Construction, Expression, and Evaluation of the Naturally Acquired Humoral Immune Response against Plasmodium vivax RMC-1, a Multistage Chimeric Protein
Abstract
1. Introduction
2. Results
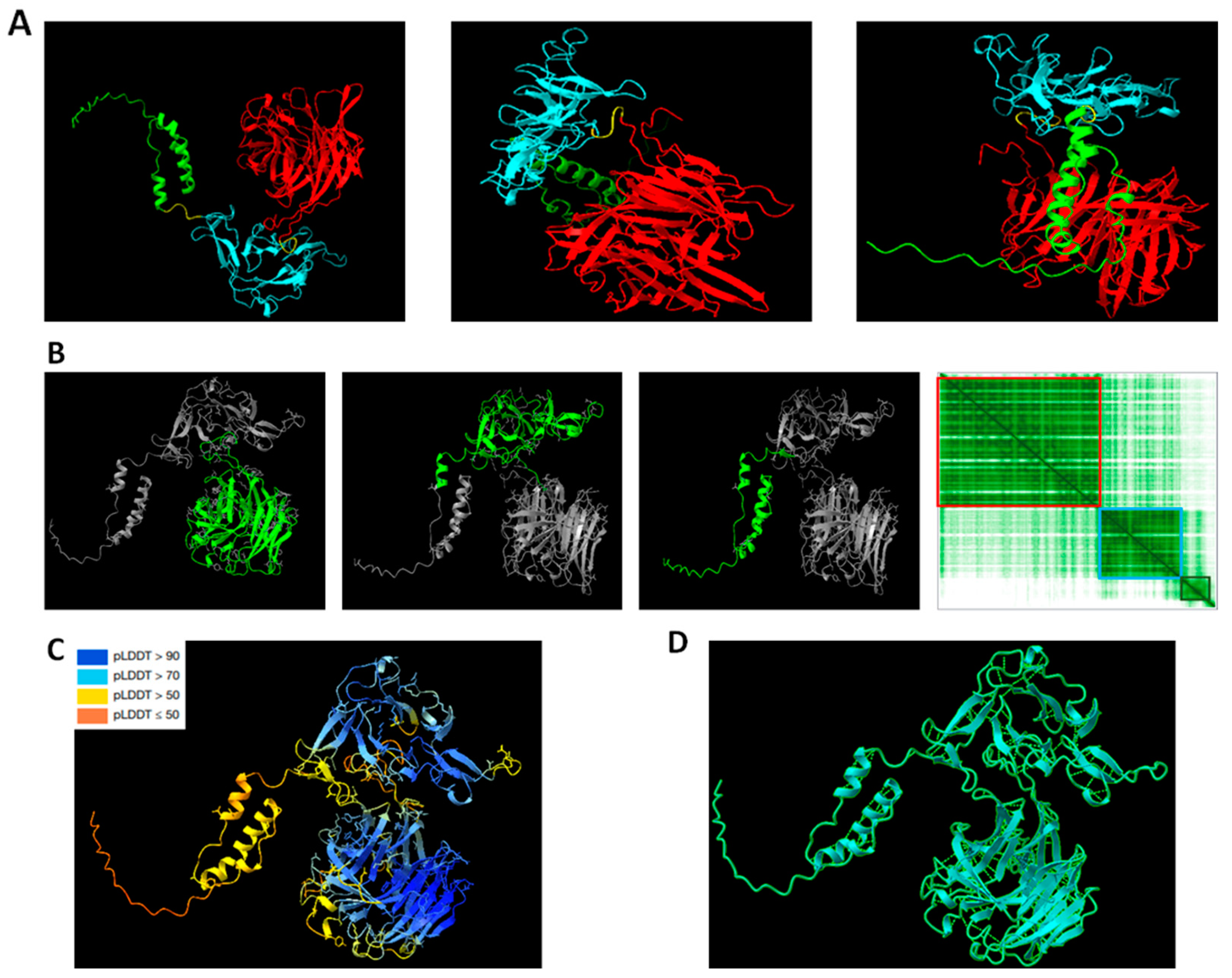
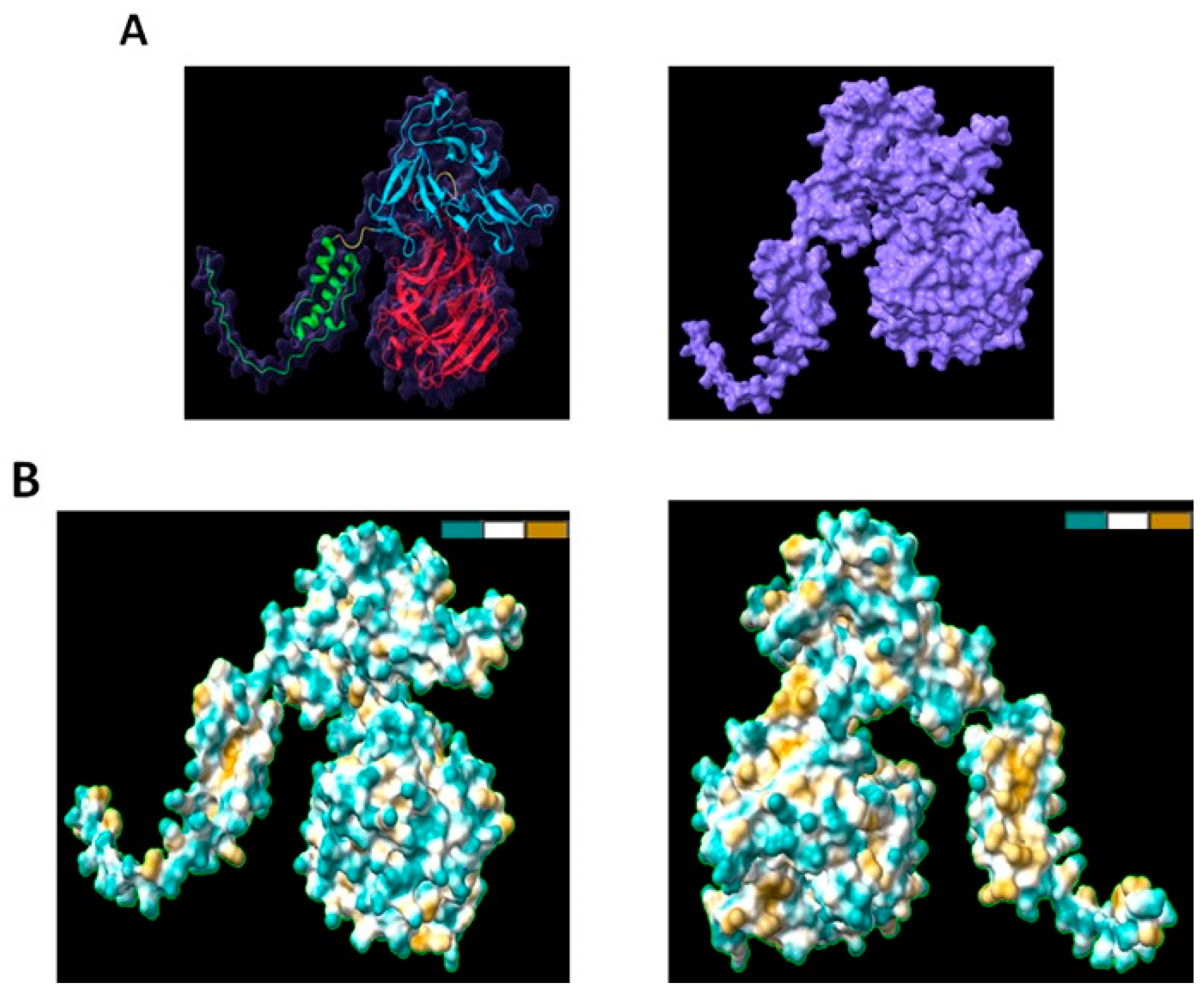
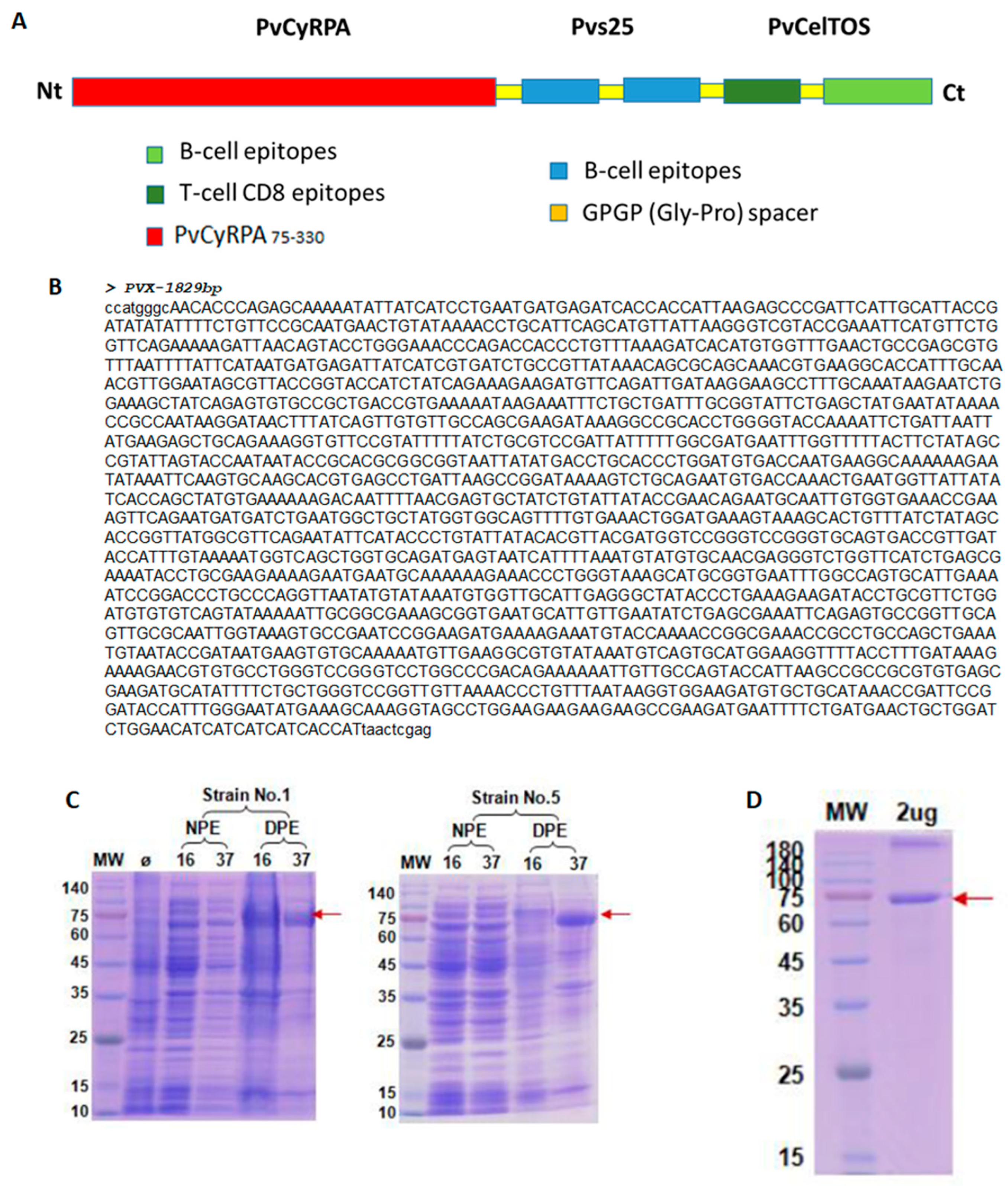
2.1. Structural Analysis of RMC-1
2.2. Epidemiological Profile of Studied Individuals
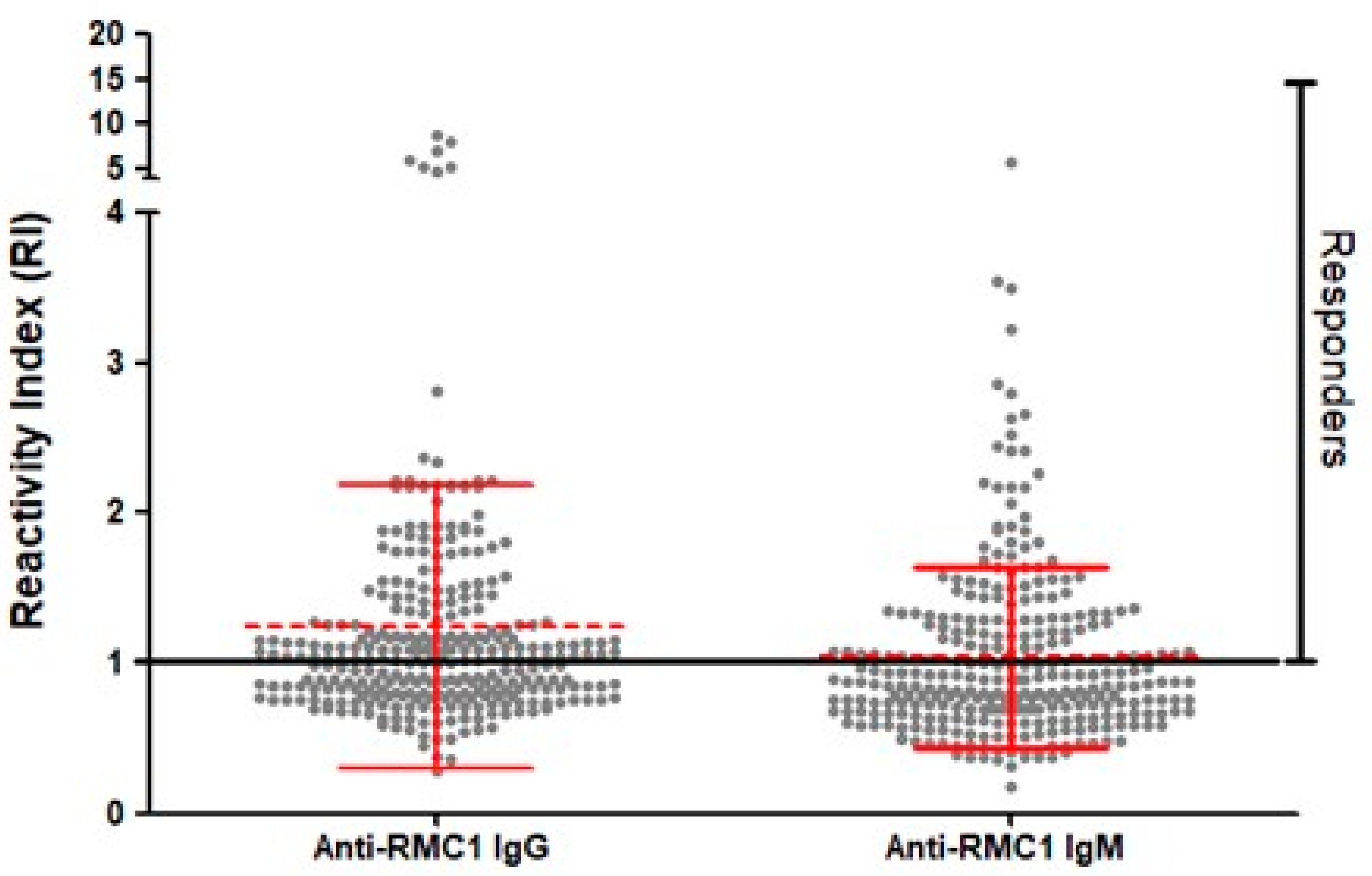
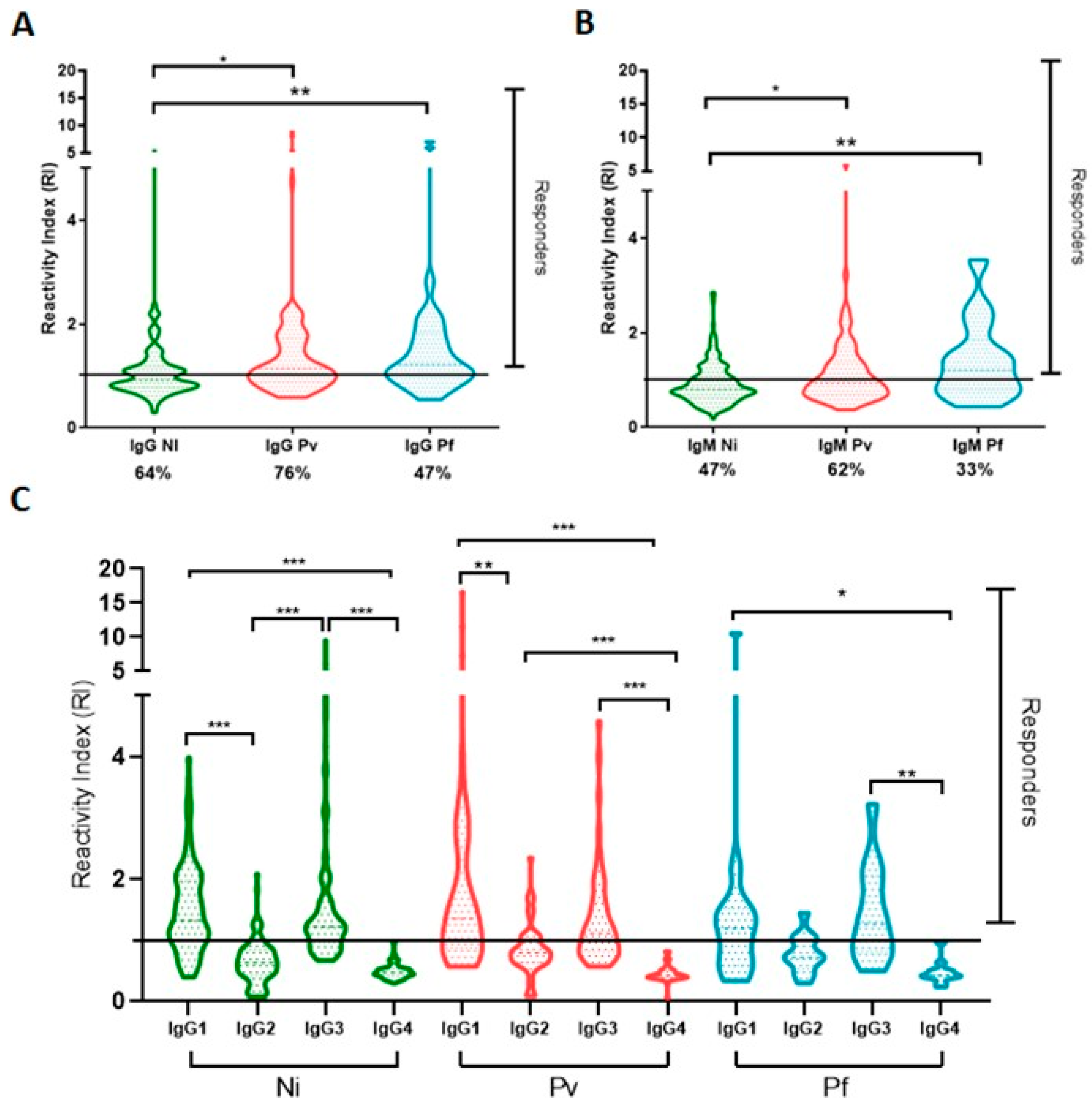
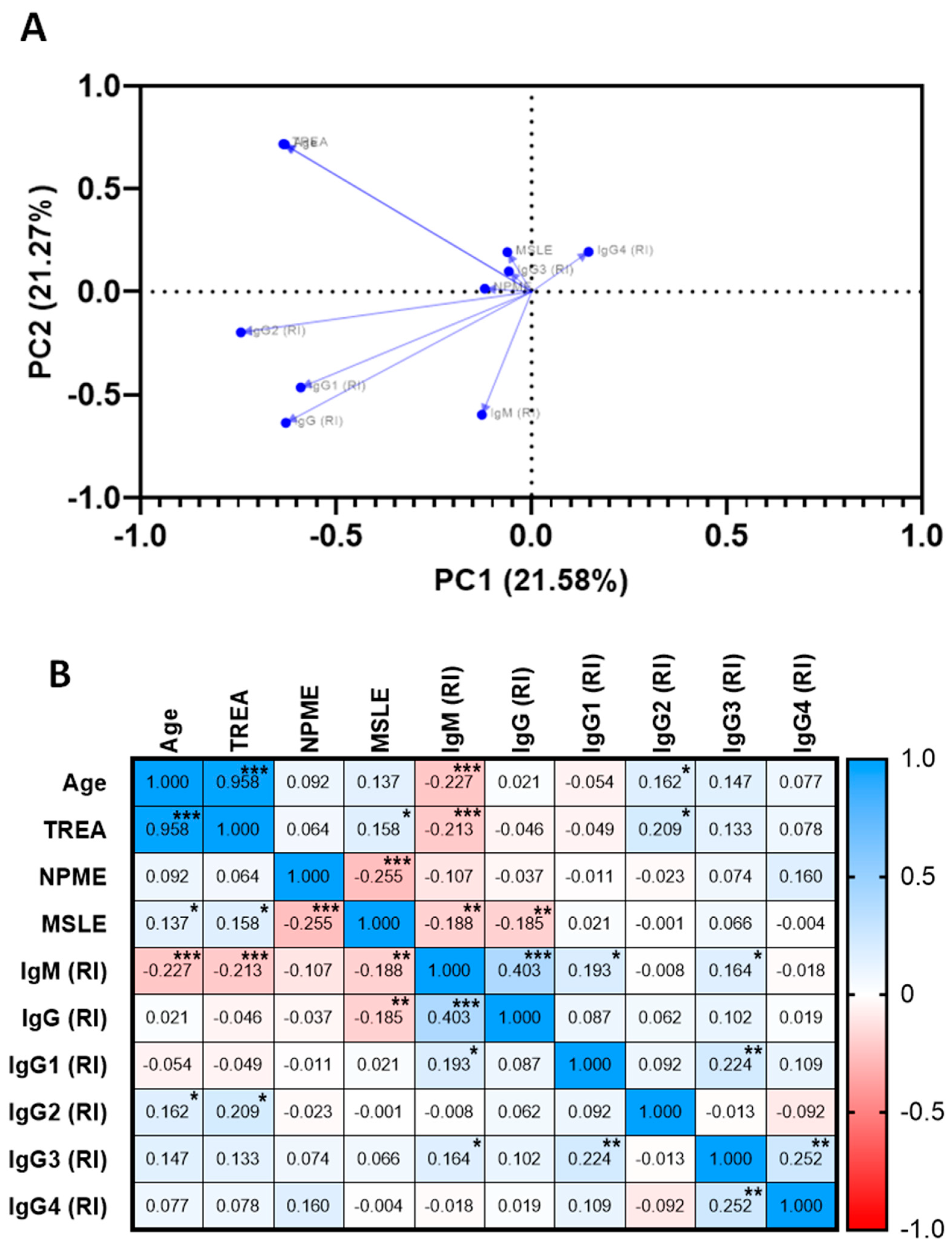
2.3. Naturally Acquired Antibodies to Recombinant PvRMC-1 and the Prevalence of Cytophilic Antibodies in Brazilian Amazon Individuals
2.4. Association of The Specific Humoral Response against PvRMC-1, Clinical, and Exposure Factors
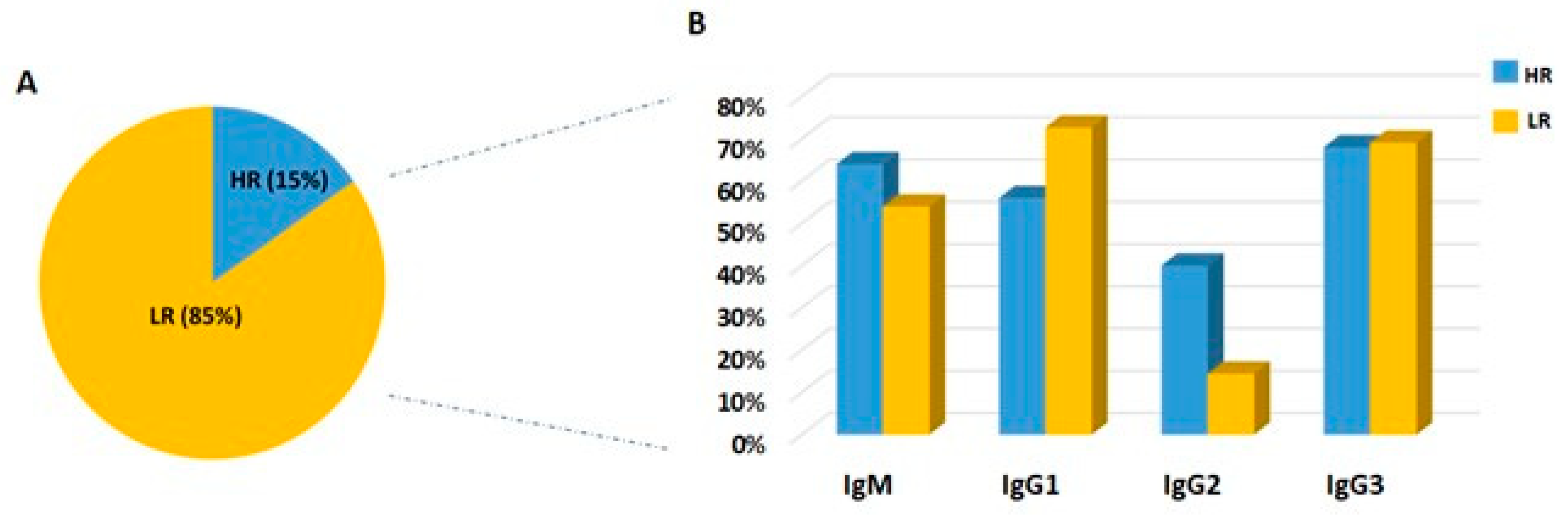
2.5. Frequency of IgM Antibodies and IgG Subclasses among High and Low Responders
3. Discussion
4. Materials and Methods
4.1. Study Area and Volunteers
4.2. Epidemiological Survey
4.3. Malaria Diagnosis and Blood Sampling
4.4. Structural Modelling
4.5. PvRMC-1 Design and Expression
4.6. ELISA
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report. Available online: https://www.malariaworld.org/blogs/world-malaria-report-2022 (accessed on 26 December 2022).
- Ministério da Saúde. Sistema de Informação da Vigilância Epidemiológica/SIVEP—Malária 2022. Available online: http://200.214.130.44/sivep_malaria/ (accessed on 20 February 2023).
- Finney, O.C.; Keitany, G.J.; Smithers, H.; Kaushansky, A.; Kappe, S.; Wang, R. Immunization with genetically attenuated P. falciparum parasites induces long-lived antibodies that efficiently block hepatocyte invasion by sporozoites. Vaccine 2014, 32, 2135–2138. [Google Scholar] [CrossRef] [PubMed]
- Behet, M.C.; Foquet, L.; van Gemert, G.-J.; Bijker, E.M.; Meuleman, P.; Leroux-Roels, G.; Hermsen, C.C.; Scholzen, A.; Sauerwein, R.W. Sporozoite immunization of human volunteers under chemoprophylaxis induces functional antibodies against pre-erythrocytic stages of Plasmodium falciparum. Malar. J. 2014, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Fabra-García, A.; Yang, A.S.; Behet, M.C.; Yap, Z.; van Waardenburg, Y.; Kaviraj, S.; Lanke, K.; van Gemert, G.J.; Jore, M.M.; Bousema, T.; et al. Human antibodies against noncircumsporozoite proteins block Plasmodium falciparum parasite development in hepatocytes. JCI Insight 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Opi, D.H.; Kurtovic, L.; Chan, J.-A.; Horton, J.L.; Feng, G.; Beeson, J.G. Multi-functional antibody profiling for malaria vaccine development and evaluation. Expert Rev. Vaccines 2021, 20, 1257–1272. [Google Scholar] [CrossRef]
- Antonelli, L.R.; Junqueira, C.; Vinetz, J.M.; Golenbock, D.T.; Ferreira, M.U.; Gazzinelli, R.T. The immunology of Plasmodium vivax malaria. Immunol. Rev. 2020, 293, 163–189. [Google Scholar] [CrossRef]
- Bergmann-Leitner, E.S.; Duncan, E.H.; Angov, E. The impact of immune responses on the asexual erythrocytic stages of plasmodium and the implication for vaccine development. In Malaria Parasites; IntechOpen: London, UK, 2012. [Google Scholar]
- Ockenhouse, C.F.; Angov, E.; Kester, K.E.; Diggs, C.; Soisson, L.; Cummings, J.F.; Stewart, A.V.; Palmer, D.R.; Mahajan, B.; Krzych, U.; et al. Phase I safety and immunogenicity trial of FMP1/AS02A, a Plasmodium falciparum MSP-1 asexual blood stage vaccine. Vaccine 2006, 24, 3009–3017. [Google Scholar] [CrossRef]
- Arora, G.; Hart, G.T.; Manzella-Lapeira, J.; Doritchamou, J.Y.A.; Narum, D.L.; Thomas, L.M.; Brzostowski, J.; Rajagopalan, S.; Doumbo, O.K.; Traore, B.; et al. NK cells inhibit Plasmodium falciparum growth in red blood cells via antibody-dependent cellular cytotoxicity. eLife 2018, 7, e36806. [Google Scholar] [CrossRef]
- Hviid, L.; Lopez-Perez, M.; Larsen, M.D.; Vidarsson, G. No sweet deal: The antibody-mediated immune response to malaria. Trends Parasitol. 2022, 38, 428–434. [Google Scholar] [CrossRef]
- Opi, D.H.; Boyle, M.J.; McLean, A.R.D.; Reiling, L.; Chan, J.-A.; Stanisic, D.I.; Ura, A.; Mueller, I.; Fowkes, F.J.I.; Rogerson, S.J.; et al. Reduced risk of placental parasitemia associated with complement fixation on Plasmodium falciparum by antibodies among pregnant women. BMC Med. 2021, 19, 201. [Google Scholar] [CrossRef]
- Kurtovic, L.; Boyle, M.J.; Opi, D.H.; Kennedy, A.T.; Tham, W.-H.; Reiling, L.; Chan, J.-A.; Beeson, J.G. Complement in malaria immunity and vaccines. Immunol. Rev. 2020, 293, 38–56. [Google Scholar] [CrossRef]
- Girard, M.P.; Reed, Z.H.; Friede, M.; Kieny, M.P. A review of human vaccine research and development: Malaria. Vaccine 2007, 25, 1567–1580. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.; Targett, G. Malaria vaccines and the new malaria agenda. Clin. Microbiol. Infect. 2011, 17, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, H.; Kusi, K.A.; Anum, D.; Hollingdale, M.R.; Peters, B.; Kim, Y.; Tetteh, J.K.A.; Ofori, M.F.; Gyan, B.A.; Koram, K.A.; et al. Measurement of ex vivo ELISpot interferon-gamma recall responses to Plasmodium falciparum AMA1 and CSP in Ghanaian adults with natural exposure to malaria. Malar. J. 2016, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Jimah, J.R.; Salinas, N.D.; Sala-Rabanal, M.; Jones, N.G.; Sibley, L.D.; Nichols, C.G.; Schlesinger, P.H.; Tolia, N.H. Malaria parasite CelTOS targets the inner leaflet of cell membranes for pore-dependent disruption. eLife 2016, 5, e20621. [Google Scholar] [CrossRef]
- Arévalo-Pinzón, G.; Garzón-Ospina, D.; Pulido, F.A.; Bermúdez, M.; Forero-Rodríguez, J.; Rodríguez-Mesa, X.M.; Reyes-Guarín, L.P.; Suárez, C.F.; Patarroyo, M.A. Plasmodium vivax Cell Traversal Protein for Ookinetes and Sporozoites (CelTOS) Functionally Restricted Regions Are Involved in Specific Host-Pathogen Interactions. Front. Cell. Infect. Microbiol. 2020, 10, 119. [Google Scholar] [CrossRef]
- Kariu, T.; Ishino, T.; Yano, K.; Chinzei, Y.; Yuda, M. CelTOS, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol. Microbiol. 2006, 59, 1369–1379. [Google Scholar] [CrossRef]
- Bergmann-Leitner, E.S.; Mease, R.M.; De La Vega, P.; Savranskaya, T.; Polhemus, M.; Ockenhouse, C.; Angov, E. Immunization with pre-erythrocytic antigen CelTOS from Plasmodium falciparum elicits cross-species protection against heterologous challenge with Plasmodium berghei. PLoS ONE 2010, 5, e12294. [Google Scholar] [CrossRef]
- Doolan, D.L.; Southwood, S.; Freilich, D.A.; Sidney, J.; Graber, N.L.; Shatney, L.; Bebris, L.; Florens, L.; Dobano, C.; Witney, A.A. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc. Natl. Acad. Sci. USA 2003, 100, 9952–9957. [Google Scholar] [CrossRef]
- Espinosa, D.A.; Vega-Rodriguez, J.; Flores-Garcia, Y.; Noe, A.R.; Muñoz, C.; Coleman, R.; Bruck, T.; Haney, K.; Stevens, A.; Retallack, D. The Plasmodium falciparum cell-traversal protein for ookinetes and sporozoites as a candidate for preerythrocytic and transmission-blocking vaccines. Infect. Immun. 2017, 85, e00498-16. [Google Scholar] [CrossRef]
- Chaves, L.B.; de Souza Perce-da-Silva, D.; Rodrigues-da-Silva, R.N.; da Silva, J.H.M.; Cassiano, G.C.; Machado, R.L.D.; Pratt-Riccio, L.R.; Banic, D.M.; da Costa Lima-Junior, J. Plasmodium vivax Cell Traversal Protein for Ookinetes and Sporozoites (PvCelTOS) gene sequence and potential epitopes are highly conserved among isolates from different regions of Brazilian Amazon. PLoS Neglected Trop. Dis. 2017, 11, e0005344. [Google Scholar]
- Rodrigues-da-Silva, R.N.; Soares, I.F.; Lopez-Camacho, C.; Martins da Silva, J.H.; Perce-da-Silva, D.d.S.; Têva, A.; Ramos Franco, A.M.; Pinheiro, F.G.; Chaves, L.B.; Pratt-Riccio, L.R. Plasmodium vivax cell-traversal protein for ookinetes and sporozoites: Naturally acquired humoral immune response and B-cell epitope mapping in brazilian amazon inhabitants. Front. Immunol. 2017, 8, 77. [Google Scholar] [CrossRef]
- Dreyer, A.M.; Matile, H.; Papastogiannidis, P.; Kamber, J.; Favuzza, P.; Voss, T.S.; Wittlin, S.; Pluschke, G. Passive immunoprotection of Plasmodium falciparum-infected mice designates the CyRPA as candidate malaria vaccine antigen. J. Immunol. 2012, 188, 6225–6237. [Google Scholar] [CrossRef]
- Favuzza, P.; Guffart, E.; Tamborrini, M.; Scherer, B.; Dreyer, A.M.; Rufer, A.C.; Erny, J.; Hoernschemeyer, J.; Thoma, R.; Schmid, G. Structure of the malaria vaccine candidate antigen CyRPA and its complex with a parasite invasion inhibitory antibody. eLife 2017, 6, e20383. [Google Scholar] [CrossRef]
- Reddy, K.S.; Amlabu, E.; Pandey, A.K.; Mitra, P.; Chauhan, V.S.; Gaur, D. Multiprotein complex between the GPI-anchored CyRPA with PfRH5 and PfRipr is crucial for Plasmodium falciparum erythrocyte invasion. Proc. Natl. Acad. Sci. USA 2015, 112, 1179–1184. [Google Scholar] [CrossRef]
- Volz, J.C.; Yap, A.; Sisquella, X.; Thompson, J.K.; Lim, N.T.; Whitehead, L.W.; Chen, L.; Lampe, M.; Tham, W.-H.; Wilson, D. Essential role of the PfRh5/PfRipr/CyRPA complex during Plasmodium falciparum invasion of erythrocytes. Cell Host Microbe 2016, 20, 60–71. [Google Scholar] [CrossRef]
- Galaway, F.; Drought, L.G.; Fala, M.; Cross, N.; Kemp, A.C.; Rayner, J.C.; Wright, G.J. P113 is a merozoite surface protein that binds the N terminus of Plasmodium falciparum RH5. Nat. Commun. 2017, 8, 14333. [Google Scholar] [CrossRef]
- Baum, J.; Chen, L.; Healer, J.; Lopaticki, S.; Boyle, M.; Triglia, T.; Ehlgen, F.; Ralph, S.A.; Beeson, J.G.; Cowman, A.F. Reticulocyte-binding protein homologue 5—An essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int. J. Parasitol. 2009, 39, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Crosnier, C.; Bustamante, L.Y.; Bartholdson, S.J.; Bei, A.K.; Theron, M.; Uchikawa, M.; Mboup, S.; Ndir, O.; Kwiatkowski, D.P.; Duraisingh, M.T. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 2011, 480, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Knuepfer, E.; Wright, K.E.; Prajapati, S.K.; Rawlinson, T.A.; Mohring, F.; Koch, M.; Lyth, O.R.; Howell, S.A.; Villasis, E.; Snijders, A.P. Divergent roles for the RH5 complex components, CyRPA and RIPR in human-infective malaria parasites. PLoS Pathog. 2019, 15, e1007809. [Google Scholar] [CrossRef] [PubMed]
- França, C.T.; White, M.T.; He, W.-Q.; Hostetler, J.B.; Brewster, J.; Frato, G.; Malhotra, I.; Gruszczyk, J.; Huon, C.; Lin, E. Identification of highly-protective combinations of Plasmodium vivax recombinant proteins for vaccine development. eLife 2017, 6, e28673. [Google Scholar] [CrossRef]
- Bitencourt Chaves, L.; Guimarães, G.d.O.; Perce-da-Silva, D.d.S.; Banic, D.M.; Totino, P.R.R.; Machado, R.L.D.; Rodrigues-da-Silva, R.N.; Pratt-Riccio, L.R.; Daniel-Ribeiro, C.T.; Lima-Junior, J.d.C. Genetic Diversity of Plasmodium vivax Cysteine-Rich Protective Antigen (PvCyRPA) in Field Isolates from Five Different Areas of the Brazilian Amazon. Genes 2021, 12, 1657. [Google Scholar] [CrossRef]
- Saxena, A.K.; Singh, K.; Su, H.P.; Klein, M.M.; Stowers, A.W.; Saul, A.J.; Long, C.A.; Garboczi, D.N. The essential mosquito-stage P25 and P28 proteins from Plasmodium form tile-like triangular prisms. Nat. Struct. Mol. Biol. 2006, 13, 90–91. [Google Scholar] [CrossRef]
- Kaslow, D.C.; Quakyi, I.A.; Syin, C.; Raum, M.G.; Keister, D.B.; Coligan, J.E.; McCutchan, T.F.; Miller, L.H. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 1988, 333, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Kaslow, D.C.; Gozar, M.M.; Tachibana, M.; Cao, Y.M.; Torii, M. Sequence polymorphism in two novel Plasmodium vivax ookinete surface proteins, Pvs25 and Pvs28, that are malaria transmission-blocking vaccine candidates. Mol. Med. 1998, 4, 772–782. [Google Scholar] [CrossRef]
- Vermeulen, A.N.; Ponnudurai, T.; Beckers, P.; Verhave, J.; Smits, M.; Meuwissen, J. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J. Exp. Med. 1985, 162, 1460–1476. [Google Scholar] [CrossRef] [PubMed]
- Kaslow, D.C.; Quakyi, I.A.; Keister, D.B. Minimal variation in a vaccine candidate from the sexual stage of Plasmodium falciparum. Mol. Biochem. Parasitol. 1989, 32, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.P.; Alpers, M.P.; Povoa, M.M.; Lal, A.A. Single amino acid variation in the ookinete vaccine antigen from field isolates of Plasmodium falciparum. Mol. Biochem. Parasitol. 1992, 50, 179–180. [Google Scholar] [CrossRef]
- Saxena, A.K.; Wu, Y.; Garboczi, D.N. Plasmodium p25 and p28 surface proteins: Potential transmission-blocking vaccines. Eukaryot. Cell 2007, 6, 1260–1265. [Google Scholar] [CrossRef]
- Chaves, L.B.; Perce-da-Silva, D.d.S.; Totino, P.R.R.; Riccio, E.K.P.; Baptista, B.d.O.; de Souza, A.B.L.; Rodrigues-da-Silva, R.N.; Machado, R.L.D.; de Souza, R.M.; Daniel-Ribeiro, C.T.; et al. Plasmodium vivax ookinete surface protein (Pvs25) is highly conserved among field isolates from five different regions of the Brazilian Amazon. Infect. Genet. Evol. 2019, 73, 287–294. [Google Scholar] [CrossRef]
- Hisaeda, H.; Stowers, A.W.; Tsuboi, T.; Collins, W.E.; Sattabongkot, J.S.; Suwanabun, N.; Torii, M.; Kaslow, D.C. Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect. Immun. 2000, 68, 6618–6623. [Google Scholar] [CrossRef]
- Malkin, E.M.; Durbin, A.P.; Diemert, D.J.; Sattabongkot, J.; Wu, Y.; Miura, K.; Long, C.A.; Lambert, L.; Miles, A.P.; Wang, J. Phase 1 vaccine trial of Pvs25H: A transmission blocking vaccine for Plasmodium vivax malaria. Vaccine 2005, 23, 3131–3138. [Google Scholar] [CrossRef]
- Wu, Y.; Ellis, R.D.; Shaffer, D.; Fontes, E.; Malkin, E.M.; Mahanty, S.; Fay, M.P.; Narum, D.; Rausch, K.; Miles, A.P. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS ONE 2008, 3, e2636. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Miles, A.P.; Wang, J.; Stowers, A.W. Expression of malaria transmission-blocking vaccine antigen Pfs25 in Pichia pastoris for use in human clinical trials. Vaccine 2003, 21, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Keister, D.B.; Muratova, O.V.; Sattabongkot, J.; Long, C.A.; Saul, A. Transmission-blocking activity induced by malaria vaccine candidates Pfs25/Pvs25 is a direct and predictable function of antibody titer. Malar. J. 2007, 6, 107. [Google Scholar] [CrossRef]
- Doolan, D.L.; Dobano, C.; Baird, J.K. Acquired immunity to malaria. Clin. Microbiol. Rev. 2009, 22, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Mariani, V.; Biasini, M.; Barbato, A.; Schwede, T. lDDT: A local superposition-free score for comparing protein structures and models using distance difference tests. Bioinformatics 2013, 29, 2722–2728. [Google Scholar] [CrossRef]
- Szebeni, J.; Kiss, B.; Bozó, T.; Turjeman, K.; Levi-Kalisman, Y.; Barenholz, Y.; Kellermayer, M. New insights into the structure of Comirnaty Covid-19 vaccine: A theory on soft nanoparticles with mRNA-lipid supercoils stabilized by hydrogen bonds. bioRxiv 2022. [Google Scholar] [CrossRef]
- Feng, H.; Zheng, L.; Zhu, X.; Wang, G.; Pan, Y.; Li, Y.; Yang, Y.; Lin, Y.; Cui, L.; Cao, Y. Genetic diversity of transmission-blocking vaccine candidates Pvs25 and Pvs28 in Plasmodium vivax isolates from Yunnan Province, China. Parasites Vectors 2011, 4, 224. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, W.M.; Liu, Y.J.; Yang, Y.M.; Cao, Y.M. Transmission-blocking vaccine candidate of Plasmodium vivax Pvs25 is highly conservative among Chinese isolates. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi Chin. J. Parasitol. Parasit. Dis. 2004, 22, 16–19. [Google Scholar]
- Yao, M.-X.; Sun, X.-D.; Gao, Y.-H.; Cheng, Z.-B.; Deng, W.-W.; Zhang, J.-J.; Wang, H. Multi-epitope chimeric antigen used as a serological marker to estimate Plasmodium falciparum transmission intensity in the border area of China-Myanmar. Infect. Dis. Poverty 2016, 5, 1–11. [Google Scholar] [CrossRef]
- Cai, Q.; Peng, G.; Bu, L.; Lin, Y.; Zhang, L.; Lustigmen, S.; Wang, H. Immunogenicity and in vitro protective efficacy of a polyepitope Plasmodium falciparum candidate vaccine constructed by epitope shuffling. Vaccine 2007, 25, 5155–5165. [Google Scholar] [CrossRef]
- McCaffery, J.N.; Fonseca, J.A.; Singh, B.; Cabrera-Mora, M.; Bohannon, C.; Jacob, J.; Arevalo-Herrera, M.; Moreno, A. A multi-stage Plasmodium vivax malaria vaccine candidate able to induce long-lived antibody responses against blood stage parasites and robust transmission-blocking activity. Front. Cell. Infect. Microbiol. 2019, 9. [Google Scholar] [CrossRef]
- Erath, J.; Djuranovic, S.; Djuranovic, S.P. Adaptation of Translational Machinery in Malaria Parasites to Accommodate Translation of Poly-Adenosine Stretches Throughout Its Life Cycle. Front. Microbiol. 2019, 10, 2823. [Google Scholar] [CrossRef]
- Kehrer, J.; Kuss, C.; Andres-Pons, A.; Reustle, A.; Dahan, N.; Devos, D.; Kudryashev, M.; Beck, M.; Mair, G.R.; Frischknecht, F. Nuclear Pore Complex Components in the Malaria Parasite Plasmodium berghei. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Mazumdar, S.; Mukherjee, P.; Yazdani, S.S.; Jain, S.; Mohmmed, A.; Chauhan, V.S. Plasmodium falciparum merozoite surface protein 1 (MSP-1)-MSP-3 chimeric protein: Immunogenicity determined with human-compatible adjuvants and induction of protective immune response. Infect. Immun. 2010, 78, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, Z.; Gu, J.; Wan, M.; Shen, Q.; Kieny, M.-P.; He, J.; Li, Z.; Zhang, Q.; Reed, Z.H. Safety and immunogenicity of a malaria vaccine, Plasmodium falciparum AMA-1/MSP-1 chimeric protein formulated in montanide ISA 720 in healthy adults. PLoS ONE 2008, 3, e1952. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Singh, B.; Cabrera-Mora, M.; Magri De Souza, A.C.; Queiroz Marques, M.T.; Porto, L.C.S.; Santos, F.; Banic, D.M.; Calvo-Calle, J.M.; Oliveira-Ferreira, J.; et al. Evaluation of Naturally Acquired IgG Antibodies to a Chimeric and Non-Chimeric Recombinant Species of Plasmodium vivax Reticulocyte Binding Protein-1: Lack of Association with HLA-DRB1*/DQB1* in Malaria Exposed Individuals from the Brazilian Amazon. PLoS ONE 2014, 9, e105828. [Google Scholar] [CrossRef]
- Fonseca, J.A.; Cabrera-Mora, M.; Singh, B.; Oliveira-Ferreira, J.; da Costa Lima-Junior, J.; Calvo-Calle, J.M.; Lozano, J.M.; Moreno, A. A chimeric protein-based malaria vaccine candidate induces robust T cell responses against Plasmodium vivax MSP1(19). Sci. Rep. 2016, 6, 34527. [Google Scholar] [CrossRef] [PubMed]
- White, M.; Chitnis, C.E. Potential role of vaccines in elimination of Plasmodium vivax. Parasitol. Int. 2022, 90, 102592. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.M.; Mirza, M.U.; Ahmad, M.A.; Saleem, M.; Froeyen, M.; Ahmad, S.; Gul, R.; Alghamdi, H.A.; Aslam, M.S.; Sajjad, M.; et al. A Putative Prophylactic Solution for COVID-19: Development of Novel Multiepitope Vaccine Candidate against SARS-CoV-2 by Comprehensive Immunoinformatic and Molecular Modelling Approach. Biology 2020, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.N.; Tolia, N. Structural vaccinology of malaria transmission-blocking vaccines. Expert Rev. Vaccines 2021, 20, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, B.; Rafique, S.; Salo-Ahen, O.M.; Ali, A.; Munir, M.; Idrees, M.; Mirza, M.U.; Vanmeert, M.; Shah, S.Z.; Jabbar, I. Antigenic peptide prediction from E6 and E7 oncoproteins of HPV types 16 and 18 for therapeutic vaccine design using immunoinformatics and MD simulation analysis. Front. Immunol. 2018, 9, 3000. [Google Scholar] [CrossRef]
- Shahid, F.; Ashfaq, U.A.; Javaid, A.; Khalid, H. Immunoinformatics guided rational design of a next generation multi epitope based peptide (MEBP) vaccine by exploring Zika virus proteome. Infect. Genet. Evol. 2020, 80, 104199. [Google Scholar] [CrossRef]
- Atapour, A.; Vosough, P.; Jafari, S.; Sarab, G.A. A multi-epitope vaccine designed against blood-stage of malaria: An immunoinformatic and structural approach. Sci. Rep. 2022, 12, 11683. [Google Scholar] [CrossRef]
- Kalita, P.; Padhi, A.K.; Zhang, K.Y.J.; Tripathi, T. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb. Pathog. 2020, 145, 104236. [Google Scholar] [CrossRef]
- Abdi, S.A.H.; Ali, A.; Sayed, S.F.; Abutahir; Ali, A.; Alam, P. Multi-Epitope-Based Vaccine Candidate for Monkeypox: An In Silico Approach. Vaccines 2022, 10, 1564. [Google Scholar] [CrossRef]
- Oluwagbemi, O.O.; Oladipo, E.K.; Kolawole, O.M.; Oloke, J.K.; Adelusi, T.I.; Irewolede, B.A.; Dairo, E.O.; Ayeni, A.E.; Kolapo, K.T.; Akindiya, O.E.; et al. Bioinformatics, Computational Informatics, and Modeling Approaches to the Design of mRNA COVID-19 Vaccine Candidates. Computation 2022, 10, 117. [Google Scholar] [CrossRef]
- Rocha, M.V.; Françoso, K.S.; Lima, L.C.; Camargo, T.M.; Machado, R.L.D.; Costa, F.T.M.; Rénia, L.; Nosten, F.; Russell, B.; Rodrigues, M.M.; et al. Generation, characterization and immunogenicity of a novel chimeric recombinant protein based on Plasmodium vivax AMA-1 and MSP1(19). Vaccine 2017, 35, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- McCaffery, J.N.; Singh, B.; Nace, D.; Moreno, A.; Udhayakumar, V.; Rogier, E. Natural infections with different Plasmodium species induce antibodies reactive to a chimeric Plasmodium vivax recombinant protein. Malar. J. 2021, 20, 1–14. [Google Scholar] [CrossRef]
- Bouharoun-Tayoun, H.; Druilhe, P. Plasmodium falciparum malaria: Evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect. Immun. 1992, 60, 1473–1481. [Google Scholar] [CrossRef]
- Roussilhon, C.; Oeuvray, C.; Müller-Graf, C.; Tall, A.; Rogier, C.; Trape, J.-F.; Theisen, M.; Balde, A.; Pérignon, J.-L.; Druilhe, P. Long-Term Clinical Protection from Falciparum Malaria Is Strongly Associated with IgG3 Antibodies to Merozoite Surface Protein 3. PLoS Med. 2007, 4, e320. [Google Scholar] [CrossRef]
- Jafarshad, A.; Dziegiel, M.H.; Lundquist, R.; Nielsen, L.K.; Singh, S.; Druilhe, P.L. A Novel Antibody-Dependent Cellular Cytotoxicity Mechanism Involved in Defense against Malaria Requires Costimulation of Monocytes FcγRII and FcγRIII. J. Immunol. 2007, 178, 3099–3106. [Google Scholar] [CrossRef]
- Druilhe, P.; Bouharoun-Tayoun, H. Antibody-Dependent Cellular Inhibition Assay. Methods Mol. Med. 2002, 72, 529–534. [Google Scholar] [CrossRef]
- Hill, D.L.; Schofield, L.; Wilson, D.W. IgG opsonization of merozoites: Multiple immune mechanisms for malaria vaccine development. Int. J. Parasitol. 2017, 47, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Shabani, S.H.; Zakeri, S.; Salmanian, A.H.; Amani, J.; Mehrizi, A.A.; Snounou, G.; Nosten, F.; Andolina, C.; Mourtazavi, Y.; Djadid, N.D. Biological, immunological and functional properties of two novel multi-variant chimeric recombinant proteins of CSP antigens for vaccine development against Plasmodium vivax infection. Mol. Immunol. 2017, 90, 158–171. [Google Scholar] [CrossRef]
- Matos, A.d.S.; Rodrigues-da-Silva, R.N.; Soares, I.F.; Baptista, B.d.O.; Souza, R.M.d.; Bitencourt-Chaves, L.; Totino, P.R.R.; Sánchez-Arcila, J.C.; Daniel-Ribeiro, C.T.; López-Camacho, C.; et al. Antibody Responses against Plasmodium vivax TRAP Recombinant and Synthetic Antigens in Naturally Exposed Individuals from the Brazilian Amazon. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Soares, I.F.; López-Camacho, C.; Rodrigues-da-Silva, R.N.; da Silva Matos, A.; de Oliveira Baptista, B.; Totino, P.R.R.; de Souza, R.M.; Harrison, K.; Gimenez, A.M.; de Freitas, E.O.; et al. Recombinant Plasmodium vivax circumsporozoite surface protein allelic variants: Antibody recognition by individuals from three communities in the Brazilian Amazon. Sci. Rep. 2020, 10, 14020. [Google Scholar] [CrossRef] [PubMed]
- Blank, A.; Fürle, K.; Jäschke, A.; Mikus, G.; Lehmann, M.; Hüsing, J.; Heiss, K.; Giese, T.; Carter, D.; Böhnlein, E.; et al. Immunization with full-length Plasmodium falciparum merozoite surface protein 1 is safe and elicits functional cytophilic antibodies in a randomized first-in-human trial. npj Vaccines 2020, 5, 10. [Google Scholar] [CrossRef]
- Dobaño, C.; Santano, R.; Vidal, M.; Jiménez, A.; Jairoce, C.; Ubillos, I.; Dosoo, D.; Aguilar, R.; Williams, N.A.; Díez-Padrisa, N.; et al. Differential Patterns of IgG Subclass Responses to Plasmodium falciparum Antigens in Relation to Malaria Protection and RTS,S Vaccination. Front. Immunol. 2019, 10, 439. [Google Scholar] [CrossRef]
- Boyle, M.J.; Chan, J.A.; Handayuni, I.; Reiling, L.; Feng, G.; Hilton, A.; Kurtovic, L.; Oyong, D.; Piera, K.A.; Barber, B.E.; et al. IgM in human immunity to Plasmodium falciparum malaria. Sci. Adv. 2019, 5, eaax4489. [Google Scholar] [CrossRef] [PubMed]
- Patgaonkar, M.; Herbert, F.; Powale, K.; Gandhe, P.; Gogtay, N.; Thatte, U.; Pied, S.; Sharma, S.; Pathak, S. Vivax infection alters peripheral B-cell profile and induces persistent serum IgM. Parasite Immunol. 2018, 40, e12580. [Google Scholar] [CrossRef]
- Longley, R.J.; White, M.T.; Brewster, J.; Liu, Z.S.J.; Bourke, C.; Takashima, E.; Harbers, M.; Tham, W.-H.; Healer, J.; Chitnis, C.E.; et al. IgG Antibody Responses Are Preferential Compared With IgM for Use as Serological Markers for Detecting Recent Exposure to Plasmodium vivax Infection. Open Forum Infect. Dis. 2021, 8, ofab228. [Google Scholar] [CrossRef] [PubMed]
- Snounou, G. Detection and identification of the four malaria parasite species infecting humans by PCR amplification. Species Diagn. Protoc. PCR Other Nucleic Acid Methods 1996, 50, 263–291. [Google Scholar]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]

| Epidemiological Features | Total (n = 301) |
|---|---|
| Male | 50.5% |
| Female | 49.5% |
| Malaria exposure | |
| Age—Mean (Sd) | 34.86 ± 16.33 |
| TREA *—Mean (Sd) | 33.83 ± 16.35 |
| TRPA *—Mean (Sd) | 26.62 ± 18.09 |
| Months since the last malaria episode— Median (IR) | 9.0 (2.0–24.0) |
| Number of malaria episodes in the last year— Median (IR) | 0.0 (0.0–1.0) |
| Number of previous malaria episodes— Median (IR) | 8.0 (3.0–18.0) |
| Previous species contracted—N (%) | |
| P. vivax | 58 (19%) |
| P. falciparum | 21 (7%) |
| P. vivax and P. falciparum | 192 (64%) |
| Never infected | 8 (3%) |
| Diagnosis (PCR)—N (%) | |
| P. vivax | 81 (27%) |
| P. falciparum | 18 (6%) |
| Mixed | 1 (0%) |
| Negative | 201 (67%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, A.d.S.; Soares, I.F.; Baptista, B.d.O.; de Souza, H.A.d.S.; Chaves, L.B.; Perce-da-Silva, D.d.S.; Riccio, E.K.P.; Albrecht, L.; Totino, P.R.R.; Rodrigues-da-Silva, R.N.; et al. Construction, Expression, and Evaluation of the Naturally Acquired Humoral Immune Response against Plasmodium vivax RMC-1, a Multistage Chimeric Protein. Int. J. Mol. Sci. 2023, 24, 11571. https://doi.org/10.3390/ijms241411571
Matos AdS, Soares IF, Baptista BdO, de Souza HAdS, Chaves LB, Perce-da-Silva DdS, Riccio EKP, Albrecht L, Totino PRR, Rodrigues-da-Silva RN, et al. Construction, Expression, and Evaluation of the Naturally Acquired Humoral Immune Response against Plasmodium vivax RMC-1, a Multistage Chimeric Protein. International Journal of Molecular Sciences. 2023; 24(14):11571. https://doi.org/10.3390/ijms241411571
Chicago/Turabian StyleMatos, Ada da Silva, Isabela Ferreira Soares, Barbara de Oliveira Baptista, Hugo Amorim dos Santos de Souza, Lana Bitencourt Chaves, Daiana de Souza Perce-da-Silva, Evelyn Kety Pratt Riccio, Letusa Albrecht, Paulo Renato Rivas Totino, Rodrigo Nunes Rodrigues-da-Silva, and et al. 2023. "Construction, Expression, and Evaluation of the Naturally Acquired Humoral Immune Response against Plasmodium vivax RMC-1, a Multistage Chimeric Protein" International Journal of Molecular Sciences 24, no. 14: 11571. https://doi.org/10.3390/ijms241411571
APA StyleMatos, A. d. S., Soares, I. F., Baptista, B. d. O., de Souza, H. A. d. S., Chaves, L. B., Perce-da-Silva, D. d. S., Riccio, E. K. P., Albrecht, L., Totino, P. R. R., Rodrigues-da-Silva, R. N., Daniel-Ribeiro, C. T., Pratt-Riccio, L. R., & Lima-Junior, J. d. C. (2023). Construction, Expression, and Evaluation of the Naturally Acquired Humoral Immune Response against Plasmodium vivax RMC-1, a Multistage Chimeric Protein. International Journal of Molecular Sciences, 24(14), 11571. https://doi.org/10.3390/ijms241411571






