Proteomics-Based Identification of Retinal Protein Networks Impacted by Elevated Intraocular Pressure in the Hypertonic Saline Injection Model of Experimental Glaucoma
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Induction of OHT and IOP Measurement
4.4. Retina Collection
4.5. Discovery-Driven Label-Free Retina Proteomics
4.6. Targeted Proteomics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kingman, S. Glaucoma is second leading cause of blindness globally. Bull. World Health Organ. 2004, 82, 887–888. [Google Scholar]
- Casson, R.J.; Chidlow, G.; Wood, J.P.M.; Crowston, J.G.; Goldberg, I. Definition of glaucoma: Clinical and experimental concepts. Clin. Exp. Ophthalmol. 2012, 40, 341–349. [Google Scholar] [CrossRef]
- Osborne, N.N.; Wood, J.P.M.; Chidlow, G.; Bae, J.-H.; Melena, J.; Nash, M.S. Ganglion cell death in glaucoma: What do we really know? Br. J. Ophthalmol. 1999, 83, 980–986. [Google Scholar] [CrossRef][Green Version]
- Quigley, H.A. Neuronal death in glaucoma. Prog. Retin. Eye Res. 1999, 18, 39–57. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma—A review. JAMA-J. Am. Med. Assoc. 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Leske, C.M. Open-angle glaucoma—An epidemiologic overview. Ophthalmic Epidemiol. 2007, 14, 166–172. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.O.; Beiser, J.A.; Brandt, J.D.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K.; Wilson, M.R.; et al. The ocular hypertension treatment study—Baseline factors that predict the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 714–720. [Google Scholar] [CrossRef]
- Prokai, L.; Zaman, K.; Prokai-Tatrai, K. Mass spectrometry-based retina proteomics. Mass Spectrom. Rev. 2023, 42, 1032–1062. [Google Scholar] [CrossRef]
- Funke, S.; Perumal, N.; Beck, S.; Gabel-Scheurich, S.; Schmelter, C.; Teister, J.; Gerbig, C.; Gramlich, O.W.; Pfeiffer, N.; Grus, F.H. Glaucoma related proteomic alterations in human retina samples. Sci. Rep. 2016, 6, 29759. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Gupta, V.B.; Chick, J.M.; Greco, T.M.; Wu, Y.; Chitranshi, N.; Wall, R.V.; Hone, E.; Deng, L.; Dheer, Y.; et al. Age-related neurodegenerative disease associated pathways identified in retinal and vitreous proteome from human glaucoma eyes. Sci. Rep. 2017, 7, 12685. [Google Scholar] [CrossRef]
- Miyara, N.; Shinzato, M.; Yamashiro, Y.; Iwamatsu, A.; Kariya, K.; Sawaguchi, S. Proteomic analysis of rat retina in a steroid-induced ocular hypertension model: Potential vulnerability to oxidative stress. Jpn. J. Ophthalmol. 2008, 52, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Kanamoto, T.; Ue, T.; Yokoyama, T.; Souchelnytskyi, N.; Kiuchi, Y. Proteomic study of DBA/2J mice retina: Down-regulation of integrin β7 correlated with retinal ganglion cell death. Proteomics 2009, 9, 4962–4969. [Google Scholar] [CrossRef] [PubMed]
- Schallenberg, M.; Prokosch, V.; Thanos, S. Regulation of retinal proteome by topical antiglaucomatous eye drops in an inherited glaucoma rat model. PLoS ONE 2012, 7, e33593. [Google Scholar] [CrossRef]
- Prokosch, V.; Schallenberg, M.; Thanos, S. Crystallins are regulated biomarkers for monitoring topical therapy of glaucomatous optic neuropathy. PLoS ONE 2012, 8, e49730. [Google Scholar] [CrossRef]
- Baggerman, G.; Vierstraete, E.; De Loof, A.; Schoofs, L. Gel-based versus gel-free proteomics: A review. Comb. Chem. High Throughput Screen. 2005, 8, 669–677. [Google Scholar] [CrossRef]
- Rabilloud, T.; Chevallet, M.; Luche, S.; Lelong, C. Two-dimensional gel electrophoresis in proteomics: Past, present and future. J. Proteomics 2010, 73, 2064–2077. [Google Scholar] [CrossRef]
- Stowell, C.; Arbogast, B.; Cioffi, G.; Burgoyne, C.; Zhou, A. Retinal proteomic changes following unilateral optic nerve transection and early experimental glaucoma in non-human primate eyes. Exp. Eye Res. 2011, 93, 13–28. [Google Scholar] [CrossRef]
- Burgoyne, C.F. The non-human primate experimental glaucoma model. Exp. Eye Res. 2015, 141, 57–73. [Google Scholar] [CrossRef]
- Johnson, T.; Tomarev, S. Animal models of glaucoma. In Animal Models of Ophthalmic Diseases; Essentials in Ophthalmology Book Series; Chan, C.C., Ed.; Springer: Cham, Switzerland, 2016; pp. 31–50. [Google Scholar]
- Biswas, S.; Wan, K.H. Review of rodent hypertensive glaucoma models. Acta Ophthalmol. 2019, 97, E331–E340. [Google Scholar] [CrossRef] [PubMed]
- Anders, F.; Teister, J.; Funke, S.; Pfeiffer, N.; Grus, F.; Solon, T.; Prokosch, V. Proteomic profiling reveals crucial retinal protein alterations in the early phase of an experimental glaucoma model. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1395–1407. [Google Scholar] [CrossRef]
- Mirzaei, M.; Gupta, V.K.; Chitranshi, N.; Deng, L.T.; Pushpitha, K.; Abbasi, M.; Chick, J.M.; Rajput, R.; Wu, Y.Q.; McKay, M.J.; et al. Retinal proteomics of experimental glaucoma model reveal intraocular pressure-induced mediators of neurodegenerative changes. J. Cell. Biochem. 2020, 121, 4931–4944. [Google Scholar] [CrossRef] [PubMed]
- Shareef, S.R.; Garcia-Valenzuela, E.; Salierno, A.; Walsh, J.; Sharma, S.C. Chronic ocular hypertension following episcleral venous occlusion in rats. Exp. Eye Res. 1995, 61, 379–382. [Google Scholar] [CrossRef]
- Sappington, R.M.; Carlson, B.J.; Crish, S.D.; Calkins, D.J. The microbead occlusion model: A paradigm for induced ocular hypertension in rats and mice. Invest. Ophthalmol. Vis. Sci. 2010, 51, 207–216. [Google Scholar] [CrossRef]
- Morrison, J.C.; Moore, C.G.; Deppmeier, L.M.; Gold, B.G.; Meshul, C.K.; Johnson, E.C. A rat model of chronic pressure-induced optic nerve damage. Exp. Eye Res. 1997, 64, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.C. Elevated intraocular pressure and optic nerve injury models in the rat. J. Glaucoma 2005, 14, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.C.; Cepurna, W.O.; Johnson, E.C. Modeling glaucoma in rats by sclerosing aqueous outflow pathways to elevate intraocular pressure. Exp. Eye Res. 2015, 141, 23–32. [Google Scholar] [CrossRef]
- Johnson, N.P.; Gregorich, S.S.; Passaglia, C.L. Spatiotemporal contrast sensitivity of Brown-Norway rats under scotopic and photopic illumination. Neuroscience 2020, 449, 63–73. [Google Scholar] [CrossRef]
- Vizcaíno, J.A.; Deutsch, E.W.; Wang, R.; Csordas, A.; Reisinger, F.; Ríos, D.; Dianes, J.A.; Sun, Z.; Farrah, T.; Bandeira, N.; et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014, 30, 223–226. [Google Scholar] [CrossRef]
- Zhang, B.; VerBerkmoes, N.C.; Langston, M.A.; Uberbacher, E.; Hettich, R.L.; Samatova, N.F. Detecting differential and correlated protein expression in label-free shotgun proteomics. J. Proteome Res. 2006, 5, 2909–2918. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Heil, L.R.; Remes, P.M.; MacCoss, M.J. Comparison of unit resolution versus high-resolution accurate mass for parallel reaction monitoring. J. Proteome Res. 2021, 20, 4435–4442. [Google Scholar] [CrossRef]
- Almasieh, M.; Levin, L.A. Neuroprotection in glaucoma: Animal models and clinical trials. Ann. Rev. Vision Sci. 2017, 3, 91–120. [Google Scholar] [CrossRef]
- Nucci, C.; Martucci, A.; Giannini, C.; Morrone, L.A.; Bagetta, C.; Mancino, R. Neuroprotective agents in the management of glaucoma. Eye 2018, 32, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Evangelho, K.; Mastronardi, C.A.; de-la-Torre, A. Experimental models of glaucoma: A powerful translational tool for the future development of new therapies for glaucoma in humans—A review of the literature. Medicina 2019, 55, 280. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.K.; McCormack, A.L.; Yates, J.R., III. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994, 5, 976–989. [Google Scholar] [CrossRef] [PubMed]
- McAlister, G.C.; Nusinow, D.P.; Jedrychowski, M.P.; Wühr, M.; Huttlin, E.L.; Erickson, B.K.; Rad, R.; Haas, W.; Gygi, S.P. MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal. Chem. 2014, 86, 7150–7158. [Google Scholar] [CrossRef]
- Ow, S.W.; Salim, M.; Noirel, J.; Evans, C.; Rehman, I.; Wright, P.C. iTRAQ underestimation in simple and complex mixtures: “The good, the bad and the ugly”. J. Proteome Res. 2009, 8, 5347–5355. [Google Scholar] [CrossRef]
- Gurdita, A.; Tan, B.; Joos, K.M.; Bizheva, K.; Choh, C. Pigmented and albino rats differ in their responses to moderate, acute and reversible intraocular pressure elevation. Doc. Ophthalmol. 2017, 134, 205–219. [Google Scholar] [CrossRef]
- Nissirios, N.; Chanis, R.; Johnson, E.; Morrison, J.; Cepurna, W.O.; Jia, L.; Mittag, T.; Danias, J. Comparison of anterior segment structures in two rat glaucoma models: An ultrasound biomicroscopic study. Invest. Ophthalmol. Vis. Sci. 2008, 49, 2478–2482. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Martinez-Rincon, T.; Subias, M.; Mendez-Martinez, S.; Pablo, L.E.; Polo, V.; Aragon-Navas, A.; Garcia-Herranz, D.; Feijoo, J.G.; Osuna, I.B.; et al. Influence of sex on neuroretinal degeneration: Six-month follow-up in rats with chronic glaucoma. Invest. Ophthalmol. Vis. Sci. 2021, 62, 9. [Google Scholar] [CrossRef]
- Joyal, J.S.; Gantner, M.L.; Smith, L.E. Retinal energy demands control vascular supply of the retina in development and disease: The role of neuronal lipid and glucose metabolism. Prog. Retin. Eye Res. 2018, 64, 131–156. [Google Scholar] [CrossRef]
- Eells, J.T. Mitochondrial dysfunction in the aging retina. Biology 2019, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Prokosch, V. Energy metabolism in the inner retina in health and glaucoma. Int. J. Mol. Sci. 2021, 22, 3689. [Google Scholar] [CrossRef] [PubMed]
- Barot, M.; Gokulgandhi, M.R.; Mitra, A.K. Mitochondrial dysfunction in retinal diseases. Curr. Eye Res. 2011, 36, 1069–1077. [Google Scholar] [CrossRef]
- Casson, R.J. Medical therapy for glaucoma: A review. Clin. Exp. Ophthalmol. 2022, 50, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. Mitochondria at work: New insights into regulation and dysregulation of cellular energy supply and metabolism. Biomedicines 2020, 8, 526. [Google Scholar] [CrossRef]
- Harrington, S.; Ryter, S.; Plataki, M.; Price, D.; Choi, A. Mitochondria in health, disease, and aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef]
- Surma, M.; Anbarasu, K.; Dutta, S.; Olivera Perez, L.J.; Huang, K.C.; Meyer, J.S.; Das, A. Enhanced mitochondrial biogenesis promotes neuroprotection in human pluripotent stem cell derived retinal ganglion cells. Commun. Biol. 2023, 6, 218. [Google Scholar] [CrossRef]
- Schmidt, M.F.; Gan, Z.Y.; Komander, D.; Dewson, G. Ubiquitin signalling in neurodegeneration: Mechanisms and therapeutic opportunities. Cell Death Differ. 2021, 28, 570–590. [Google Scholar] [CrossRef] [PubMed]
- Tatton, W.G.; Chalmers-Redman, R.M.; Sud, A.; Podos, S.M.; Mittag, T.W. Maintaining mitochondrial membrane impermeability: An opportunity for new therapy in glaucoma? Surv. Ophthalmol. 2001, 45, S277–S283. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.; Thrimawithana, T.; Little, P.J.; Xu, J.; Feng, Z.P.; Zheng, W. The nerve growth factor signaling and its potential as therapeutic target for glaucoma. Biomed Res. Int. 2014, 2014, 759473. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, C.V.; Walker, J.A.; Davidson, C. Oestrogen, ocular function and low-level vision: A review. J. Endocrinol. 2014, 223, 9–18. [Google Scholar] [CrossRef]
- Gauthier, A.C.; Liu, J. Epigenetics and signaling pathways in glaucoma. Biomed. Res. Int. 2017, 2017, 5712341. [Google Scholar] [CrossRef]
- Wang, J.S.; Kefalov, V.J. The cone-specific visual cycle. Prog. Retin. Eye Res. 2011, 30, 115–128. [Google Scholar] [CrossRef]
- Parker, R.O.; Crouch, R.K. Retinol dehydrogenases (RDHs) in the visual cycle. Exp. Eye Res. 2010, 91, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Huang, N.; Lam, D.S.; Leung, C.K. Measurement of photoreceptor layer in glaucoma: A spectral-domain optical coherence tomography study. J. Ophthalmol. 2011, 2011, 264803. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, W.; Lin, W.; Hu, W.; Tang, Z. Neuronal apoptosis, axon damage and synapse loss occur synchronously in acute ocular hypertension. Exp. Eye Res. 2019, 180, 77–85. [Google Scholar] [CrossRef]
- Rozanova, S.; Barkovits, K.; Nikolov, M.; Schmidt, C.; Urlaub, H.; Marcus, K. Quantitative mass spectrometry-based proteomics: An overview. In Quantitative Methods in Proteomics; Methods in Molecular Biology; Marcus, K., Eisenacher, M., Sitek, B., Eds.; Humana: New York, NY, USA, 2021; Volume 2228, Chapter 8; pp. 85–116. [Google Scholar] [CrossRef]
- VanGuilder, H.D.; Bixler, G.V.; Kutzler, L.; Brucklacher, R.M.; Bronson, S.K.; Kimball, S.R.; Freeman, W.R. Multi-modal proteomic analysis of retinal protein expression alterations in a rat model of diabetic retinopathy. PLoS ONE 2011, 6, e16271. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Yang, X.; Luo, C.; Cai, J.; Powell, D.W. An astrocyte-specific proteomics approach to inflammatory responses in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2012, 53, 4220–4233. [Google Scholar] [CrossRef] [PubMed]
- Prokai-Tatrai, K.; Xin, H.; Nguyen, V.; Szarka, S.; Blazics, B.; Prokai, L.; Koulen, P. 17β-Estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Mol. Pharmaceut. 2013, 10, 3253–3261. [Google Scholar] [CrossRef] [PubMed]
- Prokai, L.; Zaman, K.; Nguyen, V.; Prokai-Tatrai, K. 17β-Estradiol delivered in eye drops: Evidence of impact on protein networks and associated biological processes in the rat retina through quantitative proteomics. Pharmaceutics 2020, 12, 101. [Google Scholar] [CrossRef]
- Prokai-Tatrai, K.; Zaman, K.; Nguyen, V.; De La Cruz, D.L.; Prokai, L. Proteomics-based retinal target engagement analysis and retina-targeted delivery of 17β-estradiol by the DHED prodrug for ocular neurotherapy in males. Pharmaceutics 2021, 13, 1392. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef]
- Tutorials: Understanding the p-Value of Overlap Statistic in IPA. Available online: https://tv.qiagenbioinformatics.com/video/19605716/understanding-the-p-value-of (accessed on 29 April 2023).
- Stein, S.; NIST. Libraries of Peptide Tandem Mass Spectra; Standard Reference Database 1C; NIST: Gaithersburg, MD, USA, 2019.
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.; Liebler, D.; MacCoss, M.J. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef]
- Roepstorff, P.; Fohlman, J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed. Mass Spectrom. 1984, 11, 601. [Google Scholar] [CrossRef]

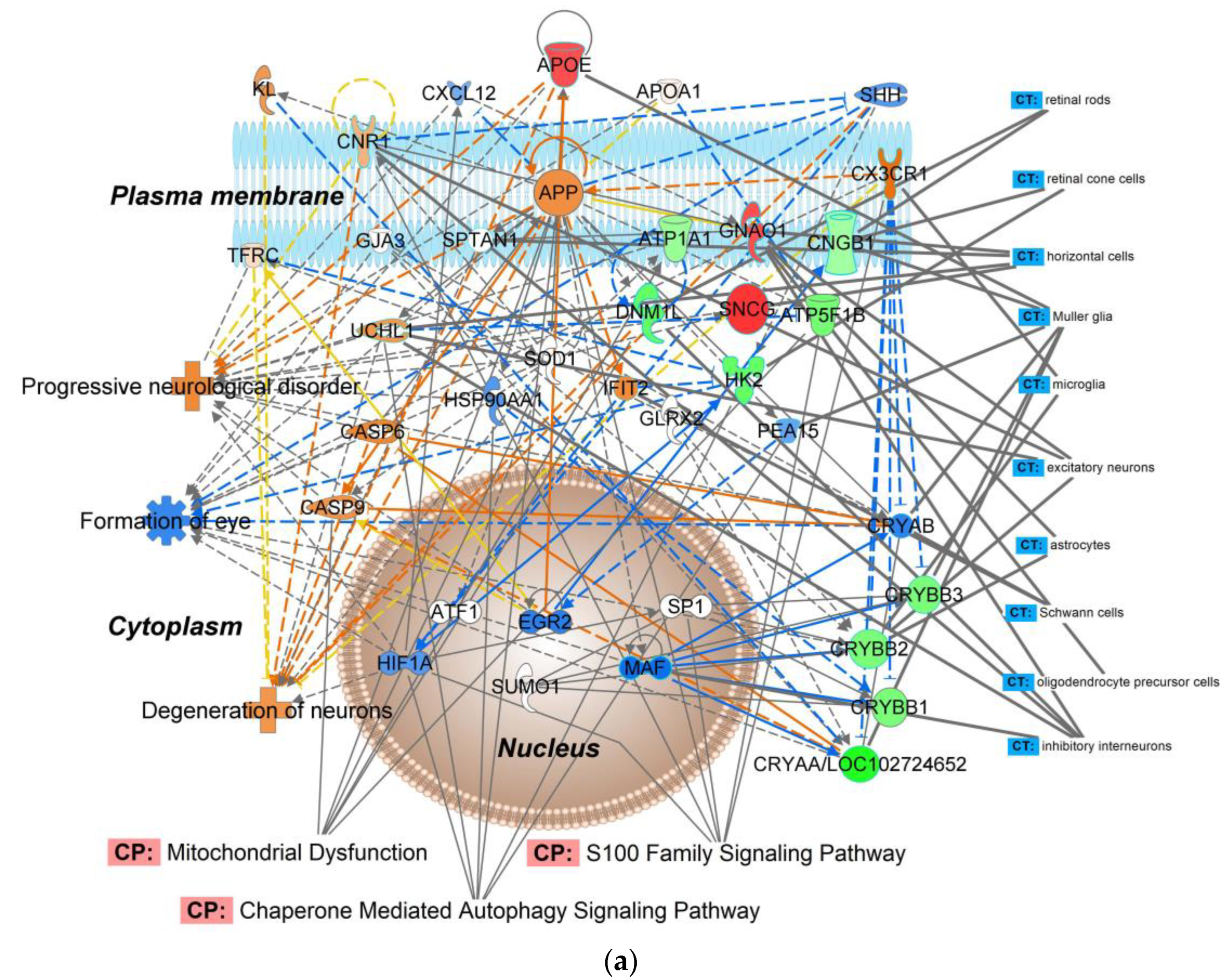
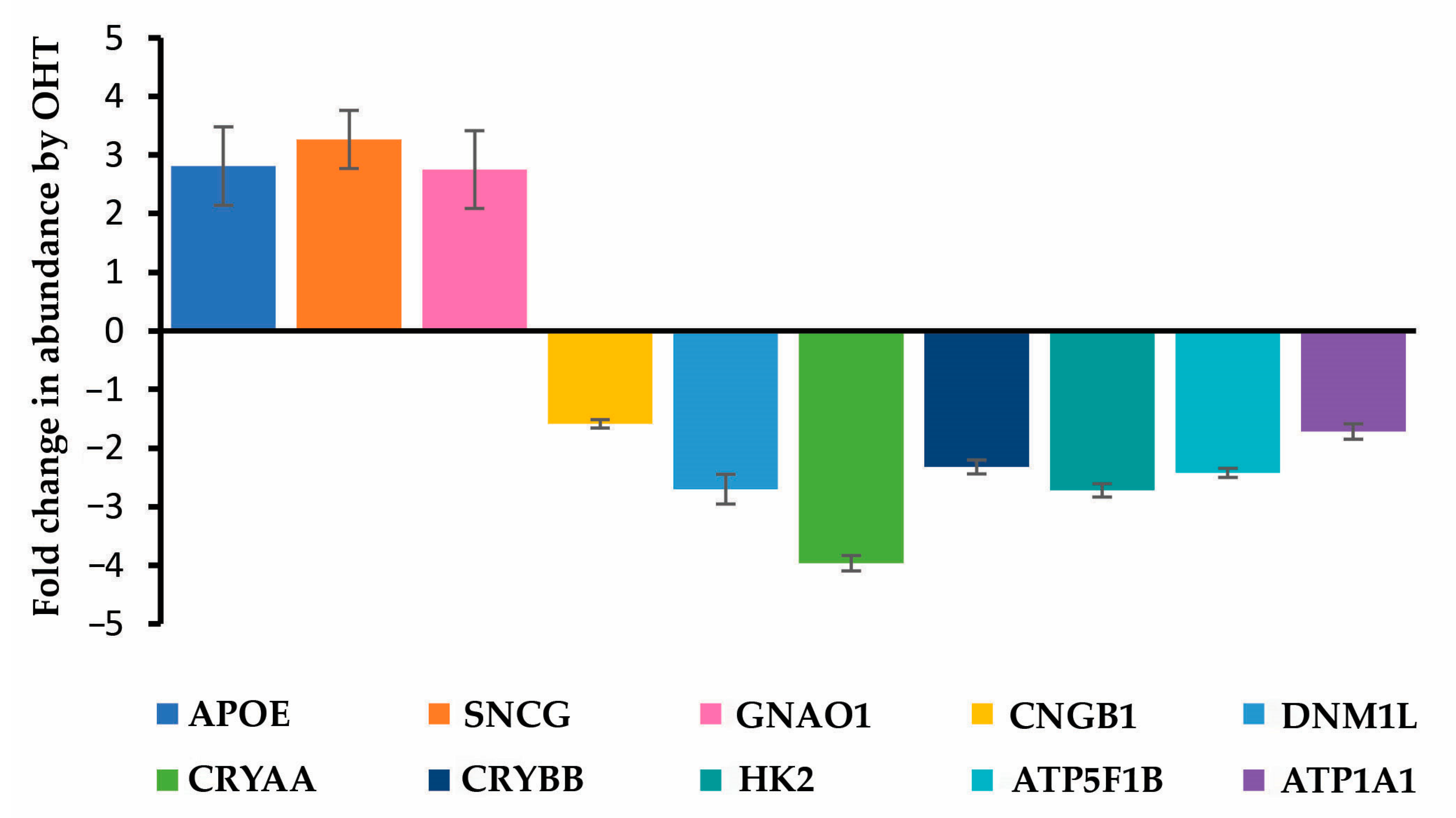
| (a) | ||
| Represented Process | Number of Associated Molecules | p-Value of Overlap |
| Cellular function and maintenance | 19 | 1.96 × 10−6–4.76 × 10−2 |
| Cellular assembly and organization | 18 | 1.96 × 10−6–4.76 × 10−2 |
| Cellular compromise | 15 | 4.99 × 10−5–4.24 × 10−2 |
| Cell death and survival | 14 | 1.93 × 10−5–4.76 × 10−2 |
| Cellular movement | 13 | 1.96 × 10−6–4.99 × 10−2 |
| (b) | ||
| Associated Disease | Number of Associated Molecules | p-Value of Overlap |
| Organismal injury and abnormalities | 31 | 1.46 × 10−6–4.33 × 10−2 |
| Neurological disease | 27 | 1.38 × 10−4–4.33 × 10−2 |
| Metabolic disease | 9 | 3.38 × 10−4–3.48 × 10−2 |
| Ophthalmic disease | 8 | 4.00 × 10−6–3.94 × 10−2 |
| Endocrine system disorder | 4 | 3.38 × 10−5–3.38 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaman, K.; Nguyen, V.; Prokai-Tatrai, K.; Prokai, L. Proteomics-Based Identification of Retinal Protein Networks Impacted by Elevated Intraocular Pressure in the Hypertonic Saline Injection Model of Experimental Glaucoma. Int. J. Mol. Sci. 2023, 24, 12592. https://doi.org/10.3390/ijms241612592
Zaman K, Nguyen V, Prokai-Tatrai K, Prokai L. Proteomics-Based Identification of Retinal Protein Networks Impacted by Elevated Intraocular Pressure in the Hypertonic Saline Injection Model of Experimental Glaucoma. International Journal of Molecular Sciences. 2023; 24(16):12592. https://doi.org/10.3390/ijms241612592
Chicago/Turabian StyleZaman, Khadiza, Vien Nguyen, Katalin Prokai-Tatrai, and Laszlo Prokai. 2023. "Proteomics-Based Identification of Retinal Protein Networks Impacted by Elevated Intraocular Pressure in the Hypertonic Saline Injection Model of Experimental Glaucoma" International Journal of Molecular Sciences 24, no. 16: 12592. https://doi.org/10.3390/ijms241612592
APA StyleZaman, K., Nguyen, V., Prokai-Tatrai, K., & Prokai, L. (2023). Proteomics-Based Identification of Retinal Protein Networks Impacted by Elevated Intraocular Pressure in the Hypertonic Saline Injection Model of Experimental Glaucoma. International Journal of Molecular Sciences, 24(16), 12592. https://doi.org/10.3390/ijms241612592









