Abstract
Cancer is a process involving cell mutation, increased proliferation, invasion, and metastasis. Over the years, this condition has represented one of the most concerning health problems worldwide due to its significant morbidity and mortality. At present, the incidence of cancer continues to grow exponentially. Thus, it is imperative to open new avenues in cancer research to understand the molecular changes driving DNA transformation, cell-to-cell interaction derangements, and immune system surveillance decay. In this regard, evidence supports the relationship between chronic inflammation and cancer. In light of this, a group of bioactive lipids derived from polyunsaturated fatty acids (PUFAs) may have a position as novel anti-inflammatory molecules known as the specialized pro-resolving mediators (SPMs), a group of pro-resolutive inflammation agents that could improve the anti-tumor immunity. These molecules have the potential role of chemopreventive and therapeutic agents for various cancer types, and their effects have been documented in the scientific literature. Thus, this review objective centers around understanding the effect of SPMs on carcinogenesis and their potential therapeutic effect.
1. Introduction
Cancer is one of the most devastating public health problems worldwide due to its significant morbidity and mortality [1]. Although the risk of developing cancer has increased, the mortality rates in a significant fraction of cancer types have decreased considerably in the last two decades, partly by introducing new and more effective screening techniques, leading to a higher tumor detection rates at early stages [2,3]. The other battlefield to control this epidemic is to develop more effective, specific, and less toxic antineoplastic agents. This movement has been a successful strategy that has paid off over the past three decades, which have seen the emergence of several new agents termed targeted antineoplastics. Monoclonal antibodies [4], tyrosine kinase inhibitors [5], mTOR inhibitors [6], retinoids [7], immunomodulatory agents (IMiDs) [8], enzymes and CRISP-R technology [9], BRAF kinase inhibitors [10], BCG vaccine [11], and phosphodiesterase-3 inhibitors [12], among others, are examples of drugs that have changed the paradigm of traditional cancer management.
Cancer is the second leading cause of death globally, and despite the aforementioned technological innovations, the cancer burden had risen to 18.1 million new cases and 9.6 million deaths in 2018 [13]. Thus, according to the World Health Organization’s International Agency for Research on Cancer Global Cancer Observatory (GLOBOCAN) forecast, 27.5 million new cancer cases worldwide are expected in 2040 [3,14]. In this context, demographic factors, such as population growth, aging, social behaviors, and economic development, will drive these projections [15]. However, the Prospective Urban Rural Epidemiology (PURE) study conducted on individuals 35–70 years of age from 21 countries worldwide has shifted our traditional view of cancer epidemiology, showing that deaths from cancer are higher when compared with cardiovascular disease (CVD) in high-income countries [16,17]. This study clearly shows a lower incidence of CVD deaths than cancer deaths when a country’s gross income stratum is high. Overall, a combination of screening, prevention, and successful treatment has resulted in a considerable increase in cancer survivors in the last five decades.
It is well known that cancer occurs due to multifactorial causes, with a substantial genetic component; however, nearly 25% of the origins of a variety of tumors are caused by chronic tissue inflammation [18,19]. In this vein, recent research has revealed that certain social, environmental, and lifestyle factors can promote chronic systemic inflammation that, in turn, can lead to several diseases, such as CVD, diabetes mellitus, chronic kidney disease, non-alcoholic fatty liver disease, autoimmune and neurodegenerative disorders, and cancer [20,21]. In this context, inflammation was first connected with cancer in 1828 by Jean Marjolin and later in 1833 by Caesar Hawkins, who described skin cancer lesions near burn wounds years after the heat damage. In 1863, Virchow was the first to hypothesize that cancer genesis relies on chronic inflammation via both irritation and injury-enhancing cell proliferation [22,23].
Today, the causal relationship of inflammation and innate immunity to cancer is more widely accepted; however, some of the molecular and cellular mechanisms mediating this relationship remain unresolved, but recent data have expanded our knowledge about the participation of inflammation as a critical component of tumor behavior. In this regard, inflammatory cells within the tumor niche are necessary for proliferation, survival, and migration enhancement. In addition, tumor cells can express some signaling molecules from the innate immune system, such as chemokines, selectins, and their receptors for invasion, migration, and metastasis, amplifying the process mentioned above. These processes open new avenues by new anti-inflammatory approaches with direct anti-cancer effects or even with the ability to interfere in normal-to-neoplastic cell transformation within a highly inflammated niche. Therefore, it is still fundamental to study the close link among these entities, where the transformation of damaged DNA, the changes in lipid and protein metabolism, and the production of free radicals constitute critical processes of carcinogenesis [24,25,26].
Therefore, cancer research has continued to look relentlessly for alternatives that can improve these therapies and offer fewer toxic events [27,28]. This fact is precisely one of the focal points of conventional cancer treatment, which involves considerable adverse effects despite having favorable results. Hence, with the emergence of molecular lipidomics, four novel lipid families have been recently discovered: Resolvins (Rvs), Maresins (MaRs), Protectins (PDs), and Lipoxins (LXs), belonging to a large group of molecules known as The Specialized Pro-resolving Lipid Mediators (SPMs) [29,30,31]. These compounds have been well-characterized since their identification as potent modulators of the immune response and for their effects on inflammation resolution. Furthermore, they have a potential effect on anti-tumor immunity [32,33]. For this reason, the objective of this review centers around the understanding of SPMs effects on carcinogenesis and their potential therapeutic effect.
2. Specialized Pro-Resolving Lipid Mediators: An Overview
2.1. Origin, Biosynthesis, and Classification
For decades, immunopathology has faced the challenge of elucidating the mechanisms of associating chronic disorders with inflammation. Within the pleiad of molecular pathways, mediators, receptors, and metabolites, the resolution of inflammation has been a well-recognized process where complex signaling downregulates the immune cascade once the noxious stimulus is eliminated by both innate and adaptative immune responses and, as a result, the recovering of tissue homeostasis occurs [34]. Upon this premise, Serhan et al. [35] were the first to identify lipid metabolism products involved in this process by describing a family of bioactive compounds later coined SPMs (Figure 1), a group of molecules derived from omega-6 polyunsaturated fatty acids (ω-6 PUFAs) metabolism, such as arachidonic acid (AA; 20:4n − 6), and omega-3 polyunsaturated fatty acids (ω-3 PUFAs), such as eicosapentaenoic acid (EPA; 20:5n − 3) and docosahexaenoic acid (DHA; 22:6n − 3) via COX/LOX pathway [36].
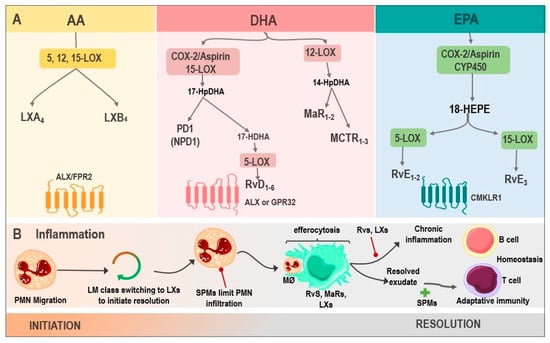
Figure 1.
Biosynthesis of SPMs and their actions in inflammation. (A) LX are generated from AA via 5, 12, 15-LOX, resulting in LXA4 and LXB4, whose receptor is ALX/FPR2. DHA, via COX-2/Aspirin as well as via 15-LOX, produces 17-HpDHA, which can be metabolized into PD1 or NPD1 and is synthesized in the nervous system or into 17-HDHA, generating RvD1-6 via 5-LOX, with activity on ALX or GPR32. Another DHA pathway is through 12-LOX, where MaR1-2 and MCTRs are produced. Finally, the biosynthesis of RvE1-3 derives from EPA, by the enzymes COX-2/Aspirin or CYP450, being their receptor CMKLR1. (B). SPMs in inflammation: the inflammatory microenvironment starts with PMN migration. Afterward, the change of pro-inflammatory LMs into pro-resolving ones occurs with the initial synthesis of LXs. PMN infiltration increases, and SPMs act at this point, reducing this influx. Moreover, the efferocytosis by MØ is stimulated and improved by Rvs, MaRs, and LXs. Adaptive immunity, stimulated by SPMs, participates during the final phase of resolution. However, whenever there is an exaggerated and chronic inflammatory response, it leads to chronic inflammation, inhibited by Rvs, and LXs. Abbreviations: SPMs: specialized pro-resolving mediator; AA: Arachidonic acid; LXs: Lipoxins; LOX: lipoxygenase; LXA4: lipoxin A4; LXB4: lipoxin B4; ALX: G protein-coupled lipoxin A4 receptor; formyl peptide receptor; DHA: docosahexaenoic acid; COX-2/Aspirin: Aspirin acetylates cyclooxygenase-2; 17-HpDHA: 17-hydroperoxyDHA; PD1: Protectin 1; NPD1: neuroprotectin 1; 17-HDHA: 17-hydroxy-DHA; Rvs: D1-6-series resolvins; GPR32: G protein-coupled receptor; MaRs1-2: Maresins 1-2; MCTR: maresin conjugates in tissue regeneration; RvE1-3: E1-3-series resolvins; EPA: Eicosapentaenoic acid; CYP450: cytochrome P450; CMKLR1: chemokine-like receptor 1; PMN: polymorphonuclear neutrophil; LM: lipid mediators; and MØ: macrophages.
SPMs belongs to G protein-coupled receptors ligands [37], and their critical role in regulating inflammation resolution has been widely documented, establishing that excessive or uncontrolled inflammation is tightly linked to a disbalance in their synthesis. Therefore, it is vital to understand every SPMs family’s role in the framework of acute and chronic inflammation [38].
2.2. Resolvins
Rvs are unique molecules synthesized from DHA and EPA by polymorphonuclear leukocytes (PMN) and macrophages during the resolution of inflammation to counter-regulate pro-inflammatory activity and promote efferocytosis [39]. In this regard, five resolvins families have been identified to date: the resolvin Ds (RvDs) 22-carbon DHA metabolites; the resolvin Es (RvEs) 20-carbon EPA metabolites; the resolvin Dn-6DPA (RvDsn-6DPA), a group of metabolites derived from the Osbond acid; the resolvin Dn-3DPA (RvDn-3DPA) that come from clupanodonic acid metabolism; and the resolvin Ts (RvTs), a clupanodonic acid metabolite [40].
RvDs are poly-hydroxyl metabolites of DHA, and to date, six RvDs (RvsD1-6), which vary in the number, position, and chirality of their hydroxyl residues and the position and cis-trans isomerism of their six double bonds, have been described [40]. Thus far, these variants have been identified in PMN and macrophages inflammatory exudates with a variety of allylic–epoxide intermediaries containing RvD1-2 at the start of the resolution, and later, RvD3-4 [41]. Furthermore, to carry out their functions, RvDs are ligands of G protein-coupled receptors (GPCR), such as ALX/FPR2, GPR18, GPR32, TRPA1, and the TRPV1 receptor [39].
On the other hand, EPA-derived E-series Rvs (RvE) includes four primary bioactive mediators (RvE1, RvE2, RvE3, and18-HEPE), synthesized by 5-LOX and 15-LOX in PMN and macrophages. These molecules exert their functions by Chem23, BLT1, and TRPV1 receptor-binding [42]. It has been reported that RvE1 regulates leukocyte adhesion molecules expression, ADP-dependent platelet activation, and PMN apoptosis stimulation [43]. In addition, RvE1, along with RvE2, increases IL-10 synthesis and phagocytosis [44]. Moreover, it is relevant to highlight the properties of RvE3 in the decrease in PMN and 18-HEPE production. These properties have been associated with a cardioprotective function in several studies since RvE1 protected against reperfusion injury in this open-chest rat model of ischemia–reperfusion [45] and protects against doxorubicin-induced cardiotoxicity by inhibiting oxidative stress, autophagy, and apoptosis by targeting AKT/mTOR signaling [46].
13-series Rvs (RvT) are also derived from ω-3 PUFAs, from docosapentaenoic acid (DPA), and synthesized by COX-2 during the resolution of acute inflammation. This family includes four mediators, RvT1, RvT2, RvT3, and RvT4 [24]. In this vein, Dalli et al. [47] described the anti-inflammatory role of RvsT1-4 in mice endothelial cells and neutrophil co-cultures during E. coli infection, demonstrating a protective response through the regulation of inflammation. Other important resolvins are the RvDsn-6DPA, a metabolite of the osbond acids, and resolvin Dn-3DPA (RvDn-3DPA), a clupanodonic acid metabolite. On the other hand, AT-RvDs are synthesized by non-native COX-2 (drug-modified cyclooxygenase 2) to form 17 (R)-hydroxyl residue named aspirin-triggered RvDs (AT-RvDs).
2.3. Lipoxins
LXs, synthesized in platelets and leukocytes from AA, are another group of bioactive lipids capable of carrying out a wide variety of functions. These include regulating the synthesis of pro-inflammatory cytokines, inhibiting angiogenesis, and reprogramming M2 macrophages by binding to ALX/FPR2 and GPR32 receptors [48]. Their variants, LXA4 derived from AA and aspirin-triggered LXA4 (ATL), are generated by 15-LOX. These compounds have protective anti-inflammatory actions on various physiologic and pathophysiologic processes as endogenous lipids acting in the resolution phase upon an inflammatory response [49], playing an essential role in the tumor microenvironment (TME) and cancer pathogenesis in several neoplasms, such as pancreatic, liver and colon cancer, melanoma, leukemia, and Kaposi’s sarcoma [50]. The healing role of these molecules has been widely demonstrated in preclinical studies. For instance, Madi et al. [51] evaluated LXA4 administration to mice with gastric ulcers, resulting in a significant histopathologic and immunohistochemical improvement in the gastric mucosa.
2.4. Maresins
MaRs, also called macrophage mediators in resolving inflammation, are an SPMs family synthesized from DHA by the 12-LOX enzyme. These have functions related to phagocytes, such as the inhibition of neutrophil recruitment and macrophage efferocytosis stimulation [52]. Moreover, they can negatively regulate the synthesis of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, to induce the resolution of inflammation and tissue regeneration [53]. The members of this family are maresin 1 (MaR1), maresin 2 (MaR2), and maresin conjugates in tissue regeneration (MCTR1-3). These compounds are synthesized by macrophages via lipooxygenation at the carbon-14 position with molecular oxygen insertion. Finally, MaRs action is achieved through BLT1 and TRPV1 receptors binding [54].
2.5. Protectins
The last group of SPMs are PDs, which, just like MaRs and RvD, are biosynthesized from DHA by the action of 15-LOX during the resolution of inflammation [55]. Protectin D1 (PD1) is the first and best-studied member of this family. This compound is synthesized by various cells, including leukocytes, such as PMN, eosinophils, and macrophages [56]. PD1 is also known as neuroprotectin D1 (NPD1) when synthesized in neural systems, and it has a protective action in the brain, retina, and in pain modulation [57]. Like other SPM family members, NPD1 exerts potent anti-inflammatory and anti-apoptotic/neuroprotective biological activities [58,59]. NPD1 actions in the central nervous system (CNS) are mediated by its interaction with the parkin-associated endothelin-like receptor (Pael-R), also known as GPR37, widely expressed in glial cells, such as astrocytes and oligodendrocytes [59].
Other neuroprotective structurally related agents exhibiting similar activity are PDX (10R, 17S-dihydroxy-4Z, 7Z, 11E, 13Z, 15E, 19Z-DHA); 20-hydroxy-PD1 (10R, 17S, 20-trihydroxy-4Z, 7Z, 11E, 13E, 15Z, 19Z-DHA); and 10-epi-PD1 (10R, 17S-Dihydroxy-4Z, 7Z, 11E, 13E, 15Z, 19Z-DHA) [60,61]. The beneficial effect of these anti-inflammatory mediators was reported by Sheets et al. [62], who demonstrated both a laser-induced choroidal neuro-vascularisation attenuation and microglia cells elongating in mouse eyes treated with NPD1. In addition, evidence in aged-mice models indicates that NPD1 is able to diminish post-operative delirium (POD). Post-surgical administration of NPD1 decreased IL-6 and TNF-α expression systemically as well as in the hippocampus and pre-frontal cortex; moreover, this SPM maintains the integrity of the blood–brain barrier (BBB) and induces macrophages polarization into an M2 phenotype, limiting neuroinflammation and cognitive decay [63].
3. From Inflammation to Cancer: A Key Topic in Carcinogenesis
Inflammation is a response to an acute process that possesses a therapeutic nature since it is a defensive mechanism against both external (e.g., bacteria and viruses) and internal agents (e.g., damaged cells and toxic compounds) [64,65]. Nevertheless, when it extends in time, it can become detrimental. Chronic inflammation is associated with various pathological entities, such as diabetes [66] and rheumatoid arthritis [67]. In fact, the existence of previous inflammatory diseases has shown an increased incidence risk for certain cancers. For instance, it has been reported that the incidence of colorectal cancer (CRC) is 4–10 times greater in individuals with ulcerative colitis (UC) in comparison with those without the inflammatory bowel disease [68]. Similarly, statistically significant evidence shows an association between prostate and ovarian cancer incidence, with the presence of previous inflammatory processes, such as prostatitis [69] and pelvic inflammatory disease [70], respectively.
Hence, chronic inflammation has proven to be a key phenomenon in cancer development (Figure 2) since it is closely related to the processes of cellular metaplasia and tumor promotion, survival, proliferation, invasion, and angiogenesis [71]. Chronic inflammation is characterized by inflammatory cell infiltration, such as macrophages, lymphocytes, and plasmatic cells, which together release cytokines (TNF-α, IL-6, TGF-β, and IL-10), growth factors, and enzymes, contributing to tissue damage and repair [72]. However, these mediators’ chronic actions have been associated with tumor initiation and progression [73]. Thus, to connect inflammation with cancer, typically two pathways are described; an intrinsic pathway depends essentially on oncogene activation and tumor suppressor gene silencing (tumor-promoting role). The other extreme is the extrinsic pathway, which is the inflammation resulting from infections or other inflammatory processes that precede cancer development (tumor-initiating role) [74].
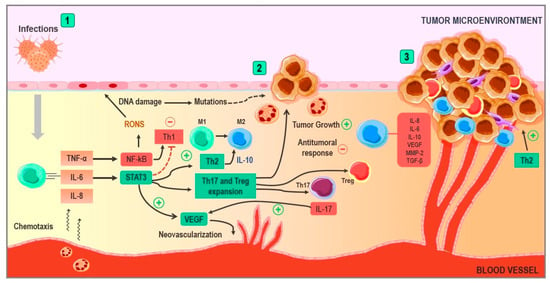
Figure 2.
Implications of chronic inflammation in cancer development. (1) In situations such as persistent infections, an immune response is triggered, and this leads to the release of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-8, inducing the chemotaxis of immune cells and the production of free radicals. When the inflammatory process becomes chronic, the reactive oxygen and nitrogen species are capable of damaging DNA. TNF-α activates the NF-KB pathway, generating free radicals and a Th1 response with certain anti-tumor properties. However, the activation of the STAT-3 pathway by IL-6 counteracts this effect, generating a Th2 response and inducing the production of IL-10. (2) The STAT-3 pathway stimulates neovascularization via the production of VEGF, as well as through the stimulation of the expansion of Th17 and Treg lymphocytes. These lymphocytes contribute to tumor growth and neovascularization via IL-17 and inhibit the mechanisms of anti-tumoral immunity. The Th2 response generates IL-10, pushing macrophages towards the M2 phenotype known for their pro-tumoral properties, such as the release of substances that favor tumor growth and survival. (3) At this point, tumor growth is stimulated by pro-inflammatory cytokines in the environment. Angiogenesis continues at the expense of VEGF, and cytokines, such as IL-10 and TGF-B, contribute to the processes of immune evasion, generating a state of tumor-induced immunosuppression. Abbreviations: TNF-α: tumor necrosis factor Alpha; IL-6: Interleukin 6; IL-8: Interleukin 8; IL-10: Interleukin 10; IL-17: Interleukin 17; NF-κB: Nuclear factor kappa B; STAT3: Signal transducer and activator of transcription 3; Treg: Regulatory T Cell; VEGF: Vascular endothelial growth factor; TGF-β: Transforming growth factor beta; and MMP-2: matrix metalloproteinase-2.
On the other hand, an inflammatory environment is prone to produce large amounts of reactive oxygen species (ROS) and reactive nitrogen species (RNS), a group of molecules collectively called RONS, produced by the inducible nitric oxide synthase (iNOS) and NADHP oxidase, resulting in phagocytic cell activation. Moreover, pro-inflammatory cytokines induce RONS production in non-phagocytic cells in the inflamed niche [75,76,77]. Multiple mechanisms have been proposed to explain the RONS and cancer association. The best-studied molecular damage mechanism by ROS and RONS is related to the high reactivity of these molecules against lipids, proteins, and DNA. Thus, when guanine reacts with ROS, 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxoDG) originates, a metabolite prone to cause G:C transversion (G➔T transversion) to T:A [78,79]. On the other hand, when 8-oxoDG reacts with ONOO−, 8-nitrodeoxyguanosine generates, causing a similar transversion [80,81].
Other critical pathways are STAT3 and NF-κB activation triggered by IL-6 and TNF-α, respectively, indispensable for perpetuating inflammation. NF-κB leads to pro-inflammatory cytokines, chemokines, RONS, and adhesion molecules induction [82] and the activation of cellular proliferation and differentiation pathways, which drives to pro-tumorigenic activity increase [83]. Additionally, it has been associated with therapy resistance, based on reports that persistent activation of the NF-κB pathway is associated with a worse prognosis [84]. Interestingly and controversially, it also holds anti-tumoral properties that lay on the expression of Th1 cytokines, such as IL-12 and IFN-γ [85,86,87]. However, STAT3 overshadowed these anti-tumoral effects, limiting this response by inducing a Th2 response with the release of IL-10, pushing macrophages into the M2 phenotype [88]. Additionally, it contributes to the expansion and development of Treg and Th17 cells, which are linked with tumor growth due to their ability to dissipate anti-tumoral immunity, enhancing both the tumor’s survival and its metastasis capacity [89,90]. In addition, STAT3 pathway activation is tightly related to tumor neovascularization via vascular endothelial growth factor (VEGF) upregulation [91]. Some studies suggest that IL-17 has a vital role in this pathway activation, potentiating the angiogenic effect [92]. Therefore, STAT3 is not only a pro-tumoral agent but also intervenes as an anti-tumoral response inhibitor [93].
During the first steps in neoplasia development, nutrient scarcity forces tumor cells to balance between apoptosis and cellular growth. This pre-angiogenic or “avascular” stage restricts growth and dissemination, reaffirming the angiogenesis importance [94] since when angiogenesis occurs, rapid tumor growth occurs, leading to poorly irrigated regions with a consequent hypoxic and acidic milieu [95], a fact having a profound impact by the VEGF release stimulated by a lower pH and, thus, enhancing angiogenesis [96]. On the other hand, low oxygen concentration stimulates hypoxia-inducible factor production (HIF), another group of angiogenic peptides involved in metabolic reprogramming, invasion, metastasis, and treatment resistance [97]. In summary, aside from stimulating tumor growth and preservation, angiogenesis is also an indispensable factor in tumor invasion and dissemination due to the wide vascular surface available for malignant cells to penetrate without mentioning the increased vascular permeability [98].
From the immunological perspective, immune evasion is a hallmark of cancer [99]. This characteristic is the result of multiple dysregulation phenomena induced by the previously mentioned tumor microenvironment. This situation is known as “tumor-induced immunosuppression”, characterized by an environment where pro-tumor immune mediators are hierarchized over anti-tumor ones: a Th2 inflammatory response dominance, M2 macrophages, Treg cells, a low capability for antigen presentation and cytotoxicity, and cytokines, such as TGF-β, IL-6, IL-8, IL-10, and VEGF [100].
Furthermore, mentioning AA derivatives is a sine qua non condition when discussing inflammation, since many studies suggest that patients who regularly take non-steroidal anti-inflammatory drugs (NSAIDs) have a lower risk of developing a wide range of neoplastic diseases and a lower risk of metastasis [101,102,103]. This affirmation makes more sense after knowing that PGE2 is the most abundant prostanoid found in several malignant lesions and that its pro-inflammatory nature contributes to tumor growth [104]. Therefore, interfering with this chain of events is considered one of the new lines of therapy against cancer [105,106,107].
Finally, it is worth mentioning that not all malignancies possess the same immunological behavior. Recent advances have suggested new methods to classify tumors according to the distribution and to quantity of lymphocytes CD3 and CD8, as well as the expression levels of B7-H1/PD-L1 [108]. Consequently, tumors can be divided into “hot”, “intermediate” (including immunosuppressed and isolated), and “cold”. On the one hand, “hot tumors” are highly infiltrated tumors with abundant anti-tumoral cells and positive responses to conventional therapies; on the other hand, “cold tumors” not only do not lack anti-tumoral cells, but are also rich in immunoevasive molecules and, therefore, associated with a worse prognosis. Additionally, other immune coordination profiles have been described for those patterns that do not correspond to the previously explained “hot” vs. “cold” classification. In this regard, the “excluded” and “immunosuppressed” phenotypes were accepted: the first one, to describe tumors with T-cells on the edge of tumoral sites or “invasive margins”—unable to infiltrate the parenchyma; the second corresponds to malignancies with low levels of immune infiltration without any physical barrier, suggesting the existence of an immunosuppressive environment within the tumor that hampers anti-tumoral functions [109,110]. This novel classification, or immunoscore, that allows the characterization of tumors beyond their expansion has been highly validated around the world not only for its greater prognostic value but for the new possibilities that arise with regard to anti-tumoral therapies based on immunology, such as T-CAR cells, immune checkpoint inhibitors, or oncolytic viruses. These are examples of techniques that could be enhanced and personalized according not only to the TNM classification but also to the immunoscore, type of cancer, and many other variables, leading to a new era in cancer therapeutics [111].
4. Anti-Tumoral Mechanisms of SPMs: An Approach to Inflammatory Responses
With better comprehension of the pathways that connect inflammation with cancer, the robust role of SPMs in the resolution of inflammation supposes a modulating mechanism in the transition to cancer. Therefore, the focus of their participation in this process has been widened in recent times, being acknowledged now that these bioactive lipids, via multimodal mechanisms, are capable of influencing diverse aspects of neoplastic development. These range from direct actions on malignant cells to the modification of functions and behaviors of multiple components in the TME (Figure 3), which also influence pro-tumor phenomena that are essential for the evolution of cancer. Hence, it is necessary to approach these potential mechanisms individually [34,37,112].
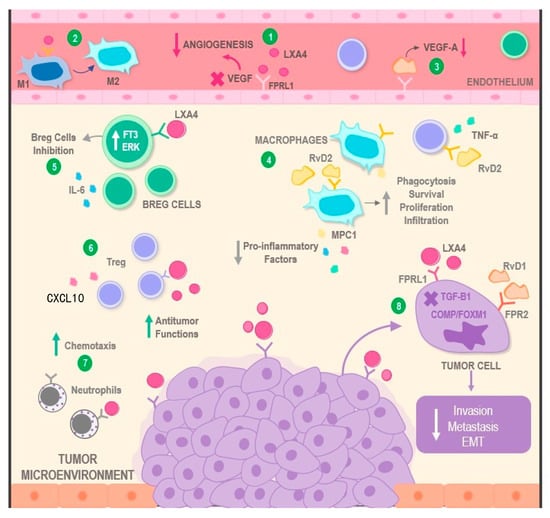
Figure 3.
Mechanisms of action involving SPMs in the modulation of the tumor microenvironment. SPMs display multimodal mechanisms of action over malignant neoplasms. On one hand, the binding of molecules, such as LXA4 and RvD1 to the FPRL1 receptor, can result in a decrease in angiogenic phenomena via VEGF inhibition (1 and 3). On the other hand, the interaction between LXA4 and macrophages may restore their anti-tumoral actions via their transition from the M1 towards the M2 phenotype (2). Similarly, RvD2 increases characteristics such as phagocytosis, infiltration, proliferation, and survival of M2, as well as simultaneous reduction of pro-inflammatory cytokines, such as MPC1, IL-6, TNF, and CXCL10, in several types of immune cells located in the TME (4). Additionally, LXA4 can exert inhibitory effects over pro-tumoral cells, such as Breg lymphocytes (5), and stimulant effects on anti-tumoral immune cells, such as Tregs and neutrophils, potentiating their antineoplastic activity (6) or increasing the levels of chemotaxis towards the tumor (7). Finally, both LXA4 and RvD1 directly lead to the decrease of essential pro-tumoral processes, such as invasion, metastasis, and EMT, by interacting with receptors located on the surface of cancer cells (8). Abbreviations: RvD2: Resolvin D2; RvD1: Resolvin D1; SPMs: Specialized Pro-Resolving Mediators; TNF-α: Tumor necrosis factor alpha; IL-6: Interleukin 6; MPC1: Mitochondrial Pyruvate Carrier 1; CXCL10: C-X-C Motif Chemokine 10; LXA4: Lipoxin 4; VEGF: Vascular Endothelial Growth Factor; FT3: Transcription Factor 3; ERK: Extracellular Signal-regulated Kinase; Breg cells: Regulatory B cells; FPRL1: Formyl Peptide Receptor-like 1; FPR2: Formyl Peptide Receptor 2; EMT: Epithelial Mesenchymal Transition; TGF-B1: Transforming Growth Factor Beta 1; COMP: Cartilage Oligomeric Matrix Protein; and FOXM1: Forkhead Box Protein M1.
4.1. SPMs and the Resolution of Inflammation
Through the collected evidence, it has been established that, for a proper resolution of inflammation, it is indispensable to inhibit the migration and activation of neutrophils toward the site of inflammation. SPMs have this mechanism of action in common, enhancing this phase [113]. At the same time, neutrophil degranulation contributes to the spread of the inflammatory response, which is why LXs exert their effect by inhibiting them [114]. It must also be taken into account that the resolution of inflammation greatly depends on the efferocytosis of apoptotic neutrophils and dead cells during the initial phase of inflammation. This is a prerequisite for effective phagocytosis [115]. Here is where Rvs and LXs come into action by promoting this mechanism effectively and favoring chemotaxis and the adhesion of non-inflammatory monocytes. They stimulate the phenotype switch of pro-inflammatory M1 macrophages into the anti-inflammatory M2 phenotype. This effect is further potentiated by MaRs that regenerate cells and tissues, particularly in certain organs, such as the heart [116,117]. Moreover, beyond its regenerative actions on various tissues, it has been reported that RvD1 exerts powerful suppressing effects over tumoral proliferation through the modulation of classic monocytes transmigration via increased MCP-1 expression in a human papillomavirus (HPV)-positive cancer cell model [118].
Moreover, it is important to add that all SPMs protect from the oxidative stress generated by pathogens and immune cells, not only by reducing ROS and RONS but also by stimulating the expression of antioxidant defenses, such as Superoxide Dismutase (SOD), Hemo-1 Oxidase (HO-1), and Nrf2, which protect against inflammation, liver toxicity, and even cancer [119,120]. Additionally, SPMs can intervene in adaptive immunity, with the recruitment of T CD4+ and CD8+ cells [121] capable of reducing the synthesis of pro-inflammatory cytokines and preventing the differentiation of T CD4+ cells into the TH1 and TH17 subsets, which are related to many diseases, via the regulation of transcription factors, without being immunosuppressive [122]. This fact was confirmed by Chiurchiu et al. [123], who found that DHA-deficient mice had high levels of these cells, and as a result, the supplementation with RvD1, RvD2, and MaR1 significantly reduced the synthesis of pro-inflammatory cytokines due to the stimulation of T cells, thus regulating chronic inflammation. While RvD1 and RvE1 influence the humoral immune response by increasing the synthesis of IgM and IgG on active B cells, they reduce the production of IgE [124]. Studies show that RvD1 expedites allergen clearance in mice models with allergic respiratory diseases, while RvE1 improves the lymphatic clearance of phagocytes and blocks platelet aggregation and activation [57,124].
It is important to point out that a considerable part of the inflammatory response is a result of the activation of transcription pathways, which allow its perpetuation. This is the reason why LXs can regulate several levels of transcription factors, such as NF-κB, in a joint action with MaRs. In addition, they control the expression of several genes linked to inflammation and inhibit the Activator protein 1 (AP-1), nerve-growth-factor-inducible protein A (NGFI-A), and the peroxisome-proliferator-activated receptor γ (PPARs) [112,125]. On the other hand, the actions of NPD1 are involved mainly in neuroinflammation. This is why Bazan et al. [62,126] pointed out that they are modulators of stress pathways that relate to cell death and the increase of cell survival.
Particular approaches have described beneficial SPM effects over tumor-promoting inflammation. These hypotheses have been sustained by the existence of tumoral progression-modulating mechanisms that exert an inflammation-promoting effect over leucocytes via apoptotic cellular debris produced as a consequence of chemotherapy [127,128]. In this context, Sulciner et al. [27] presented the inhibiting effects of RvD1, RvD2, and RvE2 on tumoral progression stimulated by cellular debris. Additionally, they reduced inflammatory responses as a result of the suppression of chemokynes and cytokines, such as TNF-α, IL-6, IL-8, CCL4, and CCL5.
4.2. SPMs and the Resolution of Tumoral Angiogenesis
It has been reported that SPMs exert an anti-tumoral effect via the inhibition of uncontrolled angiogenesis. This fact is supported by several experimental models; however, the mechanisms by which these mediators offer their anti-angiogenic activity have not been completely elucidated [129]. LXA4, by binding to its receptor (ALXR), can reduce the phosphorylation of the VEGF receptor (VEGFR) and reduce the synthesis of angiogenic mediators, such as VEGF-C. It can also attenuate the production of inflammatory mediators, such as PGE2, LTB4, IL-6, and IL-8, in malignant cells extracted from a human Kaposi sarcoma tumor [130]. In another underlying mechanism, LXA4 is also capable of diminishing the angiogenic process by lowering the synthesis of HIF-1α and, as a result, significatively reducing tumor growth [131].
For their part, RvD1 and LXB4 also generate a decrease in the expression of mRNA of pro-angiogenic factors and a reduction in VEGF-A release via a mechanism that involves STAT-3 signaling in gastric cancer cell lines. It has also been found that this antiangiogenic effect may be mediated by the activity of the formyl peptide receptor 1 (FPR1), which is a pattern recognition receptor (PRR), after seeing that the pharmacological suppression of this receptor increased the tumoral pro-angiogenic activity and diminished the activity of SPMs, their receptors, and the enzymatic components associated with their production [132]. In addition, an anti-angiogenic effect has been described for RvE1 after finding that, just like RvD1 and the ATL analog, it decreased corneal neovascularization. RvE1 exerted this effect by binding to ChemR21, which is widely expressed in this tissue. However, there is still not enough evidence of the potential effect of RvE1 in tumoral angiogenesis [133,134].
4.3. SPMs and Tumoral Immunomodulation
Even though the immune system is considered the natural defense of the body, tumoral cells have effective abilities not only at the time of evading immune responses but also in manipulating cell behavior and redirecting it to favor tumor progression. This way, they convert the immune elements inside the TME into allies responsible for maintaining chronic inflammation and ensuring neoplastic development [135,136,137]. In this context, the role of SPMs in the re-establishment of physiological anti-tumoral functions in immune cells that reside in the TME has been recently discovered to stop the evolution of cancer [32].
Firstly, studies have shown the effects these molecules have on leukocytes, such as neutrophils and PMN, by inhibiting key actions for the preservation of chronic inflammation, such as chemotaxis, migration, epithelial interactions, and the release of pro-inflammatory cytokines from these cells. In addition, they inhibit the synthesis of toxic substances, such as free radicals, which are capable of damaging nucleic acids, causing genomic instability, and, with this, potentially oncogenic mutations. They also promote their anti-tumoral activities by stimulating phagocytosis in PMN and cytotoxic actions in neutrophils [138,139].
Furthermore, the immunomodulatory effects of SPMs extend to other immune cells and, in particular, to macrophages and their precursors. Studies have shown the inhibiting role of the ATL-1 analog in the proliferation of monocytes to reduce the number of potential tumor-associated macrophages (TAMs) and, in this way, decrease the tumoral progression and increase the survival rates in patients [140]. However, similar trials performed in vivo using LXA4 show opposite results and report increases in monocyte levels, their chemotaxis, subsequent differentiation to M2, and preservation of the anti-tumoral phenotype, preventing the “switch” toward tumor-associated macrophages or M1 [141,142,143]. Even beyond maintaining physiologic activities in cells, a potentiation in phagocytic actions has been observed. This is induced by the administration of Rvs, as well as the blocking of apoptosis through mechanisms, such as the activation of the PI3K/Akt pathway. This causes the expression of the Bc12 anti-apoptotic protein, the disruption of the caspase pathway due to the absence of pro-apoptotic stimuli or signals, and the preservation of mitochondrial integrity to decrease the generation of ROS. Overall, these effects indicate a positive impact of these lipids, both in the reduction of malignant phenotypes as well as in the preservation and optimization of anti-tumoral activities [24,27,144].
Lastly, preclinical studies carried out in mice have highlighted the existing relationship between the effects caused by LXA4 and RvD1 in B, T, and NK cells. On the one hand, RvD1 is responsible for maintaining cytotoxic actions in NK cells according to what was proven in vitro, where pancreatic cancer cells were used. Similarly, researchers have listed the promoting effects of LXA4 in lymphocytic cells with altered phenotypes, especially on Treg lymphocytes in the early stages of cancer [145,146]. Contrary to the effect exerted on Tregs, there has been confirmation of suppressive effects of LXA4 on regulatory B lymphocytes (Bregs), which synthesize IL-10, due to the role these have on the promotion of tumoral development because of the negative regulation over other immune cells that they carry out. Therefore, the inhibition of Bregs via mechanisms, such as dephosphorylation of STAT3 and ERK factors by LXA4, results in a decrease in tumoral growth [49].
4.4. SPMs and Precancerous Lesions
In the continuous study of carcinogenesis, the changes provided by the inflammatory microenvironment in organs at risk must be mentioned [71]. This substantially contributes to the development of preclinical pathological changes that constitute predisposing factors for the development of an invasive lesion, whose detection is one of the cornerstones in the secondary prevention of neoplasms [147]. In this sense, SPMs offer a protection mechanism to minimize the tissue damage observed in chronic inflammatory disorders and, therefore, decrease the neoplastic transformation [148].
One of the phenomena involved in this event is the epithelial–mesenchymal transition (EMT), described as the differentiation process of epithelial cells towards an undifferentiated or mesenchymal phenotype [149]. It is associated with local tumor invasion, metastasis, premalignancy processes, and fibrosis [150]. In the EMT, there is a loss of the normal cellular structure due to a decrease in the expression of certain factors, such as E-cadherin, and an increase in the expression of vimentin. These have both been postulated as predictors of tumoral behavior and markers for the early detection of some neoplasms [151,152].
Researchers have tried to demonstrate the potential impact of SPMs on this process. In a study by Zong et al. [153], it was reported that LXA4 was capable of reverting EMT after binding to FPRL1. It was used as a treatment in a model with pancreatic cell lines, also evidencing that LXA4 influenced this process via the suppression of TGF-β1 signaling. This resulted in a decrease in local invasion and metastasis. Analogously, this fact has been explored with RvD1, depicting the important role of this mediator in EMT prevention. This was witnessed in co-cultures of hepatocellular carcinoma cells and cancer-associated fibroblasts (CAF), where RvD1 binds to its FPR2 receptor and can repress CAF-mediated EMT in liver cells through the decrease in FOXM1 and COMP expression, subsequently decreasing cellular invasion [154,155].
On the other hand, another precancerous disorder that has been linked with these bioactive lipids is inflammatory bowel disease (IBD), which has been recognized as an important risk factor for the development of CRC and colitis-associated cancer (CAC) [156]. SPMs have a role as preventive agents in this setting, after describing their effect on the resolution of inflammation in the intestinal mucosa in experimental models [157,158]. Several studies have demonstrated that RvE1 and MaR1 have a modulator effect on the inflammatory response in mice models with dextran sulfate sodium (DSS) and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis. This evidenced a decrease in the synthesis of several inflammatory mediators, such as IL-1β, TNF-m, IL-6, and IFN-γ, and in the case of MaR1, a decrease in neutrophil recruitment and ROS production was observed. This lowering effect on colon inflammation mediated by both compounds is dependent on the inhibition of NF-κB signaling [159,160]. NF-κB inhibition is also considered a regulating mechanism of progression to CRC since its increased activity promotes the aberrant production of chemokines and tumorigenic proteins responsible for the inhibition of apoptosis and an increase in cellular survival, as previously described [161].
In another order of ideas, researchers have studied the preventive effect of SPMs from the perspective of their synthesis. They have demonstrated that the suppression of 15-LOX-1, which is an important enzymatic regulator of the production of these lipid mediators, increased the incidence of CAC via a mechanism that involves the IL-6/STAT3 pathway. This established the association between the regulation of the activity of SPMs and the development of CAC [162,163].
In the context of hepatic lesions, RvD1 and RvE1 have a protective effect by relieving the liver injury caused by concanavalin A (ConA) in animal models. Pretreatment with RvD1 and RvE1 in C57BL/6 mice in whom liver damage with ConA was induced had a decrease in the production of TNF-α, IFN-γ, IL-2, IL-1β, and IL-6, as well as an inhibition of T CD4+ and CD8+ lymphocyte infiltration via a mechanism dependent on the inhibition of NF-κB and AP-1. This established the protective role of RvD1 and RvE1 in the progression from hepatitis to liver cancer [164]. The effect has also been attributed to PD1 and MaR1 since these are capable of reducing ConA-induced liver injury and, thus, attenuate the progression of the disease by inhibiting the CX3CL1/CXRCR1 axis and decreasing ROS production, respectively, via a mechanism also dependent on NF-κB [165,166].
Finally, it has been reported that prolonged exposure to ultraviolet rays (UVR) is an important carcinogenic factor by causing disturbances in the antioxidant mechanism of the skin and by triggering morphological changes in it [167]. A recent study exposes the protective effect of MaR1 by promoting the resolution of inflammation and reducing the oxidative stress caused by UVR. The administration of MaR1 reduced cutaneous edema and neutrophil recruitment, inhibited keratinocyte apoptosis, and decreased epidermal thickening and collagen degradation in the skin of hairless mice exposed to UVB radiation [168].
5. Beneficial of Supplementation with ω-3 PUFAs in Cancer: Clinical Evidence
In understanding the expanding role of the products of lipid metabolism over the regulation of the inflammatory process and, as a result, on the development of cancer, the modulation of the profile of activity of these mediators represents a therapeutic alternative to study [169,170]. Therefore, in the last few years, supplementation with foods rich in ω-3 PUFAS, such as fish oil, has gained a lot of interest after demonstrating that these lowered the incidence of cancer in several experimental models [171,172,173]. On the basis of this premise, researchers have documented a wide variety of observational studies to demonstrate the role of EPA and DHA as therapeutic and chemopreventive agents for the development of cancer and tumoral progression (Table 1) [174,175].
To this end, it is necessary to highlight clinical trials that have sought to explore this fact, in the individual context of each cancer. One has been CCR, for which studies have endeavored to explore the role of ω-3 PUFAs as chemopreventive agents or adjuvant therapy to improve its survival [43]. A study published by Cockbain et al. [176] evaluated the role of ω-3 PUFAs on patients with metastatic CCR before undergoing liver resection surgery. This phase III clinical trial reported that 18 months after surgical resection, the supplementation with EPA-FFA increased the general survival rate (SR), when compared with controls, due to its antiangiogenic properties. Similarly, the CALGB 89803 study retrospectively studied the relationship between consumption of marine-derived ω-3 PUFAs and survival in patients with completely resected stage III CCR. It showed that this supplementation increased the rate of disease-free survival (DFS), revealing that patients who consumed dark fish at least one day a week had a 35% lower risk of recurrence of death by cancer [177].
On the other hand, a multicentric study (The seAFOod Polyp Prevention Trial) evaluated the potential effect of treatment with EPA and aspirin, on their own or in combination, for the prevention of CCR. To achieve this, four groups were assigned; two of them received individually 2 g/day of EPA (as FFA or TAG) or 300 mg/day of aspirin. Another group received both simultaneously, and the last group was administered a placebo for 12 months. After the follow-up colonoscopy, results showed that the supplementation with EPA and aspirin did not reduce the ratio of individuals with one or more colorectal adenomas. However, it was established that both confer certain chemoprevention against adenoma, so this must be evaluated in the future with precision medicine [178]. Moreover, results are expected from the OMICC study (NCT03661047), which seeks to evaluate the effect of ω-3 PUFAs in the treatment with AMR101 (VASCEPA, icosapent ethyl) in the tumor microenvironment and gut microbiota in patients diagnosed with colon cancer.
Within the context of prostate cancer, there is a lot of evidence regarding the regulator effect of ω-3 PUFAs on this disease [179]. A clinical trial performed by Aronson et al. [180] explored the role of the low-fat fish oil (LFFO) diet with a reduction in the ratio of ω-6 to ω-3 in individuals subjected to radical prostatectomy. The participants consumed five capsules of fish oil (1.1 g each) per day during the study. It was evidenced that the LFFO diet did not provide statistically significant changes in the profile of biomarkers, such as IGF-1. However, secondary results showed in the Ki67 immunostaining a reduction in the proliferation of malignant tissue of 32.2% (p < 0.05). This proved the inhibitory effect of the LFFO diet in carcinogenesis and prostate cancer progression. Later, a study derived from this trial assessed the effect of this diet on the profile of serum eicosanoids. There was a decrease in the profile of 15 (S)-HETE and the cell cycle progression score (CCP) with the LFFO diet. The 15 (S)-HETE/LTB4 pathway was highlighted as a potential target in the proliferation of this type of cancer [181]. Currently, results are expected from a phase IIb trial that is evaluating the effect of supplementation with MAG-EPA over cellular proliferation, inflammation, and quality of life in patients with prostate cancer and a Gleason score ≥7 who are about to undergo a radical prostatectomy [182].
Another cancer that has been studied for the effects of PUFAs both in the therapeutic and preventive field is breast cancer [183]. Within the evidence, a small clinical trial stands out, where they explored the effect of supplementation with 2 g/day of fish oil over the nutritional and immunological parameters of patients with breast cancer without treatment, with previous chemotherapy. These results showed that supplementation led to an increase in serum levels of EPA (p = 0.004) and DHA (p = 0.007), also evidencing the preservation of serum levels of high-sensitivity C-reactive protein (hsCRP) and T CD4+ lymphocytes, showing signs of a modulator effect of ω-3 PUFAs on the immune response and inflammation of these patients [184]. In addition, the potential of DHA as neoadjuvant therapy in metastatic breast cancer has been reported. It improves the result of chemotherapy by increasing the tumor’s sensitivity to the treatment and, as a result, survival [185].
Based on this potential, a clinical trial was performed on 48 patients with locally advanced breast cancer. The intervention consisted of the administration of ω-3 PUFAs supplements (1 g/d) or placebo, along with the administration of three cycles of neoadjuvant chemotherapy with cyclophosphamide-doxorubicin-5′fluorouracil (CAF). It was then demonstrated that the supplementation significantly improved the general survival of patients when compared with controls. Moreover, there was a decrease in the expression of Ki67 (p = 0.032) and VEGF (p = 0.041), exposing the benefits of supplementation with ω-3 PUFAs in neoadjuvant chemotherapy for this type of cancer. Nevertheless, other variables must be explored further, such as the optimal dose, bioavailability, and circulating levels in future investigations [186]. On the other hand, results are expected from the DHA-WIN (NCT03831178) study, which has the objective of evaluating the potential therapeutic index (efficacy: adverse effects) of supplementation with 4.4 g/d of DHA in combination with neoadjuvant chemotherapy in patients with early breast cancer, using the Ki67 index as a marker for efficacy as well as the effect of DHA over serum phospholipids and the immune system [187]. Lastly, it is essential to mention that, even though there is an ample bibliography on the beneficial role of ω-3 PUFAs and cancer development, these results are not conclusive and must be interpreted with caution, as there are also studies showing a weak association between ω-3 PUFA supplementation and cancer suppression in certain types of cancer [188].

Table 1.
Summary of clinical evidence for the treatment of EPA and DHA in different types of cancer.
Table 1.
Summary of clinical evidence for the treatment of EPA and DHA in different types of cancer.
| Type of Cancer | Methodology | Intervention | Relevant Results | Ref. |
|---|---|---|---|---|
| Colorectal cancer | Phase III, randomized, double-blind, placebo-controlled study in 88 patients with CRCLM before undergoing liver resection surgery | Dietary supplementation with 2 g/day of EPA-FFA during the preoperative period compared with placebo. | The daily consumption of EPA-FFA increased the OS rate in patients 18 months after the surgical intervention. There were no differences in Ki67 PI, but the antiangiogenic activity of EPA-FFA was proven in vitro | [176] |
| Retrospective study on 1011 patients with stage III colon adenocarcinoma, without metastasis, prior chemotherapy, or radiotherapy. | Marine-derived ω-3 PUFAs ingestion (g/d) was calculated by multiplying the portion of each article, the frequency, and the sum of all the articles. | People who consumed dark fish ≥1 d/week obtained a higher DFS rate than those who did not (HR 0.65; CI of 95%, 0.48 to 0.87; p = 0.007). | [177] | |
| Multicentric, randomized, double-blind, placebo-controlled, two-by-two factorial study in 709 patients with sporadic CRC diagnosed with colonoscopy | The 4 study groups received: (1) 2 g/day of EPA-FFA or EPA-TAG; (2) 300 mg/day of aspirin; (3) Aspirin and EPA; or (4) Placebo | Supplementation with EPA and aspirin did not decrease the percentage of individuals with one or more colorectal adenomas. There is a need for more evidence with precision medicine. | [178] | |
| Prostate cancer | Phase II randomized study on 48 patients with localized prostate cancer, who were about to undergo radical prostatectomy. LFFO assessed for 4/6 weeks. | Subjects were assigned to 2 groups: (1) WD and a 15:1 proportion of ω-6:ω-3 PUFAs or (2) LFFO diet with a 2:1 proportion of ω-6:ω-3 PUFAs. 5 cap/day | The LFFO diet did not provide changes in biomarkers, such as IGF-1. However, the activity of Ki67 was reduced by 32.2%, demonstrating an effect on cell proliferation. | [180] |
| Post hoc analysis that used serum and prostate tissue samples obtained in a previously completed randomized clinical trial. | The levels of LBT4 and 15 (S)-HETE were assessed, as well as CCP score. FA was measured with GC and ELISA. | Levels of ω-6 PUFAs and 15 (S)-HETE were significantly decreased in comparison with controls. 15 (S)-HETE was correlated with Ki67 (p < 0.01). CCP score was significantly lower in individuals with the LFFO diet than those with WD. | [181] | |
| Breast cancer | Randomized, double-blind, clinical trial that evaluated the effect of ω-3 PUFAs over immune parameters in 45 patients with breast cancer without chemotherapy. | Provided 2 g (470 mg of EPA and 390 mg of DHA) of fish oil concentrate each day (2 caps/day) at lunch and dinner time for 30 days. | Supplementation with ω-3 PUFAs resulted in a significant increase in EPA (p = 0.004) and DHA (p = 0.007) plasma levels. The level of hsCRP and the percentage of CD4+ lymphocytes were maintained when compared with controls. | [184] |
| Randomized, double-blind, placebo-controlled clinical trial in 48 patients with locally advanced breast cancer treated with neoadjuvant CAF. | Administered 1 g of ω-3 PUFAs each day, along with 3 cycles of neoadjuvant chemotherapy with CAF for 51 days. | A significant decrease was observed in the expression of Ki67 and VEGF when compared with controls. In addition, OS and DFS rates were significantly higher: p = 0.048 and p = 0.044 | [186] |
Abbreviations: CRCLM: Colorectal cancer with liver metastasis; ω-3 PUFAs: omega-3 Polyunsaturated fatty acids; EPA: Eicosapentaenoic Acid; DHA: Docosahexaenoic Acid; EPA-FFA: Eicosapentaenoic Acid-Free Fatty Acid; OS: Overall survival; DFS: Disease-Free Survival; hsCRP: high sensitivity C-reactive protein; 15 (S)-HETE: 15-Hydroxyeicosatetraenoic acid; CCP: cell cycle progression; WD: Western diet; LFFO: Low-fat fish oil; CRC: Colorectal cancer. CAF: Cyclophosphamide, Doxorubicin, 5-Fluorouracil; and GC: Gas chromatography.
6. Conclusions
Ever since the identification of the hallmarks of cancer, the study of inflammation has gained great relevance. A considerable number of studies have highlighted the important role of inflammation as a promotor of carcinogenesis, an event where the activation of innate and adaptive immunity allows the pivotal participation of diverse types of cells and pro-inflammatory mediators that stimulate each of the stages of tumorigenesis [20,189]. This is accompanied by the elucidation of the protective actions of SPMs, the lipidic mediators that can regulate and suppress the activity of inflammatory agents, neoplastic transformation inductors, and tumoral progression. As a result, SPMs are now an attractive therapeutic target to consider in the journey of improving conventional therapy and decreasing adverse effects.
In this sense, multiple experimental studies have explored the beneficial role of SPMs in ω-3 PUFAs supplementation as a coadjuvant alternative in cancer therapy. As a result, diminished inflammation and risk of metastasis has been observed, as well as improved survival rates in patients, independent of the cancer type. However, despite substantial evidence obtained in the past years, the results remain controversial; thus, more information regarding this topic is required before introducing these supplements as a therapeutical coadjuvant and chemopreventive option in various types of cancer.
Author Contributions
Conceptualization, W.T., J.L.P. and V.B.; investigation, J.L.P., W.T, M.P.D., R.C., Y.G., P.R. and S.D.-A.; writing—original draft, J.L.P., W.T., M.P.D., R.C., Y.G., P.R. and S.D.-A.; writing—review and editing, L.D., N.V., L.A., M.C., C.C., A.C.-R. and V.B.; funding acquisition, M.C. and V.B. All authors have read and agreed to the published version of the manuscript.
Funding
Internal Funds for Research Strengthening from Universidad Simón Bolívar, Vicerrectoría de Investigación, Extensión e Innovación, Barranquilla, Colombia, and CC-0437-10-21-09-10 from Consejo de Desarrollo Científico, Humanístico y Tecnológico (CONDES), University of Zulia, and the research grant no. FZ-0058-2007 from Fundacite-Zulia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflict of interest to disclose.
References
- Nagai, H.; Kim, Y.H. Cancer Prevention from the Perspective of Global Cancer Burden Patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Battista, R.N.; Grover, S.A. Early Detection of Cancer: An Overview. Annu. Rev. Public Health 1988, 9, 21–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Parakh, S.; King, D.; Gan, H.K.; Scott, A.M. Current Development of Monoclonal Antibodies in Cancer Therapy. Recent Results Cancer Res. Fortschr. Krebsforsch. Progres. Dans Rech. Sur. Cancer 2020, 214, 1–70. [Google Scholar] [CrossRef]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. MTOR Signaling Pathway and MTOR Inhibitors in Cancer: Progress and Challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Retinoic Acids in the Treatment of Most Lethal Solid Cancers. J. Clin. Med. 2020, 9, 360. [Google Scholar] [CrossRef]
- Gao, S.; Wang, S.; Song, Y. Novel Immunomodulatory Drugs and Neo-Substrates. Biomark. Res. 2020, 8, 2. [Google Scholar] [CrossRef]
- Zhang, M.; Eshraghian, E.A.; Jammal, O.A.; Zhang, Z.; Zhu, X. CRISPR Technology: The Engine That Drives Cancer Therapy. Biomed. Pharmacother. 2021, 133, 111007. [Google Scholar] [CrossRef]
- Proietti, I.; Skroza, N.; Michelini, S.; Mambrin, A.; Balduzzi, V.; Bernardini, N.; Marchesiello, A.; Tolino, E.; Volpe, S.; Maddalena, P.; et al. BRAF Inhibitors: Molecular Targeting and Immunomodulatory Actions. Cancers 2020, 12, 1823. [Google Scholar] [CrossRef]
- Guallar-Garrido, S.; Julián, E. Bacillus Calmette-Guérin (BCG) Therapy for Bladder Cancer: An Update. ImmunoTargets Ther. 2020, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Shen, W.; Du, R.; Jiang, S.; Zhu, J.; Chen, Y.; Huang, C.; Shi, Y.; Xiang, R.; Luo, Y. Phosphodiesterase 3A Represents a Therapeutic Target That Drives Stem Cell–like Property and Metastasis in Breast Cancer. Mol. Cancer Ther. 2020, 19, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hulvat, M.C. Cancer Incidence and Trends. Surg. Clin. N. Am. 2020, 100, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hu, B.; Dehghan, M.; Mente, A.; Wang, C.; Yan, R.; Rangarajan, S.; Tse, L.A.; Yusuf, S.; Liu, X.; et al. Fruit, Vegetable, and Legume Intake and the Risk of All-Cause, Cardiovascular, and Cancer Mortality: A Prospective Study. Clin. Nutr. 2021, 40, 4316–4323. [Google Scholar] [CrossRef]
- De Souza, R.J.; Dehghan, M.; Mente, A.; Bangdiwala, S.I.; Ahmed, S.H.; Alhabib, K.F.; Altuntas, Y.; Basiak-Rasała, A.; Dagenais, G.-R.; Diaz, R.; et al. Association of Nut Intake with Risk Factors, Cardiovascular Disease, and Mortality in 16 Countries from 5 Continents: Analysis from the Prospective Urban and Rural Epidemiology (PURE) Study. Am. J. Clin. Nutr. 2020, 112, 208–219. [Google Scholar] [CrossRef]
- Murata, M. Inflammation and Cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef]
- Brostjan, C.; Oehler, R. The Role of Neutrophil Death in Chronic Inflammation and Cancer. Cell Death Discov. 2020, 6, 26. [Google Scholar] [CrossRef]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From Inflammation to Cancer. Ir. J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef]
- Shalapour, S.; Karin, M. Immunity, Inflammation, and Cancer: An Eternal Fight between Good and Evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and Cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Virchow, R. As Based upon Physiological and Pathological Histology: Cellular Pathology. Nutr. Rev. 2009, 47, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Prevete, N.; Liotti, F.; Amoresano, A.; Pucci, P.; de Paulis, A.; Melillo, R.M. New Perspectives in Cancer: Modulation of Lipid Metabolism and Inflammation Resolution. Pharmacol. Res. 2018, 128, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-Related Inflammation, the Seventh Hallmark of Cancer: Links to Genetic Instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Sulciner, M.L.; Serhan, C.N.; Gilligan, M.M.; Mudge, D.K.; Chang, J.; Gartung, A.; Lehner, K.A.; Bielenberg, D.R.; Schmidt, B.; Dalli, J.; et al. Resolvins Suppress Tumor Growth and Enhance Cancer Therapy. J. Exp. Med. 2018, 215, 115–140. [Google Scholar] [CrossRef]
- Ritter, B.; Greten, F.R. Modulating Inflammation for Cancer Therapy. J. Exp. Med. 2019, 216, 1234–1243. [Google Scholar] [CrossRef]
- Barden, A.E.; Mas, E.; Mori, T.A. N-3 Fatty Acid Supplementation and Proresolving Mediators of Inflammation. Curr. Opin. Lipidol. 2016, 27, 26–32. [Google Scholar] [CrossRef]
- Sulciner, M.L.; Gartung, A.; Gilligan, M.M.; Serhan, C.N.; Panigrahy, D. Targeting Lipid Mediators in Cancer Biology. Cancer Metastasis Rev. 2018, 37, 557–572. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.-L. Resolvins: A Family of Bioactive Products of Omega-3 Fatty Acid Transformation Circuits Initiated by Aspirin Treatment That Counter Proinflammation Signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, B.; Li, Y. Resolution of Cancer-Promoting Inflammation: A New Approach for Anticancer Therapy. Front. Immunol. 2017, 8, 71. [Google Scholar] [CrossRef]
- Hoare, A.; Soto, C.; Rojas-Celis, V.; Bravo, D. Chronic Inflammation as a Link between Periodontitis and Carcinogenesis. Mediat. Inflamm. 2019, 2019, 1029857. [Google Scholar] [CrossRef] [PubMed]
- Basil, M.C.; Levy, B.D. Specialized Pro-Resolving Mediators: Endogenous Regulators of Infection and Inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Lipoxin Biosynthesis and Its Impact in Inflammatory and Vascular Events. Biochim. Biophys. Acta 1994, 1212, 1–25. [Google Scholar] [CrossRef]
- Nicolaou, A.; Mauro, C.; Urquhart, P.; Marelli-Berg, F. Polyunsaturated Fatty Acid-Derived Lipid Mediators and T Cell Function. Front. Immunol. 2014, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Zaninelli, T.H.; Rasquel-Oliveira, F.S.; Casagrande, R.; Verri, W.A. Specialized Pro-Resolving Lipid Mediators: A New Class of Non-Immunosuppressive and Non-Opioid Analgesic Drugs. Pharmacol. Res. 2020, 151, 104549. [Google Scholar] [CrossRef]
- Greene, E.R.; Huang, S.; Serhan, C.N.; Panigrahy, D. Regulation of Inflammation in Cancer by Eicosanoids. Prostaglandins Other Lipid Mediat. 2011, 96, 27–36. [Google Scholar] [CrossRef]
- Moro, K.; Nagahashi, M.; Ramanathan, R.; Takabe, K.; Wakai, T. Resolvins and Omega Three Polyunsaturated Fatty Acids: Clinical Implications in Inflammatory Diseases and Cancer. World J. Clin. Cases 2016, 4, 155–164. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in Inflammation: Emergence of the pro-Resolving Superfamily of Mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Ye, Y.; Scheff, N.N.; Bernabé, D.; Salvo, E.; Ono, K.; Liu, C.; Veeramachaneni, R.; Viet, C.T.; Viet, D.T.; Dolan, J.C.; et al. Anti-Cancer and Analgesic Effects of Resolvin D2 in Oral Squamous Cell Carcinoma. Neuropharmacology 2018, 139, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Plastina, P.; Barone, I.; Catalano, S.; Bonofiglio, D. N-3 Polyunsaturated Fatty Acid Amides: New Avenues in the Prevention and Treatment of Breast Cancer. Int. J. Mol. Sci. 2020, 21, 2279. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Scazzocchio, B.; Vari, R.; Santangelo, C.; Giovannini, C.; Masella, R. Recent Evidence on the Role of Dietary PUFAs in Cancer Development and Prevention. Curr. Med. Chem. 2018, 25, 1818–1836. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Yacoubian, S.; Yang, R. Anti-Inflammatory and Proresolving Lipid Mediators. Annu. Rev. Pathol. 2008, 3, 279–312. [Google Scholar] [CrossRef] [PubMed]
- Keyes, K.T.; Ye, Y.; Lin, Y.; Zhang, C.; Perez-Polo, J.R.; Gjorstrup, P.; Birnbaum, Y. Resolvin E1 Protects the Rat Heart against Reperfusion Injury. Am. J. Physiol.-Heart Circ. Physiol. 2010, 299, H153–H164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, M.; Ding, W.; Zhao, M.; Ye, J.; Xu, Y.; Wang, Z.; Ye, D.; Li, D.; Liu, J.; et al. Resolvin E1 Protects against Doxorubicin-Induced Cardiotoxicity by Inhibiting Oxidative Stress, Autophagy and Apoptosis by Targeting AKT/MTOR Signaling. Biochem. Pharmacol. 2020, 180, 114188. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Chiang, N.; Serhan, C.N. Elucidation of Novel 13-Series Resolvins That Increase with Atorvastatin and Clear Infections. Nat. Med. 2015, 21, 1071–1075. [Google Scholar] [CrossRef]
- Guo, Y.; Dong, C.; Tang, J.; Deng, X.; Wang, G.; Wang, W.; Xu, H. Lipoxin A4 Alleviates Cerebral Ischemia-Reperfusion Injury through up-Regulating Nrf2. J. Neurosurg. Sci. 2018, 62, 225–226. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Q.; Tang, K.; Sun, Y.; Zhang, K.; Zhang, Y.; Luo, S.; Zhang, H.; Ye, D.; Huang, B. Lipid Mediator Lipoxin A4 Inhibits Tumor Growth by Targeting IL-10-Producing Regulatory B (Breg) Cells. Cancer Lett. 2015, 364, 118–124. [Google Scholar] [CrossRef]
- Chandrasekharan, J.A.; Huang, X.M.; Hwang, A.C.; Sharma-Walia, N. Altering the Anti-Inflammatory Lipoxin Microenvironment: A New Insight into Kaposi’s Sarcoma-Associated Herpesvirus Pathogenesis. J. Virol. 2016, 90, 11020–11031. [Google Scholar] [CrossRef]
- Madi, N.M.; Ibrahim, R.R.; Alghazaly, G.M.; Marea, K.E.; El-Saka, M.H. The Prospective Curative Role of Lipoxin A4 in Induced Gastric Ulcer in Rats: Possible Involvement of Mitochondrial Dynamics Signaling Pathway. IUBMB Life 2020, 72, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-M.; Chung, G.; Kim, Y.H.; Park, C.-K. The Role of Maresins in Inflammatory Pain: Function of Macrophages in Wound Regeneration. Int. J. Mol. Sci. 2019, 20, 5849. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lu, Y.; Morita, M.; Saito, S.; Kobayashi, Y.; Jun, B.; Bazan, N.G.; Xu, X.; Wang, Y. Stereoselective Synthesis of Maresin-like Lipid Mediators. Synlett 2019, 30, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wan, M.; Huang, W.; Stanton, R.C.; Xu, Y. Maresins: Specialized Proresolving Lipid Mediators and Their Potential Role in Inflammatory-Related Diseases. Mediat. Inflamm. 2018, 2018, 2380319. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.G. The Docosanoid Neuroprotectin D1 Induces Homeostatic Regulation of Neuroinflammation and Cell Survival. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 127–129. [Google Scholar] [CrossRef][Green Version]
- Bennett, M.; Gilroy, D.W. Lipid Mediators in Inflammation. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-Derived Resolvins, Protectins, and Maresins in Airway Inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef]
- Hong, S.; Gronert, K.; Devchand, P.R.; Moussignac, R.-L.; Serhan, C.N. Novel Docosatrienes and 17S-Resolvins Generated from Docosahexaenoic Acid in Murine Brain, Human Blood, and Glial Cells. J. Biol. Chem. 2003, 278, 14677–14687. [Google Scholar] [CrossRef]
- Bazan, N.G. Omega-3 Fatty Acids, pro-Inflammatory Signaling and Neuroprotection. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 136–141. [Google Scholar] [CrossRef]
- Shinohara, M.; Mirakaj, V.; Serhan, C.N. Functional Metabolomics Reveals Novel Active Products in the DHA Metabolome. Front. Immunol. 2012, 3, 81. [Google Scholar] [CrossRef]
- Balas, L.; Durand, T. Dihydroxylated E,E,Z-Docosatrienes. An Overview of Their Synthesis and Biological Significance. Prog. Lipid Res. 2016, 61, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sheets, K.G.; Jun, B.; Zhou, Y.; Zhu, M.; Petasis, N.A.; Gordon, W.C.; Bazan, N.G. Microglial Ramification and Redistribution Concomitant with the Attenuation of Choroidal Neovascularization by Neuroprotectin D1. Mol. Vis. 2013, 19, 1747–1759. [Google Scholar]
- Zhou, Y.; Wang, J.; Li, X.; Li, K.; Chen, L.; Zhang, Z.; Peng, M. Neuroprotectin D1 Protects Against Postoperative Delirium-Like Behavior in Aged Mice. Front. Aging Neurosci. 2020, 12, 582674. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Araki, Y.; Mimura, T. The Mechanisms Underlying Chronic Inflammation in Rheumatoid Arthritis from the Perspective of the Epigenetic Landscape. J. Immunol. Res. 2016, 2016, 6290682. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, M. Ulcerative Colitis-Associated Colorectal Cancer. World J. Gastroenterol. 2014, 20, 16389–16397. [Google Scholar] [CrossRef]
- Jiang, J.; Li, J.; Yunxia, Z.; Zhu, H.; Liu, J.; Pumill, C. The Role of Prostatitis in Prostate Cancer: Meta-Analysis. PLoS ONE 2013, 8, e85179. [Google Scholar] [CrossRef]
- Rasmussen, C.B.; Kjaer, S.K.; Albieri, V.; Bandera, E.V.; Doherty, J.A.; Høgdall, E.; Webb, P.M.; Jordan, S.J.; Rossing, M.A.; Wicklund, K.G.; et al. Pelvic Inflammatory Disease and the Risk of Ovarian Cancer and Borderline Ovarian Tumors: A Pooled Analysis of 13 Case-Control Studies. Am. J. Epidemiol. 2017, 185, 8–20. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Velázquez, M.; Ostoa-Saloma, P.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Castro, J.I.; Morales-Montor, J. The Role of Cytokines in Breast Cancer Development and Progression. J. Interferon Cytokine Res. 2015, 35, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Guven Maiorov, E.; Keskin, O.; Gursoy, A.; Nussinov, R. The Structural Network of Inflammation and Cancer: Merits and Challenges. Semin. Cancer Biol. 2013, 23, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Elner, S.G.; Bian, Z.-M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-Inflammatory Cytokines Increase Reactive Oxygen Species through Mitochondria and NADPH Oxidase in Cultured RPE Cells. Exp. Eye Res. 2007, 85, 462–472. [Google Scholar] [CrossRef]
- Kamiya, H.; Miura, K.; Ishikawa, H.; Inoue, H.; Nishimura, S.; Ohtsuka, E. C-Ha-Ras Containing 8-Hydroxyguanine at Codon 12 Induces Point Mutations at the Modified and Adjacent Positions. Cancer Res. 1992, 52, 3483–3485. [Google Scholar] [PubMed]
- Kamiya, H.; Murata-Kamiya, N.; Koizume, S.; Inoue, H.; Nishimura, S.; Ohtsuka, E. 8-Hydroxyguanine (7,8-Dihydro-8-Oxoguanine) in Hot Spots of the c-Ha- Ras Gene: Effects of Sequence Contexts on Mutation Spectra. Carcinogenesis 1995, 16, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Thanan, R.; Ma, N.; Kawanishi, S. Role of Nitrative and Oxidative DNA Damage in Inflammation-Related Carcinogenesis. J. Biomed. Biotechnol. 2012, 2012, 623019. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Ben-Neriah, Y.; Karin, M. Inflammation Meets Cancer, with NF-ΚB as the Matchmaker. Nat. Immunol. 2011, 12, 715–723. [Google Scholar] [CrossRef]
- Weichert, W.; Boehm, M.; Gekeler, V.; Bahra, M.; Langrehr, J.; Neuhaus, P.; Denkert, C.; Imre, G.; Weller, C.; Hofmann, H.-P.; et al. High Expression of RelA/P65 Is Associated with Activation of Nuclear Factor-KappaB-Dependent Signaling in Pancreatic Cancer and Marks a Patient Population with Poor Prognosis. Br. J. Cancer 2007, 97, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Markov, O.V.; Mironova, N.L.; Vlasov, V.V.; Zenkova, M.A. Molecular and Cellular Mechanisms of Antitumor Immune Response Activation by Dendritic Cells. Acta Nat. 2016, 8, 17–30. [Google Scholar] [CrossRef]
- Tugues, S.; Burkhard, S.H.; Ohs, I.; Vrohlings, M.; Nussbaum, K.; Vom Berg, J.; Kulig, P.; Becher, B. New Insights into IL-12-Mediated Tumor Suppression. Cell Death Differ. 2015, 22, 237–246. [Google Scholar] [CrossRef]
- Yu, H.; Kortylewski, M.; Pardoll, D. Crosstalk between Cancer and Immune Cells: Role of STAT3 in the Tumour Microenvironment. Nat. Rev. Immunol. 2007, 7, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Knochelmann, H.M.; Dwyer, C.J.; Bailey, S.R.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When Worlds Collide: Th17 and Treg Cells in Cancer and Autoimmunity. Cell Mol. Immunol. 2018, 15, 458–469. [Google Scholar] [CrossRef]
- Najafi, S.; Mirshafiey, A. The Role of T Helper 17 and Regulatory T Cells in Tumor Microenvironment. Immunopharmacol. Immunotoxicol. 2019, 41, 16–24. [Google Scholar] [CrossRef]
- Zhao, M.; Gao, F.-H.; Wang, J.-Y.; Liu, F.; Yuan, H.-H.; Zhang, W.-Y.; Jiang, B. JAK2/STAT3 Signaling Pathway Activation Mediates Tumor Angiogenesis by Upregulation of VEGF and BFGF in Non-Small-Cell Lung Cancer. Lung Cancer 2011, 73, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Shen, J.; Cao, J.; Zhou, Y.; Shang, L.; Jin, S.; Cao, S.; Che, D.; Liu, F.; Yu, Y. Interleukin-17 Promotes Angiogenesis by Stimulating VEGF Production of Cancer Cells via the STAT3/GIV Signaling Pathway in Non-Small-Cell Lung Cancer. Sci. Rep. 2015, 5, 16053. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Molls, M.; Radons, J. Chronic Inflammation in Cancer Development. Front. Immunol. 2011, 2, 98. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. What Is the Evidence That Tumors Are Angiogenesis Dependent? J. Natl. Cancer Inst. 1990, 82, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J.; Zhang, Z.; Zhou, W.; Wang, A.J.; Heddleston, J.M.; Pinna, C.M.A.; Hubaud, A.; Stadler, B.; Choi, M.; Bar, M.; et al. HIF Induces Human Embryonic Stem Cell Markers in Cancer Cells. Cancer Res. 2011, 71, 4640–4652. [Google Scholar] [CrossRef]
- Shi, Q.; Le, X.; Wang, B.; Abbruzzese, J.L.; Xiong, Q.; He, Y.; Xie, K. Regulation of Vascular Endothelial Growth Factor Expression by Acidosis in Human Cancer Cells. Oncogene 2001, 20, 3751–3756. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-Inducible Factors: Mediators of Cancer Progression and Targets for Cancer Therapy. Trends Pharmacol. Sci. 2012, 33, 207–214. [Google Scholar] [CrossRef]
- Zuazo-Gaztelu, I.; Casanovas, O. Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front. Oncol. 2018, 8, 248. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Burkholder, B.; Huang, R.-Y.; Burgess, R.; Luo, S.; Jones, V.S.; Zhang, W.; Lv, Z.-Q.; Gao, C.-Y.; Wang, B.-L.; Zhang, Y.-M.; et al. Tumor-Induced Perturbations of Cytokines and Immune Cell Networks. Biochim. Biophys. Acta 2014, 1845, 182–201. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, Z.; Li, H. NSAIDs Use and Reduced Metastasis in Cancer Patients: Results from a Meta-Analysis. Sci. Rep. 2017, 7, 1875. [Google Scholar] [CrossRef] [PubMed]
- Kehm, R.D.; Hopper, J.L.; John, E.M.; Phillips, K.-A.; MacInnis, R.J.; Dite, G.S.; Milne, R.L.; Liao, Y.; Zeinomar, N.; Knight, J.A.; et al. Regular Use of Aspirin and Other Non-Steroidal Anti-Inflammatory Drugs and Breast Cancer Risk for Women at Familial or Genetic Risk: A Cohort Study. Breast Cancer Res. 2019, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Huang, J.Q.; Zheng, G.F.; Lam, S.K.; Karlberg, J.; Wong, B.C.-Y. Non-Steroidal Anti-Inflammatory Drug Use and the Risk of Gastric Cancer: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2003, 95, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted Roles of PGE2 in Inflammation and Cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef]
- Wuryanti, S.; Andrijono, A.; Susworo, S.; Witjaksono, F. The Effect of High Poly Unsaturated Fatty Acid (PUFA) Dietary Supplementation on Inflammatory Status of Patients with Advanced Cervical Cancer on Radiation Treatment. Acta Med. Indones. 2015, 47, 45–49. [Google Scholar]
- Baran, Y.; Ulu, G.T.; Kiraz, Y. Role of Polyunsaturated Fatty Acids in Cancer and Their Use in Cancer Treatment. Rev. Clin. Med. 2019, 6, 84–90. [Google Scholar] [CrossRef]
- Denkins, Y.; Kempf, D.; Ferniz, M.; Nileshwar, S.; Marchetti, D. Role of Omega-3 Polyunsaturated Fatty Acids on Cyclooxygenase-2 Metabolism in Brain-Metastatic Melanoma. J. Lipid Res. 2005, 46, 1278–1284. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Ollauri-Ibáñez, C.; Ayuso-Íñigo, B.; Pericacho, M. Hot and Cold Tumors: Is Endoglin (CD105) a Potential Target for Vessel Normalization? Cancers 2021, 13, 1552. [Google Scholar] [CrossRef]
- Ren, X.; Guo, S.; Guan, X.; Kang, Y.; Liu, J.; Yang, X. Immunological Classification of Tumor Types and Advances in Precision Combination Immunotherapy. Front. Immunol. 2022, 13, 790113. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Sun, Z.-J. Turning Cold Tumors into Hot Tumors by Improving T-Cell Infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, J.A.; Sharma-Walia, N. Lipoxins: Nature’s Way to Resolve Inflammation. J. Inflamm. Res. 2015, 8, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef]
- Maderna, P.; Godson, C. Lipoxins: Resolutionary Road. Br. J. Pharmacol. 2009, 158, 947–959. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving Lipid Mediators and Mechanisms in the Resolution of Acute Inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Treating Inflammation and Infection in the 21st Century: New Hints from Decoding Resolution Mediators and Mechanisms. FASEB J. 2017, 31, 1273–1288. [Google Scholar] [CrossRef]
- Croasdell, A.; Thatcher, T.H.; Kottmann, R.M.; Colas, R.A.; Dalli, J.; Serhan, C.N.; Sime, P.J.; Phipps, R.P. Resolvins Attenuate Inflammation and Promote Resolution in Cigarette Smoke-Exposed Human Macrophages. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L888–L901. [Google Scholar] [CrossRef]
- Mattoscio, D.; Isopi, E.; Lamolinara, A.; Patruno, S.; Medda, A.; De Cecco, F.; Chiocca, S.; Iezzi, M.; Romano, M.; Recchiuti, A. Resolvin D1 Reduces Cancer Growth Stimulating a Protective Neutrophil-Dependent Recruitment of Anti-Tumor Monocytes. J. Exp. Clin. Cancer Res. 2021, 40, 129. [Google Scholar] [CrossRef]
- Leuti, A.; Maccarrone, M.; Chiurchiù, V. Proresolving Lipid Mediators: Endogenous Modulators of Oxidative Stress. Oxidative Med. Cell Longev. 2019, 2019, 8107265. [Google Scholar] [CrossRef]
- Yasui, K.; Kobayashi, N.; Yamazaki, T.; Agematsu, K.; Matsuzaki, S.; Ito, S.; Nakata, S.; Baba, A.; Koike, K. Superoxide Dismutase (SOD) as a Potential Inhibitory Mediator of Inflammation via Neutrophil Apoptosis. Free Radic. Res. 2005, 39, 755–762. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. The Resolution Code of Acute Inflammation: Novel pro-Resolving Lipid Mediators in Resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Recchiuti, A.; Mattoscio, D.; Isopi, E. Roles, Actions, and Therapeutic Potential of Specialized Pro-Resolving Lipid Mediators for the Treatment of Inflammation in Cystic Fibrosis. Front. Pharmacol. 2019, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; Maccarrone, M.; Serhan, C.N. Proresolving Lipid Mediators Resolvin D1, Resolvin D2, and Maresin 1 Are Critical in Modulating T Cell Responses. Sci. Transl. Med. 2016, 8, 353ra111. [Google Scholar] [CrossRef] [PubMed]
- Duffney, P.F.; Falsetta, M.L.; Rackow, A.R.; Thatcher, T.H.; Phipps, R.P.; Sime, P.J. Key Roles for Lipid Mediators in the Adaptive Immune Response. J. Clin. Investig. 2018, 128, 2724–2731. [Google Scholar] [CrossRef] [PubMed]
- Abdulnour, R.-E.E.; Dalli, J.; Colby, J.K.; Krishnamoorthy, N.; Timmons, J.Y.; Tan, S.H.; Colas, R.A.; Petasis, N.A.; Serhan, C.N.; Levy, B.D. Maresin 1 Biosynthesis during Platelet-Neutrophil Interactions Is Organ-Protective. Proc. Natl. Acad. Sci. USA 2014, 111, 16526–16531. [Google Scholar] [CrossRef]
- Bazan, N.G.; Eady, T.N.; Khoutorova, L.; Atkins, K.D.; Hong, S.; Lu, Y.; Zhang, C.; Jun, B.; Obenaus, A.; Fredman, G.; et al. Novel Aspirin-Triggered Neuroprotectin D1 Attenuates Cerebral Ischemic Injury after Experimental Stroke. Exp. Neurol. 2012, 236, 122–130. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Haak, V.M.; Huang, S.; Panigrahy, D. Debris-Stimulated Tumor Growth: A Pandora’s Box? Cancer Metastasis Rev. 2021, 40, 791–801. [Google Scholar] [CrossRef]
- Hisano, Y.; Hla, T. Bioactive Lysolipids in Cancer and Angiogenesis. Pharmacol. Ther. 2019, 193, 91–98. [Google Scholar] [CrossRef]
- Marginean, A.; Sharma-Walia, N. Lipoxins Exert Antiangiogenic and Anti-Inflammatory Effects on Kaposi’s Sarcoma Cells. Transl. Res. J. Lab. Clin. Med. 2015, 166, 111–133. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, H.; He, S.; Cai, L.; Li, Y.; Hu, S.; Ye, D.; Hoidal, J.; Wu, P.; Chen, X. Lipoxin A4 and Its Analogue Suppress the Tumor Growth of Transplanted H22 in Mice: The Role of Antiangiogenesis. Mol. Cancer Ther. 2010, 9, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Prevete, N.; Liotti, F.; Illiano, A.; Amoresano, A.; Pucci, P.; de Paulis, A.; Melillo, R.M. Formyl Peptide Receptor 1 Suppresses Gastric Cancer Angiogenesis and Growth by Exploiting Inflammation Resolution Pathways. Oncoimmunology 2017, 6, e1293213. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Arita, M.; Zhang, Q.; Saban, D.R.; Chauhan, S.K.; Chiang, N.; Serhan, C.N.; Dana, R. Anti-Angiogenesis Effect of the Novel Anti-Inflammatory and pro-Resolving Lipid Mediators. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4743–4752. [Google Scholar] [CrossRef] [PubMed]
- Rajasagi, N.K.; Reddy, P.B.J.; Suryawanshi, A.; Mulik, S.; Gjorstrup, P.; Rouse, B.T. Controlling Herpes Simplex Virus-Induced Ocular Inflammatory Lesions with the Lipid-Derived Mediator Resolvin E1. J. Immunol. 2011, 186, 1735–1746. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Chen, Y. Tumor Microenvironment: Sanctuary of the Devil. Cancer Lett. 2015, 368, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Lei, Y.; Li, J.-K.; Du, W.-X.; Li, R.-G.; Yang, J.; Li, J.; Li, F.; Tan, H.-B. Immune Cells within the Tumor Microenvironment: Biological Functions and Roles in Cancer Immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef]
- Scharping, N.E.; Delgoffe, G.M. Tumor Microenvironment Metabolism: A New Checkpoint for Anti-Tumor Immunity. Vaccines 2016, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, N.; Abdulnour, R.-E.E.; Walker, K.H.; Engstrom, B.D.; Levy, B.D. Specialized Proresolving Mediators in Innate and Adaptive Immune Responses in Airway Diseases. Physiol. Rev. 2018, 98, 1335–1370. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, J.; Huang, W.; Xu, Q.; Ye, D.; Sun, R.; Zhang, D. Colorectal Cancer Is Associated with a Deficiency of Lipoxin A4, an Endogenous Anti-Inflammatory Mediator. J. Cancer 2019, 10, 4719–4730. [Google Scholar] [CrossRef]
- de-Brito, N.M.; da-Costa, H.C.; Simões, R.L.; Barja-Fidalgo, C. Lipoxin-Induced Phenotypic Changes in CD115+LY6Chi Monocytes TAM Precursors Inhibits Tumor Development. Front. Oncol. 2019, 9, 540. [Google Scholar] [CrossRef]
- Simões, R.L.; De-Brito, N.M.; Cunha-Costa, H.; Morandi, V.; Fierro, I.M.; Roitt, I.M.; Barja-Fidalgo, C. Lipoxin A4 Selectively Programs the Profile of M2 Tumor-Associated Macrophages Which Favour Control of Tumor Progression. Int. J. Cancer 2017, 140, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Cuenca, J.; Través, P.G.; Fernández-Velasco, M.; Martín-Sanz, P.; Boscá, L. Lipoxin A4 Impairment of Apoptotic Signaling in Macrophages: Implication of the PI3K/Akt and the ERK/Nrf-2 Defense Pathways. Cell Death Differ. 2010, 17, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Chen, C.; Mo, Y.Y.; Hebbar, V.; Owuor, E.D.; Tan, T.H.; Kong, A.N. Activation of Mitogen-Activated Protein Kinase Pathways Induces Antioxidant Response Element-Mediated Gene Expression via a Nrf2-Dependent Mechanism. J. Biol. Chem. 2000, 275, 39907–39913. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Mohammed, A.; Rao, C.V. Role of Lipoxins, Resolvins, and Other Bioactive Lipids in Colon and Pancreatic Cancer. Cancer Metastasis Rev. 2011, 30, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.-H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 Binds Human Phagocytes with Evidence for Proresolving Receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-N.; Surh, Y.-J. Resolvin D1-Mediated NOX2 Inactivation Rescues Macrophages Undertaking Efferocytosis from Oxidative Stress-Induced Apoptosis. Biochem. Pharmacol. 2013, 86, 759–769. [Google Scholar] [CrossRef]
- Curtius, K.; Wright, N.A.; Graham, T.A. Evolution of Premalignant Disease. Cold Spring Harb. Perspect. Med. 2017, 7, a026542. [Google Scholar] [CrossRef]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev. Res. 2016, 9, 895–905. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular Mechanisms of Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Li, C.-J.; Chu, P.-Y.; Yiang, G.-T.; Wu, M.-Y. The Molecular Mechanism of Epithelial-Mesenchymal Transition for Breast Carcinogenesis. Biomolecules 2019, 9, 476. [Google Scholar] [CrossRef]
- Prasad, R.S.; Pai, A.; Shyamala, K.; Bhadranna, A.; Shenoy, S.; Yaji, A. Assessment of Epithelial-Mesenchymal Transition Signatures in Oral Submucous Fibrosis. J. Oral Maxillofac. Pathol. 2019, 23, 308. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, K.; Ara, A.; Siddiqui, S.A.; Sherwani, R.K. Transition of Immunohistochemical Expression of E-Cadherin and Vimentin from Premalignant to Malignant Lesions of Oral Cavity and Oropharynx. Oman Med. J. 2016, 31, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Chen, K.; Jiang, Z.; Chen, X.; Sun, L.; Ma, J.; Zhou, C.; Xu, Q.; Duan, W.; Han, L.; et al. Lipoxin A4 Reverses Mesenchymal Phenotypes to Attenuate Invasion and Metastasis via the Inhibition of Autocrine TGF-Β1 Signaling in Pancreatic Cancer. J. Exp. Clin. Cancer Res. 2017, 36, 181. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Wang, L.; Yao, B.; Chen, T.; Li, Q.; Liu, Z.; Liu, R.; Niu, Y.; Song, T.; et al. Resolvin D1 Prevents Epithelial-Mesenchymal Transition and Reduces the Stemness Features of Hepatocellular Carcinoma by Inhibiting Paracrine of Cancer-Associated Fibroblast-Derived COMP. J. Exp. Clin. Cancer Res. 2019, 38, 170. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, M.K.; Lee, E.J.; Lee, C.H. Resolvin D1 Inhibits TGF-Β1-Induced Epithelial Mesenchymal Transition of A549 Lung Cancer Cells via Lipoxin A4 Receptor/Formyl Peptide Receptor 2 and GPR32. Int. J. Biochem. Cell Biol. 2013, 45, 2801–2807. [Google Scholar] [CrossRef]
- Kinugasa, T.; Akagi, Y. Status of Colitis-Associated Cancer in Ulcerative Colitis. World J. Gastrointest. Oncol. 2016, 8, 351–357. [Google Scholar] [CrossRef]
- Masterson, J.C.; McNamee, E.N.; Fillon, S.A.; Hosford, L.; Harris, R.; Fernando, S.D.; Jedlicka, P.; Iwamoto, R.; Jacobsen, E.; Protheroe, C.; et al. Eosinophil-Mediated Signalling Attenuates Inflammatory Responses in Experimental Colitis. Gut 2015, 64, 1236–1247. [Google Scholar] [CrossRef]
- Nagatake, T.; Kunisawa, J. Emerging Roles of Metabolites of Ω3 and Ω6 Essential Fatty Acids in the Control of Intestinal Inflammation. Int. Immunol. 2019, 31, 569–577. [Google Scholar] [CrossRef]
- Ishida, T.; Yoshida, M.; Arita, M.; Nishitani, Y.; Nishiumi, S.; Masuda, A.; Mizuno, S.; Takagawa, T.; Morita, Y.; Kutsumi, H.; et al. Resolvin E1, an Endogenous Lipid Mediator Derived from Eicosapentaenoic Acid, Prevents Dextran Sulfate Sodium-Induced Colitis. Inflamm. Bowel Dis. 2010, 16, 87–95. [Google Scholar] [CrossRef]
- Marcon, R.; Bento, A.F.; Dutra, R.C.; Bicca, M.A.; Leite, D.F.P.; Calixto, J.B. Maresin 1, a Proresolving Lipid Mediator Derived from Omega-3 Polyunsaturated Fatty Acids, Exerts Protective Actions in Murine Models of Colitis. J. Immunol. 2013, 191, 4288–4298. [Google Scholar] [CrossRef]
- Viennois, E.; Chen, F.; Merlin, D. NF-ΚB Pathway in Colitis-Associated Cancers. Transl. Gastrointest. Cancer 2013, 2, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Zuo, X.; Jaoude, J.; Mao, F.; Colby, J.; Shureiqi, I. ALOX15 as a Suppressor of Inflammation and Cancer: Lost in the Link. Prostaglandins Other Lipid Mediat. 2017, 132, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Xu, M.; Zuo, X.; Yu, J.; Xu, W.; Moussalli, M.J.; Elias, E.; Li, H.S.; Watowich, S.S.; Shureiqi, I. 15-Lipoxygenase-1 Suppression of Colitis-Associated Colon Cancer through Inhibition of the IL-6/STAT3 Signaling Pathway. FASEB J. 2015, 29, 2359–2370. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Hua, X.; Zhou, J.; Yang, R. Resolvin D1 and E1 Alleviate the Progress of Hepatitis toward Liver Cancer in Long-Term Concanavalin A-Induced Mice through Inhibition of NF-ΚB Activity. Oncol. Rep. 2016, 35, 307–317. [Google Scholar] [CrossRef]
- Ren, J.; Meng, S.; Yan, B.; Yu, J.; Liu, J. Protectin D1 Reduces Concanavalin A-Induced Liver Injury by Inhibiting NF-ΚB-Mediated CX3CL1/CX3CR1 Axis and NLR Family, Pyrin Domain Containing 3 Inflammasome Activation. Mol. Med. Rep. 2016, 13, 3627–3638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yin, Y.; Wang, T.; Li, W.; Li, C.; Zeng, X.; Yang, W.; Zhang, R.; Tang, Y.; Shi, L.; et al. Maresin 1 Mitigates Concanavalin A-Induced Acute Liver Injury in Mice by Inhibiting ROS-Mediated Activation of NF-ΚB Signaling. Free Radic. Biol. Med. 2020, 147, 23–36. [Google Scholar] [CrossRef]
- Watson, M.; Holman, D.M.; Maguire-Eisen, M. Ultraviolet Radiation Exposure and Its Impact on Skin Cancer Risk. Semin. Oncol. Nurs. 2016, 32, 241–254. [Google Scholar] [CrossRef]
- Cezar, T.L.C.; Martinez, R.M.; Rocha, C.d.; Melo, C.P.B.; Vale, D.L.; Borghi, S.M.; Fattori, V.; Vignoli, J.A.; Camilios-Neto, D.; Baracat, M.M.; et al. Treatment with Maresin 1, a Docosahexaenoic Acid-Derived pro-Resolution Lipid, Protects Skin from Inflammation and Oxidative Stress Caused by UVB Irradiation. Sci. Rep. 2019, 9, 3062. [Google Scholar] [CrossRef]
- Panagiotopoulos, A.A.; Kalyvianaki, K.; Castanas, E.; Kampa, M. Eicosanoids in Prostate Cancer. Cancer Metastasis Rev. 2018, 37, 237–243. [Google Scholar] [CrossRef]
- Rose, D.P. Dietary Fatty Acids and Cancer. Am. J. Clin. Nutr. 1997, 66, 998S–1003S. [Google Scholar] [CrossRef]
- Trombetta, A.; Maggiora, M.; Martinasso, G.; Cotogni, P.; Canuto, R.A.; Muzio, G. Arachidonic and Docosahexaenoic Acids Reduce the Growth of A549 Human Lung-Tumor Cells Increasing Lipid Peroxidation and PPARs. Chem. Biol. Interact. 2007, 165, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Janarthan, M.; Ghosh, B.; Rana, B.; Rana, A.; Chatterjee, M. Fish Oil Regulates Cell Proliferation, Protect DNA Damages and Decrease HER-2/Neu and c-Myc Protein Expression in Rat Mammary Carcinogenesis. Clin. Nutr. 2010, 29, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Azrad, M.; Turgeon, C.; Demark-Wahnefried, W. Current Evidence Linking Polyunsaturated Fatty Acids with Cancer Risk and Progression. Front. Oncol. 2013, 3, 224. [Google Scholar] [CrossRef] [PubMed]
- Vara-Messler, M.; Buccellati, C.; Pustina, L.; Folco, G.; Rovati, G.E.; Hoxha, M. A Potential Role of PUFAs and COXIBs in Cancer Chemoprevention. Prostaglandins Other Lipid Mediat. 2015, 120, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Shan, K.; Chen, H.; Chen, Y.Q. N-3 Polyunsaturated Fatty Acids and Their Role in Cancer Chemoprevention. Curr. Pharmacol. Rep. 2015, 1, 283–294. [Google Scholar] [CrossRef]
- Cockbain, A.J.; Volpato, M.; Race, A.D.; Munarini, A.; Fazio, C.; Belluzzi, A.; Loadman, P.M.; Toogood, G.J.; Hull, M.A. Anticolorectal Cancer Activity of the Omega-3 Polyunsaturated Fatty Acid Eicosapentaenoic Acid. Gut 2014, 63, 1760–1768. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Fuchs, C.S.; Niedzwiecki, D.; Ye, X.; Zhang, S.; Song, M.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; et al. Marine ω-3 Polyunsaturated Fatty Acid and Fish Intake after Colon Cancer Diagnosis and Survival: CALGB 89803 (Alliance). Cancer Epidemiol. Biomark. Prev. 2018, 27, 438–445. [Google Scholar] [CrossRef]
- Hull, M.A.; Sprange, K.; Hepburn, T.; Tan, W.; Shafayat, A.; Rees, C.J.; Clifford, G.; Logan, R.F.; Loadman, P.M.; Williams, E.A.; et al. Eicosapentaenoic Acid and Aspirin, Alone and in Combination, for the Prevention of Colorectal Adenomas (SeAFOod Polyp Prevention Trial): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, 2 × 2 Factorial Trial. Lancet 2018, 392, 2583–2594. [Google Scholar] [CrossRef]
- Fasano, E.; Serini, S.; Cittadini, A.; Calviello, G. Long-Chain n-3 PUFA against Breast and Prostate Cancer: Which Are the Appropriate Doses for Intervention Studies in Animals and Humans? Crit. Rev. Food Sci. Nutr. 2017, 57, 2245–2262. [Google Scholar] [CrossRef]
- Aronson, W.J.; Kobayashi, N.; Barnard, R.J.; Henning, S.; Huang, M.; Jardack, P.M.; Liu, B.; Gray, A.; Wan, J.; Konijeti, R.; et al. Phase II Prospective Randomized Trial of a Low-Fat Diet with Fish Oil Supplementation in Men Undergoing Radical Prostatectomy. Cancer Prev. Res. 2011, 4, 2062–2071. [Google Scholar] [CrossRef]
- Galet, C.; Gollapudi, K.; Stepanian, S.; Byrd, J.B.; Henning, S.M.; Grogan, T.; Elashoff, D.; Heber, D.; Said, J.; Cohen, P.; et al. Effect of a Low-Fat Fish Oil Diet on Proinflammatory Eicosanoids and Cell-Cycle Progression Score in Men Undergoing Radical Prostatectomy. Cancer Prev. Res. 2014, 7, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Guertin, M.-H.; Robitaille, K.; Pelletier, J.-F.; Duchesne, T.; Julien, P.; Savard, J.; Bairati, I.; Fradet, V. Effects of Concentrated Long-Chain Omega-3 Polyunsaturated Fatty Acid Supplementation before Radical Prostatectomy on Prostate Cancer Proliferation, Inflammation, and Quality of Life: Study Protocol for a Phase IIb, Randomized, Double-Blind, Placebo-Controlled Trial. BMC Cancer 2018, 18, 64. [Google Scholar] [CrossRef]
- Fabian, C.J.; Kimler, B.F.; Hursting, S.D. Omega-3 Fatty Acids for Breast Cancer Prevention and Survivorship. Breast Cancer Res. 2015, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Paixão, E.M.d.S.; Oliveira, A.C.d.M.; Pizato, N.; Muniz-Junqueira, M.I.; Magalhães, K.G.; Nakano, E.Y.; Ito, M.K. The Effects of EPA and DHA Enriched Fish Oil on Nutritional and Immunological Markers of Treatment Naïve Breast Cancer Patients: A Randomized Double-Blind Controlled Trial. Nutr. J. 2017, 16, 71. [Google Scholar] [CrossRef]
- Bougnoux, P.; Hajjaji, N.; Ferrasson, M.N.; Giraudeau, B.; Couet, C.; Le Floch, O. Improving Outcome of Chemotherapy of Metastatic Breast Cancer by Docosahexaenoic Acid: A Phase II Trial. Br. J. Cancer 2009, 101, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Darwito, D.; Dharmana, E.; Riwanto, I.; Budijitno, S.; Suwardjo, S.; Purnomo, J.; Widodo, I.; Ghozali, A.; Aryandono, T.; Anwar, S.L. Effects of Omega-3 Supplementation on Ki-67 and VEGF Expression Levels and Clinical Outcomes of Locally Advanced Breast Cancer Patients Treated with Neoadjuvant CAF Chemotherapy: A Randomized Controlled Trial Report. Asian Pac. J. Cancer Prev. 2019, 20, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Newell, M.; Mackey, J.R.; Bigras, G.; Alvarez-Camacho, M.; Goruk, S.; Ghosh, S.; Schmidt, A.; Miede, D.; Chisotti, A.; Postovit, L.; et al. Comparing Docosahexaenoic Acid (DHA) Concomitant with Neoadjuvant Chemotherapy versus Neoadjuvant Chemotherapy Alone in the Treatment of Breast Cancer (DHA WIN): Protocol of a Double-Blind, Phase II, Randomised Controlled Trial. BMJ Open 2019, 9, e030502. [Google Scholar] [CrossRef]
- Lee, K.H.; Seong, H.J.; Kim, G.; Jeong, G.H.; Kim, J.Y.; Park, H.; Jung, E.; Kronbichler, A.; Eisenhut, M.; Stubbs, B.; et al. Consumption of Fish and ω-3 Fatty Acids and Cancer Risk: An Umbrella Review of Meta-Analyses of Observational Studies. Adv. Nutr. 2020, 11, 1134–1149. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the Hallmarks of Cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).