An Overview of the Enhanced Effects of Curcumin and Chemotherapeutic Agents in Combined Cancer Treatments
Abstract
1. Introduction
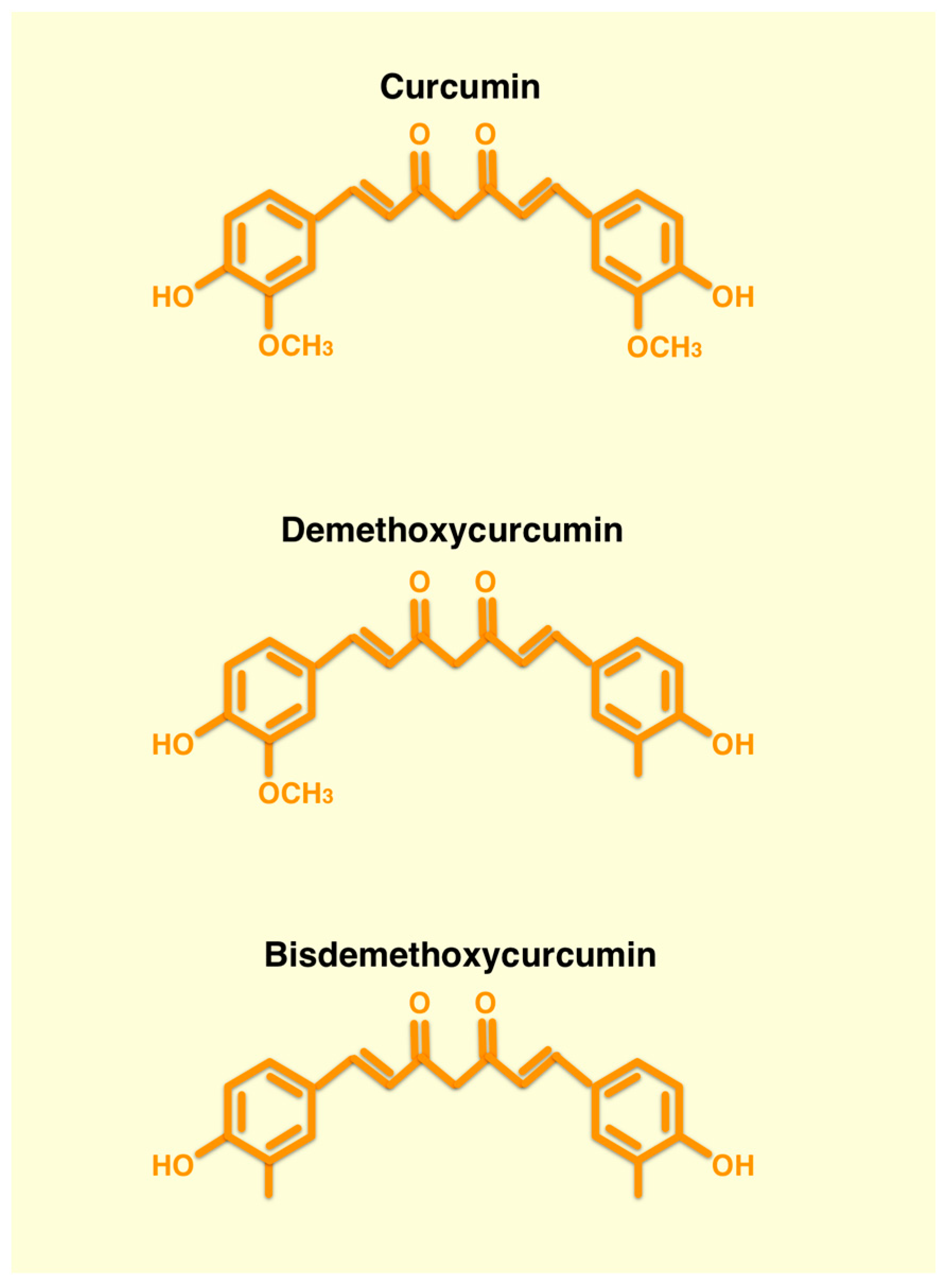
2. Curcumin
2.1. Chemical Structure and Anticancer Effects
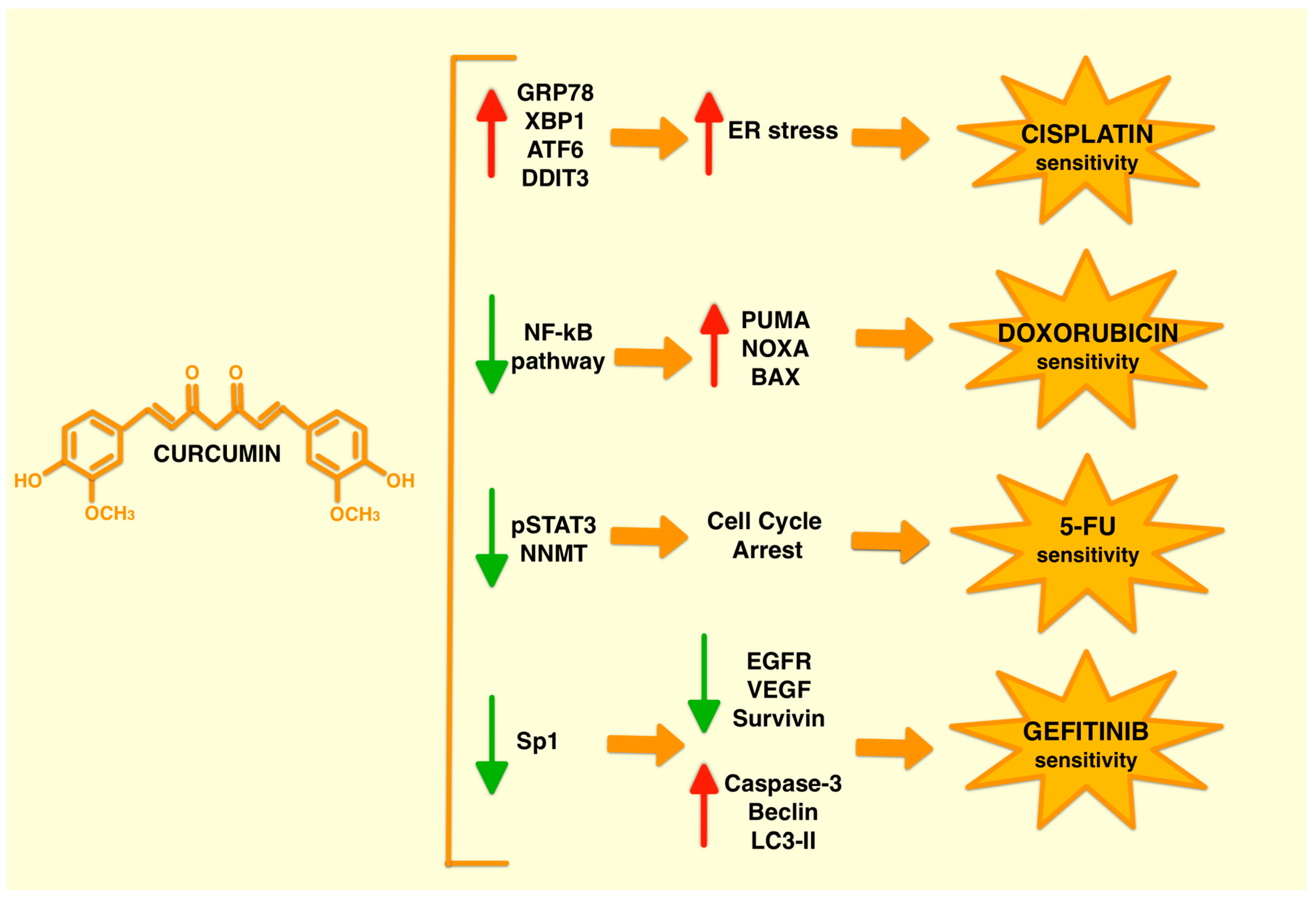
2.2. Curcumin Combined Treatments Affect Different Pathways
3. The Combination of Curcumin and Chemotherapy Drugs in Cancer Therapy
3.1. Curcumin and Cisplatin Combined Treatment
3.2. Curcumin and Doxorubicin Combined Treatment
3.3. Curcumin and 5-Fluorouracil (5-FU) Combined Treatment
3.4. Curcumin and Gefitinib Combined Treatment
4. Combined Treatments in Clinical Trials
5. Curcumin: Benefits and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos, P.; Kyriakidis, M.; Perpinia, A.; Karavidas, A.; Zimeras, S.; Mamalis, N.; Kouvela, M.; Charpidou, A. The Role of Metoprolol and Enalapril in the Prevention of Doxorubicin-induced Cardiotoxicity in Lymphoma Patients. Anticancer. Res. 2019, 39, 5703–5707. [Google Scholar] [CrossRef] [PubMed]
- Aktaş, I.; Özmen, Ö.; Tutun, H.; Yalçın, A.; Türk, A. Artemisinin attenuates doxorubicin induced cardiotoxicity and hepatotoxicity in rats. Biotech. Histochem. 2020, 95, 121–128. [Google Scholar] [CrossRef]
- Wang, Y.; Chao, X.; Ahmad, F.U.D.; Shi, H.; Mehboob, H.; Hassan, W. Protects against Doxorubicin-Induced Cardiotoxicity and Nephrotoxicity. Cardiol. Res. Pract. 2019, 2019, 7395239. [Google Scholar] [CrossRef]
- Wang, W.; Shanmugam, M.K.; Xiang, P.; Yam, T.Y.A.; Kumar, V.; Chew, W.S.; Chang, J.K.; Ali, M.Z.B.; Reolo, M.J.Y.; Peh, Y.X.; et al. Sphingosine 1-Phosphate Receptor 2 Induces Otoprotective Responses to Cisplatin Treatment. Cancers 2020, 12, 211. [Google Scholar] [CrossRef]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Ahmad, J.; Akhter, S.; Greig, N.H.; Kamal, M.A.; Midoux, P.; Pichon, C. Engineered Nanoparticles Against MDR in Cancer: The State of the Art and its Prospective. Curr. Pharm. Des. 2016, 22, 4360–4373. [Google Scholar] [CrossRef]
- Piccolo, M.T.; Menale, C.; Crispi, S. Combined anticancer therapies: An overview of the latest applications. Anticancer. Agents Med. Chem. 2015, 15, 408–422. [Google Scholar] [CrossRef]
- Grkovic, T.; Akee, R.; Thornburg, C.; Trinh, S.; Britt, J.; Harris, M.; Evans, J.; Kang, U.; Ensel, S.; Henrich, C.; et al. National Cancer Institute (NCI) Program for Natural Products Discovery: Rapid Isolation and Identification of Biologically Active Natural Products from the NCI Prefractionated Library. ACS Chem. Biol. 2020, 15, 1104–1114. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef]
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules 2020, 25, 4553. [Google Scholar] [CrossRef] [PubMed]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef]
- Abraham, A.; Kattoor, A.J.; Saldeen, T.; Mehta, J.L. Vitamin E and its anticancer effects. Crit. Rev. Food Sci. Nutr. 2019, 59, 2831–2838. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Filosa, S.; Madonna, M.; Giello, G.; Di Pardo, A.; Maglione, V.; Baldi, A.; Crispi, S. Curcumin C3 complex®/Bioperine® has antineoplastic activity in mesothelioma: An in vitro and in vivo analysis. J. Exp. Clin. Cancer Res. 2019, 38, 360. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Reyes, P.K.; Hernández-Ramírez, N.; Cortés, J.; Poquet, L.; Redeuil, K.; Rangel-Escareño, C.; Kussmann, M.; Silva-Zolezzi, I.; Tejero, M.E. Gene expression changes by high-polyphenols cocoa powder intake: A randomized crossover clinical study. Eur. J. Nutr. 2019, 58, 1887–1898. [Google Scholar] [CrossRef]
- Joven, J.; Micol, V.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menéndez, J.A.; Platform, B.F.C. Polyphenols and the modulation of gene expression pathways: Can we eat our way out of the danger of chronic disease? Crit. Rev. Food Sci. Nutr. 2014, 54, 985–1001. [Google Scholar] [CrossRef]
- Darvesh, A.S.; Bishayee, A. Chemopreventive and therapeutic potential of tea polyphenols in hepatocellular cancer. Nutr. Cancer 2013, 65, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Evangelopoulos, A.; Schizas, N.; Kazazis, C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer. Res. 2015, 35, 645–651. [Google Scholar]
- Mokhtari-Zaer, A.; Marefati, N.; Atkin, S.L.; Butler, A.E.; Sahebkar, A. The protective role of curcumin in myocardial ischemia-reperfusion injury. J. Cell. Physiol. 2018, 234, 214–222. [Google Scholar] [CrossRef]
- Hedayati-Moghadam, M.; Hosseinian, S.; Paseban, M.; Shabgah, A.G.; Gholizadeh, J.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. The Role of Chemokines in Cardiovascular Diseases and the Therapeutic Effect of Curcumin on CXCL8 and CCL2 as Pathological Chemokines in Atherosclerosis. Adv. Exp. Med. Biol. 2021, 1328, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Abdollahi, E.; Nikfar, B.; Chaichian, S.; Ekhlasi-Hundrieser, M. Curcumin as a potential modulator of M1 and M2 macrophages: New insights in atherosclerosis therapy. Heart Fail. Rev. 2019, 24, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef]
- Jadhav, P.; Jiang, Y.; Jarr, K.; Layton, C.; Ashouri, J.F.; Sinha, S.R. Efficacy of Dietary Supplements in Inflammatory Bowel Disease and Related Autoimmune Diseases. Nutrients 2020, 12, 2156. [Google Scholar] [CrossRef]
- Strimpakos, A.S.; Sharma, R.A. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox Signal. 2008, 10, 511–545. [Google Scholar] [CrossRef]
- Calibasi-Kocal, G.; Pakdemirli, A.; Bayrak, S.; Ozupek, N.M.; Sever, T.; Basbinar, Y.; Ellidokuz, H.; Yigitbasi, T. Curcumin effects on cell proliferation, angiogenesis and metastasis in colorectal cancer. J. BUON 2019, 24, 1482–1487. [Google Scholar] [PubMed]
- Hu, C.; Li, M.; Guo, T.; Wang, S.; Huang, W.; Yang, K.; Liao, Z.; Wang, J.; Zhang, F.; Wang, H. Anti-metastasis activity of curcumin against breast cancer via the inhibition of stem cell-like properties and EMT. Phytomedicine 2019, 58, 152740. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Mandal, D.; Saha, B.; Sen, G.; Das, T.; Sa, G. Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. J. Biol. Chem. 2007, 282, 15954–15964. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Cicero, A.F.G.; Blesso, C.N.; Pirro, M.; Majeed, M.; Sahebkar, A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2019, 59, 89–101. [Google Scholar] [CrossRef]
- Chen, H.W.; Yu, S.L.; Chen, J.J.; Li, H.N.; Lin, Y.C.; Yao, P.L.; Chou, H.Y.; Chien, C.T.; Chen, W.J.; Lee, Y.T.; et al. Anti-invasive gene expression profile of curcumin in lung adenocarcinoma based on a high throughput microarray analysis. Mol. Pharmacol. 2004, 65, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, C.; Rodriguez, S.; Ramachandran, R.; Raveendran Nair, P.K.; Fonseca, H.; Khatib, Z.; Escalon, E.; Melnick, S.J. Expression profiles of apoptotic genes induced by curcumin in human breast cancer and mammary epithelial cell lines. Anticancer. Res. 2005, 25, 3293–3302. [Google Scholar] [PubMed]
- Ye, M.; Zhang, J.; Miao, Q.; Yao, L. Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015, 357, 196–205. [Google Scholar] [CrossRef]
- Sun, M.; Estrov, Z.; Ji, Y.; Coombes, K.R.; Harris, D.H.; Kurzrock, R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol. Cancer Ther. 2008, 7, 464–473. [Google Scholar] [CrossRef]
- Link, A.; Balaguer, F.; Shen, Y.; Lozano, J.J.; Leung, H.C.; Boland, C.R.; Goel, A. Curcumin modulates DNA methylation in colorectal cancer cells. PLoS ONE 2013, 8, e57709. [Google Scholar] [CrossRef]
- Rajitha, B.; Belalcazar, A.; Nagaraju, G.P.; Shaib, W.L.; Snyder, J.P.; Shoji, M.; Pattnaik, S.; Alam, A.; El-Rayes, B.F. Inhibition of NF-κB translocation by curcumin analogs induces G0/G1 arrest and downregulates thymidylate synthase in colorectal cancer. Cancer Lett. 2016, 373, 227–233. [Google Scholar] [CrossRef]
- Lim, T.G.; Lee, S.Y.; Huang, Z.; Lim, D.Y.; Chen, H.; Jung, S.K.; Bode, A.M.; Lee, K.W.; Dong, Z. Curcumin suppresses proliferation of colon cancer cells by targeting CDK2. Cancer Prev. Res. 2014, 7, 466–474. [Google Scholar] [CrossRef]
- Guo, L.D.; Chen, X.J.; Hu, Y.H.; Yu, Z.J.; Wang, D.; Liu, J.Z. Curcumin inhibits proliferation and induces apoptosis of human colorectal cancer cells by activating the mitochondria apoptotic pathway. Phytother. Res. 2013, 27, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.Z.; Wang, J.; Li, X.D.; Wang, G.L.; Liu, F.N.; Cheng, M.S.; Li, F. Curcumin suppresses proliferation and invasion in human gastric cancer cells by downregulation of PAK1 activity and cyclin D1 expression. Cancer Biol. Ther. 2009, 8, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.L.; Tang, Q.F.; Zhou, W.C.; Qiu, Y.Y.; Hu, S.J.; Yin, P.H. Ras/ERK signaling pathway is involved in curcumin-induced cell cycle arrest and apoptosis in human gastric carcinoma AGS cells. J. Asian Nat. Prod. Res. 2015, 17, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ngai, S.C. Curcumin Sensitizes Cancers Towards TRAIL-induced Apoptosis via Extrinsic and Intrinsic Apoptotic Pathways. Curr. Drug Targets 2020, 21, 849–854. [Google Scholar] [CrossRef]
- He, Y.C.; He, L.; Khoshaba, R.; Lu, F.G.; Cai, C.; Zhou, F.L.; Liao, D.F.; Cao, D. Curcumin Nicotinate Selectively Induces Cancer Cell Apoptosis and Cycle Arrest through a P53-Mediated Mechanism. Molecules 2019, 24, 4179. [Google Scholar] [CrossRef]
- Zhou, H.; Ning, Y.; Zeng, G.; Zhou, C.; Ding, X. Curcumin promotes cell cycle arrest and apoptosis of acute myeloid leukemia cells by inactivating AKT. Oncol. Rep. 2021, 45, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rishi, A.K.; Wu, W.; Polin, L.; Sharma, S.; Levi, E.; Albelda, S.; Pass, H.I.; Wali, A. Curcumin suppresses growth of mesothelioma cells in vitro and in vivo, in part, by stimulating apoptosis. Mol. Cell. Biochem. 2011, 357, 83–94. [Google Scholar] [CrossRef]
- Chearwae, W.; Shukla, S.; Limtrakul, P.; Ambudkar, S.V. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Mol. Cancer Ther. 2006, 5, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Chearwae, W.; Wu, C.P.; Chu, H.Y.; Lee, T.R.; Ambudkar, S.V.; Limtrakul, P. Curcuminoids purified from turmeric powder modulate the function of human multidrug resistance protein 1 (ABCC1). Cancer Chemother. Pharmacol. 2006, 57, 376–388. [Google Scholar] [CrossRef]
- Hsieh, Y.W.; Huang, C.Y.; Yang, S.Y.; Peng, Y.H.; Yu, C.P.; Chao, P.D.; Hou, Y.C. Oral intake of curcumin markedly activated CYP 3A4: In vivo and ex-vivo studies. Sci. Rep. 2014, 4, 6587. [Google Scholar] [CrossRef]
- Koe, X.F.; Tengku Muhammad, T.S.; Chong, A.S.; Wahab, H.A.; Tan, M.L. Cytochrome P450 induction properties of food and herbal-derived compounds using a novel multiplex RT-qPCR in vitro assay, a drug-food interaction prediction tool. Food Sci. Nutr. 2014, 2, 500–520. [Google Scholar] [CrossRef]
- Kusuhara, H.; Furuie, H.; Inano, A.; Sunagawa, A.; Yamada, S.; Wu, C.; Fukizawa, S.; Morimoto, N.; Ieiri, I.; Morishita, M.; et al. Pharmacokinetic interaction study of sulphasalazine in healthy subjects and the impact of curcumin as an in vivo inhibitor of BCRP. Br. J. Pharmacol. 2012, 166, 1793–1803. [Google Scholar] [CrossRef]
- Riddell, I.A. Cisplatin and Oxaliplatin: Our Current Understanding of Their Actions. Met. Ions Life Sci. 2018, 18, 1–42. [Google Scholar] [CrossRef]
- Skowron, M.A.; Melnikova, M.; van Roermund, J.G.H.; Romano, A.; Albers, P.; Thomale, J.; Schulz, W.A.; Niegisch, G.; Hoffmann, M.J. Multifaceted Mechanisms of Cisplatin Resistance in Long-Term Treated Urothelial Carcinoma Cell Lines. Int. J. Mol. Sci. 2018, 19, 590. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, R.; Dai, A. Curcumin Increased the Sensitivity of Non-Small-Cell Lung Cancer to Cisplatin through the Endoplasmic Reticulum Stress Pathway. Evid. Based Complement. Altern. Med. 2022, 2022, 6886366. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhao, P.; Wu, S.; Yang, T.; Chen, Y.; Zhang, X.; He, C.; Zheng, C.; Li, K.; Ma, X.; et al. Cisplatin and curcumin co-loaded nano-liposomes for the treatment of hepatocellular carcinoma. Int. J. Pharm. 2018, 545, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.H.; You, H.Y.; Feng, Y.J.; Zhang, Z.T. LncRNA KCNQ1OT1 is a key factor in the reversal effect of curcumin on cisplatin resistance in the colorectal cancer cells. Mol. Cell. Biochem. 2021, 476, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Meredith, A.M.; Dass, C.R. Increasing role of the cancer chemotherapeutic doxorubicin in cellular metabolism. J. Pharm. Pharmacol. 2016, 68, 729–741. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, N.; Sharma, S. ATP binding cassette transporters and cancer: Revisiting their controversial role. Pharmacogenomics 2021, 22, 1211–1235. [Google Scholar] [CrossRef]
- Huang, J.F.; Wen, C.J.; Zhao, G.Z.; Dai, Y.; Li, Y.; Wu, L.X.; Zhou, H.H. Overexpression of ABCB4 contributes to acquired doxorubicin resistance in breast cancer cells in vitro. Cancer Chemother. Pharmacol. 2018, 82, 199–210. [Google Scholar] [CrossRef]
- Abdin, S.M.; Tolba, M.F.; Zaher, D.M.; Omar, H.A. Nuclear factor-κB signaling inhibitors revert multidrug-resistance in breast cancer cells. Chem. Biol. Interact. 2021, 340, 109450. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes. Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Shafiee, M.; Banikazemi, Z.; Pourhanifeh, M.H.; Khanbabaei, H.; Shamshirian, A.; Amiri Moghadam, S.; ArefNezhad, R.; Sahebkar, A.; Avan, A.; et al. Curcumin inhibits NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathol. Res. Pract. 2019, 215, 152556. [Google Scholar] [CrossRef]
- Sen, G.S.; Mohanty, S.; Hossain, D.M.S.; Bhattacharyya, S.; Banerjee, S.; Chakraborty, J.; Saha, S.; Ray, P.; Bhattacharjee, P.; Mandal, D.; et al. Curcumin enhances the efficacy of chemotherapy by tailoring p65NFκB-p300 cross-talk in favor of p53-p300 in breast cancer. J. Biol. Chem. 2011, 286, 42232–42247. [Google Scholar] [CrossRef]
- Vértessy, B.G.; Tóth, J. Keeping uracil out of DNA: Physiological role, structure and catalytic mechanism of dUTPases. Acc. Chem. Res. 2009, 42, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fang, S.; Shao, X.; Li, Y.; Tong, Q.; Kong, B.; Chen, L.; Wang, Y.; Yang, J.; Yu, H.; et al. Curcumin Reverses NNMT-Induced 5-Fluorouracil Resistance via Increasing ROS and Cell Cycle Arrest in Colorectal Cancer Cells. Biomolecules 2021, 11, 1295. [Google Scholar] [CrossRef]
- Ichiki, K.; Mitani, N.; Doki, Y.; Hara, H.; Misaki, T.; Saiki, I. Regulation of activator protein-1 activity in the mediastinal lymph node metastasis of lung cancer. Clin. Exp. Metastasis 2000, 18, 539–545. [Google Scholar] [CrossRef]
- Lev-Ari, S.; Starr, A.; Katzburg, S.; Berkovich, L.; Rimmon, A.; Ben-Yosef, R.; Vexler, A.; Ron, I.; Earon, G. Curcumin induces apoptosis and inhibits growth of orthotopic human non-small cell lung cancer xenografts. J. Nutr. Biochem. 2014, 25, 843–850. [Google Scholar] [CrossRef]
- Montopoli, M.; Ragazzi, E.; Froldi, G.; Caparrotta, L. Cell-cycle inhibition and apoptosis induced by curcumin and cisplatin or oxaliplatin in human ovarian carcinoma cells. Cell Prolif. 2009, 42, 195–206. [Google Scholar] [CrossRef]
- Nessa, M.U.; Beale, P.; Chan, C.; Yu, J.Q.; Huq, F. Studies on combination of platinum drugs cisplatin and oxaliplatin with phytochemicals anethole and curcumin in ovarian tumour models. Anticancer. Res. 2012, 32, 4843–4850. [Google Scholar]
- Zou, J.; Zhu, L.; Jiang, X.; Wang, Y.; Wang, X.; Chen, B. Curcumin increases breast cancer cell sensitivity to cisplatin by decreasing FEN1 expression. Oncotarget 2018, 9, 11268–11278. [Google Scholar] [CrossRef] [PubMed]
- Vanhecke, E.; Adriaenssens, E.; Verbeke, S.; Meignan, S.; Germain, E.; Berteaux, N.; Nurcombe, V.; Le Bourhis, X.; Hondermarck, H. Brain-derived neurotrophic factor and neurotrophin-4/5 are expressed in breast cancer and can be targeted to inhibit tumor cell survival. Clin. Cancer Res. 2011, 17, 1741–1752. [Google Scholar] [CrossRef]
- Kumar, P.; Barua, C.C.; Sulakhiya, K.; Sharma, R.K. Curcumin Ameliorates Cisplatin-Induced Nephrotoxicity and Potentiates Its Anticancer Activity in SD Rats: Potential Role of Curcumin in Breast Cancer Chemotherapy. Front. Pharmacol. 2017, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Rodríguez, N.C.; Alvarez-Quezada, O.A.; Benavides, P.Z.; Vargas-Alanís, G.; Franco-Molina, M.; Zamora-Ávila, D.; Arellano-Rodríguez, M.; Saavedra-Alonso, S.; Izaguirre-Álvarez, J.M.; Rodríguez-Padilla, C. Curcumin Sensitizes 4T1 Murine Breast Cancer Cells to Cisplatin Through PAR4 Secretion. In Vivo 2022, 36, 2767–2773. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Wang, Z.; Zhao, Y.; Wang, D.; Li, Y.; Ma, L.; Li, X.; Li, J.; Xiao, N.; Tian, J.; et al. Effects of curcumin on bladder cancer cells and development of urothelial tumors in a rat bladder carcinogenesis model. Cancer Lett. 2008, 264, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Lim, J.; Jeon, H.; Seo, S.; Lee, H.; Choi, H.; Jeon, S.; Jeong, B. Curcumin potentiates antitumor activity of cisplatin in bladder cancer cell lines via ROS-mediated activation of ERK1/2. Oncotarget 2016, 7, 63870–63886. [Google Scholar] [CrossRef]
- Kao, C.; Cheng, Y.; Yang, M.; Cha, T.; Sun, G.; Ho, C.; Lin, Y.; Wang, H.; Wu, S.; Way, T. Demethoxycurcumin induces apoptosis in HER2 overexpressing bladder cancer cells through degradation of HER2 and inhibiting the PI3K/Akt pathway. Environ. Toxicol. 2021, 36, 2186–2195. [Google Scholar] [CrossRef]
- Gao, X.; Deeb, D.; Jiang, H.; Liu, Y.; Dulchavsky, S.; Gautam, S. Curcumin differentially sensitizes malignant glioma cells to TRAIL/Apo2L-mediated apoptosis through activation of procaspases and release of cytochrome c from mitochondria. J. Exp. Ther. Oncol. 2005, 5, 39–48. [Google Scholar]
- Kim, J.M.; Noh, E.M.; Kwon, K.B.; Kim, J.S.; You, Y.O.; Hwang, J.K.; Hwang, B.M.; Kim, B.S.; Lee, S.H.; Lee, S.J.; et al. Curcumin suppresses the TPA-induced invasion through inhibition of PKCα-dependent MMP-expression in MCF-7 human breast cancer cells. Phytomedicine 2012, 19, 1085–1092. [Google Scholar] [CrossRef]
- Dhandapani, K.; Mahesh, V.; Brann, D. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NF kappa B transcription factors. J. Neurochem. 2007, 102, 522–538. [Google Scholar] [CrossRef]
- Zanotto, A.; Braganhol, E.; Edelweiss, M.; Behr, G.; Zanin, R.; Schroder, R.; Simoes-Pires, A.; Battastini, A.; Moreira, J. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J. Nutr. Biochem. 2012, 23, 591–601. [Google Scholar] [CrossRef]
- Gökçe Kütük, S.; Gökçe, G.; Kütük, M.; Gürses Cila, H.E.; Nazıroğlu, M. Curcumin enhances cisplatin-induced human laryngeal squamous cancer cell death through activation of TRPM2 channel and mitochondrial oxidative stress. Sci. Rep. 2019, 9, 17784. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, T.; Wen, L.; Wang, H.; Fei, D.; Jin, C. Curcumin enhances the effectiveness of cisplatin by suppressing CD133. Exp. Ther. Med. 2013, 6, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.P.; Zhou, Q.; Li, Y.P.; Zhang, S.C.; Xu, H.W.; Wu, S.; Shen, B.X.; Ding, L.C.; Xue, J.; Chen, Z.S.; et al. Curcumin ameliorates cisplatin-induced cystopathy via activating NRF2 pathway. Neurourol. Urodyn. 2018, 37, 2470–2479. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Gutiérrez, H.J.; Bobadilla-Morales, L.; Barba-Barba, C.C.; Ortega-De-La-Torre, C.; Sánchez-Zubieta, F.A.; Corona-Rivera, J.R.; González-Quezada, B.A.; Armendáriz-Borunda, J.S.; Silva-Cruz, R.; Corona-Rivera, A. Curcumin potentiates the effect of chemotherapy against acute lymphoblastic leukemia cells via downregulation of NF-κB. Oncol. Lett. 2016, 12, 4117–4124. [Google Scholar] [CrossRef] [PubMed]
- Chueahongthong, F.; Tima, S.; Chiampanichayakul, S.; Berkland, C.; Anuchapreeda, S. Co-Treatments of Edible Curcumin from Turmeric Rhizomes and Chemotherapeutic Drugs on Cytotoxicity and FLT3 Protein Expression in Leukemic Stem Cells. Molecules 2021, 26, 5785. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, D.; Tang, P.; Zuo, Y. Curcumin increases the sensitivity of K562/DOX cells to doxorubicin by targeting S100 calcium-binding protein A8 and P-glycoprotein. Oncol. Lett. 2020, 19, 83–92. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Q.; Li, Y.; Tang, H.; Liu, W.; Yang, X. Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur. J. Pharm. Biopharm. 2015, 93, 27–36. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Shi, Z.; Yang, Y.; Xie, X.; Lee, S.M.; Wang, Y.; Leong, K.W.; Chen, M. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 2017, 58, 349–364. [Google Scholar] [CrossRef]
- Yu, L.L.; Wu, J.G.; Dai, N.; Yu, H.G.; Si, J.M. Curcumin reverses chemoresistance of human gastric cancer cells by downregulating the NF-κB transcription factor. Oncol. Rep. 2011, 26, 1197–1203. [Google Scholar] [CrossRef]
- Firouzi Amoodizaj, F.; Baghaeifar, S.; Taheri, E.; Farhoudi Sefidan Jadid, M.; Safi, M.; Seyyed Sani, N.; Hajazimian, S.; Isazadeh, A.; Shanehbandi, D. Enhanced anticancer potency of doxorubicin in combination with curcumin in gastric adenocarcinoma. J. Biochem. Mol. Toxicol. 2020, 34, e22486. [Google Scholar] [CrossRef]
- Namkaew, J.; Jaroonwitchawan, T.; Rujanapun, N.; Saelee, J.; Noisa, P. Combined effects of curcumin and doxorubicin on cell death and cell migration of SH-SY5Y human neuroblastoma cells. In Vitro Cell. Dev. Biol. Anim. 2018, 54, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 2015, 36, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer 2015, 15, 250. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, X.; Lin, J.; Song, F.; Shao, Y. Low curcumin concentration enhances the anticancer effect of 5-fluorouracil against colorectal cancer. Phytomedicine 2021, 85, 153547. [Google Scholar] [CrossRef] [PubMed]
- Vinod, B.S.; Antony, J.; Nair, H.H.; Puliyappadamba, V.T.; Saikia, M.; Narayanan, S.S.; Bevin, A.; Anto, R.J. Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis. 2013, 4, e505. [Google Scholar] [CrossRef] [PubMed]
- James, M.I.; Iwuji, C.; Irving, G.; Karmokar, A.; Higgins, J.A.; Griffin-Teal, N.; Thomas, A.; Greaves, P.; Cai, H.; Patel, S.R.; et al. Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett. 2015, 364, 135–141. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.; Zhang, Z.; Jia, Y.; Zhang, C.; Peng, L. Hydrazinocurcumin and 5-fluorouracil enhance apoptosis and restrain tumorigenicity of HepG2 cells via disrupting the PTEN-mediated PI3K/Akt signaling pathway. Biomed. Pharmacother. 2020, 129, 109851. [Google Scholar] [CrossRef]
- Tian, F.; Fan, T.; Zhang, Y.; Jiang, Y.; Zhang, X. Curcumin potentiates the antitumor effects of 5-FU in treatment of esophageal squamous carcinoma cells through downregulating the activation of NF-κB signaling pathway in vitro and in vivo. Acta Biochim Biophys. Sin. 2012, 44, 847–855. [Google Scholar] [CrossRef]
- Pendleton, E.G.; Jamasbi, R.J.; Geusz, M.E. Tetrahydrocurcumin, Curcumin, and 5-Fluorouracil Effects on Human Esophageal Carcinoma Cells. Anticancer. Agents Med. Chem. 2019, 19, 1012–1020. [Google Scholar] [CrossRef]
- Yang, H.; Huang, S.; Wei, Y.; Cao, S.; Pi, C.; Feng, T.; Liang, J.; Zhao, L.; Ren, G. Curcumin Enhances the Anticancer Effect Of 5-fluorouracil against Gastric Cancer through Down-Regulation of COX-2 and NF- κB Signaling Pathways. J. Cancer 2017, 8, 3697–3706. [Google Scholar] [CrossRef]
- Ham, I.H.; Wang, L.; Lee, D.; Woo, J.; Kim, T.H.; Jeong, H.Y.; Oh, H.J.; Choi, K.S.; Kim, T.M.; Hur, H. Curcumin inhibits the cancer-associated fibroblast-derived chemoresistance of gastric cancer through the suppression of the JAK/STAT3 signaling pathway. Int. J. Oncol. 2022, 61, 85. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Huang, H.P.; Wang, Y.; Jin, J.; Long, W.G.; Chen, K.; Zhao, X.H.; Chen, C.G.; Li, J. Curcumin overcome primary gefitinib resistance in non-small-cell lung cancer cells through inducing autophagy-related cell death. J. Exp. Clin. Cancer Res. 2019, 38, 254. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.; Yang, C.; Ueda, M.; Kristen, A.; Tournev, I.; Schmidt, H.; Coelho, T.; Berk, J.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Fu, L.; Huang, J.; Dai, Y.; Wang, B.; Xu, G.; Wu, L.; Zhou, H. Curcumin reverses doxorubicin resistance via inhibition the efflux function of ABCB4 in doxorubicin-resistant breast cancer cells. Mol. Med. Rep. 2019, 19, 5162–5168. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Yu, N.; Jiao, Y.; Hong, W.; Zhou, K.; Ji, X.; Yuan, H.; Wang, H.; Li, A.; Wang, G.; et al. Star polyester-based folate acid-targeting nanoparticles for doxorubicin and curcumin co-delivery to combat multidrug-resistant breast cancer. Drug Deliv. 2021, 28, 1709–1721. [Google Scholar] [CrossRef]
- Sohail, M.; Yu, B.; Sun, Z.; Liu, J.; Li, Y.; Zhao, F.; Chen, D.; Yang, X.; Xu, H. Complex polymeric nanomicelles co-delivering doxorubicin and dimethoxycurcumin for cancer chemotherapy. Drug Deliv. 2022, 29, 1523–1535. [Google Scholar] [CrossRef]
- Zhou, Q.; Fu, Z. In vitro and in vivo Study of a Novel Liposome-Mediated Dual Drug Delivery for Synergistic Lung Cancer Therapy via Oral Administration. Onco Targets Ther. 2020, 13, 12695–12703. [Google Scholar] [CrossRef]
- Jeon, Y.; Sym, S.J.; Yoo, B.K.; Baek, J.H. Long-term Survival, Tolerability, and Safety of First-Line Bevacizumab and FOLFIRI in Combination with Ginsenoside-Modified Nanostructured Lipid Carrier Containing Curcumin in Patients with Unresectable Metastatic Colorectal Cancer. Integr. Cancer Ther. 2022, 21, 15347354221105498. [Google Scholar] [CrossRef]
- De Jaeghere, E.A.; Tuyaerts, S.; Van Nuffel, A.M.T.; Belmans, A.; Bogaerts, K.; Baiden-Amissah, R.; Lippens, L.; Vuylsteke, P.; Henry, S.; Trinh, X.B.; et al. Pembrolizumab, radiotherapy, and an immunomodulatory five-drug cocktail in pretreated patients with persistent, recurrent, or metastatic cervical or endometrial carcinoma: Results of the phase II PRIMMO study. Cancer Immunol. Immunother. 2022, 72, 475–491. [Google Scholar] [CrossRef]
- Tuyaerts, S.; Van Nuffel, A.M.T.; Naert, E.; Van Dam, P.A.; Vuylsteke, P.; De Caluwé, A.; Aspeslagh, S.; Dirix, P.; Lippens, L.; De Jaeghere, E.; et al. PRIMMO study protocol: A phase II study combining PD-1 blockade, radiation and immunomodulation to tackle cervical and uterine cancer. BMC Cancer 2019, 19, 506. [Google Scholar] [CrossRef]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Irving, G.R.; Iwuji, C.O.; Morgan, B.; Berry, D.P.; Steward, W.P.; Thomas, A.; Brown, K.; Howells, L.M. Combining curcumin (C3-complex, Sabinsa) with standard care FOLFOX chemotherapy in patients with inoperable colorectal cancer (CUFOX): Study protocol for a randomised control trial. Trials 2015, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, A.; Baskaran, R.; Maeng, H.J.; Yoo, B.K. Ginsenoside improves physicochemical properties and bioavailability of curcumin-loaded nanostructured lipid carrier. Arch. Pharm. Res. 2017, 40, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, A.; Baskaran, R.; Baek, J.H.; Sundaramoorthy, P.; Yoo, B.K. In Vitro Cytotoxicity and Bioavailability of Ginsenoside-Modified Nanostructured Lipid Carrier Containing Curcumin. AAPS PharmSciTech 2019, 20, 88. [Google Scholar] [CrossRef]
- de Man, F.M.; Goey, A.K.L.; van Schaik, R.H.N.; Mathijssen, R.H.J.; Bins, S. Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics. Clin. Pharmacokinet. 2018, 57, 1229–1254. [Google Scholar] [CrossRef]
- Gbolahan, O.B.; O’Neil, B.H.; McRee, A.J.; Sanoff, H.K.; Fallon, J.K.; Smith, P.C.; Ivanova, A.; Moore, D.T.; Dumond, J.; Asher, G.N. A phase I evaluation of the effect of curcumin on dose-limiting toxicity and pharmacokinetics of irinotecan in participants with solid tumors. Clin. Transl. Sci. 2022, 15, 1304–1315. [Google Scholar] [CrossRef]
- Liang, Q.; Zhuo, Y.; Wu, X.; Zheng, S.; Zhuang, J.; Wang, K.; Chen, S. Curcumin combining temozolomide formed localized nanogel for inhibition of postsurgical chemoresistant glioblastoma. Nanomedcine 2023, 18. [Google Scholar] [CrossRef]
- Semlali, A.; Beji, S.; Ajala, I.; Al-Zharani, M.; Rouabhia, M. Synergistic Effects of New Curcumin Analog (PAC) and Cisplatin on Oral Cancer Therapy. Curr. Issues Mol. Biol. 2023, 45, 5018–5035. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Hegde, M.; Parama, D.; Girisa, S.; Kumar, A.; Daimary, U.D.; Garodia, P.; Yenisetti, S.C.; Oommen, O.V.; Aggarwal, B.B. Role of Turmeric and Curcumin in Prevention and Treatment of Chronic Diseases: Lessons Learned from Clinical Trials. ACS Pharmacol. Transl. Sci. 2023, 6, 447–518. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Alipour, M.; Dalir Abdolahinia, E.; Ahmadian, E.; Eftekhari, A.; Forouhandeh, H.; Rahbar Saadat, Y.; Sharifi, S.; Zununi Vahed, S. Curcumin nanoformulations: Beneficial nanomedicine against cancer. Phytother. Res. 2022, 36, 1156–1181. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
| Drug Combined to Curcumin | Tumour | References |
|---|---|---|
Cisplatin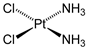 | Lung cancer | [66,67] |
| Ovarian cancer | [68,69] | |
| Breast cancer | [70,71,72,73] | |
| Bladder cancer | [74,75,76] | |
| Glioblastoma | [77,78,79,80] | |
| Laryngeal | [81,82,83] | |
Doxorubicin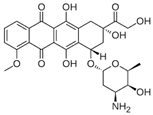 | Glioblastoma | [80] |
| Leukaemia | [84,85,86] | |
| Hepatocellular | [87,88] | |
| Gastric | [89,90] | |
| Neuroblastoma | [91] | |
5-Fluorouracil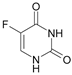 | Colorectal | [92,93,94] |
| Breast | [95] | |
| Liver | [96,97] | |
| Oesophageal | [98,99] | |
| Gastric | [100,101] | |
Gefitinib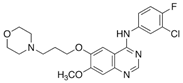 | Lung cancer | [102,103] |
| NCT Number | Study Title | Drug in Combination to Curcumin | Curcumin Used | Condition | Status | Phase | References |
|---|---|---|---|---|---|---|---|
| NCT02439385 | Avastin/FOLFIRI in Combination with Curcumin in Colorectal Cancer Patients With Unresectable Metastasis | FOLFIRI: Folinic acid, 5-FU and Irinotecan | Ginsenoside-modified nanostructured lipid carrier containing Curcumin. Curcumin Sigma-Aldrich | Colorectal Cancer | Completed | Phase 2 | [108] |
| NCT03192059 | Study of Pembrolizumab, Radiation and Immune Modulatory Cocktail in Cervical/Uterine Cancer | Cyclophosphamide, Aaspirin, Lansoprazole, Vitamin D, Pembrolizumab, Radiotherapy | Curcumin, CurcuPhyt | Cervical Cancer, Endometrial Cancer, Uterine Cancer | Completed | Phase 2 | [109,110] |
| NCT00192842 | Gemcitabine with Curcumin for Pancreatic Cancer | Gemcitabine | Curcumin C3—complex, SabinsaCorporation (Piscataway NJ) | Pancreatic Cancer | Completed | Phase 2 | [111] |
| NCT01490996 | Combining Curcumin with FOLFOX Chemotherapy in Patients with Inoperable Colorectal Cancer | FOLFOX: Folinic acid, 5-FU and oxaliplatin | Curcumin C3—complex, SabinsaCorporation (Piscataway NJ) | Colon cancer metastasis | Completed | Phase 1–2 | [96,112,113] |
| NCT01859858 | Effect of Curcumin on Dose Limiting Toxicity and Pharmacokinetics of Irinotecan in Patients with Solid Tumors | Irinotecan | Curcumin phosphatidylcholine complex, Meriva | Advanced colorectal cancer | Completed | Phase 1 | [110] |
| NCT00852332 | Docetaxel with or without a Phytochemical in Treating Patients With Breast Cancer | Docetaxel | Curcumin capsules | Breast Cancer | Terminated | Phase 2 | |
| NCT02095717 | Multicenter Study Comparing Taxotere Plus Curcumin Versus Taxotere Plus Placebo Combination in First-line Treatment of Prostate Cancer Metastatic Castration Resistant (CURTAXEL) | Taxotere | Curcumin formulated in 500 mg capsules | Prostate Cancer Metastatic Castration Resistant | Terminated | Phase 2 | |
| NCT01608139 | Study of Curcumin, Vorinostat, and Sorafenib | Vorinostat (SAHA), and Sorafenib | Curcumin powder | Advanced cancers | Withdrawn | Phase 1 | |
| NCT01048983 | Reducing Symptom Burden—Non Small Cell Lung Cancer (NSCLC) | Armodafinil, Bupropion, and Minocycline | Curcumin stick pack | Non-Small Cell Lung Cancer | Withdrawn | Phase 1–2 | |
| NCT02321293 | A Open-label Prospective Cohort Trial of Curcumin Plus Tyrosine Kinase Inhibitors (TKI) for EGFR-Mutant Advanced NSCLC | Gefitinib and Erlotinib | CurcuVIVA™ | Lung cancer | Unknown status | Phase 1 | |
| NCT00295035 | Phase III Trial of Gemcitabine, Curcumin and Celebrex in Patients with Metastatic Colon Cancer | CELECOXIB | Curcumin | Colon cancer | Unknown status | Phase 3 | |
| NCT02724202 | Curcumin in Combination with 5FU for Colon Cancer | 5-flurorouracil | Curcumin BCM-95 | Metastatic colon cancer | Unknown status | Early Phase 1 | |
| NCT00486460 | Phase III Trial of Gemcitabine, Curcumin and Celebrex in Patients with Advance or Inoperable Pancreatic Cancer | Gemcitabine and Celebrex | Curcumin | Pancreatic Cancer | Unknown status | Phase 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacciola, N.A.; Cuciniello, R.; Petillo, G.D.; Piccioni, M.; Filosa, S.; Crispi, S. An Overview of the Enhanced Effects of Curcumin and Chemotherapeutic Agents in Combined Cancer Treatments. Int. J. Mol. Sci. 2023, 24, 12587. https://doi.org/10.3390/ijms241612587
Cacciola NA, Cuciniello R, Petillo GD, Piccioni M, Filosa S, Crispi S. An Overview of the Enhanced Effects of Curcumin and Chemotherapeutic Agents in Combined Cancer Treatments. International Journal of Molecular Sciences. 2023; 24(16):12587. https://doi.org/10.3390/ijms241612587
Chicago/Turabian StyleCacciola, Nunzio Antonio, Rossana Cuciniello, Gianluigi Daniele Petillo, Miriam Piccioni, Stefania Filosa, and Stefania Crispi. 2023. "An Overview of the Enhanced Effects of Curcumin and Chemotherapeutic Agents in Combined Cancer Treatments" International Journal of Molecular Sciences 24, no. 16: 12587. https://doi.org/10.3390/ijms241612587
APA StyleCacciola, N. A., Cuciniello, R., Petillo, G. D., Piccioni, M., Filosa, S., & Crispi, S. (2023). An Overview of the Enhanced Effects of Curcumin and Chemotherapeutic Agents in Combined Cancer Treatments. International Journal of Molecular Sciences, 24(16), 12587. https://doi.org/10.3390/ijms241612587







