Extracellular Vesicles in Young Serum Contribute to the Restoration of Age-Related Brain Transcriptomes and Cognition in Old Mice
Abstract
1. Introduction
2. Results
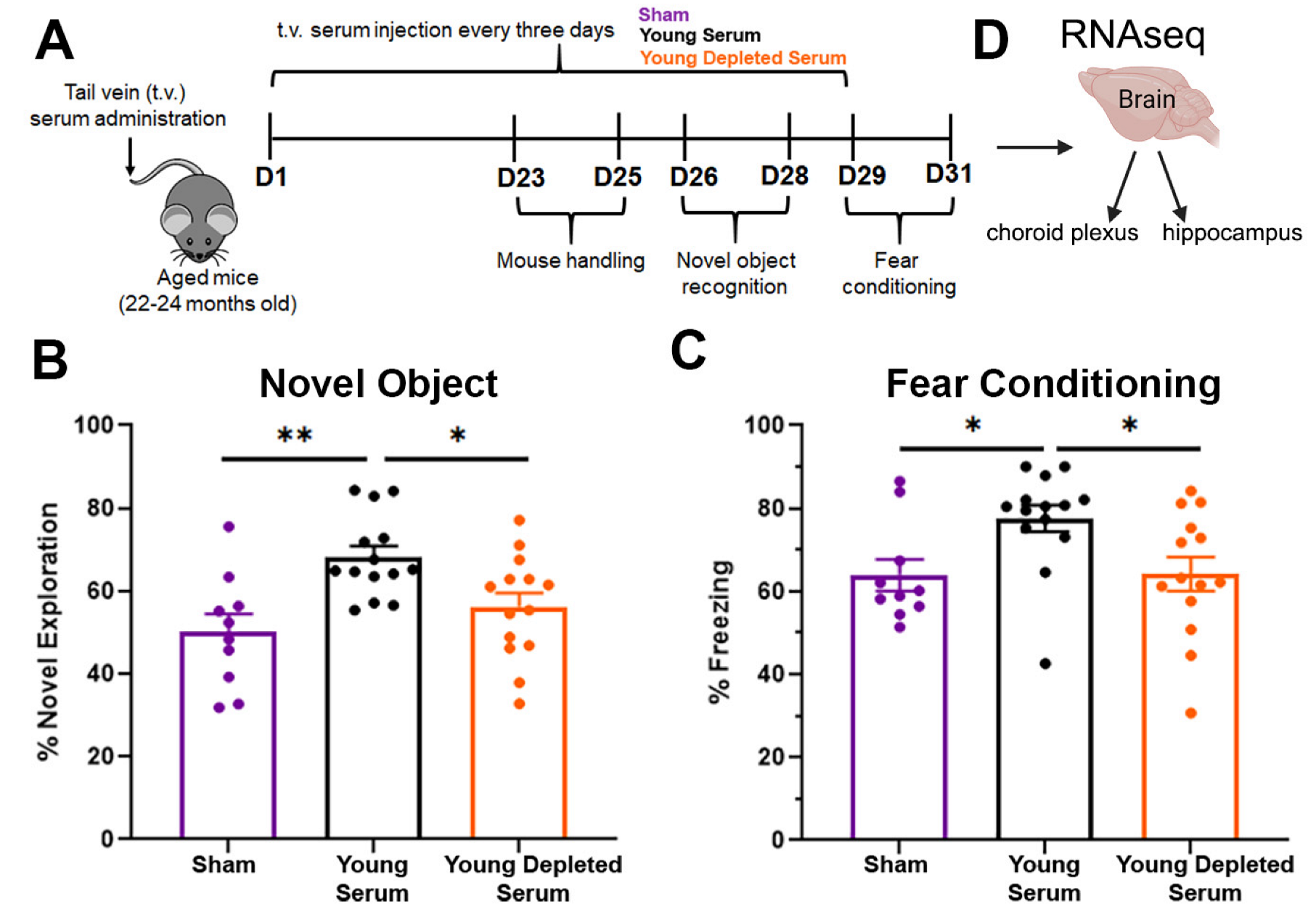
2.1. Circulating EVs Contribute to the Beneficial Effect of Young Serum on Cognitive Functions
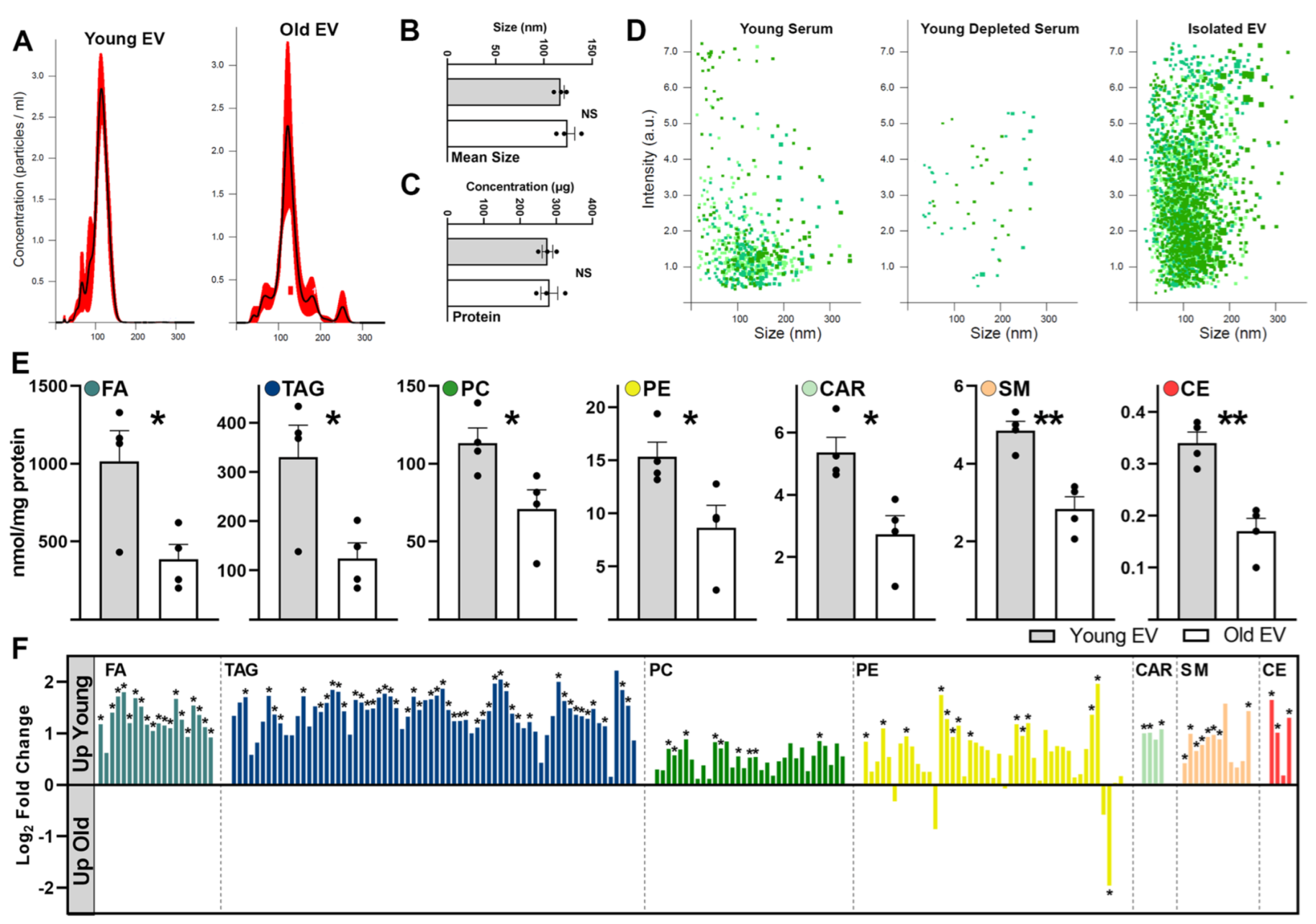
2.2. Aging Shifts Phospholipid Profiles of Circulating Serum EVs
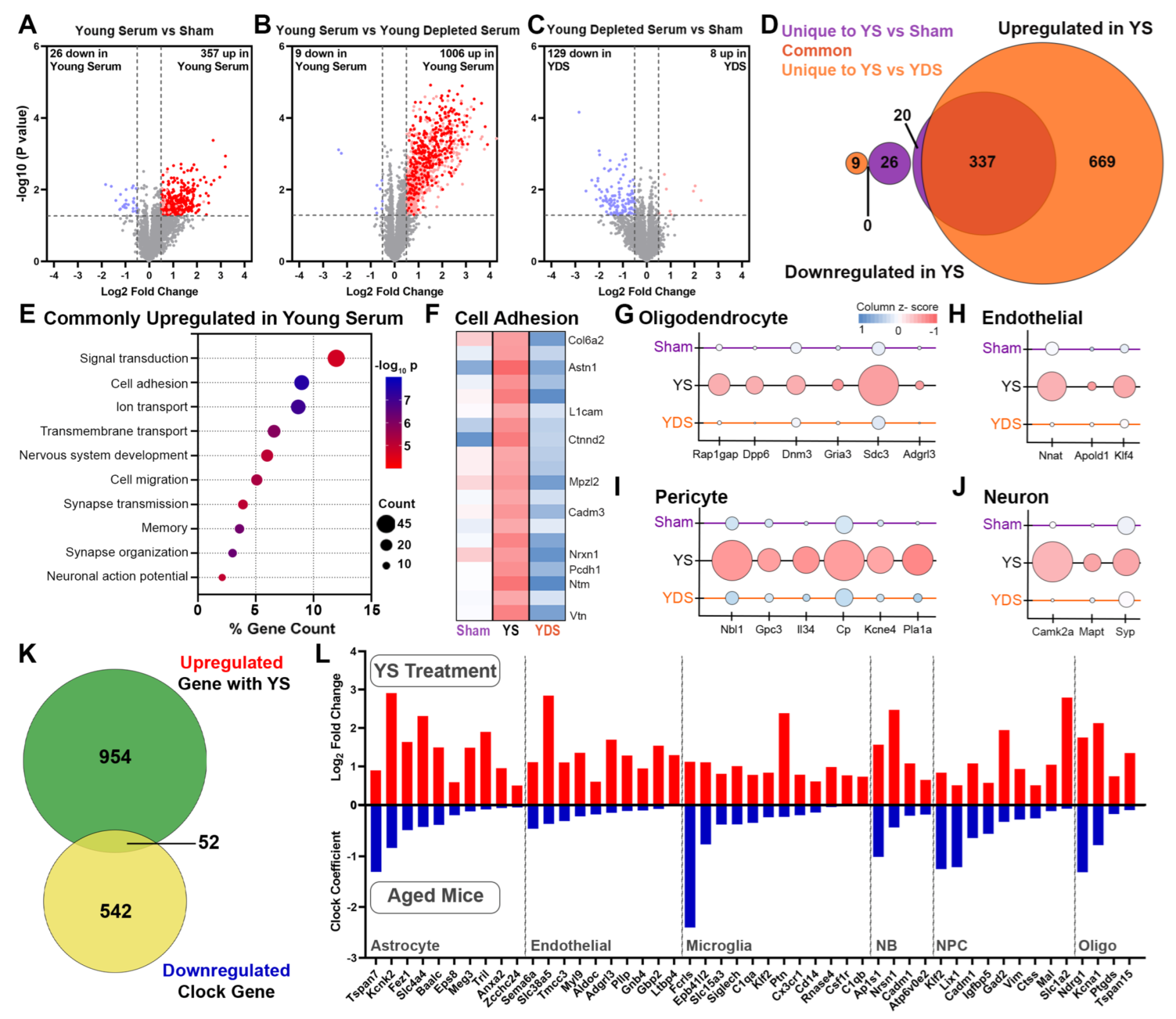
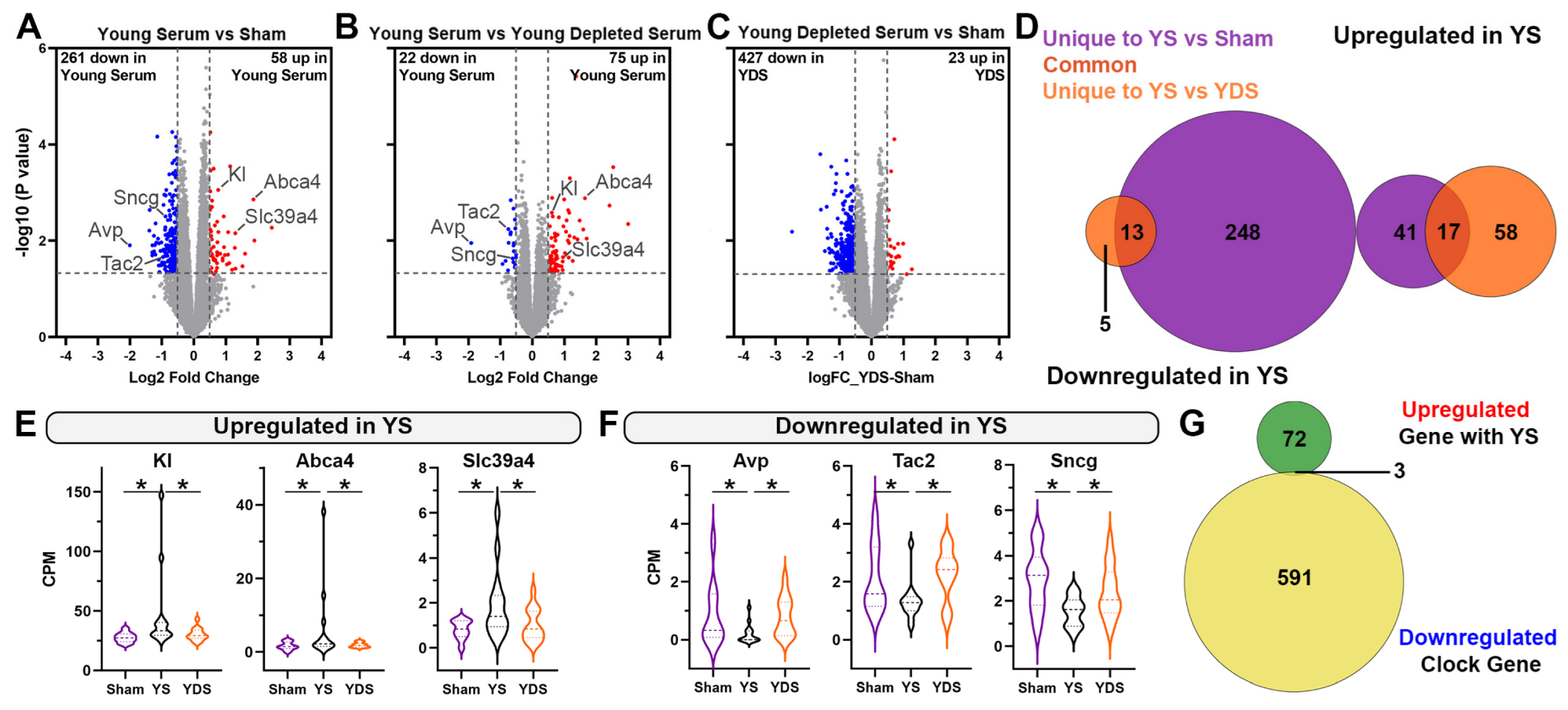
2.3. Specific Effect of Young Serum on Gene Expression of Aged Choroid Plexus
2.4. Unique Gene Expression Changes in the Aged Hippocampus of Young Serum-Treated Mice
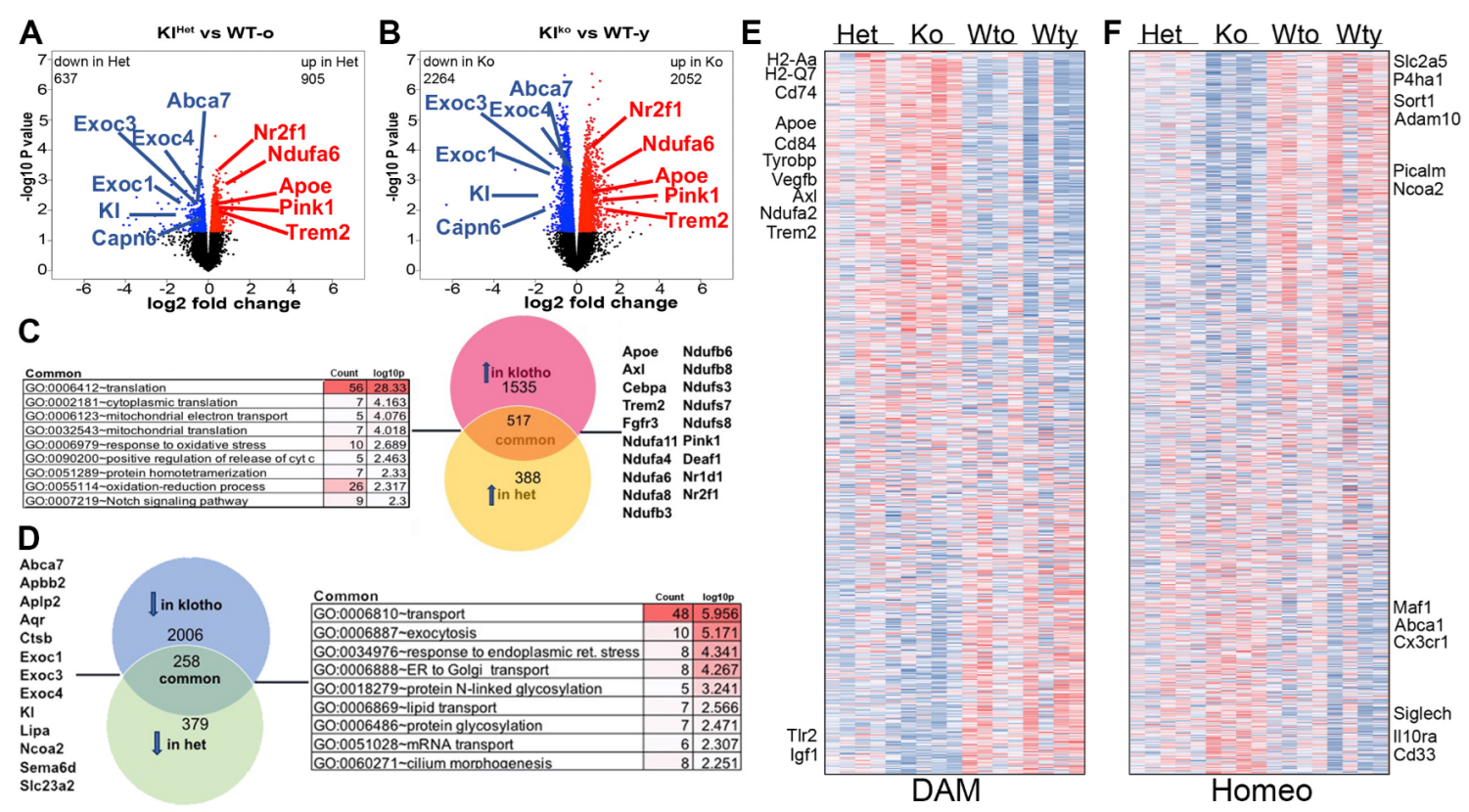
2.5. Klotho Deficiency Significantly Affects Hippocampal Gene Expression
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Serum Treatments
4.3. Extracellular Vesicle Characterization
4.4. Multi-Dimensional Mass Spectrometry Shotgun Lipidomics (MDMS-SL)
4.5. Behavioral Testing
4.6. Animal Tissue Processing
4.7. Bulk mRNA-seq Data
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dar, G.H.; Badierah, R.; Nathan, E.G.; Bhat, M.A.; Dar, A.H.; Redwan, E.M. Extracellular vesicles: A new paradigm in understanding, diagnosing and treating neurodegenerative disease. Front. Aging Neurosci. 2022, 14, 967231. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga-Valderrama, L.R.; Sheridan, G.K. Extracellular vesicles and intercellular communication in the central nervous system. FEBS Lett. 2021, 595, 1391–1410. [Google Scholar] [CrossRef] [PubMed]
- Vanherle, S.; Haidar, M.; Irobi, J.; Bogie, J.F.J.; Hendriks, J.J.A. Extracellular vesicle-associated lipids in central nervous system disorders. Adv. Drug Deliv. Rev. 2020, 159, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Ashapkin, V.V.; Kutueva, L.I.; Vanyushin, B.F. The Effects of Parabiosis on Aging and Age-Related Diseases; Springer International Publishing: Cham, Switzerland, 2020; pp. 107–122. [Google Scholar]
- Conboy, M.J.; Conboy, I.M.; Rando, T.A. Heterochronic parabiosis: Historical perspective and methodological considerations for studies of aging and longevity. Aging Cell 2013, 12, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Clemens, Z.J.; Shinde, S.N.; Sivakumar, S.; Pius, A.; Bhatia, A.; Picciolini, S.; Carlomagno, C.; Gualerzi, A.; Bedoni, M.; et al. Regulation of aged skeletal muscle regeneration by circulating extracellular vesicles. Nat. Aging 2021, 1, 1148–1161. [Google Scholar] [CrossRef]
- Willis, C.M.; Nicaise, A.M.; Krzak, G.; Ionescu, R.-B.; Pappa, V.; D’Angelo, A.; Agarwal, R.; Repollés-de-Dalmau, M.; Peruzzotti-Jametti, L.; Pluchino, S. Soluble factors influencing the neural stem cell niche in brain physiology, inflammation, and aging. Exp. Neurol. 2022, 355, 114124. [Google Scholar] [CrossRef]
- Mehdipour, M.; Amiri, P.; Liu, C.; Decastro, J.; Kato, C.; Skinner, C.M.; Conboy, M.J.; Aran, K.; Conboy, I.M. Small-animal blood exchange is an emerging approach for systemic aging research. Nat. Protoc. 2022, 17, 2469–2493. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef]
- De Miguel, Z.; Khoury, N.; Betley, M.J.; Lehallier, B.; Willoughby, D.; Olsson, N.; Yang, A.C.; Hahn, O.; Lu, N.; Vest, R.T.; et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature 2021, 600, 494–499. [Google Scholar] [CrossRef]
- Horowitz, A.M.; Fan, X.; Bieri, G.; Smith, L.K.; Sanchez-Diaz, C.I.; Schroer, A.B.; Gontier, G.; Casaletto, K.B.; Kramer, J.H.; Williams, K.E.; et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 2020, 369, 167–173. [Google Scholar] [CrossRef]
- Villeda, S.A.; Plambeck, K.E.; Middeldorp, J.; Castellano, J.M.; Mosher, K.I.; Luo, J.; Smith, L.K.; Bieri, G.; Lin, K.; Berdnik, D.; et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 2014, 20, 659–663. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Banks, W.A.; Bullock, K.M.; Haney, M.; Batrakova, E.; Kabanov, A.V. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lananna, B.V.; Imai, S.I. Friends and foes: Extracellular vesicles in aging and rejuvenation. FASEB BioAdvances 2021, 3, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.R.; Li, A. Aging-suppressor Klotho: Prospects in diagnostics and therapeutics. Ageing Res. Rev. 2022, 82, 101766. [Google Scholar] [CrossRef]
- Buckley, M.T.; Sun, E.D.; George, B.M.; Liu, L.; Schaum, N.; Xu, L.; Reyes, J.M.; Goodell, M.A.; Weissman, I.L.; Wyss-Coray, T.; et al. Cell-type-specific aging clocks to quantify aging and rejuvenation in neurogenic regions of the brain. Nat. Aging 2022, 3, 121–137. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Lobas, M.A.; Helsper, L.; Vernon, C.G.; Schreiner, D.; Zhang, Y.; Holtzman, M.J.; Thedens, D.R.; Weiner, J.A. Molecular heterogeneity in the choroid plexus epithelium: The 22-member gamma-protocadherin family is differentially expressed, apically localized, and implicated in CSF regulation. J. Neurochem. 2012, 120, 913–927. [Google Scholar] [CrossRef]
- Dilling, C.; Roewer, N.; Forster, C.Y.; Burek, M. Multiple protocadherins are expressed in brain microvascular endothelial cells and might play a role in tight junction protein regulation. J. Cereb. Blood Flow Metab. 2017, 37, 3391–3400. [Google Scholar] [CrossRef]
- Szmydynger-Chodobska, J.; Pascale, C.L.; Pfeffer, A.N.; Coulter, C.; Chodobski, A. Expression of junctional proteins in choroid plexus epithelial cell lines: A comparative study. Cereb. Fluid Res. 2007, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Wang, L.C.; Su, Y.T.; Wei, W.Y.; Tsai, K.J. Synthetic alpha5beta1 integrin ligand PHSRN is proangiogenic and neuroprotective in cerebral ischemic stroke. Biomaterials 2018, 185, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Ayloo, S.; Lazo, C.G.; Sun, S.; Zhang, W.; Cui, B.; Gu, C. Pericyte-to-endothelial cell signaling via vitronectin-integrin regulates blood-CNS barrier. Neuron 2022, 110, 1641–1655.e6. [Google Scholar] [CrossRef]
- Zeisel, A.; Munoz-Manchado, A.B.; Codeluppi, S.; Lonnerberg, P.; La Manno, G.; Jureus, A.; Marques, S.; Munguba, H.; He, L.; Betsholtz, C.; et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 2015, 347, 1138–1142. [Google Scholar] [CrossRef]
- Fitz, N.F.; Nam, K.N.; Wolfe, C.M.; Letronne, F.; Playso, B.E.; Iordanova, B.E.; Kozai, T.D.Y.; Biedrzycki, R.J.; Kagan, V.E.; Tyurina, Y.Y.; et al. Phospholipids of APOE lipoproteins activate microglia in an isoform-specific manner in preclinical models of Alzheimer’s disease. Nat. Commun. 2021, 12, 3416. [Google Scholar] [CrossRef] [PubMed]
- Regard, J.B.; Scheek, S.; Borbiev, T.; Lanahan, A.A.; Schneider, A.; Demetriades, A.M.; Hiemisch, H.; Barnes, C.A.; Verin, A.D.; Worley, P.F. Verge: A novel vascular early response gene. J. Neurosci. 2004, 24, 4092–4103. [Google Scholar] [CrossRef]
- Krizhanovsky, V.; Ben-Arie, N. A novel role for the choroid plexus in BMP-mediated inhibition of differentiation of cerebellar neural progenitors. Mech. Dev. 2006, 123, 67–75. [Google Scholar] [CrossRef]
- Akkermans, O.; Delloye-Bourgeois, C.; Peregrina, C.; Carrasquero-Ordaz, M.; Kokolaki, M.; Berbeira-Santana, M.; Chavent, M.; Reynaud, F.; Raj, R.; Agirre, J.; et al. GPC3-Unc5 receptor complex structure and role in cell migration. Cell 2022, 185, 3931–3949.e26. [Google Scholar] [CrossRef]
- Abbott, G.W.; Jepps, T.A. Kcne4 Deletion Sex-Dependently Alters Vascular Reactivity. J. Vasc. Res. 2016, 53, 138–148. [Google Scholar] [CrossRef]
- Ryan, F.; Zarruk, J.G.; Losslein, L.; David, S. Ceruloplasmin Plays a Neuroprotective Role in Cerebral Ischemia. Front. Neurosci. 2018, 12, 988. [Google Scholar] [CrossRef]
- Zanardi, A.; Alessio, M. Ceruloplasmin Deamidation in Neurodegeneration: From Loss to Gain of Function. Int. J. Mol. Sci. 2021, 22, 663. [Google Scholar] [CrossRef] [PubMed]
- Kunis, G.; Baruch, K.; Miller, O.; Schwartz, M. Immunization with a Myelin-Derived Antigen Activates the Brain’s Choroid Plexus for Recruitment of Immunoregulatory Cells to the CNS and Attenuates Disease Progression in a Mouse Model of ALS. J. Neurosci. 2015, 35, 6381–6393. [Google Scholar] [CrossRef] [PubMed]
- Higgins-Chen, A.T.; Thrush, K.L.; Levine, M.E. Aging biomarkers and the brain. Semin. Cell Dev. Biol. 2021, 116, 180–193. [Google Scholar] [CrossRef]
- Sala Frigerio, C.; Wolfs, L.; Fattorelli, N.; Thrupp, N.; Voytyuk, I.; Schmidt, I.; Mancuso, R.; Chen, W.T.; Woodbury, M.E.; Srivastava, G.; et al. The Major Risk Factors for Alzheimer’s Disease: Age, Sex, and Genes Modulate the Microglia Response to Abeta Plaques. Cell Rep. 2019, 27, 1293–1306.e6. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Zhu, L.; Stein, L.R.; Kim, D.; Ho, K.; Yu, G.Q.; Zhan, L.; Larsson, T.E.; Mucke, L. Klotho controls the brain-immune system interface in the choroid plexus. Proc. Natl. Acad. Sci. USA 2018, 115, E11388–E11396. [Google Scholar] [CrossRef]
- De Benedictis, C.A.; Haffke, C.; Hagmeyer, S.; Sauer, A.K.; Grabrucker, A.M. Expression Analysis of Zinc Transporters in Nervous Tissue Cells Reveals Neuronal and Synaptic Localization of ZIP4. Int. J. Mol. Sci. 2021, 22, 4511. [Google Scholar] [CrossRef] [PubMed]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; van der Lee, S.J.; Amlie-Wolf, A.; et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef]
- Colt, E.W.D.; Wardlaw, S.L.; Frantz, A.G. The effect of running on plasma β-endorphin. Life Sci. 1981, 28, 1637–1640. [Google Scholar] [CrossRef]
- Iram, T.; Kern, F.; Kaur, A.; Myneni, S.; Morningstar, A.R.; Shin, H.; Garcia, M.A.; Yerra, L.; Palovics, R.; Yang, A.C.; et al. Young CSF restores oligodendrogenesis and memory in aged mice via Fgf17. Nature 2022, 605, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Llorente, A.; Skotland, T.; Sylvänne, T.; Kauhanen, D.; Róg, T.; Orłowski, A.; Vattulainen, I.; Ekroos, K.; Sandvig, K. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2013, 1831, 1302–1309. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Hough, K.P.; Wilson, L.S.; Trevor, J.L.; Strenkowski, J.G.; Maina, N.; Kim, Y.I.; Spell, M.L.; Wang, Y.; Chanda, D.; Dager, J.R.; et al. Unique Lipid Signatures of Extracellular Vesicles from the Airways of Asthmatics. Sci. Rep. 2018, 8, 10340. [Google Scholar] [CrossRef]
- Sun, Y.; Saito, K.; Saito, Y. Lipidomic Analysis of Extracellular Vesicles Isolated from Human Plasma and Serum; Springer: New York, NY, USA, 2022; pp. 157–173. [Google Scholar]
- Zhang, X.; Takeuchi, T.; Takeda, A.; Mochizuki, H.; Nagai, Y. Comparison of serum and plasma as a source of blood extracellular vesicles: Increased levels of platelet-derived particles in serum extracellular vesicle fractions alter content profiles from plasma extracellular vesicle fractions. PLoS ONE 2022, 17, e0270634. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Nie, C.; Li, Y.; Li, R.; Yan, Y.; Zhang, D.; Li, T.; Li, Z.; Sun, Y.; Zhen, H.; Ding, J.; et al. Distinct biological ages of organs and systems identified from a multi-omics study. Cell Rep. 2022, 38, 110459. [Google Scholar] [CrossRef]
- Peters, M.J.; Joehanes, R.; Pilling, L.C.; Schurmann, C.; Conneely, K.N.; Powell, J.; Reinmaa, E.; Sutphin, G.L.; Zhernakova, A.; Schramm, K.; et al. The transcriptional landscape of age in human peripheral blood. Nat. Commun. 2015, 6, 8570. [Google Scholar] [CrossRef]
- Li, X.; Gibson, A.W.; Kimberly, R.P. Human FcR polymorphism and disease. Curr. Top Microbiol. Immunol. 2014, 382, 275–302. [Google Scholar]
- Perot, B.P.; Ménager, M.M. Tetraspanin 7 and its closest paralog tetraspanin 6: Membrane organizers with key functions in brain development, viral infection, innate immunity, diabetes and cancer. Med. Microbiol. Immunol. 2020, 209, 427–436. [Google Scholar] [CrossRef]
- Wittner, J.; Schuh, W. Krüppel-like Factor 2 (KLF2) in Immune Cell Migration. Vaccines 2021, 9, 1171. [Google Scholar] [CrossRef]
- Zhang, G.; Qin, Q.; Zhang, C.; Sun, X.; Kazama, K.; Yi, B.; Cheng, F.; Guo, Z.F.; Sun, J. NDRG1 Signaling is Essential for Endothelial Inflammation and Vascular Remodeling. Circ. Res. 2022, 132, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Helwa, I.; Cai, J.; Drewry, M.D.; Zimmerman, A.; Dinkins, M.B.; Khaled, M.L.; Seremwe, M.; Dismuke, W.M.; Bieberich, E.; Stamer, W.D.; et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS ONE 2017, 12, e0170628. [Google Scholar] [CrossRef]
- Baek, R.; Varming, K.; Jorgensen, M.M. Does smoking, age or gender affect the protein phenotype of extracellular vesicles in plasma? Transfus. Apher. Sci. 2016, 55, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Enjeti, A.K.; Ariyarajah, A.; D’Crus, A.; Seldon, M.; Lincz, L.F. Circulating microvesicle number, function and small RNA content vary with age, gender, smoking status, lipid and hormone profiles. Thromb. Res. 2017, 156, 65–72. [Google Scholar] [CrossRef]
- Han, X.; Yang, K.; Gross, R.W. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom. Rev. 2012, 31, 134–178. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Cheng, H.; Gross, R.W.; Han, X. Automated lipid identification and quantification by multidimensional mass spectrometry-based shotgun lipidomics. Anal. Chem. 2009, 81, 4356–4368. [Google Scholar] [CrossRef]
- Fitz, N.F.; Wolfe, C.M.; Playso, B.E.; Biedrzycki, R.J.; Lu, Y.; Nam, K.N.; Lefterov, I.; Koldamova, R. Trem2 deficiency differentially affects phenotype and transcriptome of human APOE3 and APOE4 mice. Mol. Neurodegener. 2020, 15, 41. [Google Scholar] [CrossRef]
- Nam, K.N.; Wolfe, C.M.; Fitz, N.F.; Letronne, F.; Castranio, E.L.; Mounier, A.; Schug, J.; Lefterov, I.; Koldamova, R. Integrated approach reveals diet, APOE genotype and sex affect immune response in APP mice. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 152–161. [Google Scholar] [CrossRef]
- Castranio, E.L.; Wolfe, C.M.; Nam, K.N.; Letronne, F.; Fitz, N.F.; Lefterov, I.; Koldamova, R. ABCA1 haplodeficiency affects the brain transcriptome following traumatic brain injury in mice expressing human APOE isoforms. Acta Neuropathol. Commun. 2018, 6, 69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitz, N.F.; Sahu, A.; Lu, Y.; Ambrosio, F.; Lefterov, I.; Koldamova, R. Extracellular Vesicles in Young Serum Contribute to the Restoration of Age-Related Brain Transcriptomes and Cognition in Old Mice. Int. J. Mol. Sci. 2023, 24, 12550. https://doi.org/10.3390/ijms241612550
Fitz NF, Sahu A, Lu Y, Ambrosio F, Lefterov I, Koldamova R. Extracellular Vesicles in Young Serum Contribute to the Restoration of Age-Related Brain Transcriptomes and Cognition in Old Mice. International Journal of Molecular Sciences. 2023; 24(16):12550. https://doi.org/10.3390/ijms241612550
Chicago/Turabian StyleFitz, Nicholas F., Amrita Sahu, Yi Lu, Fabrisia Ambrosio, Iliya Lefterov, and Radosveta Koldamova. 2023. "Extracellular Vesicles in Young Serum Contribute to the Restoration of Age-Related Brain Transcriptomes and Cognition in Old Mice" International Journal of Molecular Sciences 24, no. 16: 12550. https://doi.org/10.3390/ijms241612550
APA StyleFitz, N. F., Sahu, A., Lu, Y., Ambrosio, F., Lefterov, I., & Koldamova, R. (2023). Extracellular Vesicles in Young Serum Contribute to the Restoration of Age-Related Brain Transcriptomes and Cognition in Old Mice. International Journal of Molecular Sciences, 24(16), 12550. https://doi.org/10.3390/ijms241612550





