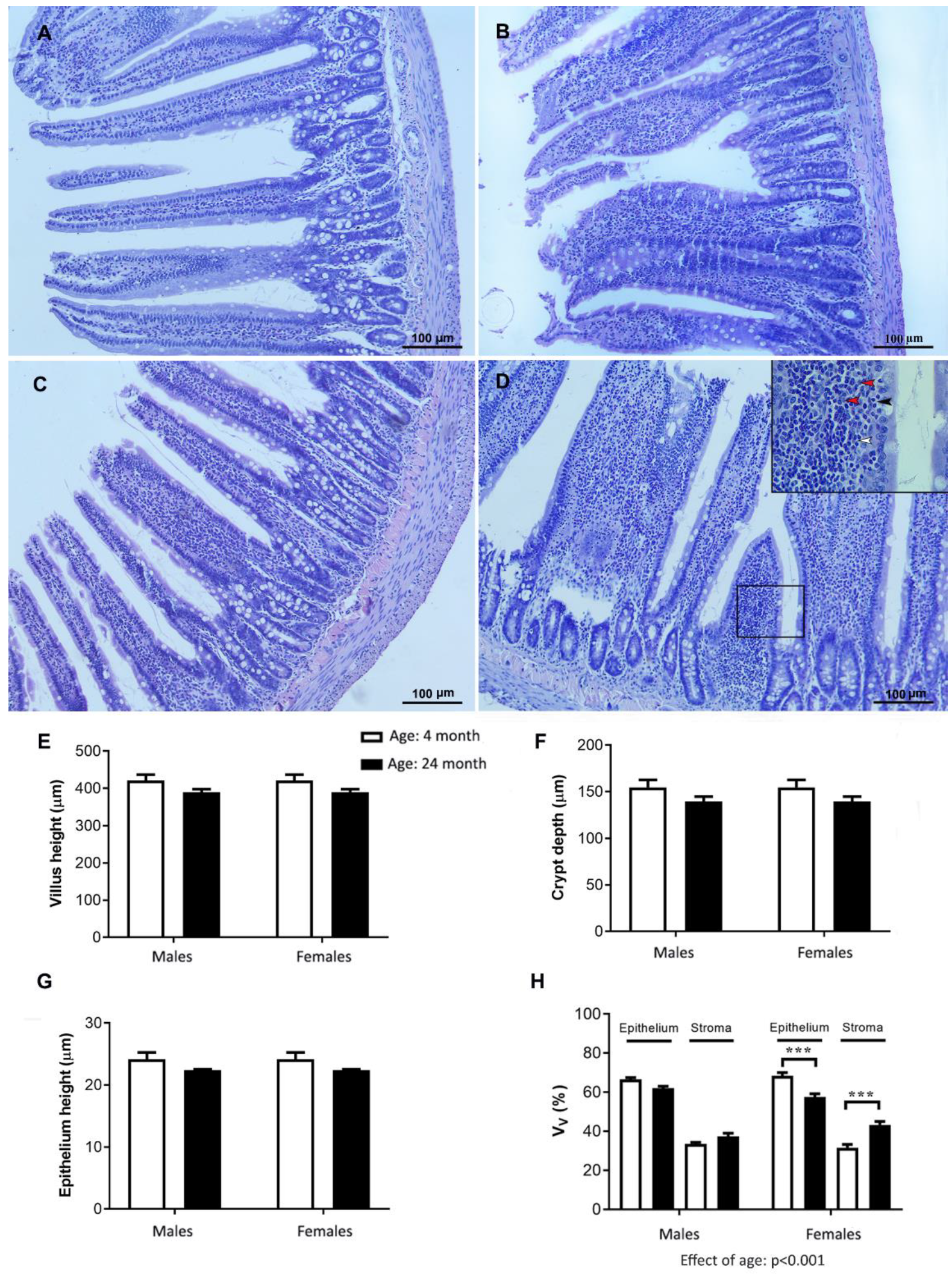
2.8. Histology of the Jejunum
2.9. Oxidative Stress Parameters in the Jejunum
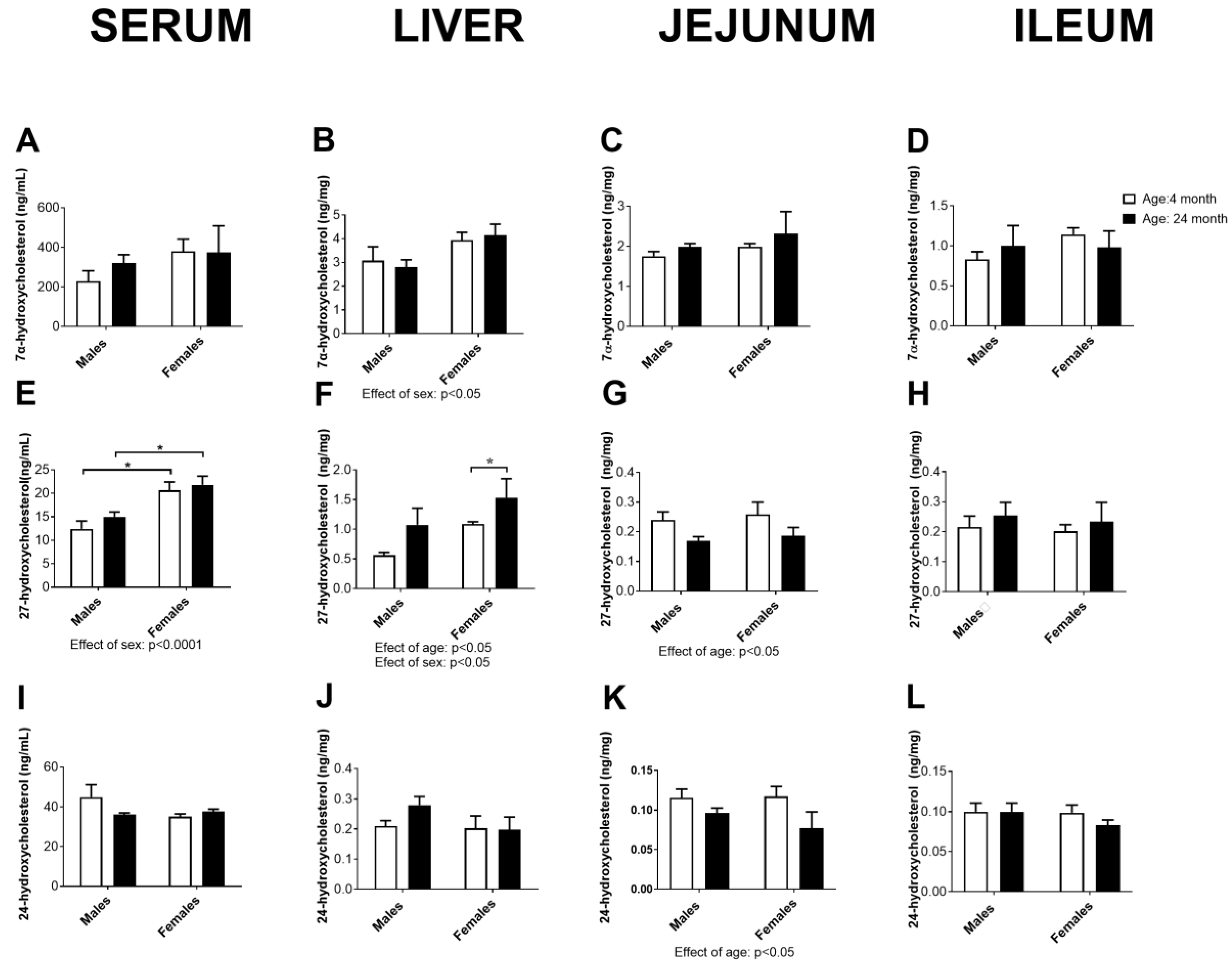
2.10. Concentration of Cholesterol Degradation Intermediates in Serum, the Liver, Jejunum, and Ileum
2.11. Gene and Immunohistochemical Analysis of CYP27A1 in the Liver
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Collection of Serum, Liver, and Small Intestine Tissues
4.3. Histological, Immunohistochemical, and Morphometric Analyses
4.4. Quantification of Sterols and Oxysterols in Serum, the Liver, Jejunum, and Ileum
4.5. Determination of Oxidative Stress Biomarkers
4.6. Gene Expression Analyses
4.7. Serum Measurements of Biochemical Parameters
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef]
- Alfonso, F.; Rivero, F.; Sánchez-Madrid, F. Variability in atherogenic lipoproteins and coronary artery disease progression. Eur. Heart J. 2018, 39, 2559–2561. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.-P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Younossi, Y.; Golabi, P.; Mishra, A.; Rafiq, N.; Henry, L. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2019, 69, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kawakami, R.; Sakamoto, A.; Cornelissen, A.; Mori, M.; Kawai, K.; Ghosh, S.; Romero, M.E.; Kolodgie, F.D.; Finn, A.V.; et al. Sex Differences in Coronary Atherosclerosis. Curr. Atheroscler. Rep. 2022, 24, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Man, J.J.; Beckman, J.A.; Jaffe, I.Z. Sex as a Biological Variable in Atherosclerosis. Circ. Res. 2020, 126, 1297–1319. [Google Scholar] [CrossRef]
- Malhotra, P.; Gill, R.K.; Saksena, S.; Alrefai, W.A. Disturbances in Cholesterol Homeostasis and Non-alcoholic Fatty Liver Diseases. Front. Med. 2020, 7, 467. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Boekholdt, S.M.; Arsenault, B.J.; Mora, S.; Pedersen, T.R.; LaRosa, J.C.; Nestel, P.J.; Simes, R.J.; Durrington, P.; Hitman, G.A.; Welch, K.M.A.; et al. Association of LDL Cholesterol, Non–HDL Cholesterol, and Apolipoprotein B Levels with Risk of Cardiovascular Events Among Patients Treated with Statins: A Meta-Analysis. JAMA 2012, 307, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Lütjohann, D.; Klör, H.-U.; Stellaard, F. Measurement of Serum Low Density Lipoprotein Cholesterol and Triglyceride-Rich Remnant Cholesterol as Independent Predictors of Atherosclerotic Cardiovascular Disease: Possibilities and Limitations. Nutrients 2023, 15, 2202. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Berthold, H.K.; Campesi, I.; Carrero, J.-J.; Dhakal, S.; Franconi, F.; Gouni-Berthold, I.; Heiman, M.L.; Kautzky-Willer, A.; Klein, S.L.; et al. Sex- and Gender-Based Pharmacological Response to Drugs. Pharmacol. Rev. 2021, 73, 730–762. [Google Scholar] [CrossRef]
- Sayaf, K.; Gabbia, D.; Russo, F.P.; De Martin, S. The Role of Sex in Acute and Chronic Liver Damage. Int. J. Mol. Sci. 2022, 23, 10654. [Google Scholar] [CrossRef]
- Waxman, D.J.; O’connor, C. Growth Hormone Regulation of Sex-Dependent Liver Gene Expression. Mol. Endocrinol. 2006, 20, 2613–2629. [Google Scholar] [CrossRef]
- DiStefano, J.K. NAFLD and NASH in Postmenopausal Women: Implications for Diagnosis and Treatment. Endocrinology 2020, 161, bqaa134. [Google Scholar] [CrossRef]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2016, 21, 1024–1032. [Google Scholar] [CrossRef]
- Aydın, S.; Atukeren, P.; Çakatay, U.; Uzun, H.; Altuğ, T. Gender-dependent oxidative variations in liver of aged rats. Biogerontology 2009, 11, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Zakari, M.; Nee, J.; Hirsch, W.; Kuo, B.; Lembo, A.; Staller, K. Gender differences in chronic constipation on anorectal motility. Neurogastroenterol. Motil. 2016, 29, e12980. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Toner, B.B.; Fukudo, S.; Guthrie, E.; Locke, G.R.; Norton, N.J.; Sperber, A.D. Gender, Age, Society, Culture, and the Patient’s Perspective in the Functional Gastrointestinal Disorders. Gastroenterology 2006, 130, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.A.; Heitkemper, M.; Crowell, M.D.; Emmanuel, A.; Halpert, A.; McRoberts, J.A.; Toner, B. Age, Gender, and Women’s Health and the Patient. Gastroenterology 2016, 150, 1332–1343.e4. [Google Scholar] [CrossRef]
- Barros, P.R.; Costa, T.J.; Akamine, E.H.; Tostes, R.C. Vascular Aging in Rodent Models: Contrasting Mechanisms Driving the Female and Male Vascular Senescence. Front. Aging 2021, 2, 727604. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Correa, E.; González-Pérez, I.; Clavel-Pérez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and nutritional overview of diet-induced metabolic syndrome models in rats: What is the best choice? Nutr. Diabetes 2020, 10, 24. [Google Scholar] [CrossRef]
- Brinton, R.D. Minireview: Translational Animal Models of Human Menopause: Challenges and Emerging Opportunities. Endocrinology 2012, 153, 3571–3578. [Google Scholar] [CrossRef]
- Félix-Redondo, F.J.; Grau, M.; Baena-Díez, J.M.; Dégano, I.R.; de León, A.C.; Guembe, M.J.; Alzamora, M.T.; Vega-Alonso, T.; Robles, N.R.; Ortiz, H.; et al. Prevalence of obesity and associated cardiovascular risk: The DARIOS study. BMC Public Health 2013, 13, 542. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Chung, H.-F.; Dobson, A.J.; Pandeya, N.; Brunner, E.J.; Kuh, D.; Greenwood, D.C.; Hardy, R.; Cade, J.E.; Giles, G.G.; et al. Type of menopause, age of menopause and variations in the risk of incident cardiovascular disease: Pooled analysis of individual data from 10 international studies. Hum. Reprod. 2020, 35, 1933–1943. [Google Scholar] [CrossRef]
- Patel, S.B.; Zablocki, C.J. Plant sterols and stanols: Their role in health and disease. J. Clin. Lipidol. 2008, 2, S11–S19. [Google Scholar] [CrossRef]
- Kruit, J.K.; Groen, A.K.; van Berkel, T.J.; Kuipers, F. Emerging roles of the intestine in control of cholesterol metabolism. World J. Gastroenterol. 2006, 12, 6429–6439. [Google Scholar] [CrossRef]
- Duan, L.-P.; Wang, H.H.; Wang, D.Q.-H. Cholesterol absorption is mainly regulated by the jejunal and ileal ATP-binding cassette sterol efflux transporters Abcg5 and Abcg8 in mice. J. Lipid Res. 2004, 45, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.-P.; Wang, H.H.; Ohashi, A.; Wang, D.Q.-H. Role of intestinal sterol transporters Abcg5, Abcg8, and Npc1l1 in cholesterol absorption in mice: Gender and age effects. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G269–G276. [Google Scholar] [CrossRef] [PubMed]
- Pascot, A.; Lemieux, I.; Bergeron, J.; Tremblay, A.; Nadeau, A.; Prud’Homme, D.; Couillard, C.; Lamarche, B.; Després, J.-P. HDL particle size: A marker of the gender difference in the metabolic risk profile. Atherosclerosis 2002, 160, 399–406. [Google Scholar] [CrossRef]
- Clifton, P.M.; Nestel, P.J. Influence of gender, body mass index, and age on response of plasma lipids to dietary fat plus cholesterol. Arterioscler. Thromb. J. Vasc. Biol. 1992, 12, 955–962. [Google Scholar] [CrossRef]
- Abbott, R.D.; Garrison, R.J.; Wilson, P.W.; Epstein, F.H.; Castelli, W.P.; Feinleib, M.; LaRue, C. Joint distribution of lipoprotein cholesterol classes. The Framingham study. Arteriosclerosis 1983, 3, 260–272. [Google Scholar] [CrossRef]
- Parini, P.; Angelin, B.; Rudling, M. Cholesterol and Lipoprotein Metabolism in Aging. Arter. Thromb. Vasc. Biol. 1999, 19, 832–839. [Google Scholar] [CrossRef]
- Van Lenten, B.J.; Jenkins, C.H.; Roheim, P.S. Plasma apolipoprotein profiles of male and female rats. Atherosclerosis 1980, 37, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Mast, N.; Pikuleva, I.A. Drugs and Scaffold That Inhibit Cytochrome P450 27A1 In Vitro and In Vivo. Mol. Pharmacol. 2017, 93, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kucera, O.; Cervinkova, Z. Experimental models of non-alcoholic fatty liver disease in rats. World J. Gastroenterol. 2014, 20, 8364–8376. [Google Scholar] [CrossRef]
- Schmucker, D.L. Age-related changes in liver structure and function: Implications for disease? Exp. Gerontol. 2005, 40, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.X.; Dhahbi, J.M.; Mote, P.L.; Spindler, S.R. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc. Natl. Acad. Sci. USA 2001, 98, 10630–10635. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, L.-L.; Liu, T.-Y.; Wang, Z.-T. Evaluation of Gender-Related Differences in Various Oxidative Stress Enzymes in Mice. Chin. J. Physiol. 2012, 54, 385–390. [Google Scholar] [CrossRef]
- Engels, K.; Rakov, H.; Zwanziger, D.; Moeller, L.C.; Homuth, G.; Köhrle, J.; Brix, K.; Führer, D. Differences in Mouse Hepatic Thyroid Hormone Transporter Expression with Age and Hyperthyroidism. Eur. Thyroid. J. 2015, 4, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Shi, H. Sex Hormones and Their Receptors Regulate Liver Energy Homeostasis. Int. J. Endocrinol. 2015, 2015, 294278. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, S.; Lolli, F.; Ciana, P.; Maggi, A. Sexual Dimorphism and Estrogen Action in Mouse Liver. Adv. Exp. Med. Biol. 2017, 1043, 141–151. [Google Scholar] [CrossRef]
- Waxman, D.J.; Holloway, M.G. Sex Differences in the Expression of Hepatic Drug Metabolizing Enzymes. Mol. Pharmacol. 2009, 76, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Vranckx, R.; Rouaze-Romet, M.; Savu, L.; Mechighel, P.; Maya, M.; Nunez, E.A. Regulation of rat thyroxine-binding globulin and transthyretin: Studies in thyroidectomized and hypophysectomized rats given tri-iodothyronine or/and growth hormone. J. Endocrinol. 1994, 142, 77–84. [Google Scholar] [CrossRef]
- Ren, J.; Xu, N.; Zheng, H.; Tian, W.; Li, H.; Li, Z.; Wang, Y.; Tian, Y.; Kang, X.; Liu, X. Expression of Thyroid Hormone Responsive SPOT 14 Gene Is Regulated by Estrogen in Chicken (Gallus gallus). Sci. Rep. 2017, 7, 10243. [Google Scholar] [CrossRef]
- Drozdowski, L.; Thomson, A.B. Aging and the intestine. World J. Gastroenterol. 2006, 12, 7578–7584. [Google Scholar] [CrossRef]
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34. [Google Scholar] [CrossRef]
- Haghikia, A.; Zimmermann, F.; Schumann, P.; Jasina, A.; Roessler, J.; Schmidt, D.; Heinze, P.; Kaisler, J.; Nageswaran, V.; Aigner, A.; et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur. Heart J. 2021, 43, 518–533. [Google Scholar] [CrossRef]
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016, 7, 313–322. [Google Scholar] [CrossRef]
- Ide, T.; Tsutsui, H.; Ohashi, N.; Hayashidani, S.; Suematsu, N.; Tsuchihashi, M.; Tamai, H.; Takeshita, A. Greater Oxidative Stress in Healthy Young Men Compared with Premenopausal Women. Arter. Thromb. Vasc. Biol. 2002, 22, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Saint-Denis, M.; Labrot, F.; Narbonne, J.F.; Ribera, D. Glutathione, Glutathione-Related Enzymes, and Catalase Activities in the Earthworm Eisenia fetida andrei. Arch. Environ. Contam. Toxicol. 1998, 35, 602–614. [Google Scholar] [CrossRef]
- Barrett, K.E.; McCole, D.F. Hydrogen peroxide scavenger, catalase, alleviates ion transport dysfunction in murine colitis. Clin. Exp. Pharmacol. Physiol. 2016, 43, 1097–1106. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Olkkonen, V.M.; Béaslas, O.; Nissilä, E. Oxysterols and Their Cellular Effectors. Biomolecules 2012, 2, 76–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-R.; Ishikawa, T.; Umetani, M. The interaction between metabolism, cancer and cardiovascular disease, connected by 27-hydroxycholesterol. Clin. Lipidol. 2014, 9, 617–624. [Google Scholar] [CrossRef] [PubMed]
- DuSell, C.D.; Umetani, M.; Shaul, P.W.; Mangelsdorf, D.J.; McDonnell, D.P. 27-Hydroxycholesterol Is an Endogenous Selective Estrogen Receptor Modulator. Mol. Endocrinol. 2008, 22, 65–77. [Google Scholar] [CrossRef]
- Umetani, M.; Ghosh, P.; Ishikawa, T.; Umetani, J.; Ahmed, M.; Mineo, C.; Shaul, P.W. The Cholesterol Metabolite 27-Hydroxycholesterol Promotes Atherosclerosis via Proinflammatory Processes Mediated by Estrogen Receptor Alpha. Cell Metab. 2014, 20, 172–182. [Google Scholar] [CrossRef]
- Pandak, W.M.; Ren, S.; Marques, D.; Hall, E.; Redford, K.; Mallonee, D.; Bohdan, P.; Heuman, D.; Gil, G.; Hylemon, P. Transport of Cholesterol into Mitochondria Is Rate-limiting for Bile Acid Synthesis via the Alternative Pathway in Primary Rat Hepatocytes. J. Biol. Chem. 2002, 277, 48158–48164. [Google Scholar] [CrossRef]
- Šošić-Jurjević, B.; Trifunović, S.; Živanović, J.; Ajdžanović, V.; Miler, M.; Ristić, N.; Filipović, B. Vitamin D3 Treatment Alters Thyroid Functional Morphology in Orchidectomized Rat Model of Osteoporosis. Int. J. Mol. Sci. 2022, 23, 791. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An Open Source Plugin for the Quantitative Evaluation and Automated Scoring of Immunohistochemistry Images of Human Tissue Samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef]
- Mackay, D.S.; Jones, P.J.; Myrie, S.B.; Plat, J.; Lütjohann, D. Methodological considerations for the harmonization of non-cholesterol sterol bio-analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 957, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Šošić-Jurjević, B.; Lütjohann, D.; Renko, K.; Filipović, B.; Radulović, N.; Ajdžanović, V.; Trifunović, S.; Nestorović, N.; Živanović, J.; Stojanoski, M.M.; et al. The isoflavones genistein and daidzein increase hepatic concentration of thyroid hormones and affect cholesterol metabolism in middle-aged male rats. J. Steroid Biochem. Mol. Biol. 2019, 190, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Reprint of: Oxygen Free Radicals and Iron in Relation to Biology and Medicine: Some Problems and Concepts. Arch. Biochem. Biophys. 2022, 726, 109246. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase Activity. In CRC Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Tamura, M.; Oshino, N.; Chance, B. Some Characteristics of Hydrogen- and Alkylhydroperoxides Metabolizing Systems in Cardiac Tissue. J. Biochem. 1982, 92, 1019–1031. [Google Scholar] [CrossRef]
- Glatzle, D.; Vuilleumier, J.P.; Weber, F.; Decker, K. Glutathione reductase test with whole blood, a convenient procedure for the assessment of the riboflavin status in humans. Experientiay 1974, 30, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Rehncrona, S.; Smith, D.S.; Åkesson, B.; Westerberg, E.; Siesjö, B.K. Peroxidative Changes in Brain Cortical Fatty Acids and Phospholipids, as Characterized During Fe2+- and Ascorbic Acid-Stimulated Lipid Peroxidation In Vitro. J. Neurochem. 1980, 34, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef]




| LIVER | ||||
|---|---|---|---|---|
| Male | Female | |||
| 4 Months Old | 24 Months Old | 4 Months Old | 24 Months Old | |
| SOD | 4229.21 ± 238.03 | 3422.69 ± 148.96 * | 5240.94 ± 141.14 # | 4067.30 ± 496.44 * |
| CAT | 45,205.96 ± 3565.04 | 56,429.24 ± 5534.05 * | 68,532.40 ± 6549.49 # | 40,356.88 ± 1236.01 *# |
| GSH-Px | 110,017.7 ± 7828.8 | 106,351.8 ± 2635.98 | 162,364.9 ± 17,537.1 # | 141,762.0 ± 2812.06 # |
| GR | 4085.2 ± 423.71 | 6753.10 ± 676.68 * | 6967.84 ± 696.30 # | 7321.54 ± 552.21 |
| GST | 186,862.5 ± 18,052.3 | 177,522.3 ± 12,200.20 | 139,906.3 ± 6629.87 # | 159,316.7 ± 17,128.6 |
| GSH | 5770.6 ± 144.92 | 4679.30 ± 366.86 * | 2870.46 ± 195.63 # | 3087.54 ± 119.97 # |
| SH | 3990.14 ± 189.64 | 3968.20 ± 241.74 | 3292.61 ± 201.85 | 4076.74 ± 153.81 * |
| LPO | 11.53 ± 0.47 | 11.52 ± 1.01 | 13.99 ± 0.40 # | 13.71 ± 0.30 # |
| PCO | 4791.26 ± 428.16 | 3469.29 ± 202.72 * | 3468.53 ± 74.29 # | 3672.75 ± 133.57 |
| JEJUNUM | ||||
|---|---|---|---|---|
| Male | Female | |||
| 4 Months Old | 24 Months Old | 4 Months Old | 24 Months Old | |
| SOD | 970.91 ± 71.62 | 998.08 ± 70.66 | 1055.48 ± 88.14 | 1139.35 ± 79.6 |
| CAT | 266.87 ± 40.36 | 270.18 ± 55.99 | 821.97 ± 87.25 # | 525.69 ± 52.75 *# |
| GSH-Px | 3077.86 ± 277.89 | 2305.47 ± 165.04 * | 1895.50 ± 147.60 # | 1823.15 ± 372.38 # |
| GR | 5149.06 ± 445.14 | 3040.19 ± 409.55 * | 5810.93 ± 377.83 | 4987.14 ± 332.52 *# |
| GST | 17,394.64 ± 1624.05 | 11,041.67 ± 736.54 * | 14,172.50 ± 1531.93 # | 12,312.50 ± 934.77 * |
| GSH | 543.94 ± 70.50 | 600.45 ± 33.48 | 804.87 ± 12.90 # | 936.13 ± 76.11 # |
| SH | 1194.24 ± 90.95 | 1118.26 ± 119.23 | 966.66 ± 49.49 # | 818.52 ± 13.49 *# |
| LPO | 6.84 ± 1.11 | 8.68 ± 1.22 | 7.52 ± 0.37 | 7.79 ± 0.07 # |
| PCO | 1462.00 ± 84.22 | 1172.97 ± 79.44 * | 1313.07 ± 54.53 | 1278.91 ± 55.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šošić-Jurjević, B.; Lütjohann, D.; Trifunović, S.; Pavlović, S.; Borković Mitić, S.; Jovanović, L.; Ristić, N.; Marina, L.; Ajdžanović, V.; Filipović, B. Differences in Cholesterol Metabolism, Hepato-Intestinal Aging, and Hepatic Endocrine Milieu in Rats as Affected by the Sex and Age. Int. J. Mol. Sci. 2023, 24, 12624. https://doi.org/10.3390/ijms241612624
Šošić-Jurjević B, Lütjohann D, Trifunović S, Pavlović S, Borković Mitić S, Jovanović L, Ristić N, Marina L, Ajdžanović V, Filipović B. Differences in Cholesterol Metabolism, Hepato-Intestinal Aging, and Hepatic Endocrine Milieu in Rats as Affected by the Sex and Age. International Journal of Molecular Sciences. 2023; 24(16):12624. https://doi.org/10.3390/ijms241612624
Chicago/Turabian StyleŠošić-Jurjević, Branka, Dieter Lütjohann, Svetlana Trifunović, Slađan Pavlović, Slavica Borković Mitić, Ljubiša Jovanović, Nataša Ristić, Ljiljana Marina, Vladimir Ajdžanović, and Branko Filipović. 2023. "Differences in Cholesterol Metabolism, Hepato-Intestinal Aging, and Hepatic Endocrine Milieu in Rats as Affected by the Sex and Age" International Journal of Molecular Sciences 24, no. 16: 12624. https://doi.org/10.3390/ijms241612624
APA StyleŠošić-Jurjević, B., Lütjohann, D., Trifunović, S., Pavlović, S., Borković Mitić, S., Jovanović, L., Ristić, N., Marina, L., Ajdžanović, V., & Filipović, B. (2023). Differences in Cholesterol Metabolism, Hepato-Intestinal Aging, and Hepatic Endocrine Milieu in Rats as Affected by the Sex and Age. International Journal of Molecular Sciences, 24(16), 12624. https://doi.org/10.3390/ijms241612624







