DHEA Induces Sex-Associated Differential Patterns in Cytokine and Antibody Levels in Mice Infected with Plasmodium berghei ANKA
Abstract
1. Introduction
2. Results
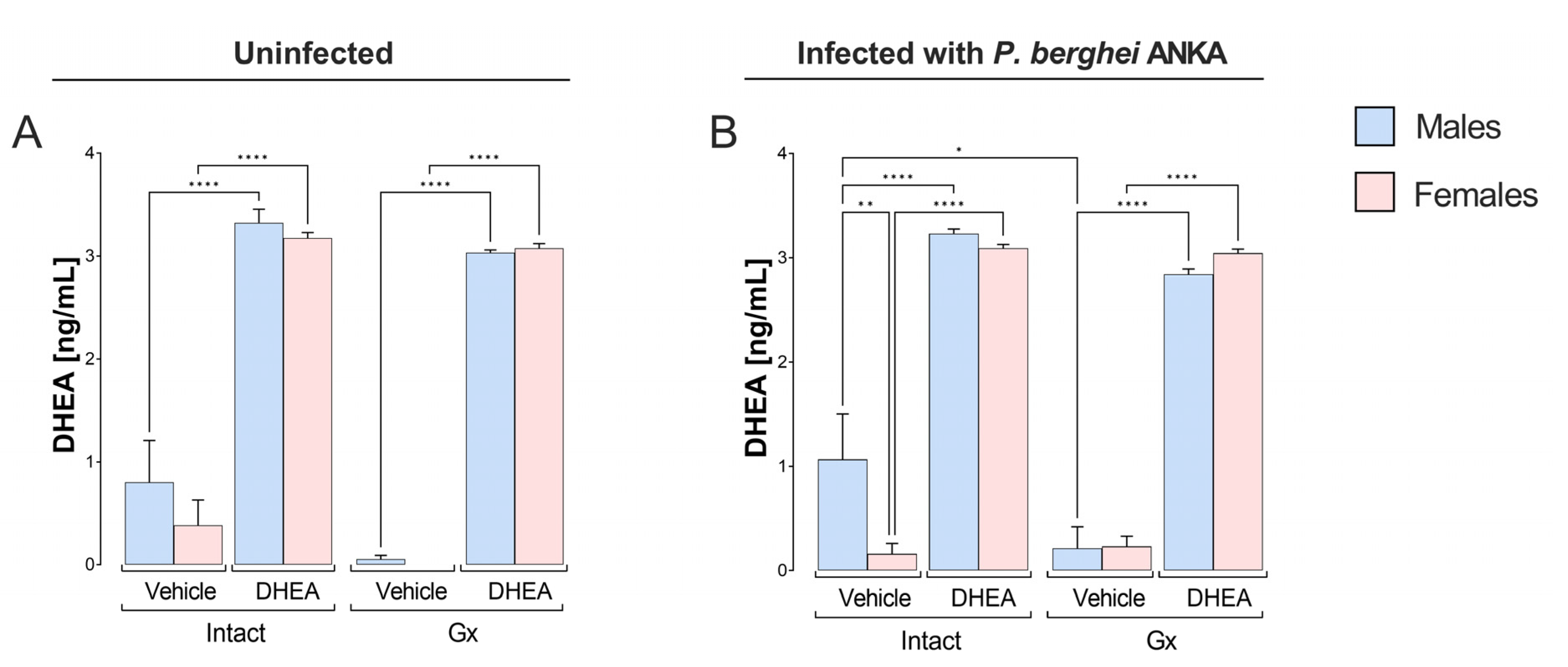
2.1. DHEA Administration Dramatically Increased DHEA Levels in Mice Infected with P. berghei ANKA
2.2. Effects of Modifying DHEA Concentration on Temperature and Hemoglobin Concentration in Mice Infected with P. berghei ANKA
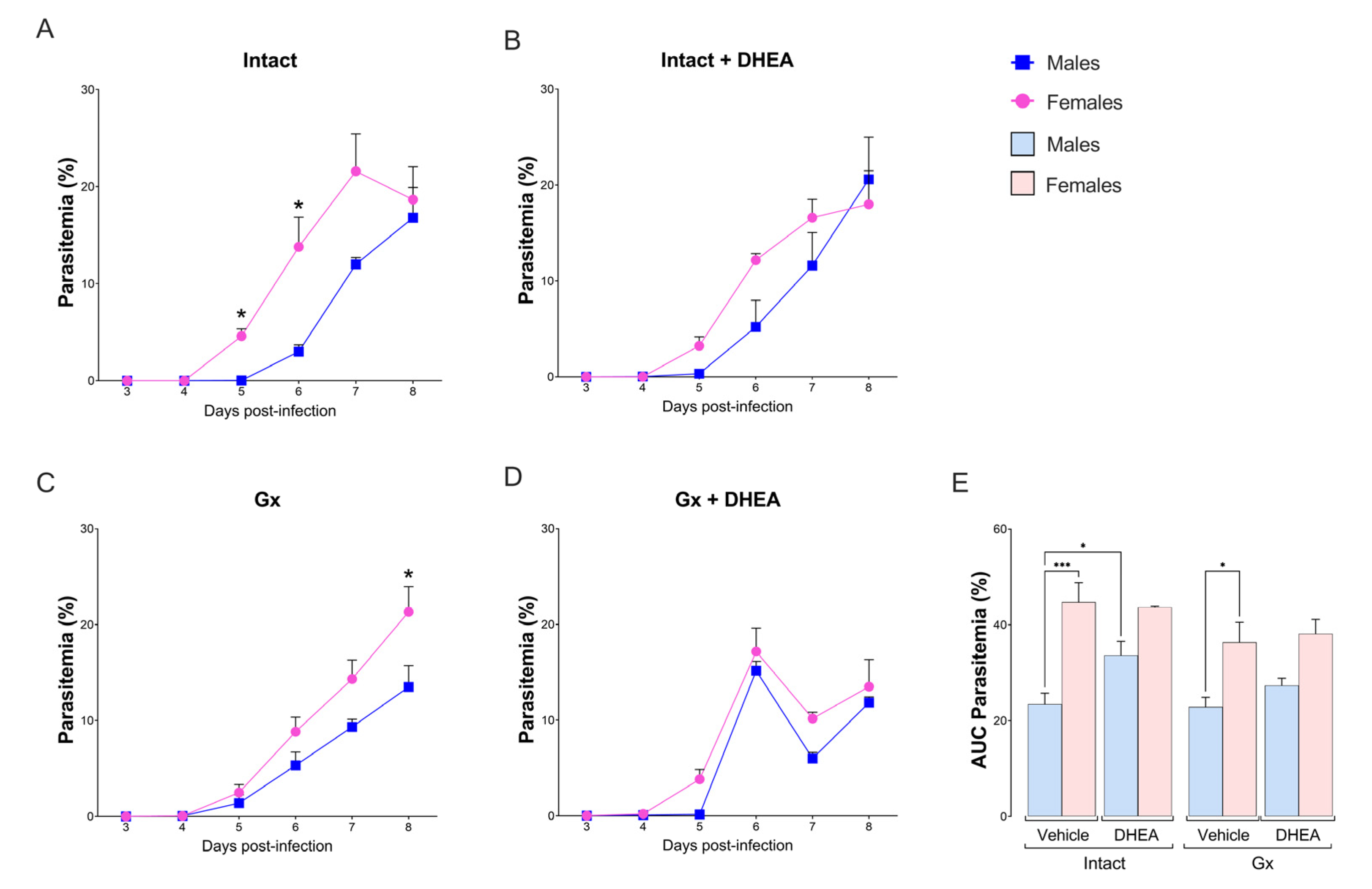
2.3. DHEA Administration to Intact Male Mice Increased Parasitemia
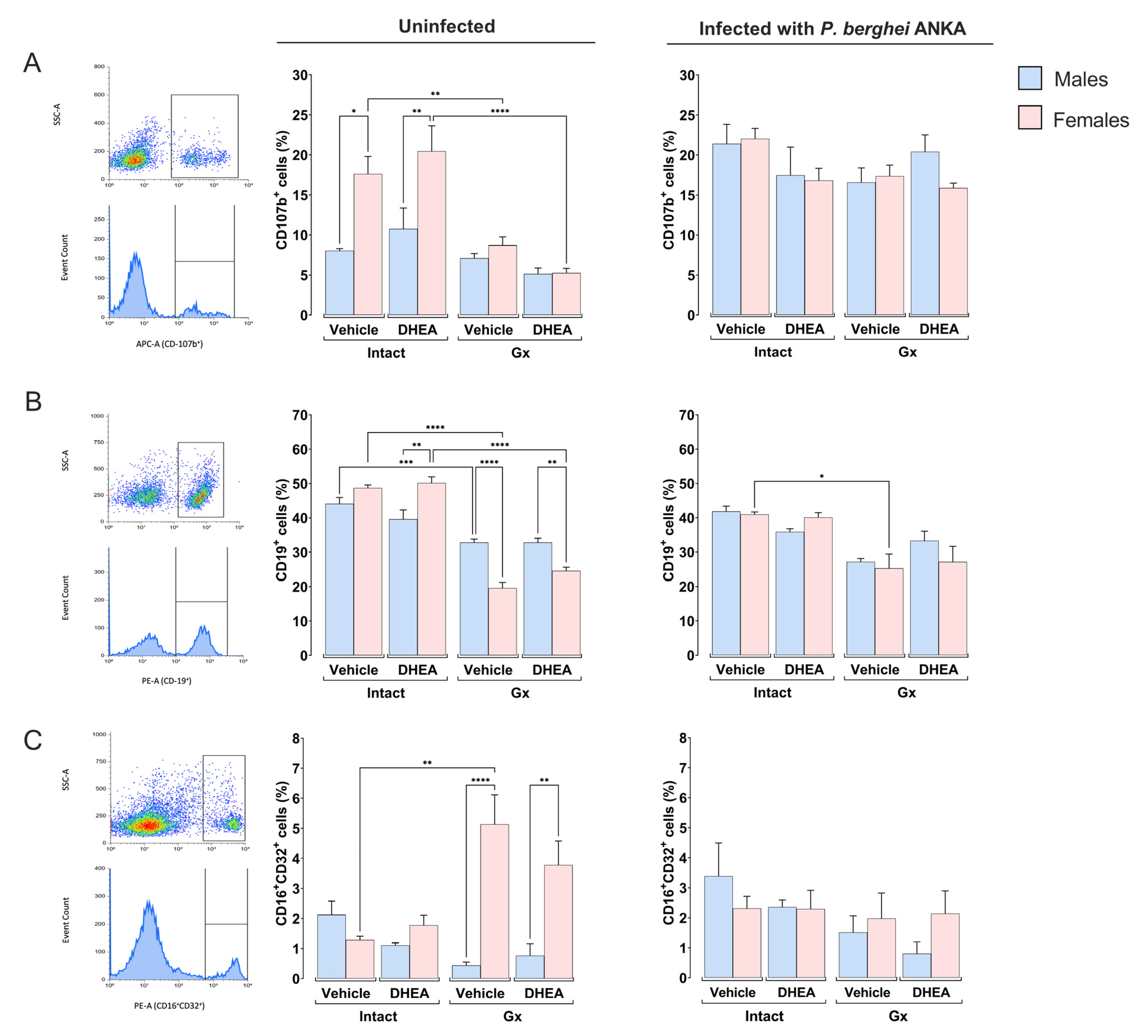
2.4. Reducing the DHEA Concentration Generated a Dimorphic Pattern in the CD3+, CD19+ and NK Cell Populations of Uninfected Mice
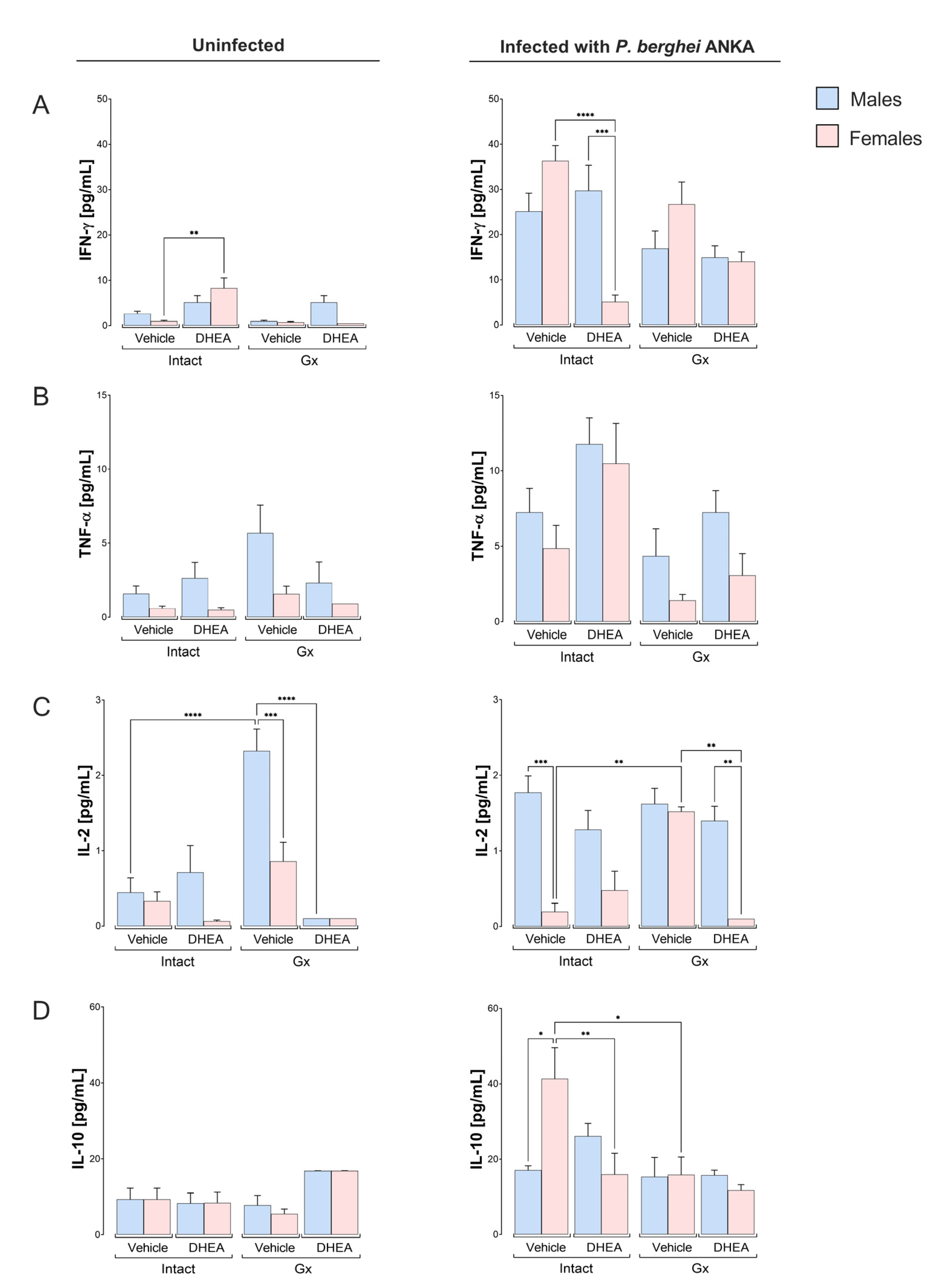
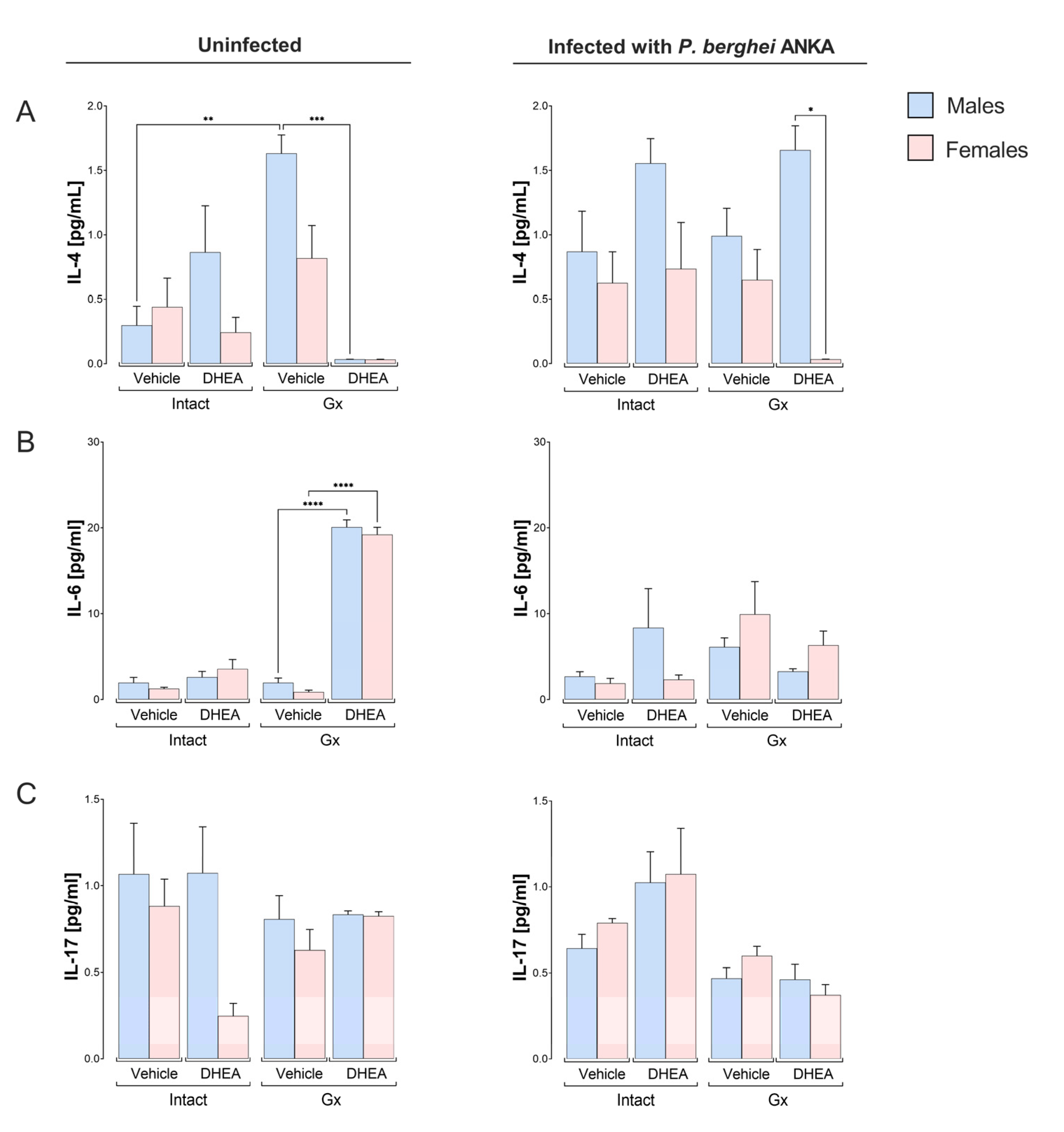
2.5. DHEA Generated a Dimorphic Pattern in the Concentrations of IFN-γ, IL-2 and IL-4
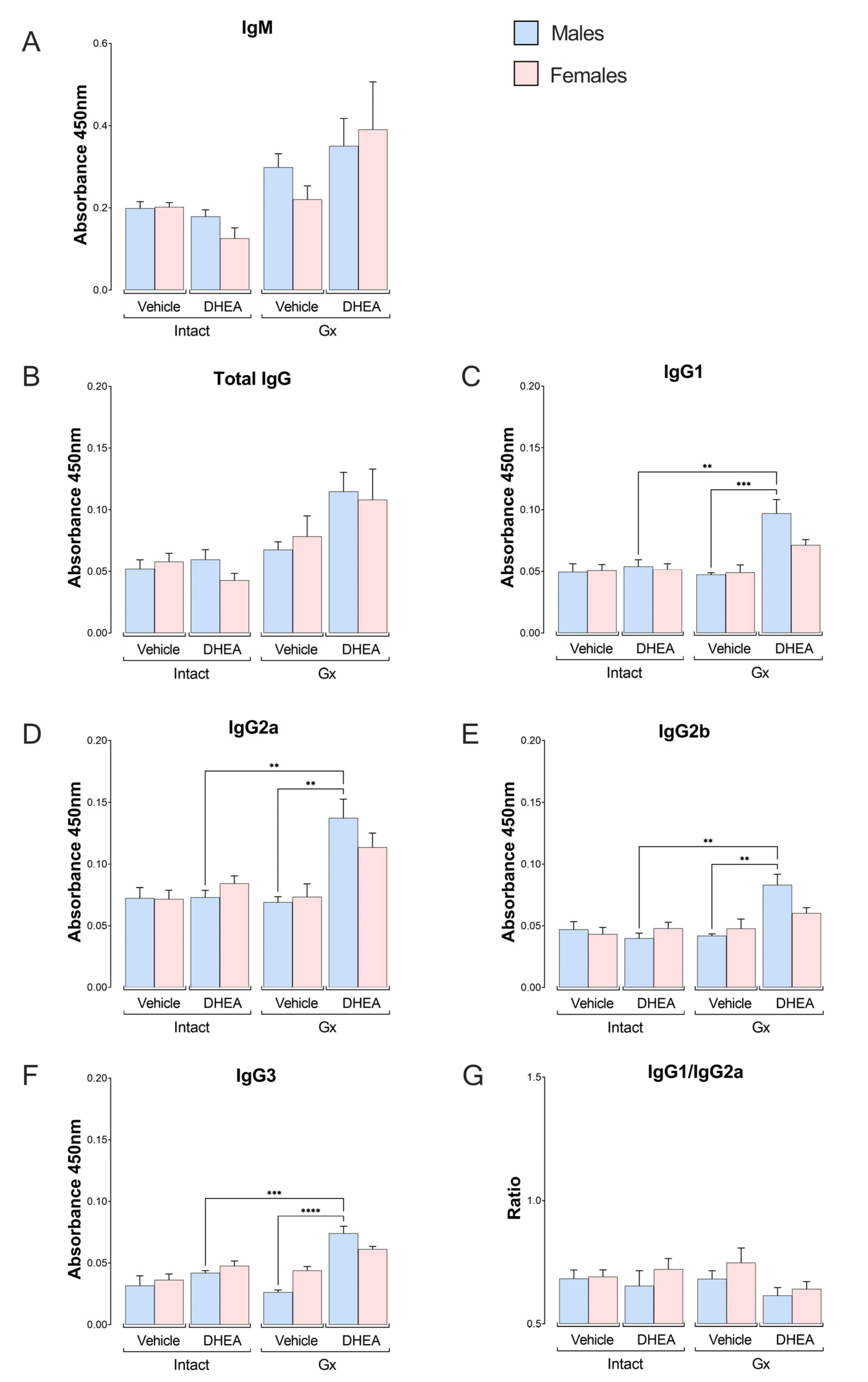
2.6. Reconstitution of Gx Males with DHEA Increased the Concentration of the IgG Subclass
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Gonadectomy
4.2.1. Orchidectomy
4.2.2. Ovariectomy
4.3. DHEA Administration
4.4. Parasite, Infection and Parasitemia
4.5. Body Weight and Temperature
4.6. Hemoglobin Concentration
4.7. Extraction of Sex Hormones from Plasma Samples
4.8. Quantification of DHEA in Plasma
4.9. Quantification of Cell Populations in the Spleen
4.10. Plasma Th1/Th2/Th17 Cytokine Quantification
4.11. Quantification of IgM and IgG Antibody Concentrations
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Landgraf, B.; Kollaritsch, H.; Wiedermann, G.; Wernsdorfer, W.H. Parasite density of Plasmodium falciparum malaria in Ghanaian schoolchildren: Evidence for influence of sex hormones? Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Wildling, E.; Winkler, S.; Kremsner, P.G.; Brandts, C.; Jenne, L.; Wernsdorfer, W.H. Malaria epidemiology in the province of Moyen Ogoov, Gabon. Trop. Med. Parasitol. 1995, 46, 77–82. [Google Scholar] [PubMed]
- Klein, S.L. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004, 26, 247–264. [Google Scholar] [CrossRef]
- Aguilar-Castro, J.; Cervantes-Candelas, L.A.; Buendia-Gonzalez, F.O.; Nolasco-Perez, T.J.; Lopez-Padilla, M.S.; Fernandez-Rivera, O.; Cervantes-Sandoval, A.; Legorreta-Herrera, M. Dimorphic effect of 17beta-oestradiol on pathology and oxidative stress in experimental malaria. Immunobiology 2020, 225, 151873. [Google Scholar] [CrossRef]
- Cervantes-Candelas, L.A.; Aguilar-Castro, J.; Buendia-Gonzalez, F.O.; Fernandez-Rivera, O.; Nolasco-Perez, T.J.; Lopez-Padilla, M.S.; Chavira-Ramirez, D.R.; Legorreta-Herrera, M. 17beta-Estradiol Is Involved in the Sexual Dimorphism of the Immune Response to Malaria. Front. Endocrinol. 2021, 12, 643851. [Google Scholar] [CrossRef]
- Aguilar-Castro, J.; Cervantes-Candelas, L.A.; Buendia-Gonzalez, F.O.; Fernandez-Rivera, O.; Nolasco-Perez, T.J.; Lopez-Padilla, M.S.; Chavira-Ramirez, D.R.; Cervantes-Sandoval, A.; Legorreta-Herrera, M. Testosterone induces sexual dimorphism during infection with Plasmodium berghei ANKA. Front. Cell Infect. Microbiol. 2022, 12, 968325. [Google Scholar] [CrossRef] [PubMed]
- Legorreta-Herrera, M.; Mosqueda-Romo, N.A.; Nava-Castro, K.E.; Morales-Rodriguez, A.L.; Buendia-Gonzalez, F.O.; Morales-Montor, J. Sex hormones modulate the immune response to Plasmodium berghei ANKA in CBA/Ca mice. Parasitol. Res. 2015, 114, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Cernetich, A.; Garver, L.S.; Jedlicka, A.E.; Klein, P.W.; Kumar, N.; Scott, A.L.; Klein, S.L. Involvement of gonadal steroids and gamma interferon in sex differences in response to blood-stage malaria infection. Infect. Immun. 2006, 74, 3190–3203. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, K.; Sowa, P.; Rutkowska-Talipska, J.; Kuryliszyn-Moskal, A.; Rutkowski, R. Dehydroepiandrosterone (DHEA): Hypes and hopes. Drugs 2014, 74, 1195–1207. [Google Scholar] [CrossRef]
- Knoferl, M.W.; Angele, M.K.; Catania, R.A.; Diodato, M.D.; Bland, K.I.; Chaudry, I.H. Immunomodulatory effects of dehydroepiandrosterone in proestrus female mice after trauma-hemorrhage. J. Appl. Physiol. 2003, 95, 529–535. [Google Scholar] [CrossRef]
- Solerte, S.B.; Fioravanti, M.; Vignati, G.; Giustina, A.; Cravello, L.; Ferrari, E. Dehydroepiandrosterone sulfate enhances natural killer cell cytotoxicity in humans via locally generated immunoreactive insulin-like growth factor I. J. Clin. Endocrinol. Metab. 1999, 84, 3260–3267. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yu, L.; Zhao, J.; Ma, H. Effect of dehydroepiandrosterone on the immune function of mice in vivo and in vitro. Mol. Immunol. 2019, 112, 283–290. [Google Scholar] [CrossRef]
- Leplina, O.Y.; Tikhonova, M.A.; Sakchno, L.V.; Tyrinova, T.V.; Ostanin, A.A.; Chernykh, E.R. Effect of dehydroepiandrosterone sulfate on maturation and functional properties of interferon-alpha-induced dendritic cells. Bull. Exp. Biol. Med. 2009, 148, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nathan, D.; Padgett, D.A.; Loria, R.M. Androstenediol and dehydroepiandrosterone protect mice against lethal bacterial infections and lipopolysaccharide toxicity. J. Med. Microbiol. 1999, 48, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Loria, R.M.; Inge, T.H.; Cook, S.S.; Szakal, A.K.; Regelson, W. Protection against acute lethal viral infections with the native steroid dehydroepiandrosterone (DHEA). J. Med. Virol. 1988, 26, 301–314. [Google Scholar] [CrossRef]
- Filipin Mdel, V.; Caetano, L.C.; Brazao, V.; Santello, F.H.; Toldo, M.P.; do Prado, J.C., Jr. DHEA and testosterone therapies in Trypanosoma cruzi-infected rats are associated with thymic changes. Res. Vet. Sci. 2010, 89, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Kurtis, J.D.; Friedman, J.F.; Leenstra, T.; Langdon, G.C.; Wu, H.W.; Manalo, D.L.; Su, L.; Jiz, M.; Jarilla, B.; Pablo, A.O.; et al. Pubertal development predicts resistance to infection and reinfection with Schistosoma japonicum. Clin. Infect. Dis. 2006, 42, 1692–1698. [Google Scholar] [CrossRef]
- Fallon, P.G.; Richardson, E.J.; Jones, F.M.; Dunne, D.W. Dehydroepiandrosterone sulfate treatment of mice modulates infection with Schistosoma mansoni. Clin. Diagn. Lab. Immunol. 1998, 5, 251–253. [Google Scholar] [CrossRef]
- Galindo-Sevilla, N.; Soto, N.; Mancilla, J.; Cerbulo, A.; Zambrano, E.; Chavira, R.; Huerto, J. Low serum levels of dehydroepiandrosterone and cortisol in human diffuse cutaneous leishmaniasis by Leishmania mexicana. Am. J. Trop. Med. Hyg. 2007, 76, 566–572. [Google Scholar] [CrossRef]
- Freilich, D.; Ferris, S.; Wallace, M.; Leach, L.; Kallen, A.; Frincke, J.; Ahlem, C.; Hacker, M.; Nelson, D.; Hebert, J. 16alpha-bromoepiandrosterone, a dehydroepiandrosterone (DHEA) analogue, inhibits Plasmodium falciparum and Plasmodium berghei growth. Am. J. Trop. Med. Hyg. 2000, 63, 280–283. [Google Scholar] [CrossRef]
- Bernin, H.; Lotter, H. Sex bias in the outcome of human tropical infectious diseases: Influence of steroid hormones. J. Infect. Dis. 2014, 209 (Suppl. S3), S107–S113. [Google Scholar] [CrossRef]
- Neunzig, J.; Bernhardt, R. Dehydroepiandrosterone sulfate (DHEAS) stimulates the first step in the biosynthesis of steroid hormones. PLoS ONE 2014, 9, e89727. [Google Scholar] [CrossRef][Green Version]
- Buendia-Gonzalez, F.O.; Legorreta-Herrera, M. The Similarities and Differences between the Effects of Testosterone and DHEA on the Innate and Adaptive Immune Response. Biomolecules 2022, 12, 1768. [Google Scholar] [CrossRef]
- Kurtis, J.D.; Mtalib, R.; Onyango, F.K.; Duffy, P.E. Human resistance to Plasmodium falciparum increases during puberty and is predicted by dehydroepiandrosterone sulfate levels. Infect. Immun. 2001, 69, 123–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leenstra, T.; ter Kuile, F.O.; Kariuki, S.K.; Nixon, C.P.; Oloo, A.J.; Kager, P.A.; Kurtis, J.D. Dehydroepiandrosterone sulfate levels associated with decreased malaria parasite density and increased hemoglobin concentration in pubertal girls from western Kenya. J. Infect. Dis. 2003, 188, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Villavicencio, J.A.; Larralde, C.; Morales-Montor, J. Treatment with dehydroepiandrosterone in vivo and in vitro inhibits reproduction, growth and viability of Taenia crassiceps metacestodes. Int. J. Parasitol. 2008, 38, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Stumhofer, J.S. The spleen: “epicenter” in malaria infection and immunity. J. Leukoc. Biol. 2021, 110, 753–769. [Google Scholar] [CrossRef]
- Lyke, K.E.; Burges, R.; Cissoko, Y.; Sangare, L.; Dao, M.; Diarra, I.; Kone, A.; Harley, R.; Plowe, C.V.; Doumbo, O.K.; et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect. Immun. 2004, 72, 5630–5637. [Google Scholar] [CrossRef]
- Awandare, G.A.; Goka, B.; Boeuf, P.; Tetteh, J.K.; Kurtzhals, J.A.; Behr, C.; Akanmori, B.D. Increased levels of inflammatory mediators in children with severe Plasmodium falciparum malaria with respiratory distress. J. Infect. Dis. 2006, 194, 1438–1446. [Google Scholar] [CrossRef][Green Version]
- Powell, J.M.; Sonnenfeld, G. The effects of dehydroepiandrosterone (DHEA) on in vitro spleen cell proliferation and cytokine production. J. Interferon Cytokine Res. 2006, 26, 34–39. [Google Scholar] [CrossRef]
- Langhorne, J.; Cross, C.; Seixas, E.; Li, C.; Von Der Weid, T. A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc. Natl. Acad. Sci. USA 1998, 95, 1730–1734. [Google Scholar] [CrossRef]
- Sudo, N.; Yu, X.N.; Kubo, C. Dehydroepiandrosterone attenuates the spontaneous elevation of serum IgE level in NC/Nga mice. Immunol. Lett. 2001, 79, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.C.; Souza, L.C.; Caperuto, L.C.; Anhe, G.F.; Amanso, A.M.; Teixeira, V.P.; Bordin, S.; Carpinelli, A.R.; Britto, L.R.; Barbieri, R.L.; et al. Dehydroepiandrosterone increases beta-cell mass and improves the glucose-induced insulin secretion by pancreatic islets from aged rats. FEBS Lett. 2006, 580, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Dalla Valle, L.; Couet, J.; Labrie, Y.; Simard, J.; Belvedere, P.; Simontacchi, C.; Labrie, F.; Colombo, L. Occurrence of cytochrome P450c17 mRNA and dehydroepiandrosterone biosynthesis in the rat gastrointestinal tract. Mol. Cell. Endocrinol. 1995, 111, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Moore, T.M.; Drew, B.G.; Ribas, V.; Wanagat, J.; Civelek, M.; Segawa, M.; Wolf, D.M.; Norheim, F.; Seldin, M.M.; et al. Estrogen receptor alpha controls metabolism in white and brown adipocytes by regulating Polg1 and mitochondrial remodeling. Sci. Transl. Med. 2020, 12, eaax8096. [Google Scholar] [CrossRef]
- Bobyleva, V.; Kneer, N.; Bellei, M.; Battelli, D.; Lardy, H.A. Concerning the mechanism of increased thermogenesis in rats treated with dehydroepiandrosterone. J. Bioenerg. Biomembr. 1993, 25, 313–321. [Google Scholar] [CrossRef]
- Peschle, C.; Rappaport, I.A.; Sasso, G.F.; Condorelli, M.; Gordon, A.S. The role of estrogen in the regulation of erythropoietin production. Endocrinology 1973, 92, 358–362. [Google Scholar] [CrossRef]
- Voegeli, T.A.; Kurtz, A.; Grimm, M.O.; Effert, P.; Eckardt, K.U. Anemia under androgen deprivation: Influence of flutamide, cyproteroneacetate and orchiectomy on the erythropoietin system. Horm. Metab. Res. 2005, 37, 89–93. [Google Scholar] [CrossRef]
- Chen, F.; Knecht, K.; Birzin, E.; Fisher, J.; Wilkinson, H.; Mojena, M.; Moreno, C.T.; Schmidt, A.; Harada, S.; Freedman, L.P.; et al. Direct agonist/antagonist functions of dehydroepiandrosterone. Endocrinology 2005, 146, 4568–4576. [Google Scholar] [CrossRef]
- dos Santos, C.D.; Toldo, M.P.A.; do Prado Júnior, J.C. Trypanosoma cruzi: The effects of dehydroepiandrosterone (DHEA) treatment during experimental infection. Acta Trop. 2005, 95, 109–115. [Google Scholar] [CrossRef]
- Lu, S.F.; McKenna, S.E.; Cologer-Clifford, A.; Nau, E.A.; Simon, N.G. Androgen receptor in mouse brain: Sex differences and similarities in autoregulation. Endocrinology 1998, 139, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Azzi, L.; El-Alfy, M.; Labrie, F. Gender differences and effects of sex steroids and dehydroepiandrosterone on androgen and oestrogen alpha receptors in mouse sebaceous glands. Br. J. Dermatol. 2006, 154, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Refaeli, Y.; Van Parijs, L.; London, C.A.; Tschopp, J.; Abbas, A.K. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity 1998, 8, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Torres, L.; Rodriguez-Ropon, A.; Aguilar-Medina, M.; Favila-Castillo, L. Mouse splenic CD4+ and CD8+ T cells undergo extensive apoptosis during a Plasmodium chabaudi chabaudi AS infection. Parasite Immunol. 2001, 23, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Arsenovic-Ranin, N.; Kosec, D.; Pilipovic, I.; Nacka-Aleksic, M.; Bufan, B.; Stojic-Vukanic, Z.; Leposavic, G. Sex and age as determinants of rat T-cell phenotypic characteristics: Influence of peripubertal gonadectomy. Mol. Cell. Biochem. 2017, 431, 169–185. [Google Scholar] [CrossRef]
- Bruder, J.M.; Sobek, L.; Oettel, M. Dehydroepiandrosterone stimulates the estrogen response element. J. Steroid Biochem. Mol. Biol. 1997, 62, 461–466. [Google Scholar] [CrossRef]
- Priyanka, H.P.; Krishnan, H.C.; Singh, R.V.; Hima, L.; Thyagarajan, S. Estrogen modulates in vitro T cell responses in a concentration- and receptor-dependent manner: Effects on intracellular molecular targets and antioxidant enzymes. Mol. Immunol. 2013, 56, 328–339. [Google Scholar] [CrossRef]
- Legorreta-Herrera, M.; Rivas-Contreras, S.; Ventura-Gallegos, J.; Zentella-Dehesa, A. Nitric oxide is involved in the upregulation of IFN-gamma and IL-10 mRNA expression by CD8(+) T cells during the blood stages of P. chabaudi AS infection in CBA/Ca mice. Int. J. Biol. Sci. 2011, 7, 1401–1411. [Google Scholar]
- Belnoue, E.; Potter, S.M.; Rosa, D.S.; Mauduit, M.; Gruner, A.C.; Kayibanda, M.; Mitchell, A.J.; Hunt, N.H.; Renia, L. Control of pathogenic CD8+ T cell migration to the brain by IFN-gamma during experimental cerebral malaria. Parasite Immunol. 2008, 30, 544–553. [Google Scholar] [CrossRef]
- Druzd, D.; Matveeva, O.; Ince, L.; Harrison, U.; He, W.; Schmal, C.; Herzel, H.; Tsang, A.H.; Kawakami, N.; Leliavski, A. Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity 2017, 46, 120–132. [Google Scholar] [CrossRef]
- Francelin, C.; Paulino, L.C.; Gameiro, J.; Verinaud, L. Effects of Plasmodium berghei on thymus: High levels of apoptosis and premature egress of CD4(+)CD8(+) thymocytes in experimentally infected mice. Immunobiology 2011, 216, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, C.M.; Cleary, J.; Dagtas, A.S.; Moussai, D.; Diamond, B. Estrogen alters thresholds for B cell apoptosis and activation. J. Clin. Investig. 2002, 109, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Das, A. IL-2 mediates NK cell proliferation but not hyperactivity. Immunol. Res. 2018, 66, 151–157. [Google Scholar] [CrossRef]
- Legorreta-Herrera, M.; Nava-Castro, K.E.; Palacios-Arreola, M.I.; Hernandez-Cervantes, R.; Aguilar-Castro, J.; Cervantes-Candelas, L.A.; Morales-Montor, J. Sex-Associated Differential mRNA Expression of Cytokines and Its Regulation by Sex Steroids in Different Brain Regions in a Plasmodium berghei ANKA Model of Cerebral Malaria. Mediators Inflamm. 2018, 2018, 5258797. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.D.; Toldo, M.P.; Santello, F.H.; Filipin Mdel, V.; Brazao, V.; do Prado Junior, J.C. Dehydroepiandrosterone increases resistance to experimental infection by Trypanosoma cruzi. Vet. Parasitol. 2008, 153, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cao, J.; Yu, L.; Ma, H. Dehydroepiandrosterone alleviates E. Coli O157:H7-induced inflammation by preventing the activation of p38 MAPK and NF-kappaB pathways in mice peritoneal macrophages. Mol. Immunol. 2019, 114, 114–122. [Google Scholar] [CrossRef]
- Pratschke, S.; von Dossow-Hanfstingl, V.; Dietz, J.; Schneider, C.P.; Tufman, A.; Albertsmeier, M.; Winter, H.; Angele, M.K. Dehydroepiandrosterone modulates T-cell response after major abdominal surgery. J. Surg. Res. 2014, 189, 117–125. [Google Scholar] [CrossRef]
- Chang, D.M.; Chu, S.J.; Chen, H.C.; Kuo, S.Y.; Lai, J.H. Dehydroepiandrosterone suppresses interleukin 10 synthesis in women with systemic lupus erythematosus. Ann. Rheum. Dis. 2004, 63, 1623–1626. [Google Scholar] [CrossRef]
- Medina, T.S.; Costa, S.P.; Oliveira, M.D.; Ventura, A.M.; Souza, J.M.; Gomes, T.F.; Vallinoto, A.C.; Povoa, M.M.; Silva, J.S.; Cunha, M.G. Increased interleukin-10 and interferon-gamma levels in Plasmodium vivax malaria suggest a reciprocal regulation which is not altered by IL-10 gene promoter polymorphism. Malar. J. 2011, 10, 264. [Google Scholar] [CrossRef]
- Claser, C.; De Souza, J.B.; Thorburn, S.G.; Grau, G.E.; Riley, E.M.; Rénia, L.; Hafalla, J.C. Host resistance to Plasmodium-induced acute immune pathology is regulated by interleukin-10 receptor signaling. Infect. Immun. 2017, 85, e00941-16. [Google Scholar] [CrossRef]
- Ho, A.; Liu, Y.; Khan, T.A.; Hsu, D.-H.; Bazan, J.F.; Moore, K.W. A receptor for interleukin 10 is related to interferon receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 11267–11271. [Google Scholar] [CrossRef] [PubMed]
- Omer, F.M.; Riley, E.M. Transforming growth factor beta production is inversely correlated with severity of murine malaria infection. J. Exp. Med. 1998, 188, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.Q. TGF-beta: The sword, the wand, and the shield of FOXP3(+) regulatory T cells. J. Mol. Cell Biol. 2012, 4, 29–37. [Google Scholar] [CrossRef]
- Coles, A.J.; Thompson, S.; Cox, A.L.; Curran, S.; Gurnell, E.M.; Chatterjee, V.K. Dehydroepiandrosterone replacement in patients with Addison’s disease has a bimodal effect on regulatory (CD4+CD25hi and CD4+FoxP3+) T cells. Eur. J. Immunol. 2005, 35, 3694–3703. [Google Scholar] [CrossRef] [PubMed]
- Raju, K.; Rabinovich, B.A.; Radvanyi, L.G.; Spaner, D.; Miller, R.G. A central role for IL-2 in fate determination of mature T cells--I: Role in determining the Th1/Th2 profile in primary T cell cultures. Int. Immunol. 2001, 13, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Tabata, N.; Tagami, H.; Terui, T. Dehydroepiandrosterone may be one of the regulators of cytokine production in atopic dermatitis. Arch. Dermatol. Res. 1997, 289, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Son, B.K.; Kojima, T.; Ogawa, S.; Akishita, M. Testosterone inhibits aneurysm formation and vascular inflammation in male mice. J. Endocrinol. 2019, 241, 307–317. [Google Scholar] [CrossRef]
- Cheng, G.F.; Tseng, J. Regulation of murine interleukin-10 production by dehydroepiandrosterone. J. Interferon Cytokine Res. 2000, 20, 471–478. [Google Scholar] [CrossRef]
- Huang, B.; Huang, S.; Liu, M.; Chen, Y.; Wu, B.; Guo, H.; Su, X.-Z.; Lu, F. T cell Ig and mucin-1 and-3 in Plasmodium berghei ANKA infection. Parasitol. Res. 2013, 112, 2713. [Google Scholar] [CrossRef][Green Version]
- Hofman, F.M.; Brock, M.; Taylor, C.R.; Lyons, B. IL-4 regulates differentiation and proliferation of human precursor B cells. J. Immunol. 1988, 141, 1185–1190. [Google Scholar] [CrossRef]
- Yone, C.L.; Kremsner, P.G.; Luty, A.J. Immunoglobulin G isotype responses to erythrocyte surface-expressed variant antigens of Plasmodium falciparum predict protection from malaria in African children. Infect. Immun. 2005, 73, 2281–2287. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, K.K.; Al-Rayyan, N.; Ivanova, M.M.; Mattingly, K.A.; Ripp, S.L.; Klinge, C.M.; Prough, R.A. DHEA metabolites activate estrogen receptors alpha and beta. Steroids 2013, 78, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Nephew, K.P.; Sheeler, C.Q.; Dudley, M.D.; Gordon, S.; Nayfield, S.G.; Khan, S.A. Studies of dehydroepiandrosterone (DHEA) with the human estrogen receptor in yeast. Mol. Cell. Endocrinol. 1998, 143, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Legorreta-Herrera, M.; Ventura-Ayala, M.L.; Licona-Chavez, R.N.; Soto-Cruz, I.; Hernandez-Clemente, F.F. Early treatment during a primary malaria infection modifies the development of cross immunity. Parasite Immunol. 2004, 26, 7–17. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buendía-González, F.O.; Cervantes-Candelas, L.A.; Aguilar-Castro, J.; Fernández-Rivera, O.; Nolasco-Pérez, T.d.J.; López-Padilla, M.S.; Chavira-Ramírez, D.R.; Cervantes-Sandoval, A.; Legorreta-Herrera, M. DHEA Induces Sex-Associated Differential Patterns in Cytokine and Antibody Levels in Mice Infected with Plasmodium berghei ANKA. Int. J. Mol. Sci. 2023, 24, 12549. https://doi.org/10.3390/ijms241612549
Buendía-González FO, Cervantes-Candelas LA, Aguilar-Castro J, Fernández-Rivera O, Nolasco-Pérez TdJ, López-Padilla MS, Chavira-Ramírez DR, Cervantes-Sandoval A, Legorreta-Herrera M. DHEA Induces Sex-Associated Differential Patterns in Cytokine and Antibody Levels in Mice Infected with Plasmodium berghei ANKA. International Journal of Molecular Sciences. 2023; 24(16):12549. https://doi.org/10.3390/ijms241612549
Chicago/Turabian StyleBuendía-González, Fidel Orlando, Luis Antonio Cervantes-Candelas, Jesús Aguilar-Castro, Omar Fernández-Rivera, Teresita de Jesús Nolasco-Pérez, Monserrat Sofía López-Padilla, David Roberto Chavira-Ramírez, Armando Cervantes-Sandoval, and Martha Legorreta-Herrera. 2023. "DHEA Induces Sex-Associated Differential Patterns in Cytokine and Antibody Levels in Mice Infected with Plasmodium berghei ANKA" International Journal of Molecular Sciences 24, no. 16: 12549. https://doi.org/10.3390/ijms241612549
APA StyleBuendía-González, F. O., Cervantes-Candelas, L. A., Aguilar-Castro, J., Fernández-Rivera, O., Nolasco-Pérez, T. d. J., López-Padilla, M. S., Chavira-Ramírez, D. R., Cervantes-Sandoval, A., & Legorreta-Herrera, M. (2023). DHEA Induces Sex-Associated Differential Patterns in Cytokine and Antibody Levels in Mice Infected with Plasmodium berghei ANKA. International Journal of Molecular Sciences, 24(16), 12549. https://doi.org/10.3390/ijms241612549






