Fallopia japonica Root Extract Ameliorates Ovalbumin-Induced Airway Inflammation in a CARAS Mouse Model by Modulating the IL-33/TSLP/NF-κB Signaling Pathway
Abstract
1. Introduction
2. Results
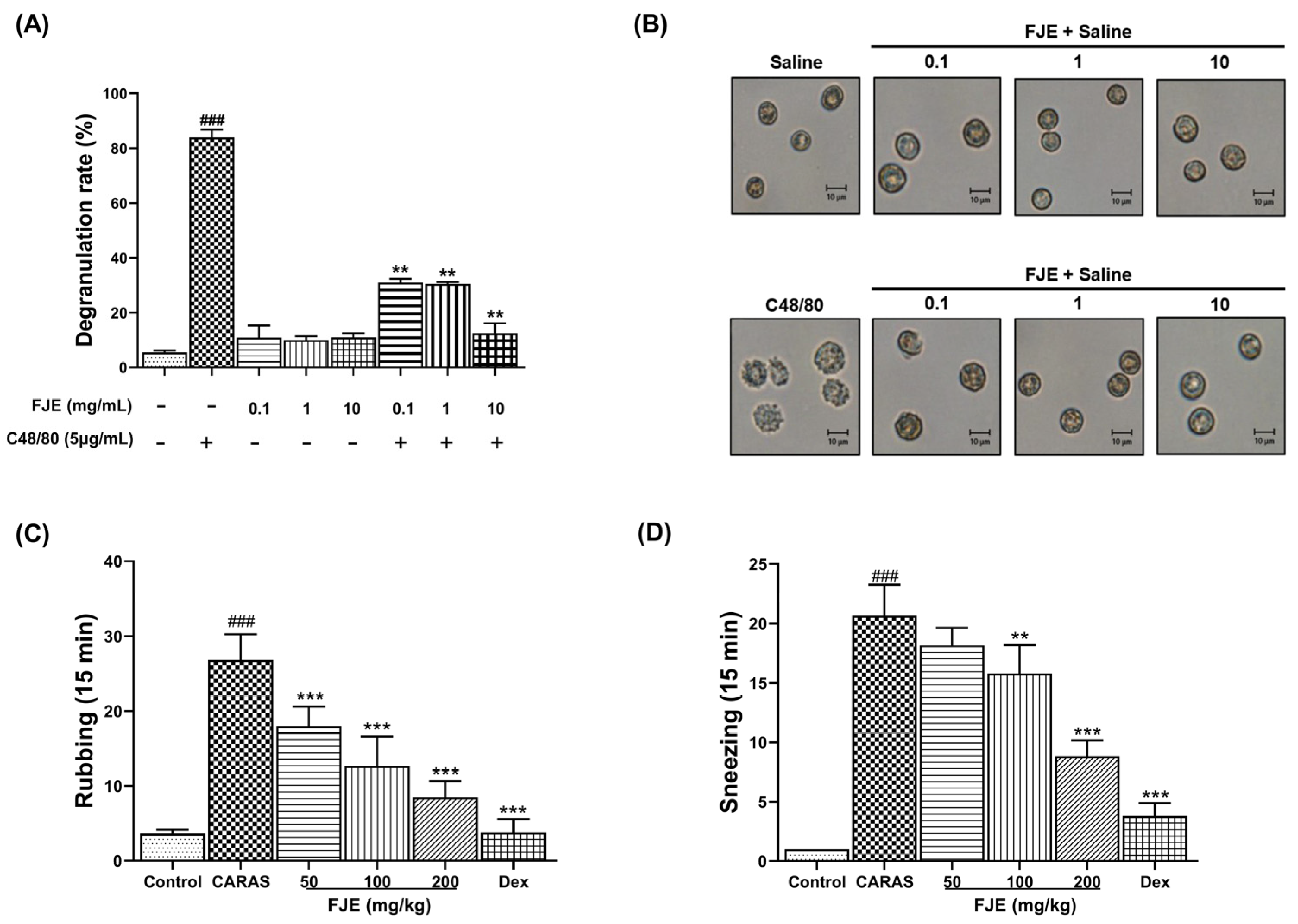
2.1. FJE Suppressed Rat Peritoneal Mast Cell (RPMC) Degranulation
2.2. FJE Inhibited Nasal Symptoms in the OVA-Induced CARAS Mouse Model
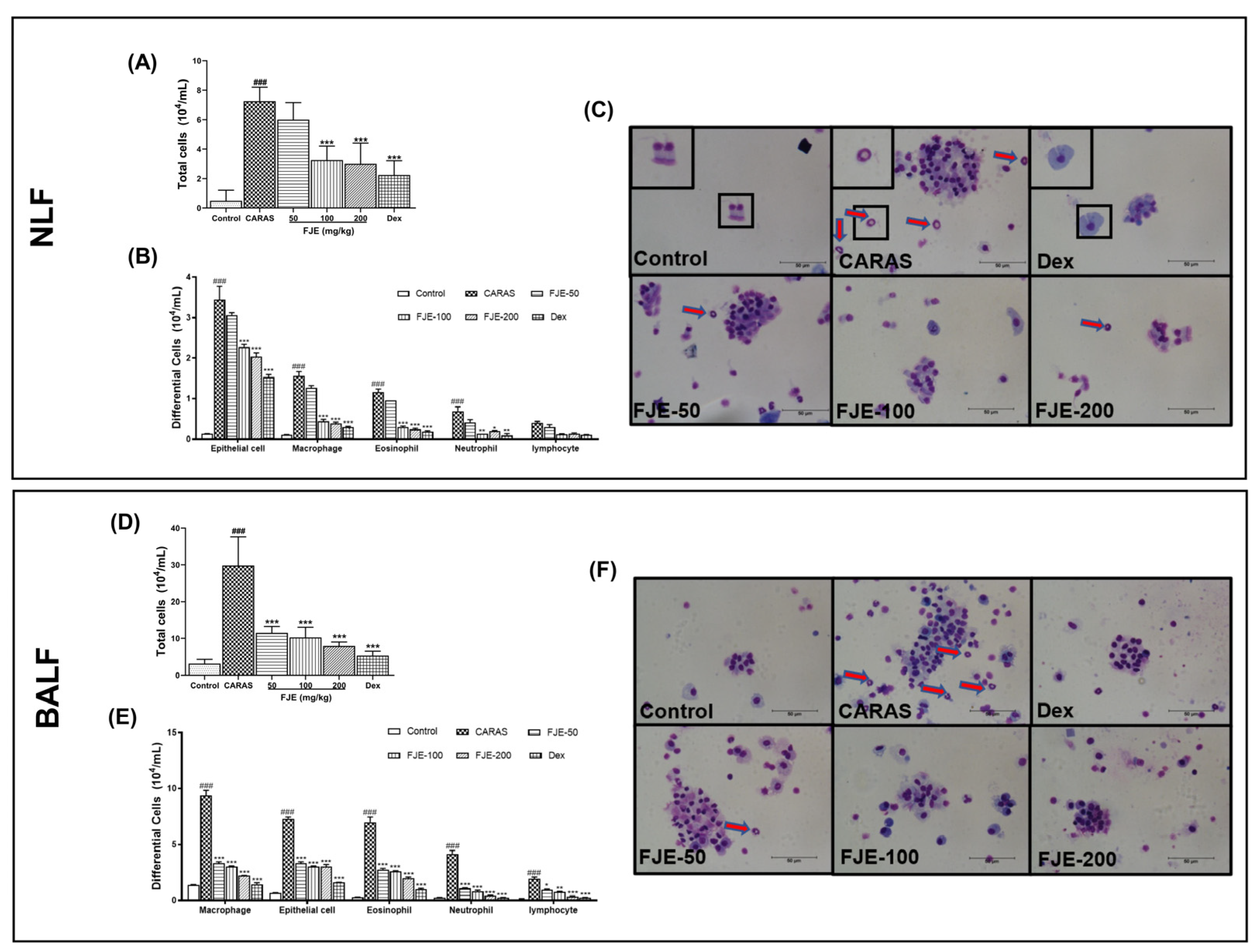
2.3. FJE Reduced Infiltration of Inflammatory Cells in the Nasal Lavage Fluid (NLF) and BALF of the OVA-Induced CARAS Mouse Model
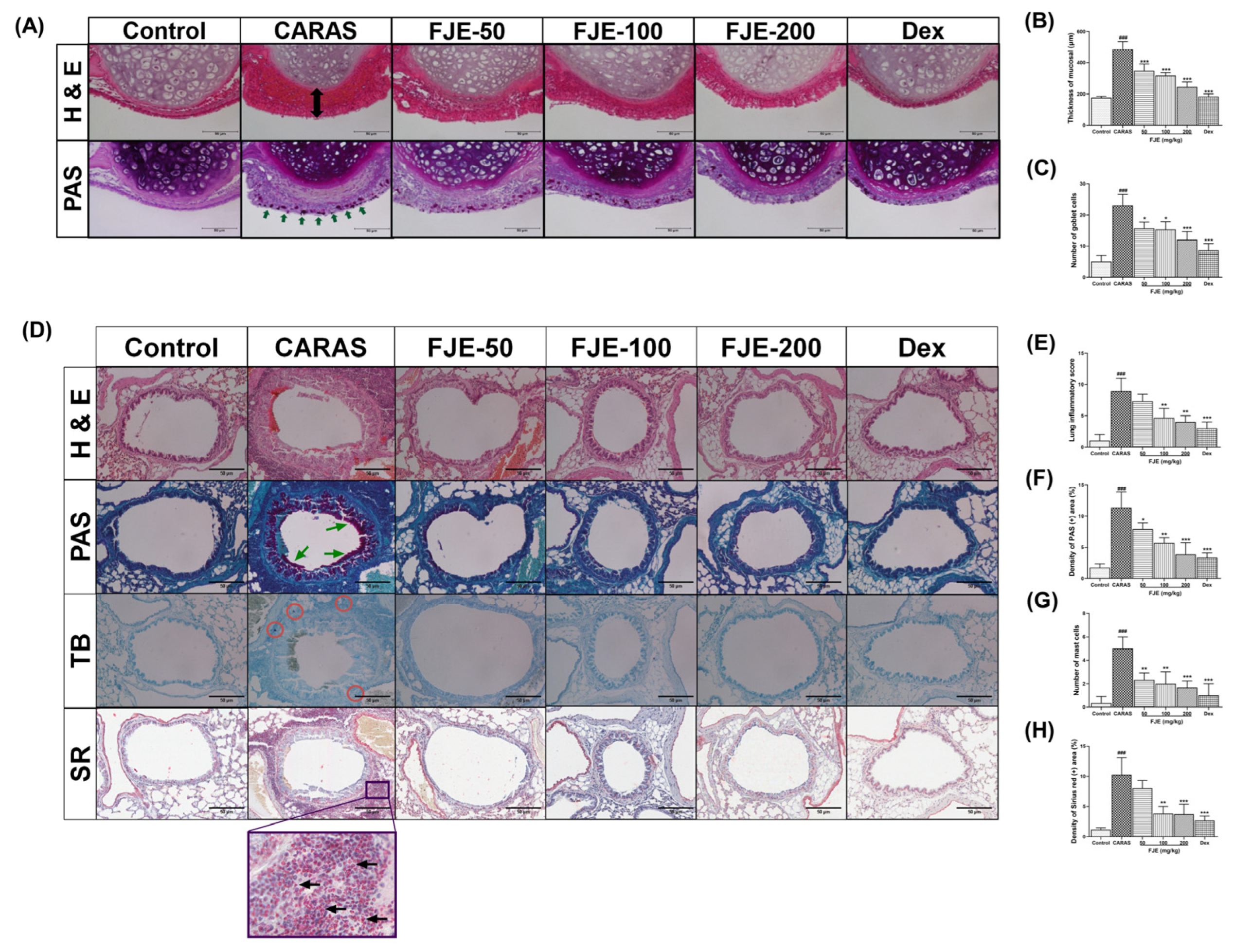
2.4. FJE Suppressed Histopathological Alterations in the Nasal Mucosa and Lung Tissue of the OVA-Induced CARAS Mouse Model
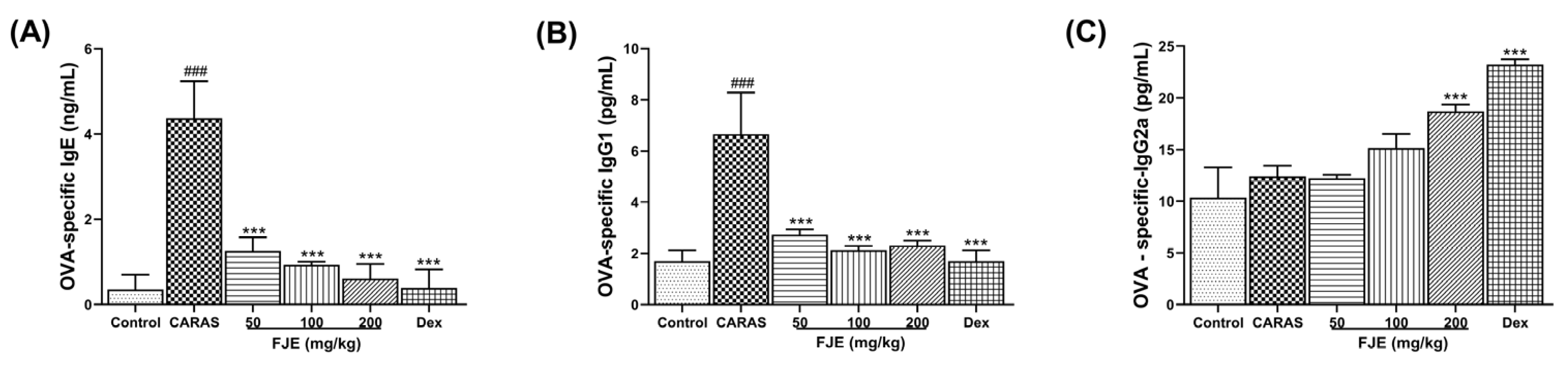
2.5. FJE Suppressed Immunoglobulin Levels in Serum of the OVA-Induced CARAS Mouse Model
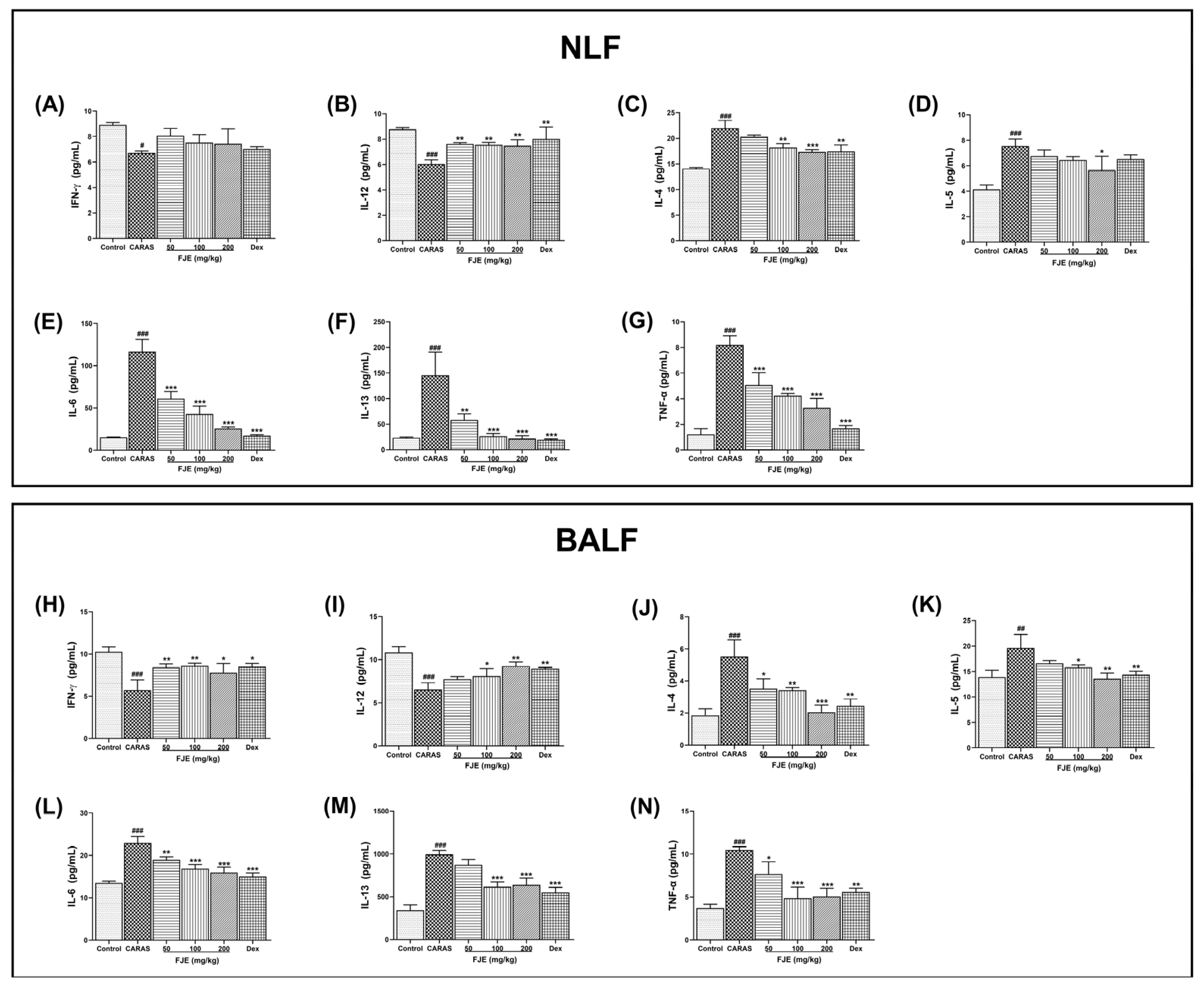
2.6. FJE Regulated T Helper–Associated Cytokine Production in NLF and BALF of the OVA-Induced CARAS Mouse Model
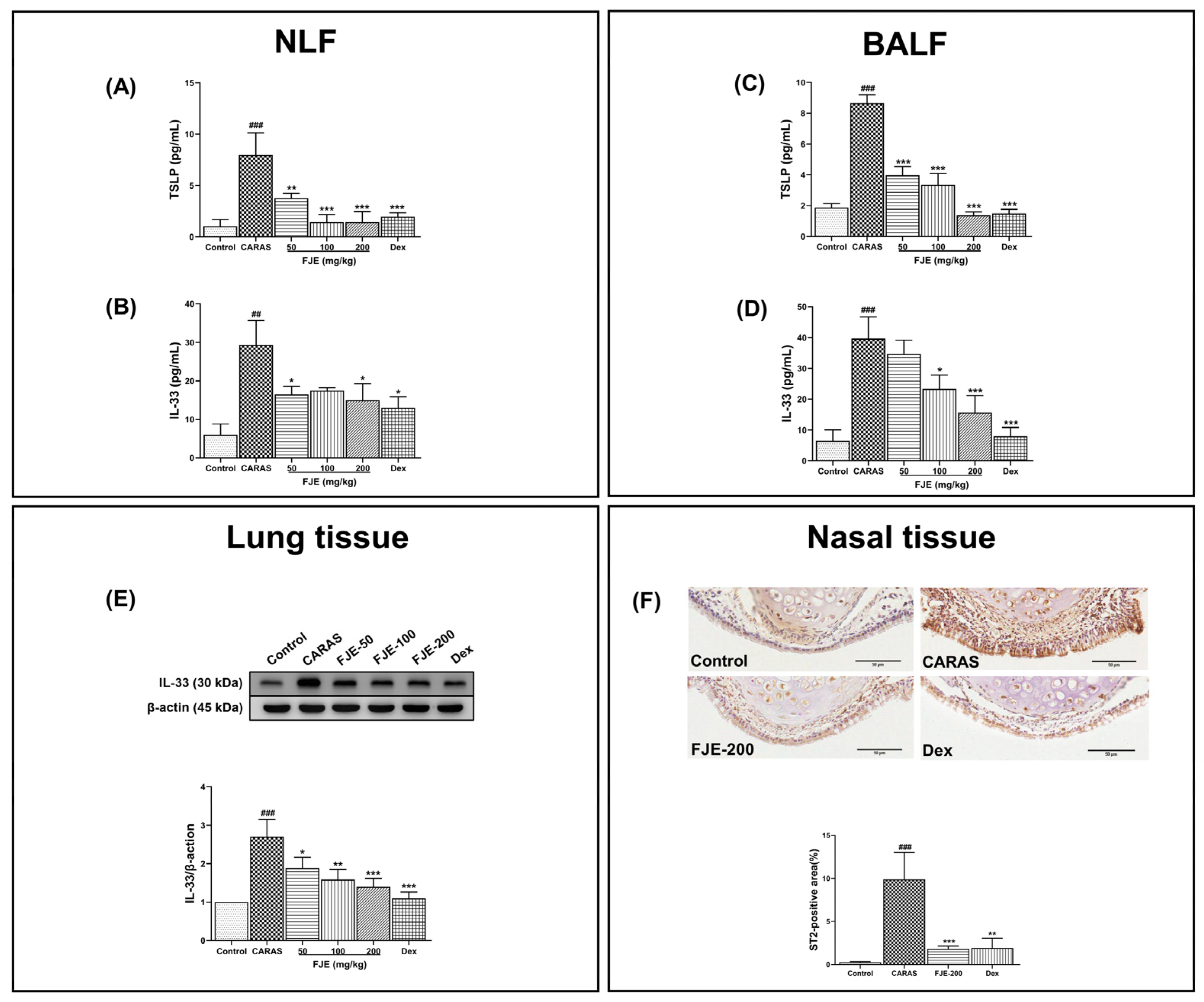
2.7. FJE Regulates Epithelium-Derived Cytokines in NLF and BALF of the OVA-Induced CARAS Mouse Model
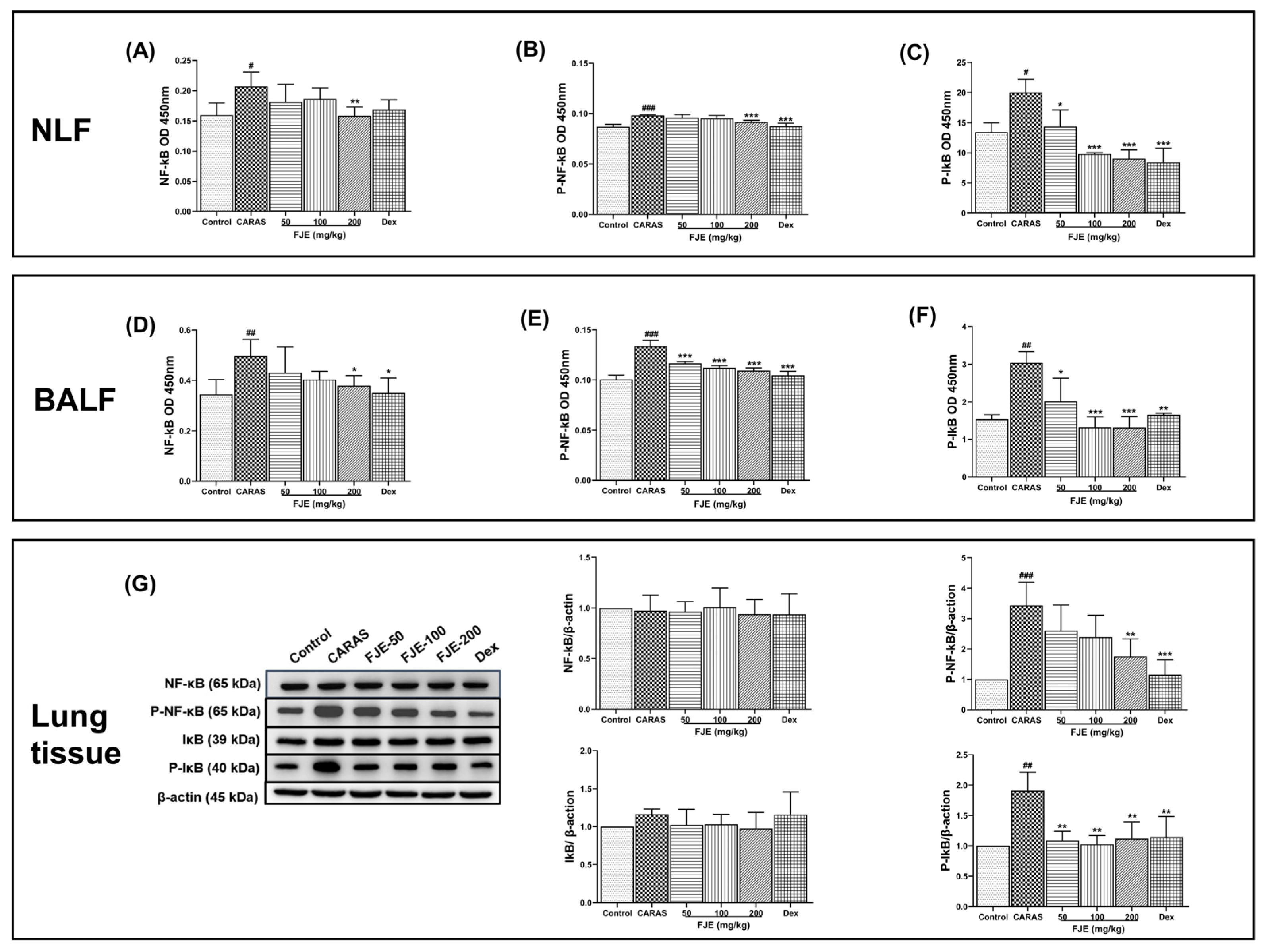
2.8. FJE Regulates the Inflammatory Response via the NF-κB Signaling Pathway in the OVA-Induced CARAS Mouse Model
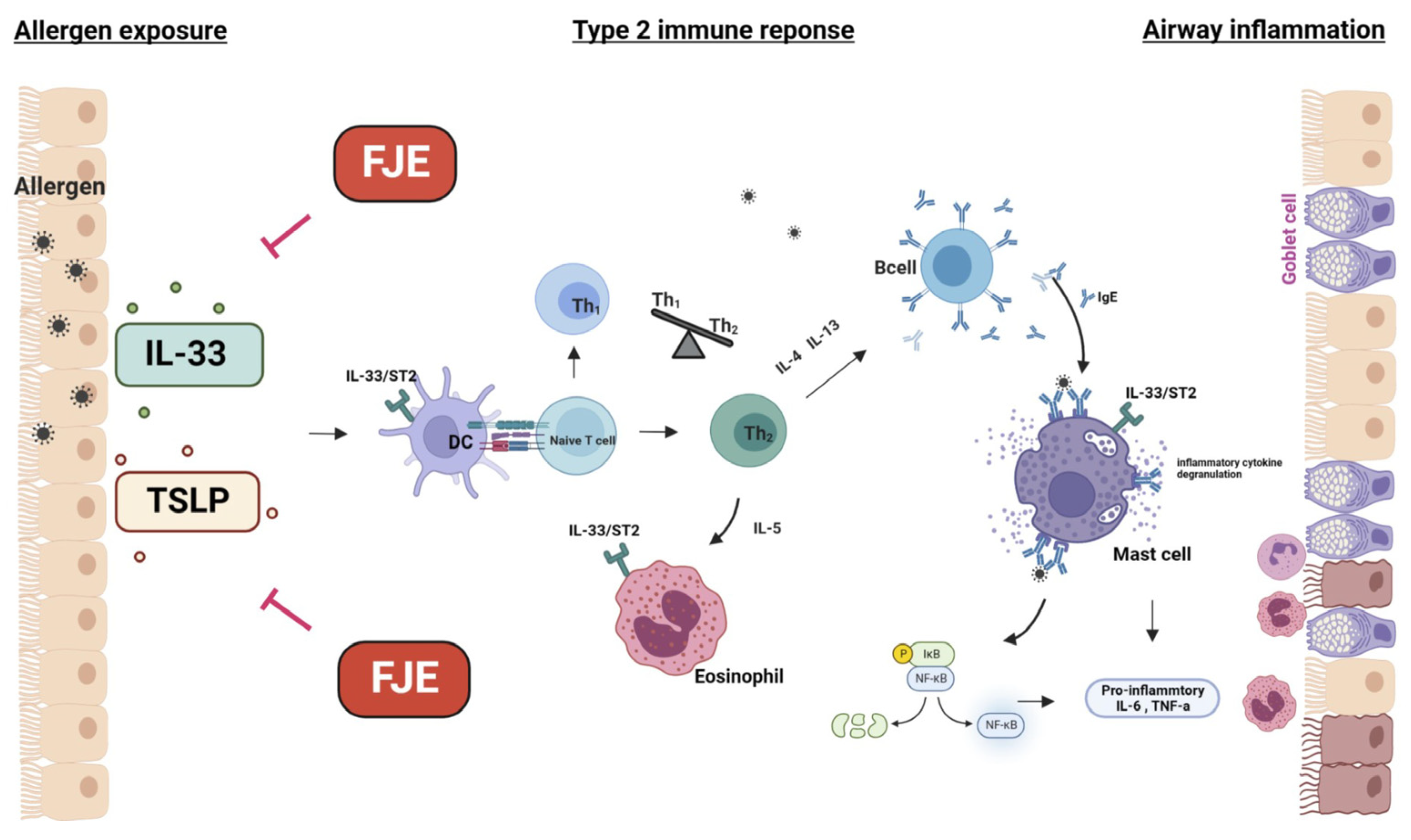
3. Discussion
4. Materials and Methods
4.1. Preparation of Fallopia Japonica Root Extract
4.2. Animal Studies
4.3. CARAS Model and Treatment
4.4. Preparation of RPMC and Degranulation Assay
4.5. Collection of Serum, NLF, and BALF
4.6. Histological Analysis
4.7. Immunohistochemistry
4.8. Evaluation of Cytokine Levels and Immunoglobulins
4.9. Western Blot Anlaysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Asher, I. The Global Asthma Report 2018. Global Asthma Network 2018, 1–92. Available online: http://globalasthmanetwork.org/Global%20asthma%20Report%202018%20Embargo.pdf (accessed on 3 August 2023).
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; van Weel, C.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008, 63 (Suppl. 86), 8–160. [Google Scholar] [CrossRef]
- Brozek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Paiva Ferreira, L.K.D.; Paiva Ferreira, L.A.M.; Monteiro, T.M.; Bezerra, G.C.; Bernardo, L.R.; Piuvezam, M.R. Combined allergic rhinitis and asthma syndrome (CARAS). Int. Immunopharmacol. 2019, 74, 105718. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Jung, J.K.; Park, Y.K. Comparison of the efficacy of KOB03, ketotifen, and montelukast in an experimental mouse model of allergic rhinitis. Int. Immunopharmacol. 2013, 16, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Hox, V.; Lourijsen, E.; Jordens, A.; Aasbjerg, K.; Agache, I.; Alobid, I.; Bachert, C.; Boussery, K.; Campo, P.; Fokkens, W.; et al. Benefits and harm of systemic steroids for short- and long-term use in rhinitis and rhinosinusitis: An EAACI position paper. Clin. Transl. Allergy 2020, 10, 1. [Google Scholar] [CrossRef]
- Lipworth, B.J.; Jackson, C.M. Safety of inhaled and intranasal corticosteroids: Lessons for the new millennium. Drug Saf. 2000, 23, 11–33. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Mihindukulasooriya, S.P.; Kim, H.J.; Kim, A.; Kim, H.J.; Jeon, Y.-J.; Jee, Y. Oral administration of polyphenol-rich Sargassum horneri suppresses particulate matter exacerbated airway inflammation in murine allergic asthma: Relevance to the TLR mediated NF-κB pathway inhibition. J. Funct. Foods 2020, 71, 103991. [Google Scholar] [CrossRef]
- Franova, S.; Joskova, M.; Sutovska, M.; Pechanova, O.; Nosalova, G. Polyphenolic compounds and experimentally induced allergic asthma. Eur. Respir. J. 2011, 38, p4099. [Google Scholar]
- Joskova, M.; Sadlonova, V.; Nosalova, G.; Novakova, E.; Franova, S. Polyphenols and Their Components in Experimental Allergic Asthma. Adv. Exp. Med. Biol. 2013, 756, 91–98. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, J.Y.; Cho, C.H.; Kim, C.J. Anti-asthmatic action of quercetin and rutin in conscious guinea-pigs challenged with aerosolized ovalbumin. Arch. Pharmacal Res. 2007, 30, 1599. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.M.; McGhie, T.K.; Cooney, J.M.; Jensen, D.J.; Gould, E.M.; Lyall, K.A.; Hurst, R.D. Blackcurrant proanthocyanidins augment IFN-gamma-induced suppression of IL-4 stimulated CCL26 secretion in alveolar epithelial cells. Mol. Nutr. Food Res. 2010, 54 (Suppl. 2), S159–S170. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, C.; Shinoda, K.; Yoshimura, M.; Watanabe, Y.; Obata, A.; Nakayama, T. Naringenin chalcone suppresses allergic asthma by inhibiting the type-2 function of CD4 T cells. Allergol. Int. 2010, 59, 67–73. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Trill, J.; Tan, L.-L.; Chang, W.-J.; Zhang, Y.; Willcox, M.; Xia, R.-Y.; Jiang, Y.; Moore, M.; Liu, J.-P.; et al. Reynoutria japonica Houtt for Acute Respiratory Tract Infections in Adults and Children: A Systematic Review. Front. Pharmacol. 2022, 13, 787032. [Google Scholar] [CrossRef]
- Zhou, Z.; Miwa, M.; Nara, K.; Wu, B.; Nakaya, H.; Lian, C.; Miyashita, N.; Oishi, R.; Maruta, E.; Hogetsu, T. Patch establishment and development of a clonal plant, Polygonum cuspidatum, on Mount Fuji. Mol. Ecol. 2003, 12, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-S.; Lee, Y.-C.; Kim, J.-D.; Kim, H.-M.; Kim, C.-H. Inhibitory effects of Polygonum cuspidatum water extract (PCWE) and its component rasveratrol on acyl-coenzyme A–cholesterol acyltransferase activity for cholesteryl ester synthesis in HepG2 cells. Vasc. Pharmacol. 2004, 40, 279–284. [Google Scholar] [CrossRef]
- Zahedi, H.S.; Jazayeri, S.; Ghiasvand, R.; Djalali, M.; Eshraghian, M.R. Effects of polygonum cuspidatum containing resveratrol on inflammation in male professional basketball players. Int. J. Prev. Med. 2013, 4, S1–S4. [Google Scholar]
- Cucu, A.-A.; Baci, G.-M.; Dezsi, Ş.; Nap, M.-E.; Beteg, F.I.; Bonta, V.; Bobiş, O.; Caprio, E.; Dezmirean, D.S. New Approaches on Japanese Knotweed (Fallopia japonica) Bioactive Compounds and Their Potential of Pharmacological and Beekeeping Activities: Challenges and Future Directions. Plants 2021, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Chen, Y.T.; Chiu, C.C.; Liao, W.T.; Liu, Y.C.; David Wang, H.M. Polygonum cuspidatum extracts as bioactive antioxidaion, anti-tyrosinase, immune stimulation and anticancer agents. J. Biosci. Bioeng. 2015, 119, 464–469. [Google Scholar] [CrossRef]
- Shen, M.-Y.; Liu, Y.-J.; Don, M.-J.; Liu, H.-Y.; Chen, Z.-W.; Mettling, C.; Corbeau, P.; Chiang, C.-K.; Jang, Y.-S.; Li, T.-H.; et al. Combined phytochemistry and chemotaxis assays for identification and mechanistic analysis of anti-inflammatory phytochemicals in Fallopia japonica. PLoS ONE 2011, 6, e27480. [Google Scholar] [CrossRef]
- Lee, B.; Moon, S.K. Resveratrol inhibits TNF-alpha-induced proliferation and matrix metalloproteinase expression in human vascular smooth muscle cells. J. Nutr. 2005, 135, 2767–2773. [Google Scholar] [CrossRef] [PubMed]
- Divekar, R.; Kita, H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liao, H.; Zhang, Y.; Liu, L. Regulation of the expression of interleukin-4 and interleukin-5 by the signal pathway of PKC-NF-kappaB in T lymphocyte of allergic rhinitis. Lin Chuang Er Bi Yan Hou Ke Za Zhi 2006, 20, 197–200. [Google Scholar] [PubMed]
- Li, Y.; Wang, W.; Lv, Z.; Li, Y.; Chen, Y.; Huang, K.; Corrigan, C.J.; Ying, S. Elevated Expression of IL-33 and TSLP in the Airways of Human Asthmatics In Vivo: A Potential Biomarker of Severe Refractory Disease. J. Immunol. 2018, 200, 2253–2262. [Google Scholar] [CrossRef]
- Enomoto, T.; Nagasako-Akazome, Y.; Kanda, T.; Ikeda, M.; Dake, Y. Clinical effects of apple polyphenols on persistent allergic rhinitis: A randomized double-blind placebo-controlled parallel arm study. J. Investig. Allergol. Clin. Immunol. 2006, 16, 283–289. [Google Scholar] [PubMed]
- Lv, C.; Zhang, Y.; Shen, L. Preliminary Clinical Effect Evaluation of Resveratrol in Adults with Allergic Rhinitis. Int. Arch. Allergy Immunol. 2018, 175, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, E.W. Inflammatory mediators in allergic rhinitis. J. Allergy Clin. Immunol. 2004, 114, S135–S138. [Google Scholar] [CrossRef]
- Kennedy-Feitosa, E.; Okuro, R.T.; Pinho Ribeiro, V.; Lanzetti, M.; Barroso, M.V.; Zin, W.A.; Porto, L.C.; Brito-Gitirana, L.; Valenca, S.S. Eucalyptol attenuates cigarette smoke-induced acute lung inflammation and oxidative stress in the mouse. Pulm. Pharmacol. Ther. 2016, 41, 11–18. [Google Scholar] [CrossRef]
- Piao, C.H.; Bui, T.T.; Fan, Y.J.; Nguyen, T.V.; Shin, D.U.; Song, C.H.; Lee, S.Y.; Shin, H.S.; Kim, H.T.; Chai, O.H. In vivo and in vitro antiallergic and antiinflammatory effects of Dryopteris crassirhizoma through the modulation of the NFkB signaling pathway in an ovalbumininduced allergic asthma mouse model. Mol. Med. Rep. 2020, 22, 3597–3606. [Google Scholar] [CrossRef]
- Lewkowich, I.P.; Rempel, J.D.; HayGlass, K.T. Antigen-specific versus total immunoglobulin synthesis: Total IgE and IgG1, but not IgG2a levels predict murine antigen-specific responses. Int. Arch. Allergy Immunol. 2004, 133, 145–153. [Google Scholar] [CrossRef]
- Mountford, A.P.; Fisher, A.; Wilson, R.A. The profile of IgG1 and IgG2a antibody responses in mice exposed to Schistosoma mansoni. Parasite Immunol. 1994, 16, 521–527. [Google Scholar] [CrossRef]
- Rostamian, M.; Sohrabi, S.; Kavosifard, H.; Niknam, H.M. Lower levels of IgG1 in comparison with IgG2a are associated with protective immunity against Leishmania tropica infection in BALB/c mice. J. Microbiol. Immunol. Infect. 2017, 50, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Skoner, D.P. Allergic rhinitis: Definition, epidemiology, pathophysiology, detection, and diagnosis. J. Allergy Clin. Immunol. 2001, 108, S2–S8. [Google Scholar] [CrossRef]
- Hofmann, A.M.; Abraham, S.N. New roles for mast cells in modulating allergic reactions and immunity against pathogens. Curr. Opin. Immunol. 2009, 21, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Foster, P.S.; Hogan, S.P.; Matthaei, K.I.; Young, I.G. Interleukin-4 and interleukin-5 as targets for the inhibition of eosinophilic inflammation and allergic airways hyperreactivity. Mem. Inst. Oswaldo Cruz 1997, 92 (Suppl. 2), 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kuperman, D.A.; Huang, X.; Koth, L.L.; Chang, G.H.; Dolganov, G.M.; Zhu, Z.; Elias, J.A.; Sheppard, D.; Erle, D.J. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 2002, 8, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Palomares, O.; Yaman, G.; Azkur, A.K.; Akkoc, T.; Akdis, M.; Akdis, C.A. Role of Treg in immune regulation of allergic diseases. Eur. J. Immunol. 2010, 40, 1232–1240. [Google Scholar] [CrossRef]
- Marques, C.R.; Costa, R.S.; Costa, G.N.O.; da Silva, T.M.; Teixeira, T.O.; de Andrade, E.M.M.; Galvao, A.A.; Carneiro, V.L.; Figueiredo, C.A. Genetic and epigenetic studies of FOXP3 in asthma and allergy. Asthma Res. Pract. 2015, 1, 10. [Google Scholar] [CrossRef]
- Mueller, M.; Beck, V.; Jungbauer, A. PPARalpha activation by culinary herbs and spices. Planta Med. 2011, 77, 497–504. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Deng, M.X.; He, J.; Zeng, Q.X.; Wen, W.; Wong, D.S.; Tse, H.F.; Xu, G.; Lian, Q.; Shi, J.; et al. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells 2012, 30, 2692–2699. [Google Scholar] [CrossRef]
- Peterson, J.D.; Herzenberg, L.A.; Vasquez, K.; Waltenbaugh, C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Chung, F. Anti-inflammatory cytokines in asthma and allergy: Interleukin-10, interleukin-12, interferon-gamma. Mediat. Inflamm. 2001, 10, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Dorrington, M.G.; Fraser, I.D.C. NF-kappaB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Edwards, M.R.; Bartlett, N.W.; Clarke, D.; Birrell, M.; Belvisi, M.; Johnston, S.L. Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol. Ther. 2009, 121, 1–13. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Fan, Y.; Nguyen, T.V.; Piao, C.H.; Shin, H.S.; Song, C.H.; Chai, O.H. Fructus Amomi extract attenuates nasal inflammation by restoring Th1/Th2 balance and down-regulation of NF-kappaB phosphorylation in OVA-induced allergic rhinitis. Biosci. Rep. 2022, 42, BSR20212681. [Google Scholar] [CrossRef]
- West, E.E.; Kashyap, M.; Leonard, W.J. TSLP: A Key Regulator of Asthma Pathogenesis. Drug Discov. Today Dis. Mech. 2012, 9, e83–e88. [Google Scholar] [CrossRef]
- Lee, H.C.; Ziegler, S.F. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc. Natl. Acad. Sci. USA 2007, 104, 914–919. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Kaur, D.; Gomez, E.; Doe, C.; Berair, R.; Woodman, L.; Saunders, R.; Hollins, F.; Rose, F.R.; Amrani, Y.; May, R.; et al. IL-33 drives airway hyper-responsiveness through IL-13-mediated mast cell: Airway smooth muscle crosstalk. Allergy 2015, 70, 556–567. [Google Scholar] [CrossRef]
- Patel, N.N.; Kohanski, M.A.; Maina, I.W.; Workman, A.D.; Herbert, D.R.; Cohen, N.A. Sentinels at the wall: Epithelial-derived cytokines serve as triggers of upper airway type 2 inflammation. Int. Forum Allergy Rhinol. 2019, 9, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Liu, F.; Wang, M. Emodin Alleviates the Airway Inflammation of Cough Variant Asthma in Mice by Regulating the Notch Pathway. Med. Sci. Monit. 2019, 25, 5621–5629. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.D.; Li, X.Z.; Wu, Y.X.; Shen, Y.; Liu, F.F.; Gao, P.P.; Sun, L.; Qian, F. Emodin alleviates alternatively activated macrophage and asthmatic airway inflammation in a murine asthma model. Acta Pharmacol. Sin. 2018, 39, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhong, X.-G.; Li, Y.-H.; Jia, X.; Zhang, S.-J.; Gao, Y.-S.; Liu, M.; Wu, R.-H. Protective effect of emodin against airway inflammation in the ovalbumin-induced mouse model. Chin. J. Integr. Med. 2015, 21, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.L.; Hurst, R.D.; Sawyer, G.M.; Kruger, M.C. The in vitro evaluation of isolated procyanidins as modulators of cytokine-induced eotaxin production in human alveolar epithelial cells. J. Berry Res. 2016, 6, 115–124. [Google Scholar] [CrossRef]
- Sawyer, G.M.; Stevenson, D.E.; McGhie, T.K.; Hurst, R.D. Suppression of CCL26 and CCL11 generation in human alveolar epithelial cells by apple extracts containing procyanidins. J. Funct. Foods 2017, 31, 141–151. [Google Scholar] [CrossRef]
- Tie, S.; Zhang, L.; Li, B.; Xing, S.; Wang, H.; Chen, Y.; Cui, W.; Gu, S.; Tan, M.J.F.S.; Wellness, H. Effect of dual targeting procyanidins nanoparticles on metabolomics of lipopolysaccharide-stimulated inflammatory macrophages. Food Sci. Hum. Wellness 2023, 12, 2252–2262. [Google Scholar] [CrossRef]
- Chen, F.; Wang, H.; Zhao, J.; Yan, J.; Meng, H.; Zhan, H.; Chen, L.; Yuan, L. Grape seed proanthocyanidin inhibits monocrotaline-induced pulmonary arterial hypertension via attenuating inflammation: In vivo and in vitro studies. J. Nutr. Biochem. 2019, 67, 72–77. [Google Scholar] [CrossRef]
- Georgieva, E.; Ivanova, D.; Zhelev, Z.; Bakalova, R.; Gulubova, M.; Aoki, I. Mitochondrial dysfunction and redox imbalance as a diagnostic marker of “free radical diseases”. Anticancer Res. 2017, 37, 5373–5381. [Google Scholar]
- Zhu, W.; Li, M.C.; Wang, F.R.; Mackenzie, G.G.; Oteiza, P.I. The inhibitory effect of ECG and EGCG dimeric procyanidins on colorectal cancer cells growth is associated with their actions at lipid rafts and the inhibition of the epidermal growth factor receptor signaling. Biochem. Pharmacol. 2020, 175, 113923. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Piao, C.H.; Song, C.H.; Chai, O.H. Skullcapflavone II attenuates ovalbumin-induced allergic rhinitis through the blocking of Th2 cytokine production and mast cell histamine release. Int. Immunopharmacol. 2017, 52, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Argun Baris, S.; Vural, C.; Yaprak, B.; Onyilmaz, T.; Tuncel, D.; Vatansever, S.; Isken, T.; Basyigit, I.; Boyaci, H.; Yildiz, F. The effects of sildenafil on smoke induced lung inflammation in rats. Malays. J. Pathol. 2016, 38, 39–44. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Fan, Y.J.; Nguyen, T.V.; Yu, Z.N.; Song, C.H.; Lee, S.-Y.; Shin, H.S.; Chai, O.H. Fallopia japonica Root Extract Ameliorates Ovalbumin-Induced Airway Inflammation in a CARAS Mouse Model by Modulating the IL-33/TSLP/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 12514. https://doi.org/10.3390/ijms241512514
Jin J, Fan YJ, Nguyen TV, Yu ZN, Song CH, Lee S-Y, Shin HS, Chai OH. Fallopia japonica Root Extract Ameliorates Ovalbumin-Induced Airway Inflammation in a CARAS Mouse Model by Modulating the IL-33/TSLP/NF-κB Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(15):12514. https://doi.org/10.3390/ijms241512514
Chicago/Turabian StyleJin, Juan, Yan Jing Fan, Thi Van Nguyen, Zhen Nan Yu, Chang Ho Song, So-Yong Lee, Hee Soon Shin, and Ok Hee Chai. 2023. "Fallopia japonica Root Extract Ameliorates Ovalbumin-Induced Airway Inflammation in a CARAS Mouse Model by Modulating the IL-33/TSLP/NF-κB Signaling Pathway" International Journal of Molecular Sciences 24, no. 15: 12514. https://doi.org/10.3390/ijms241512514
APA StyleJin, J., Fan, Y. J., Nguyen, T. V., Yu, Z. N., Song, C. H., Lee, S.-Y., Shin, H. S., & Chai, O. H. (2023). Fallopia japonica Root Extract Ameliorates Ovalbumin-Induced Airway Inflammation in a CARAS Mouse Model by Modulating the IL-33/TSLP/NF-κB Signaling Pathway. International Journal of Molecular Sciences, 24(15), 12514. https://doi.org/10.3390/ijms241512514






