Differential Cytotoxicity of Curcumin-Loaded Micelles on Human Tumor and Stromal Cells
Abstract
1. Introduction
2. Results
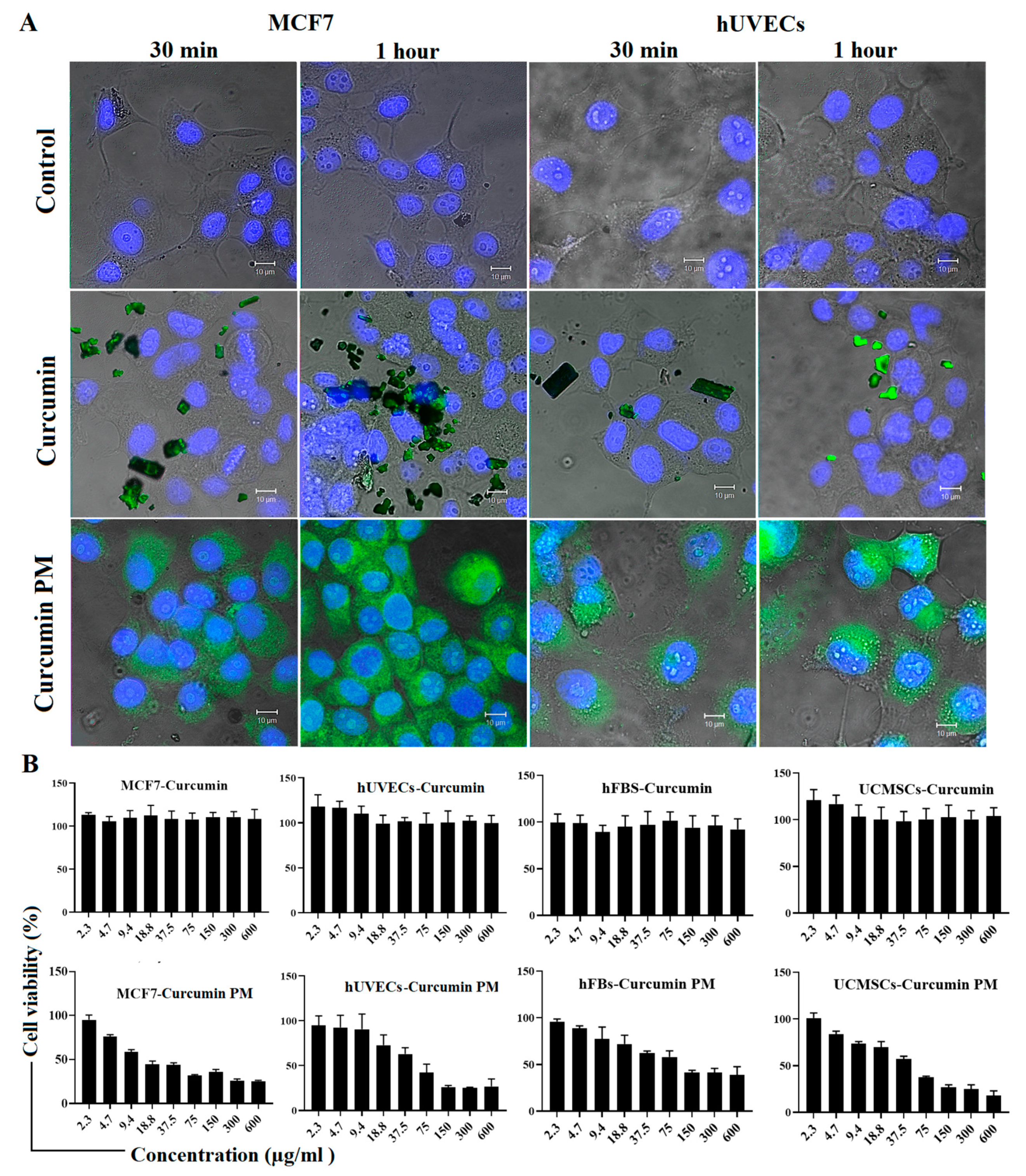
2.1. Curcumin-Loaded Micelles Enhances Intracellular Uptake and Cytotoxicity
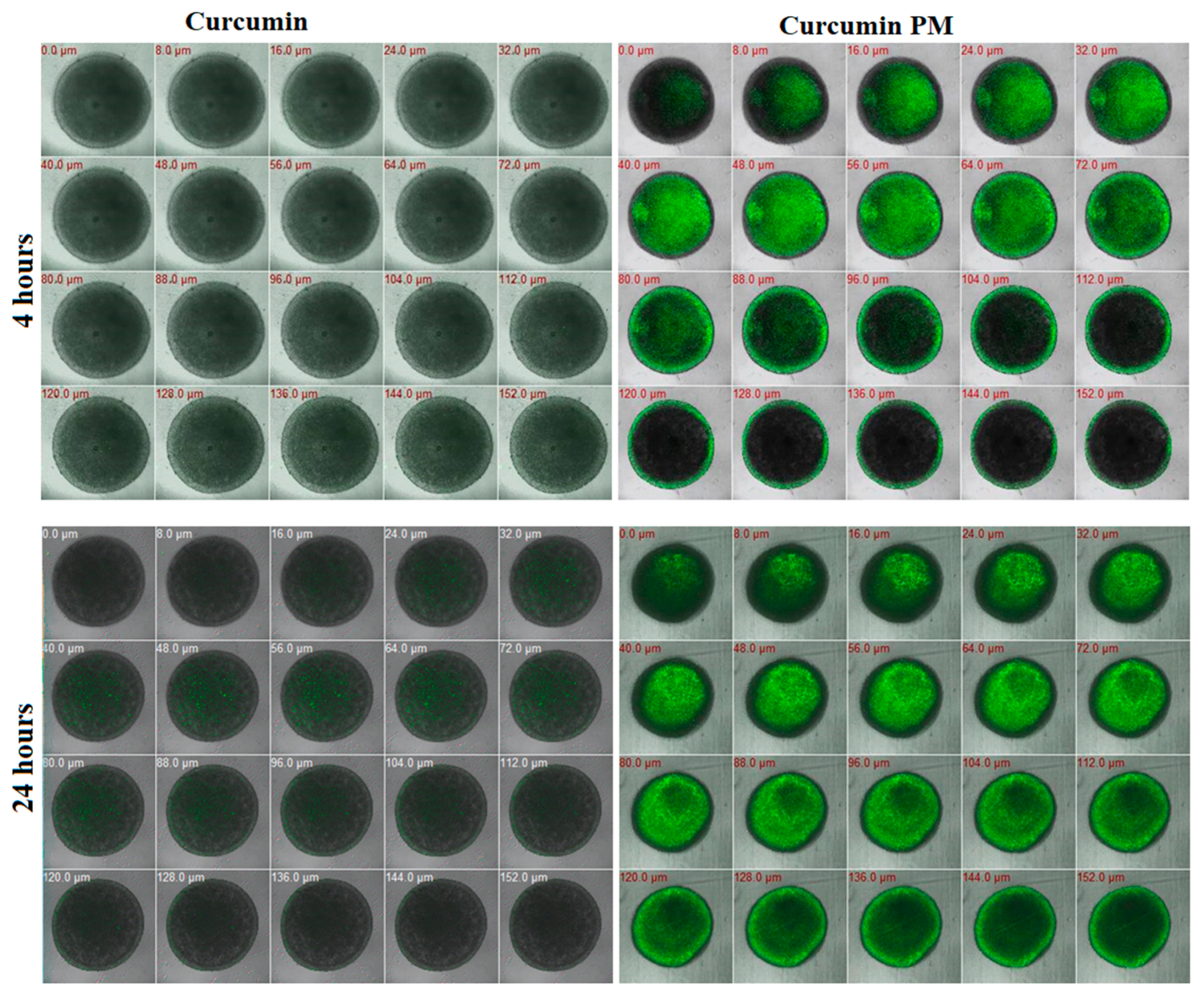
2.2. Curcumin-Loaded Micelles Infiltrated and Attenuated the Growth of MCF7 Spheroids
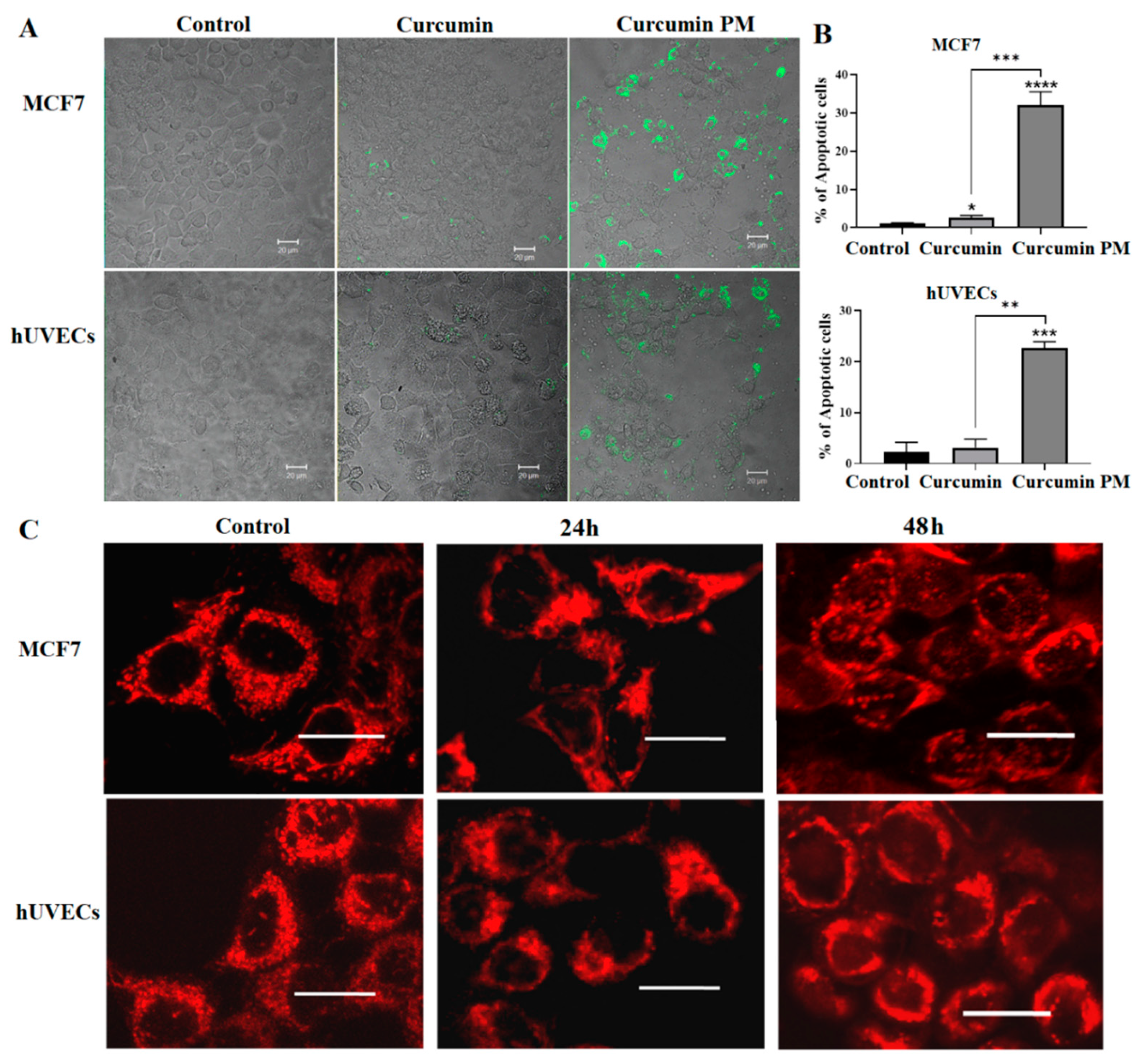
2.3. Curcumin-Loaded Micelles Induced Apoptosis
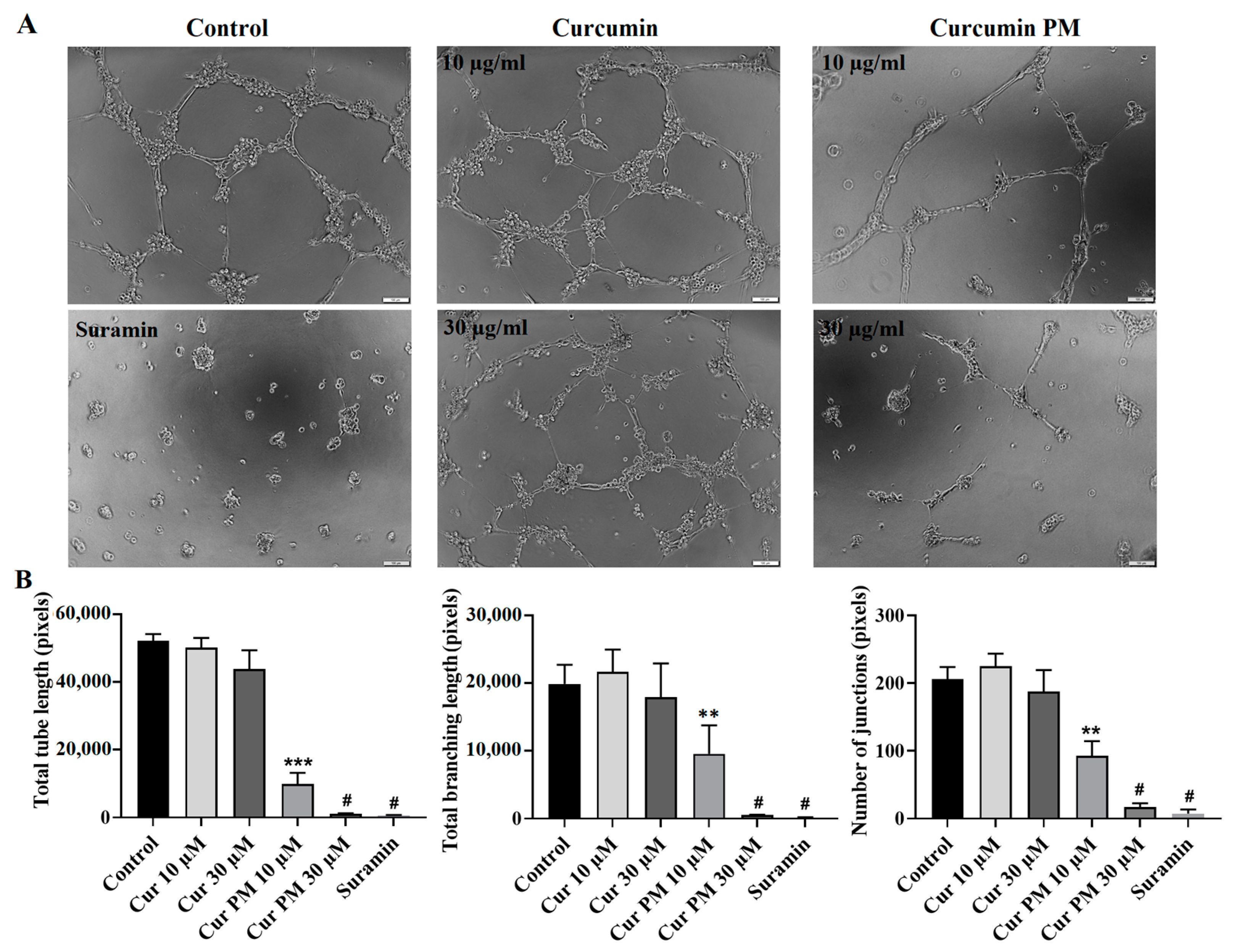
2.4. Curcumin PM Attenuated the Tube Formation of hUVECs
2.5. Curcumin PM Prevented the Migration of hFBs and UCMSCs
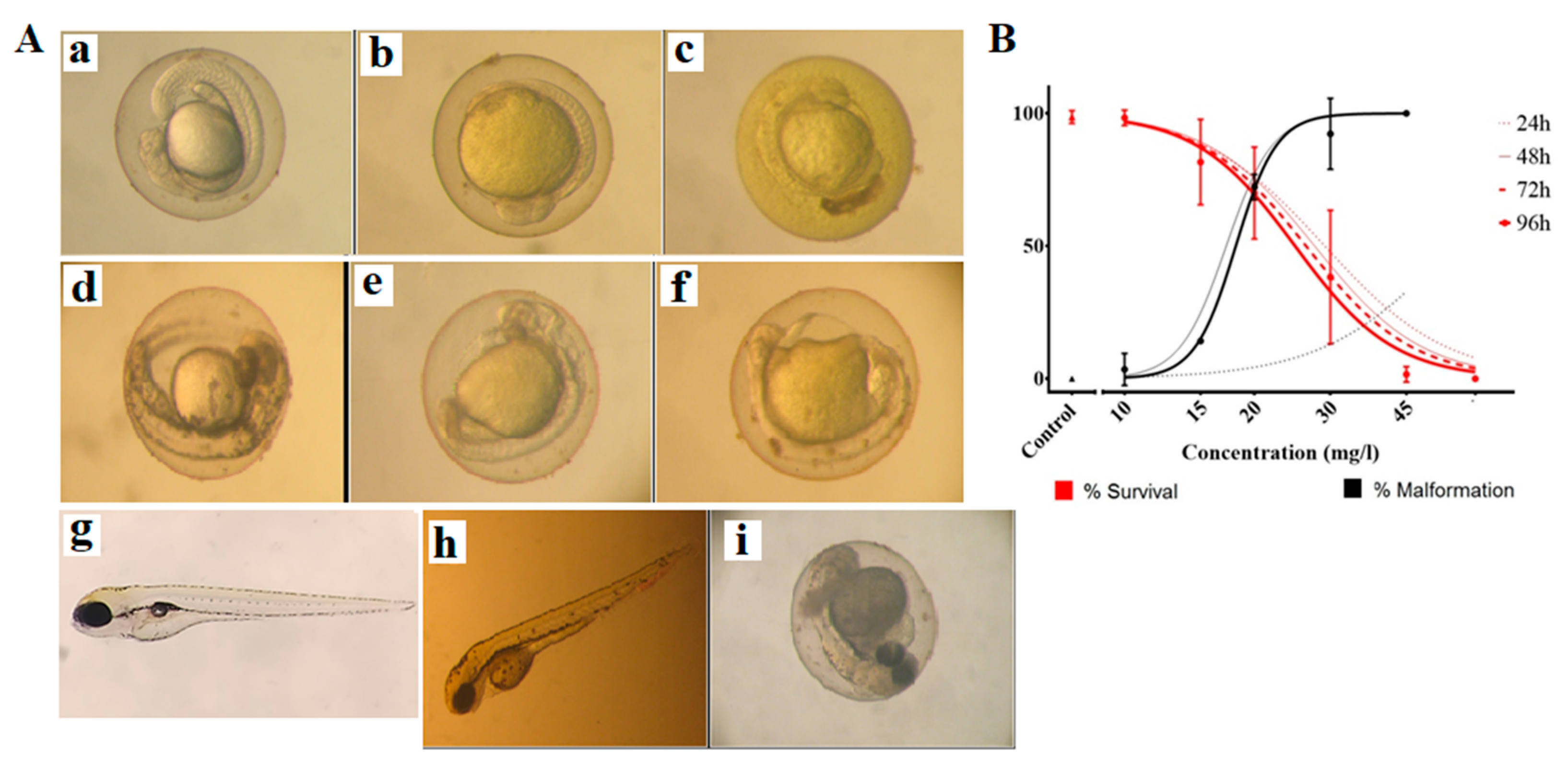
2.6. Toxicity of Curcumin-Loaded Micelles in Zebrafish Embryos
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Three-Dimensional Culture Cell Formation
4.4. Cellular Uptake Study
4.5. Cytotoxicity Assay
4.6. Multicellular Tumor Spheroid Assay
4.7. MitoTracker Assay
4.8. Wound-Healing Assay
4.9. Angiogenesis Assay
4.10. Substance Treatment and Embryo Assessment
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, M.; Ikemot, K.; Kawauchi, S.; Furuya, T.; Yamamoto, S.; Oka, M.; Oga, A.; Nagashima, Y.; Sasaki, K. Luminal A and luminal B (HER2 negative) subtypes of breast cancer consist of a mixture of tumors with different genotype. BMC Res. Notes 2012, 5, 376. [Google Scholar] [CrossRef] [PubMed]
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Parton, M.; Dowsett, M.; Smith, I. Studies of apoptosis in breast cancer. BMJ 2001, 322, 1528–1532. [Google Scholar] [CrossRef] [PubMed]
- Jerusalem, G.; Rorive, A.; Collignon, J. Chemotherapy options for patients suffering from heavily pretreated metastatic breast cancer. Future Oncol. 2015, 11, 1775–1789. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Toft, D.J.; Cryns, V.L. Minireview: Basal-like breast cancer: From molecular profiles to targeted therapies. JME 2011, 25, 199–211. [Google Scholar] [CrossRef]
- Karunagaran, D.; Rashmi, R.; Kumar, R.S.T. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr. Cancer Drug Targets 2005, 5, 117–129. [Google Scholar] [CrossRef]
- Cridge, B.J.; Larsen, L.; Rosengren, R.J. Curcumin and its derivatives in breast cancer: Current developments and potential for the treatment of drug-resistant cancers. Oncol. Discov. 2013, 1, 1–9. [Google Scholar] [CrossRef]
- Ramachandran, C.; Rodriguez, S.; Ramachandran, R.; Nair, P.K.R.; Fonseca, H.; Khatib, Z.; Escalon, E.; Melnick, S.J. Expression profiles of apoptotic genes induced by curcumin in human breast cancer and mammary epithelial cell lines. Anticancer Res. 2005, 25, 3293–3302. [Google Scholar] [PubMed]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Sahu, A.; Kasoju, N.; Goswami, P.; Bora, U. Encapsulation of curcumin in pluronic block copolymer micelles for drug delivery applications. J. Biomater. Appl. 2011, 2, 619–639. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, C.; Sahoo, S.K. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials 2010, 31, 6597–6611. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A promising candidate for therapeutic applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Ha, P.T.; Nguyen, A.S.; Nguyen, D.T.; Do, H.D.; Thi, Q.N.; Thi, M.N.H. Curcumin as fluorescent probe for directly monitoring in vitro uptake of curcumin combined paclitaxel loaded PLA-TPGS nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 025001. [Google Scholar] [CrossRef]
- Mogharbel, B.F.; Francisco, J.C.; Irioda, A.C.; Dziedzic, D.S.M.; Ferreira, P.E.; de Souza, D.; de Souza, C.; Neto, N.B.; Guarita-Souza, L.C.; Franco, C.R.C.; et al. Fluorescence properties of curcumin-loaded nanoparticles for cell tracking. Int. J. Nanomed. 2018, 13, 5823–5836. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Qi, L.L.; Zheng, S.D.; Wu, T.X. Curcumin induces apoptosis through the mitochondria-mediated apoptotic pathway in HT-29 cells. J. Zhejiang Univ. Sci. B 2009, 10, 93–102. [Google Scholar] [CrossRef]
- Keshavarz, R.; Bakhshinejad, B.; Babashah, S.; Baghi, N.; Sadeghizadeh, M. Dendrosomal nanocurcumin and p53 overexpression synergistically trigger apoptosis in glioblastoma cells. Iran. J. Basic Med. Sci. 2016, 19, 1353–1362. [Google Scholar]
- Cao, Z.; He, S.; Peng, Y.; Liao, X.; Lu, H. Nanocurcumin inhibits angiogenesis via down-regulating hif1a/VEGF-A signaling in zebrafish. Curr. Neurovasc. Res. 2020, 17, 147–154. [Google Scholar] [CrossRef]
- Kamble, S.S.; Utage, B.; Mogle, P.P.; Kamble, R.D.; Hese, S.V.; Dawane, B.S.; Gacche, R.N. Evaluation of curcumin capped copper nanoparticles as possible inhibitors of human breast cancer cells and angiogenesis: A comparative study with native curcumin. AAPS PharmSciTech 2015, 17, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Chen, X.; Guan, S.; Yan, Y.; Lin, H.; Hua, Z.C. Curcumin inhibits angiogenesis and improves defective hematopoiesis induced by tumor-derived VEGF in tumor model through modulating VEGF-VEGFR2 signaling pathway. Oncotarget 2015, 6, 19469–19482. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F. The essential medicinal chemistry of curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Jasial, S.; Hu, Y.; Bajorath, J. How frequently are pan-assay interference compounds active? Large-scale analysis of screening data reveals diverse activity profiles, low global hit frequency, and many consistently inactive compounds. J. Med. Chem. 2017, 60, 3879–3886. [Google Scholar] [CrossRef] [PubMed]
- Bajorath, J. Evolution of assay interference concepts in drug discovery. Expert Opin. Drug Discov. 2021, 16, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.X.; Cui, W.W.; Yang, X.; Tao, A.B.; Lan, T.; Li, T.S. Mesenchymal stem cells for mitigating radiotherapy side effects. Cells 2021, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Portas, M.; Mansilla, E.; Drago, H.; Dubner, D.; Radl, A.; Coppola, A.; di Giorgio, M. Use of human cadaveric mesenchymal stem cells for cell therapy of a chronic radiation-induced skin lesion: A case report. Radiat. Prot. Dosim. 2016, 171, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Chapel, A.; Francois, S.; Douay, L.; Benderitter, M.; Voswinkel, J. New insights for pelvic radiation disease treatment: Multipotent stromal cell is a promise mainstay treatment for the restoration of abdominopelvic severe chronic damages induced by radiotherapy. World J. Stem. Cells 2013, 5, 106–111. [Google Scholar] [CrossRef]
- Kursova, L.V.; Konoplyannikov, A.G.; Pasov, V.V.; Ivanova, I.; Poluektova, M.; Konoplyannikova, O.A. Possibilities for the use of autologous mesenchymal stem cells in the therapy of radiation-induced lung injuries. Bull. Exp. Biol. Med. 2009, 147, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Velpula, N.; Ugrappa, S.; Kodangal, S. A role of radioprotective agents in cancer therapeutics: A review. Int. J. Basic Clin. Pharmacol. 2013, 2, 677–682. [Google Scholar] [CrossRef]
- Hosseini, S.; Chamani, J.; Hadipanah, M.R.; Ebadpour, N.; Hojjati, A.S.; Mohammadzadeh, M.H.; Rahimi, H.R. Nano-curcumin’s suppression of breast cancer cells (MCF7) through the inhibition of cyclinD1 expression. Breast Cancer Dove Med. Press 2019, 11, 137–142. [Google Scholar] [PubMed]
- Barbosa, M.A.G.; Xavier, C.P.R.; Pereira, R.F.; Petrikaitė, V.; Vasconcelos, M.H. 3D cell culture models as recapitulators of the tumor microenvironment for the screening of anti-cancer drugs. Cancers 2021, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Rodenhizer, D.; Dean, T.A.; D’Arcangelo, E.; McGuigan, A.P. The current landscape of 3D in vitro tumor models: What cancer hallmarks are accessible for drug discovery? Adv. Healthc. Mater. 2018, 7, 1701174. [Google Scholar] [CrossRef]
- Hoang, N.T.T.; Phuong, T.T.; Nguyen, T.T.; Tran, Y.T.H.; Nguyen, A.T.T.; Nguyen, T.L.; Bui, K.T.V. In vitro characterization of derrone as an aurora kinase inhibitor. Biol. Pharm. Bull. 2016, 39, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Thoma, C.R.; Zimmermann, M.; Agarkova, I.; Kelm, J.M.; Krek, W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv. Drug Deliv. Rev. 2014, 69–70, 29–41. [Google Scholar] [CrossRef] [PubMed]
- LaBarbera, D.V.; Reid, B.G.; Yoo, B.H. The multicellular tumor spheroid model for high-throughput cancer drug discovery. Expert Opin. Drug Discov. 2012, 7, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Jahani, M.; Rezazadeh, D.; Mohammadi, P.; Abdolmaleki, A.; Norooznezhad, A.; Mansouri, K. Regenerative medicine and angiogenesis; challenges and opportunities. Adv. Pharm Bull. 2020, 10, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Sagaradze, G.D.; Basalova, N.A.; Efimenko, A.Y.; Tkachuk, V.A. Mesenchymal stromal cells as critical contributors to tissue regeneration. Front. Cell Dev. Biol. 2020, 8, 576176. [Google Scholar] [CrossRef]
- Stappenbeck, T.S.; Miyoshi, H. The role of stromal stem cells in tissue regeneration and wound repair. Science 2009, 324, 1666–1669. [Google Scholar] [CrossRef]
- Ganiger, S.; Malleshappa, H.N.; Krishnappa, H.; Rajashekhar, G.; Rao, V.R.; Sullivan, F. A two generation reproductive toxicity study with curcumin, turmeric yellow, in Wistar rats. Food Chem. Toxicol. 2007, 45, 64–69. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Lin, C.-Y.; Lin, T.-W.; Ken, C.-F.; Wen, Y.-D. Curcumin affects development of zebrafish embryo. Biol. Pharm. Bull. 2007, 30, 1336–1339. [Google Scholar] [CrossRef]
- Coradini, K.; de Andrade, D.F.; Altenhofen, S.; Reolon, G.K.; Nery, L.R.; Silva, N.E.; Vianna, M.R.M.R.; Bonan, C.D.; Beck, R.C.R. Free and nanoencapsulated curcumin prevents scopolamine-induced cognitive impairment in adult zebrafish. J. Drug Deliv. Sci. Technol. 2021, 66, 102781. [Google Scholar] [CrossRef]
- Coradini, K.; Friedrich, R.B.; Fonseca, F.N.; Vencato, M.S.; Andrade, D.F.; Oliveira, C.M.; Battistel, A.P.; Guterres, S.S.; da Rocha, M.I.U.M.; Pohlmann, A.R.; et al. A novel approach to arthritis treatment based on resveratrol and curcumin co-encapsulated in lipid-core nanocapsules: In vivo studies. Eur. J. Pharm. Sci. 2015, 78, 163–170. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2013. [Google Scholar]



| MCF7 | hUVECs | hFBs | UCMSCs | |
|---|---|---|---|---|
| Curcumin | NA | NA | NA | NA |
| Curcumin PM (µg/mL) | 13.9 ± 0.5 | 36.96 ± 1.2 | 28.34 ± 2.4 | 26.62 ± 5.1 |
| Time of Exposure (h) | LC50 (mg/L) | EC50 (mg/L) |
|---|---|---|
| 24 | 29.1 | 57.4 |
| 48 | 27.6 | 17.2 |
| 72 | 26.4 | |
| 96 | 24.9 | 18.1 |
| (p > 0.05) | (p < 0.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, X.-H.; Hoang, M.H.T.; Vu, A.-T.; Nguyen, L.-T.; Bui, D.T.T.; Dinh, D.-T.; Nguyen, X.-H.; Than, U.T.T.; Mai, H.T.; To, T.T.; et al. Differential Cytotoxicity of Curcumin-Loaded Micelles on Human Tumor and Stromal Cells. Int. J. Mol. Sci. 2022, 23, 12362. https://doi.org/10.3390/ijms232012362
Do X-H, Hoang MHT, Vu A-T, Nguyen L-T, Bui DTT, Dinh D-T, Nguyen X-H, Than UTT, Mai HT, To TT, et al. Differential Cytotoxicity of Curcumin-Loaded Micelles on Human Tumor and Stromal Cells. International Journal of Molecular Sciences. 2022; 23(20):12362. https://doi.org/10.3390/ijms232012362
Chicago/Turabian StyleDo, Xuan-Hai, My Hanh Thi Hoang, Anh-Tuan Vu, Lai-Thanh Nguyen, Dung Thi Thuy Bui, Duy-Thanh Dinh, Xuan-Hung Nguyen, Uyen Thi Trang Than, Hien Thi Mai, Thuy Thanh To, and et al. 2022. "Differential Cytotoxicity of Curcumin-Loaded Micelles on Human Tumor and Stromal Cells" International Journal of Molecular Sciences 23, no. 20: 12362. https://doi.org/10.3390/ijms232012362
APA StyleDo, X.-H., Hoang, M. H. T., Vu, A.-T., Nguyen, L.-T., Bui, D. T. T., Dinh, D.-T., Nguyen, X.-H., Than, U. T. T., Mai, H. T., To, T. T., Nguyen, T. N. H., & Hoang, N. T. M. (2022). Differential Cytotoxicity of Curcumin-Loaded Micelles on Human Tumor and Stromal Cells. International Journal of Molecular Sciences, 23(20), 12362. https://doi.org/10.3390/ijms232012362







