Cytotoxic and Apoptotic Effects of Pinostilbene and Bortezomib Combination Treatment on Human Multiple Myeloma Cells
Abstract
1. Introduction
2. Results
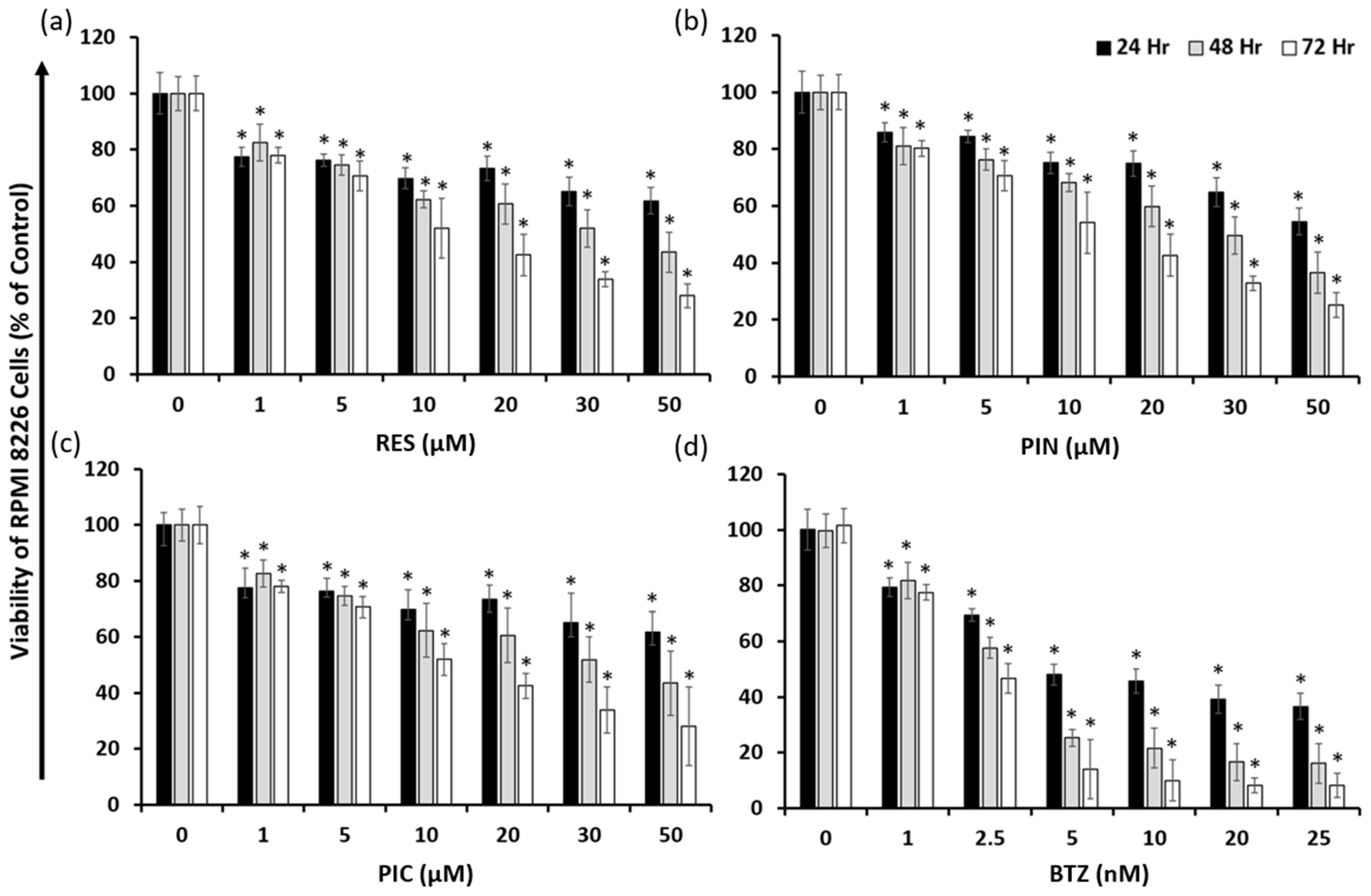
2.1. Resveratrol, Pinostilbene, Piceatannol, and Bortezomib Inhibit Multiple Myeloma RPMI 8226 Cell Growth
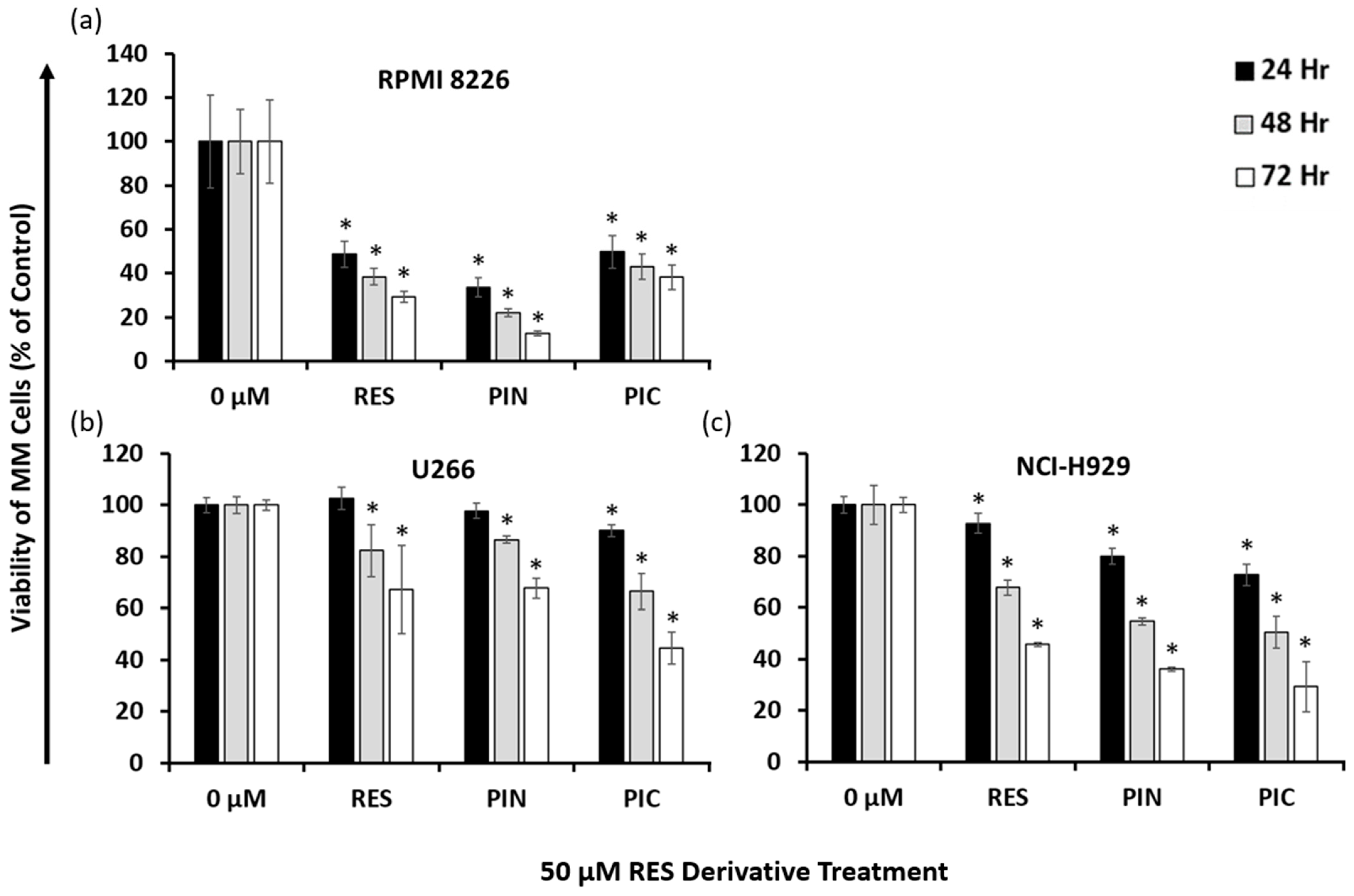
2.2. Resveratrol Derivatives Have Varying Cytotoxic Effects on Different Human MM Cell Lines
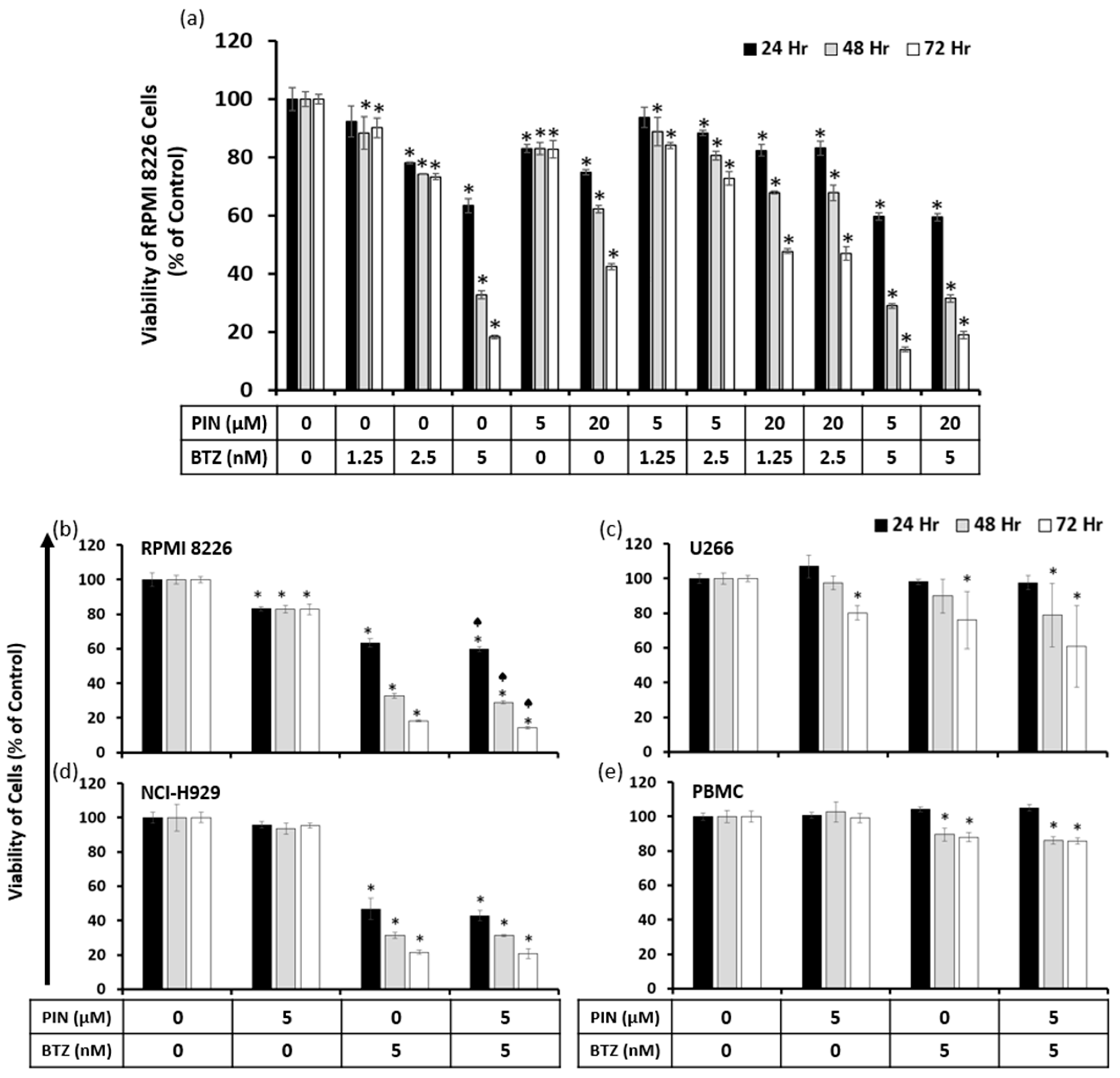
2.3. A Combination of 5 μM PIN and 5 nM BTZ Is Synergistically Cytotoxic to MM Cells and Is Minimally Toxic to Normal Human Peripheral Blood Mononuclear Cells (PBMC)
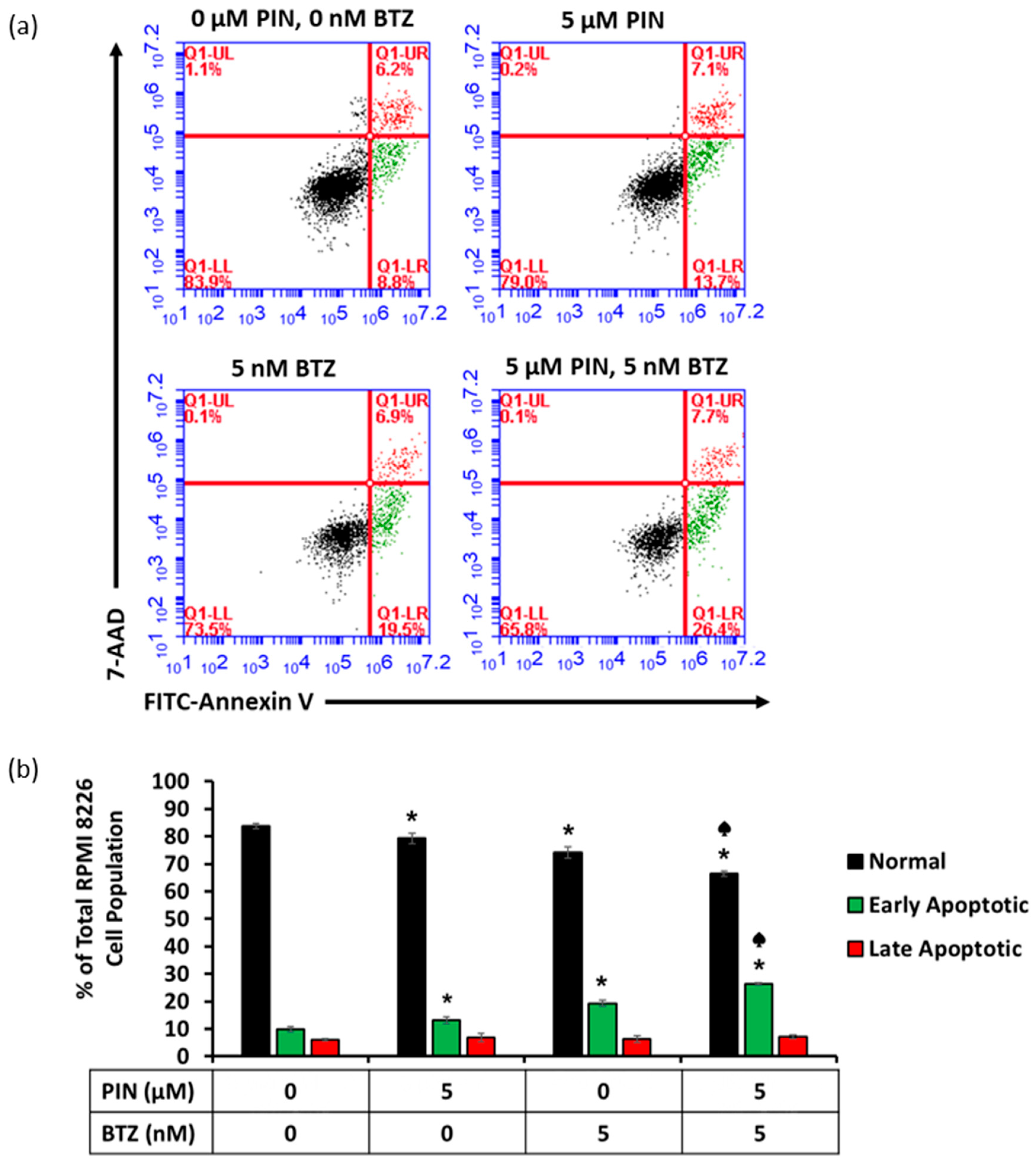
2.4. The 5 μM PIN and 5 nM BTZ Combination Treatment Increases Percentages of Apoptotic RPMI 8226 Cells
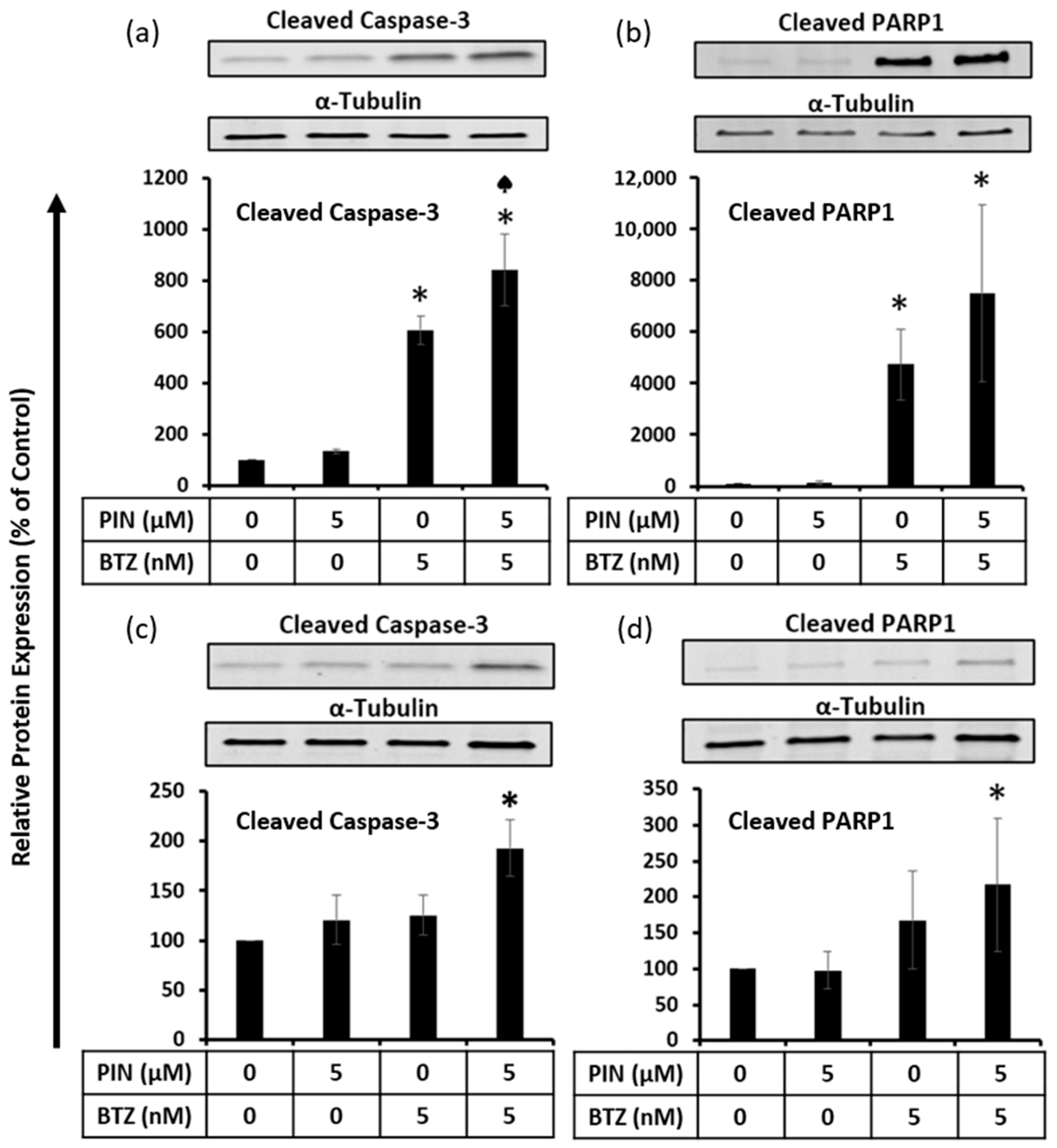
2.5. The 5 μM PIN and 5 nM BTZ Combination Treatment Increases Proapoptotic Protein Expression in MM RPMI 8226 Cells Cultured Individually and Co-Cultured with Human Bone Marrow Stromal HS-5 Cells
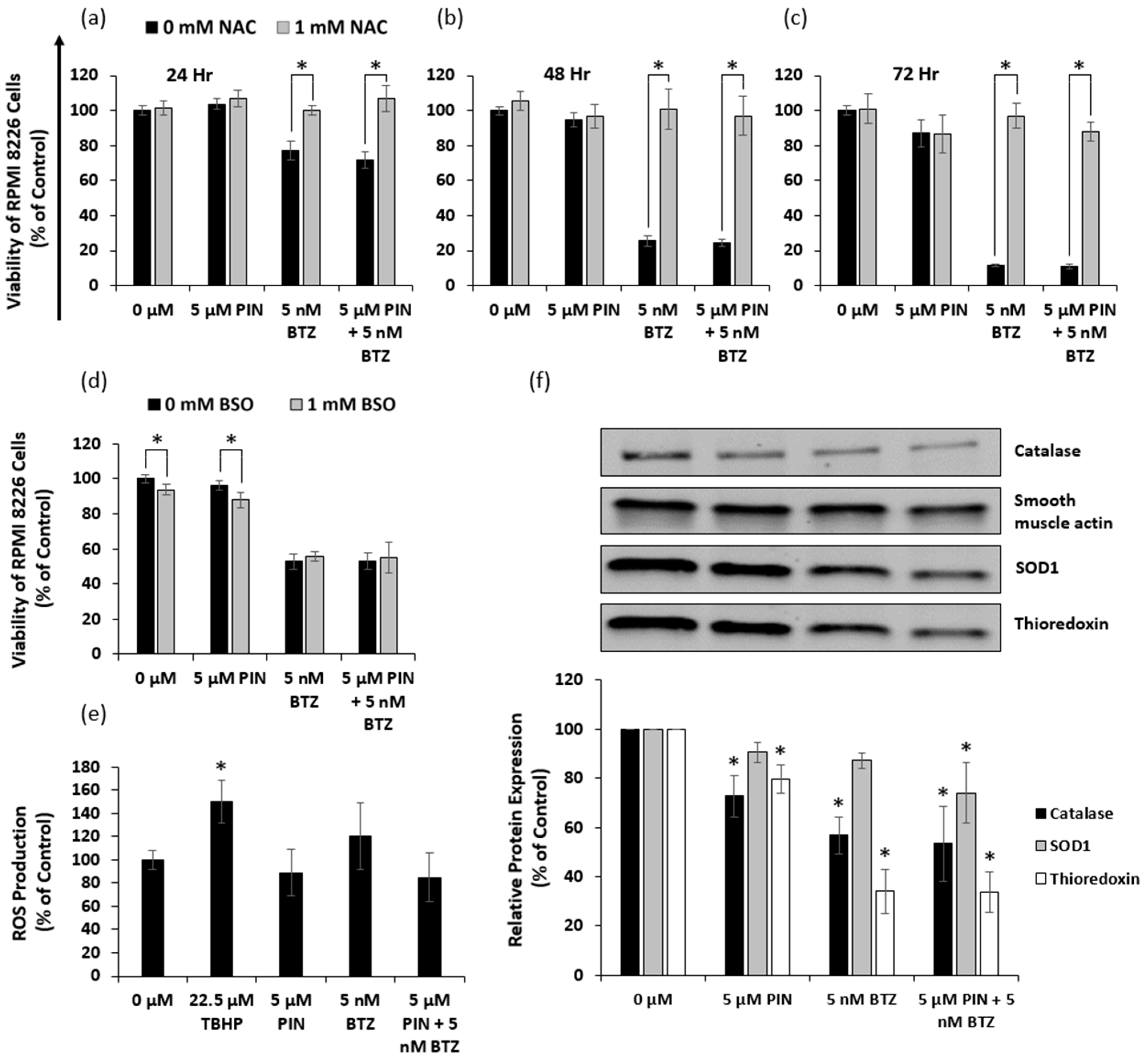
2.6. The 5 μM PIN and 5 nM BTZ Combination Treatment Affects the Oxidative State of MM RPMI 8226 Cells
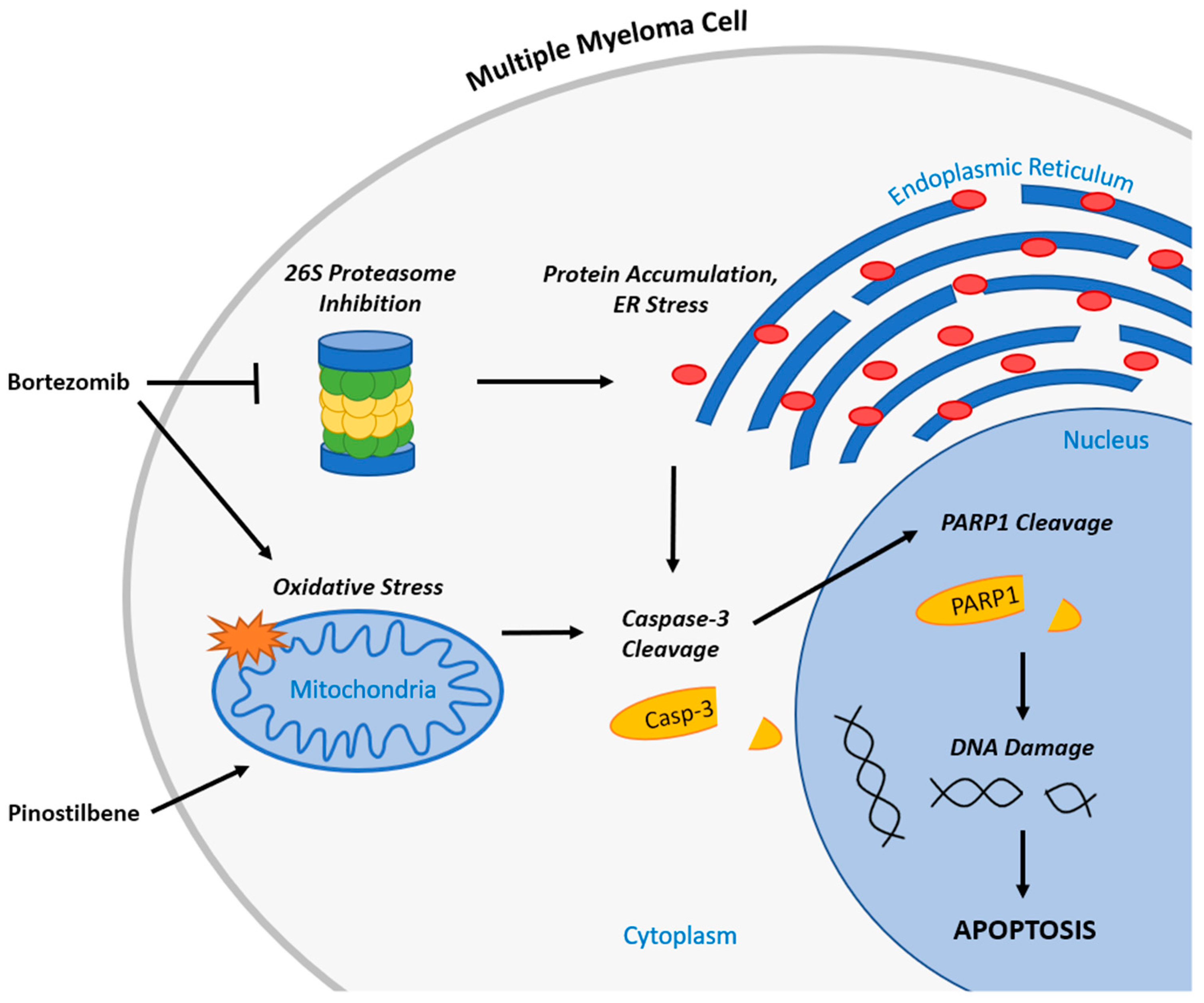
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Viability Assays
4.4. Synergism Identification
4.5. Western Blotting
4.6. Flow Cytometry Analysis
4.7. Oxidative Stress Analysis
4.8. Transwell Model
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Kyle, R.A.; Pfeiffer, R.M.; Katzmann, J.A.; Caporaso, N.E.; Hayes, R.B.; Dispenzieri, A.; Kumar, S.; Clark, R.J.; Baris, D.; et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood 2009, 113, 5412–5417. [Google Scholar] [CrossRef]
- Nakaya, A.; Fujita, S.; Satake, A.; Nakanishi, T.; Azuma, Y.; Tsubokura, Y.; Hotta, M.; Yoshimura, H.; Ishii, K.; Ito, T.; et al. Impact of CRAB Symptoms in Survival of Patients with Symptomatic Myeloma in Novel Agent Era. Hematol. Rep. 2017, 9, 6887. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, Z.; Cheng, H.; Mao, X.; Sui, W.; Deng, S.; Wei, X.; Lv, J.; Du, C.; Xu, J.; et al. Multiple myeloma hinders erythropoiesis and causes anaemia owing to high levels of CCL3 in the bone marrow microenvironment. Sci. Rep. 2020, 10, 20508. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Dimopoulos, M.A. Pathogenesis of bone disease in multiple myeloma: From bench to bedside. Blood Cancer J. 2018, 8, 7. [Google Scholar] [CrossRef]
- Oyajobi, B.O. Multiple myeloma/hypercalcemia. Arthritis Res. Ther. 2007, 9, S4. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Kastritis, E.; Rosinol, L.; Bladé, J.; Ludwig, H. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia 2008, 22, 1485–1493. [Google Scholar] [CrossRef]
- Gulla, A.; Anderson, K.C. Multiple myeloma: The (r)evolution of current therapy and a glance into the future. Haematologica 2020, 105, 2358–2367. [Google Scholar] [CrossRef]
- Talamo, G.; Dimaio, C.; Abbi, K.K.S.; Pandey, M.K.; Malysz, J.; Creer, M.H.; Zhu, J.; Mir, M.A.; Varlotto, J.M. Current Role of Radiation Therapy for Multiple Myeloma. Front. Oncol. 2015, 5, 40. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef]
- Bazarbachi, A.H.; Al Hamed, R.; Malard, F.; Harousseau, J.-L.; Mohty, M. Relapsed refractory multiple myeloma: A comprehensive overview. Leukemia 2019, 33, 2343–2357. [Google Scholar] [CrossRef] [PubMed]
- Fu, J. Cx43 expressed on bone marrow stromal cells plays an essential role in multiple myeloma cell survival and drug resistance. Arch. Med. Sci. 2017, 13, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Zeldenrust, S.R.; Dingli, D.; Russell, S.J.; Lust, J.A.; et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008, 111, 2516–2520. [Google Scholar] [CrossRef] [PubMed]
- Field-Smith, A.; Morgan, G.J.; Davies, F.E. Bortezomib (Velcade™) in the Treatment of Multiple Myeloma. Ther. Clin. Risk Manag. 2006, 2, 271–279. [Google Scholar] [CrossRef]
- Ito, S. Proteasome Inhibitors for the Treatment of Multiple Myeloma. Cancers 2020, 12, 265. [Google Scholar] [CrossRef]
- Lopez, J.; Tait, S.W.G. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef]
- Richardson, P.G.; Barlogie, B.; Berenson, J.; Singhal, S.; Jagannath, S.; Irwin, D.; Rajkumar, S.V.; Srkalovic, G.; Alsina, M.; Alexanian, R.; et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N. Engl. J. Med. 2003, 348, 2609–2617. [Google Scholar] [CrossRef]
- Mohan, M.; Matin, A.; Davies, F.E. Update on the optimal use of bortezomib in the treatment of multiple myeloma. Cancer Manag. Res. 2017, 9, 51–63. [Google Scholar] [CrossRef]
- Kalantari, H.; Das, D.K. Physiological effects of resveratrol. BioFactors 2010, 36, 401–406. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res./Rev. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Boissy, P.; Andersen, T.L.; Abdallah, B.M.; Kassem, M.; Plesner, T.; Delaissé, J.-M. Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Cancer Res. 2005, 65, 9943–9952. [Google Scholar] [CrossRef] [PubMed]
- Schmeel, F.C.; Schmeel, L.C.; Kim, Y.; Schmidt-Wolf, I.G.H. Piceatannol exhibits selective toxicity to multiple myeloma cells and influences the Wnt/ beta-catenin pathway. Hematol. Oncol. 2014, 32, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, X.; Cai, X.; Song, M.; Zheng, J.; Pan, C.; Qiu, P.; Zhang, L.; Zhou, S.; Tang, Z.; et al. Identification of pinostilbene as a major colonic metabolite of pterostilbene and its inhibitory effects on colon cancer cells. Mol. Nutr. Food Res. 2016, 60, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-J.; Chin, M.-C.; Lin, C.-C.; His, Y.-T.; Lo, Y.-S.; Chuang, Y.-C.; Chen, M.-K. Pinostilbene Hydrate Suppresses Human Oral Cancer Cell Metastasis by Downregulation of Matrix Metalloproteinase-2 Through the Mitogen-Activated Protein Kinase Signaling Pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 50, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-G.; Wu, G.-Z.; Wu, G.-H.; Bao, Y.-G. Combining the mammalian target of rapamycin inhibitor, rapamycin, with resveratrol has a synergistic effect in multiple myeloma. Oncol. Lett. 2018, 15, 6257–6264. [Google Scholar] [CrossRef]
- Li, Q.; Yue, Y.; Chen, L.; Xu, C.; Wang, Y.; Du, L.; Xue, X.; Liu, Q.; Wang, Y.; Fan, F. Resveratrol Sensitizes Carfilzomib-Induced Apoptosis via Promoting Oxidative Stress in Multiple Myeloma Cells. Front. Pharmacol. 2018, 9, 334. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Sethi, G.; Vadhan-Raj, S.; Bueso-Ramos, C.; Takada, Y.; Gaur, U.; Nair, A.S.; Shishodia, S.; Aggarwal, B.B. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood 2007, 109, 2293–2302. [Google Scholar] [CrossRef]
- Miki, H.; Uehara, N.; Kimura, A.; Sasaki, T.; Yuri, T.; Yoshizawa, K.; Tsubura, A. Resveratrol induces apoptosis via ROS-triggered autophagy in human colon cancer cells. Int. J. Oncol. 2012, 40, 1020–1028. [Google Scholar] [CrossRef]
- Lang, F.; Qin, Z.; Li, F.; Zhang, H.; Fang, Z.; Hao, E. Apoptotic Cell Death Induced by Resveratrol Is Partially Mediated by the Autophagy Pathway in Human Ovarian Cancer Cells. PLoS ONE 2015, 10, e0129196. [Google Scholar] [CrossRef]
- Zhou, R.; Ma, R.; Jin, Z.; Tang, L.; Zhou, Y.; Zhang, Y. Resveratrol increases the sensitivity of multiple myeloma cells against bortezomib via Hedgehog signaling pathway. Trop. J. Pharm. Res. 2019, 18, 2133–2138. [Google Scholar] [CrossRef]
- Raab, M.S.; Podar, K.; Breitkreutz, I.; Richardson, P.G.; Anderson, K.C. Multiple myeloma. Lancet 2009, 374, 324–339. [Google Scholar] [CrossRef]
- Kazandjian, D. Multiple myeloma epidemiology and survival, a unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, L.; Zhu, F.; Han, X.; Sun, L.; Chen, K. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4T1 Cells. Toxins 2019, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, Z.; Zhang, X. Resveratrol Induces Apoptosis in Murine Prostate Cancer Cells via Hypoxia-Inducible Factor 1-alpha (HIF-1α)/Reactive Oxygen Species (ROS)/P53 Signaling. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 8970–8976. [Google Scholar] [CrossRef]
- Cheng, L.; Yan, B.; Chen, K.; Jiang, Z.; Zhou, C.; Cao, J.; Qian, W.; Li, J.; Sun, L.; Ma, J.; et al. Resveratrol-Induced Downregulation of NAF-1 Enhances the Sensitivity of Pancreatic Cancer Cells to Gemcitabine via the ROS/Nrf2 Signaling Pathways. Oxidative Med. Cell. Longev. 2018, 2018, 9482018. [Google Scholar] [CrossRef] [PubMed]
- Sergides, C.; Chirilă, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717. [Google Scholar] [CrossRef]
- Lai, X.; Huang, C.; Nie, X.; Chen, Q.; Tang, Y.; Fu, X.; Lin, Y.; Nie, C.; Xu, X.; Wang, X.; et al. Bortezomib Inhibits Multiple Myeloma Cells by Transactivating ATF3 to Trigger miR-135a-5p-Dependent Apoptosis. Front. Oncol. 2021, 11, 720261. [Google Scholar] [CrossRef]
- Que, W.; Li, S.; Chen, J. NS-398 enhances the efficacy of bortezomib against RPMI8226 human multiple myeloma cells. Mol. Med. Rep. 2013, 7, 1641–1645. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Hu, Y.; Guo, T.; Wang, H.-F.; Zhang, X.-P.; He, W.-J.; Tan, H. Resveratrol as a novel agent for treatment of multiple myeloma with matrix metalloproteinase inhibitory activity. Acta Pharmacol. Sin. 2006, 27, 1447–1452. [Google Scholar] [CrossRef]
- Matsui, W.; Huff, C.A.; Wang, Q.; Malehorn, M.T.; Barber, J.; Tanhehco, Y.; Smith, B.D.; Civin, C.I.; Jones, R.J. Characterization of clonogenic multiple myeloma cells. Blood 2004, 103, 2332–2336. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.; Mager, D.E. Pharmacodynamic Models of Differential Bortezomib Signaling Across Several Cell Lines of Multiple Myeloma. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Kleiveland, C.R. Peripheral Blood Mononuclear Cells. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer International Publishing: Cham, Switzerland, 2015; pp. 161–167. [Google Scholar] [CrossRef]
- Hideshima, T.; Ikeda, H.; Chauhan, D.; Okawa, Y.; Raje, N.; Podar, K.; Mitsiades, C.; Munshi, N.C.; Richardson, P.G.; Carrasco, R.D.; et al. Bortezomib induces canonical nuclear factor-κB activation in multiple myeloma cells. Blood 2009, 114, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, A.M.; Leleu, X.; Sacco, A.; Moreau, A.-S.; Hatjiharissi, E.; Jia, X.; Xu, L.; Ciccarelli, B.; Patterson, C.J.; Ngo, H.T.; et al. Resveratrol Exerts Antiproliferative Activity and Induces Apoptosis in Waldenstrom’s macroglobulinemia. Clin. Cancer Res. 2008, 14, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Anson, F.; Thayumanavan, S.; Hardy, J.A. Exogenous Introduction of Initiator and Executioner Caspases Results in Different Apoptotic Outcomes. JACS Au 2021, 1, 1240–1256. [Google Scholar] [CrossRef]
- Shi, Y. Mechanisms of Caspase Activation and Inhibition during Apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- McStay, G.P.; Salvesen, G.S.; Green, D.R. Overlapping cleavage motif selectivity of caspases: Implications for analysis of apoptotic pathways. Cell Death Differ. 2008, 15, 322–331. [Google Scholar] [CrossRef]
- Chaitanya, G.V.; Alexander, J.S.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef]
- Lipchick, B.C.; Fink, E.E.; Nikiforov, M.A. Oxidative stress and proteasome inhibitors in multiple myeloma. Pharmacol. Res. 2016, 105, 210–215. [Google Scholar] [CrossRef]
- Caillot, M.; Dakik, H.; Mazurier, F.; Sola, B. Targeting Reactive Oxygen Species Metabolism to Induce Myeloma Cell Death. Cancers 2021, 13, 2411. [Google Scholar] [CrossRef]
- Min, J.Y.; Lim, S.-O.; Jung, G. Downregulation of catalase by reactive oxygen species via hypermethylation of CpG island II on the catalase promoter. FEBS Lett. 2010, 584, 2427–2432. [Google Scholar] [CrossRef]
- Wang, W.; Adachi, M.; Kawamura, R.; Sakamoto, H.; Hayashi, T.; Ishida, T.; Imai, K.; Shinomura, Y. Parthenolide-induced apoptosis in multiple myeloma cells involves reactive oxygen species generation and cell sensitivity depends on catalase activity. Apoptosis 2006, 11, 2225–2235. [Google Scholar] [CrossRef] [PubMed]
- Raninga, P.V.; Di Trapani, G.; Vuckovic, S.; Bhatia, M.; Tonissen, K.F. Inhibition of thioredoxin 1 leads to apoptosis in drug-resistant multiple myeloma. Oncotarget 2015, 6, 15410–15424. [Google Scholar] [CrossRef] [PubMed]
- Nerini-Molteni, S.; Ferrarini, M.; Cozza, S.; Caligaris-Cappio, F.; Sitia, R. Redox homeostasis modulates the sensitivity of myeloma cells to bortezomib. Br. J. Haematol. 2008, 141, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Avet-Loiseau, H.; Facon, T.; Attal, M.; Tiab, M.; Hulin, C.; Doyen, C.; Garderet, L.; Randriamalala, E.; Araujo, C.; et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood 2011, 118, 5752–5758. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
| 24 h | 48 h | 72 h | |
|---|---|---|---|
| RES | 51.49 ± 5.09 μM | 26.95 ± 1.92 μM | 13.19 ± 0.82 μM |
| PIN | 51.38 ± 3.47 μM | 25.78 ± 1.70 μM | 13.21 ± 0.69 μM |
| PIC | 32.33 ± 2.83 μM | 18.57 ± 1.25 μM | 13.82 ± 0.88 μM |
| BTZ | 8.86 ± 0.82 nM | 3.31 ± 0.33 nM | 2.05 ± 0.22 nM |
| PIN (μM) | BTZ (nM) | 24 h | 48 h | 72 h |
|---|---|---|---|---|
| 5 | 1.25 | 23.1299 | 2.84814 | 1.82595 |
| 5 | 2.5 | 4.98265 | 2.19354 | 1.8127 |
| 5 | 5 | 0.97987 | 0.89462 | 0.90181 |
| 20 | 1.25 | 4.04991 | 1.85988 | 1.55874 |
| 20 | 2.5 | 5.21424 | 2.34567 | 1.91552 |
| 20 | 5 | 1.07322 | 1.10315 | 1.3671 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staskiewicz, A.; Wong, E.; Tucker, M.; Farhin, R.; Park, J.; Saade, R.; Alkhazali, T.; Dang, T.; Wang, X. Cytotoxic and Apoptotic Effects of Pinostilbene and Bortezomib Combination Treatment on Human Multiple Myeloma Cells. Int. J. Mol. Sci. 2023, 24, 12590. https://doi.org/10.3390/ijms241612590
Staskiewicz A, Wong E, Tucker M, Farhin R, Park J, Saade R, Alkhazali T, Dang T, Wang X. Cytotoxic and Apoptotic Effects of Pinostilbene and Bortezomib Combination Treatment on Human Multiple Myeloma Cells. International Journal of Molecular Sciences. 2023; 24(16):12590. https://doi.org/10.3390/ijms241612590
Chicago/Turabian StyleStaskiewicz, Anna, Erica Wong, Michael Tucker, Riya Farhin, Jonathan Park, Rana Saade, Tina Alkhazali, Tu Dang, and Xinyu Wang. 2023. "Cytotoxic and Apoptotic Effects of Pinostilbene and Bortezomib Combination Treatment on Human Multiple Myeloma Cells" International Journal of Molecular Sciences 24, no. 16: 12590. https://doi.org/10.3390/ijms241612590
APA StyleStaskiewicz, A., Wong, E., Tucker, M., Farhin, R., Park, J., Saade, R., Alkhazali, T., Dang, T., & Wang, X. (2023). Cytotoxic and Apoptotic Effects of Pinostilbene and Bortezomib Combination Treatment on Human Multiple Myeloma Cells. International Journal of Molecular Sciences, 24(16), 12590. https://doi.org/10.3390/ijms241612590




_Wang.png)


