Thymic Stromal Lymphopoietin (TSLP), Its Isoforms and the Interplay with the Epithelium in Allergy and Asthma
Abstract
1. Introduction
2. TSLP, Its Receptors and Molecular Interactions
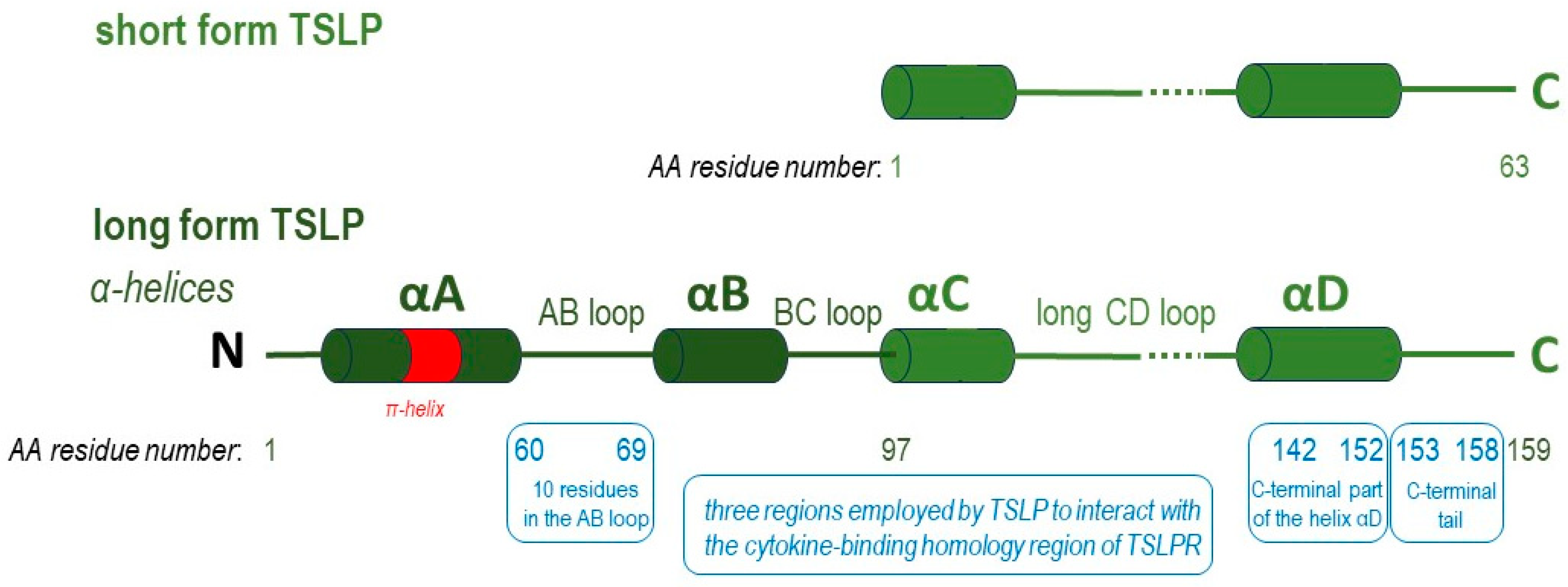
3. TSLP Isoforms
3.1. The Long Isoform of TSLP
3.2. The Short Form TSLP
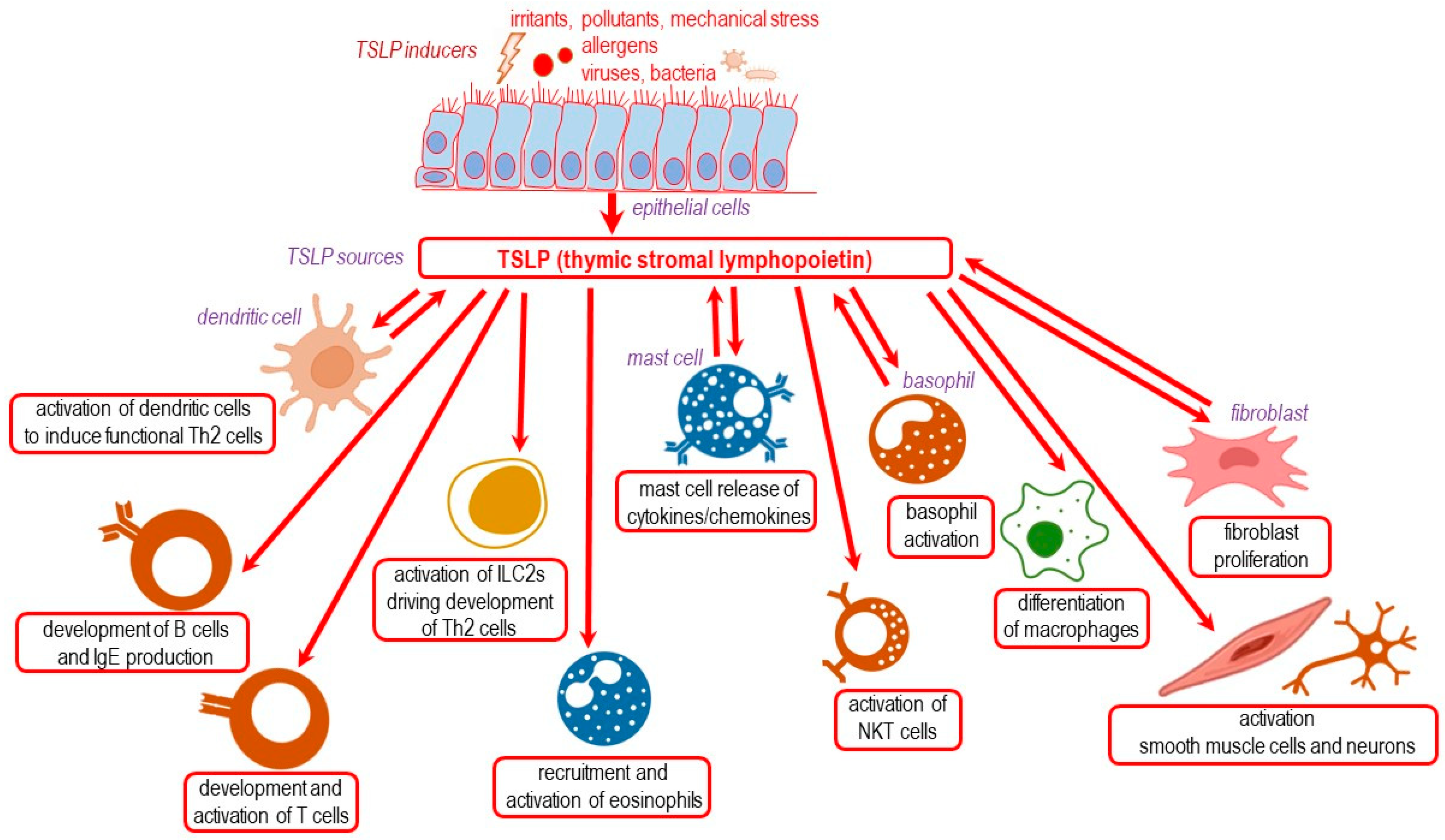
4. TSLP as a Critical Cytokine in Epithelium Interactions and T2 Responses
5. TSLP in Asthma, Nasal Polyposis, Allergic Rhinitis and Ocular Allergy
6. TSLP in Urticaria, Atopic Dermatitis and Food Allergy
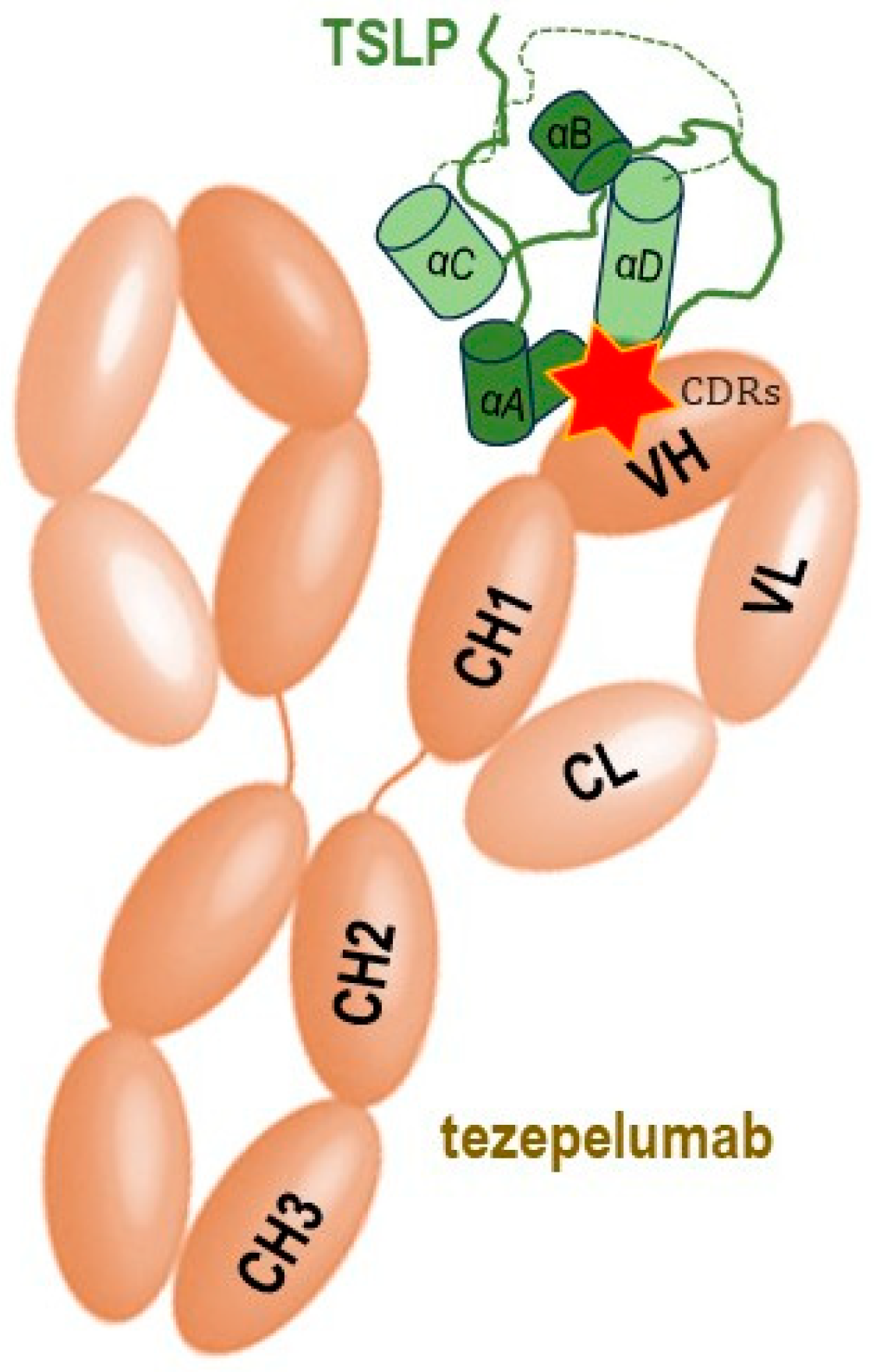
7. Overview of the Pharmacological Anti-TSLP Strategies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ebina-Shibuya, R.; Leonard, W.J. Role of thymic stromal lymphopoietin in allergy and beyond. Nat. Rev. Immunol. 2022, 23, 24–37. [Google Scholar] [CrossRef]
- Quentmeier, H.; Drexler, H.G.; Fleckenstein, D.; Zaborski, M.; Armstrong, A.; Sims, J.E.; Lyman, S.D. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia 2001, 15, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdi, Z.; Comeau, M.R.; Jessup, H.K.; Yoon, B.-R.P.; Brewer, A.; Chartier, S.; Paquette, N.; Ziegler, S.F.; Sarfati, M.; Delespesse, G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 2007, 204, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Yu, Q.; Lv, J.; Di, C.; Lin, X.; Su, W.; Wu, M.; Xia, Z. Airway epithelial TSLP production of TLR2 drives type 2 immunity in allergic airway inflammation. Eur. J. Immunol. 2018, 48, 1838–1850. [Google Scholar] [CrossRef]
- Lee, H.-C.; Headley, M.B.; Loo, Y.-M.; Berlin, A.; Gale, M.; Debley, J.S.; Lukacs, N.W.; Ziegler, S.F. Thymic stromal lymphopoietin is induced by respiratory syncytial virus–infected airway epithelial cells and promotes a type 2 response to infection. J. Allergy Clin. Immunol. 2012, 130, 1187–1196.e5. [Google Scholar] [CrossRef]
- Stier, M.T.; Bloodworth, M.H.; Toki, S.; Newcomb, D.C.; Goleniewska, K.; Boyd, K.L.; Quitalig, M.; Hotard, A.L.; Moore, M.L.; Hartert, T.V.; et al. Respiratory syncytial virus infection activates IL-13–producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J. Allergy Clin. Immunol. 2016, 138, 814–824.e11. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Coira, J.; Villaseñor, A.; Izquierdo, E.; Huang, M.; Barker-Tejeda, T.C.; Radzikowska, U.; Sokolowska, M.; Barber, D. The Importance of Metabolism for Immune Homeostasis in Allergic Diseases. Front. Immunol. 2021, 12, 692004. [Google Scholar] [CrossRef]
- Hristova, M.; Habibovic, A.; Veith, C.; Janssen-Heininger, Y.M.; Dixon, A.E.; Geiszt, M.; van der Vliet, A. Airway epithelial dual oxidase 1 mediates allergen-induced IL-33 secretion and activation of type 2 immune responses. J. Allergy Clin. Immunol. 2016, 137, 1545–1556.e11. [Google Scholar] [CrossRef]
- Boldogh, I.; Bacsi, A.; Choudhury, B.K.; Dharajiya, N.; Alam, R.; Hazra, T.K.; Mitra, S.; Goldblum, R.M.; Sur, S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J. Clin. Investig. 2005, 115, 2169–2179. [Google Scholar] [CrossRef]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell–mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef]
- Bell, B.D.; Kitajima, M.; Larson, R.P.; Stoklasek, T.A.; Dang, K.; Sakamoto, K.; Wagner, K.-U.; Kaplan, D.H.; Reizis, B.; Hennighausen, L.; et al. The transcription factor STAT5 is critical in dendritic cells for the development of TH2 but not TH1 responses. Nat. Immunol. 2013, 14, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, P.P.; Tan, Z.; Page, B.D.; Hedtrich, S. TSLP as druggable target—A silver-lining for atopic diseases? Pharmacol. Ther. 2020, 217, 107648. [Google Scholar] [CrossRef]
- Pandey, A.; Ozaki, K.; Baumann, H.; Levin, S.D.; Puel, A.; Farr, A.G.; Ziegler, S.F.; Leonard, W.J.; Lodish, H.F. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat. Immunol. 2000, 1, 59–64. [Google Scholar] [CrossRef]
- Park, L.S.; Martin, U.; Garka, K.E.; Gliniak, B.; Di Santo, J.P.; Muller, W.; Largaespada, D.A.; Copeland, N.G.; Jenkins, N.A.; Farr, A.G.; et al. Cloning of the Murine Thymic Stromal Lymphopoietin (Tslp) Receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 2000, 192, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, K.; Peelman, F.; Braun, H.; Lopez, J.; Van Rompaey, D.; Dansercoer, A.; Vandenberghe, I.; Pauwels, K.; Tavernier, J.; Lambrecht, B.N.; et al. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat. Commun. 2017, 8, 14937. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Pelaia, G.; Crimi, C.; Maglio, A.; Gallelli, L.; Terracciano, R.; Vatrella, A. Tezepelumab: A Potential New Biological Therapy for Severe Refractory Asthma. Int. J. Mol. Sci. 2021, 22, 4369. [Google Scholar] [CrossRef] [PubMed]
- Fornasa, G.; Tsilingiri, K.; Caprioli, F.; Botti, F.; Mapelli, M.; Meller, S.; Kislat, A.; Homey, B.; Di Sabatino, A.; Sonzogni, A.M.; et al. Dichotomy of short and long thymic stromal lymphopoietin isoforms in inflammatory disorders of the bowel and skin. J. Allergy Clin. Immunol. 2015, 136, 413–422. [Google Scholar] [CrossRef]
- Li, Y.; Lund, C.; Nervik, I.; Loevenich, S.; Døllner, H.; Anthonsen, M.W.; Johnsen, I.B. Characterization of signaling pathways regulating the expression of pro-inflammatory long form thymic stromal lymphopoietin upon human metapneumovirus infection. Sci. Rep. 2018, 8, 883. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Hirota, T.; Jodo, A.I.; Doi, S.; Kameda, M.; Fujita, K.; Miyatake, A.; Enomoto, T.; Noguchi, E.; Yoshihara, S.; et al. Functional Analysis of the Thymic Stromal Lymphopoietin Variants in Human Bronchial Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2009, 40, 368–374. [Google Scholar] [CrossRef]
- Varricchi, G.; Pecoraro, A.; Marone, G.; Criscuolo, G.; Spadaro, G.; Genovese, A.; Marone, G. Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer. Front. Immunol. 2018, 9, 1595. [Google Scholar] [CrossRef]
- Gounni, A.S.; Koussih, L. SUMO Wrestling in the Airway Epithelium: Does It Regulate Thymic Stromal Lymphopoietin? Am. J. Respir. Cell Mol. Biol. 2022, 66, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Poposki, J.A.; Klingler, A.I.; Stevens, W.W.; Peters, A.T.; Hulse, K.E.; Grammer, L.C.; Schleimer, R.P.; Welch, K.C.; Smith, S.S.; Sidle, D.M.; et al. Proprotein convertases generate a highly functional heterodimeric form of thymic stromal lymphopoietin in humans. J. Allergy Clin. Immunol. 2017, 139, 1559–1567.e8. [Google Scholar] [CrossRef] [PubMed]
- Melum, G.R.; Farkas, L.; Scheel, C.; Van Dieren, B.; Gran, E.; Liu, Y.-J.; Johansen, F.-E.; Jahnsen, F.L.; Baekkevold, E.S. A thymic stromal lymphopoietin–responsive dendritic cell subset mediates allergic responses in the upper airway mucosa. J. Allergy Clin. Immunol. 2014, 134, 613–621.e7. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Wang, Y.H.; Duramad, O.; Hori, T.; Delespesse, G.J.; Watanabe, N.; Qin, F.X.; Yao, Z.; Cao, W.; Liu, Y.J. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005, 202, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Takai, T.; Chen, X.; Okumura, K.; Ogawa, H. Long TSLP transcript expression and release of TSLP induced by TLR ligands and cytokines in human keratinocytes. J. Dermatol. Sci. 2012, 66, 233–237. [Google Scholar] [CrossRef]
- Datta, A.; Alexander, R.; Sulikowski, M.G.; Nicholson, A.G.; Maher, T.M.; Scotton, C.J.; Chambers, R.C. Evidence for a Functional Thymic Stromal Lymphopoietin Signaling Axis in Fibrotic Lung Disease. J. Immunol. 2013, 191, 4867–4879. [Google Scholar] [CrossRef]
- Mena, A.M.; Langlois, A.; Speca, S.; Schneider, L.; Desreumaux, P.; Dubuquoy, L.; Bertin, B. The Expression of the Short Isoform of Thymic Stromal Lymphopoietin in the Colon Is Regulated by the Nuclear Receptor Peroxisome Proliferator Activated Receptor-Gamma and Is Impaired during Ulcerative Colitis. Front. Immunol. 2017, 8, 1052. [Google Scholar] [CrossRef]
- Dong, H.; Hu, Y.; Liu, L.; Zou, M.; Huang, C.; Luo, L.; Yu, C.; Wan, X.; Zhao, H.; Chen, J.; et al. Distinct roles of short and long thymic stromal lymphopoietin isoforms in house dust mite-induced asthmatic airway epithelial barrier disruption. Sci. Rep. 2016, 6, 39559. [Google Scholar] [CrossRef]
- Gandolfo, S.; Bulfoni, M.; Fabro, C.; Russi, S.; Sansonno, D.; Di Loreto, C.; Cesselli, D.; De Vita, S. Thymic stromal lymphopoietin expression from benign lymphoproliferation to malignant B-cell lymphoma in primary Sjogren’s syndrome. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S118), 55–64. [Google Scholar]
- Cultrone, A.; de Wouters, T.; Lakhdari, O.; Kelly, D.; Mulder, I.; Logan, E.; Lapaque, N.; Doré, J.; Blottière, H.M. The NF-κB binding site located in the proximal region of the TSLP promoter is critical for TSLP modulation in human intestinal epithelial cells. Eur. J. Immunol. 2013, 43, 1053–1062. [Google Scholar] [CrossRef]
- Harada, M.; Hirota, T.; Jodo, A.I.; Hitomi, Y.; Sakashita, M.; Tsunoda, T.; Miyagawa, T.; Doi, S.; Kameda, M.; Fujita, K.; et al. Thymic Stromal Lymphopoietin Gene Promoter Polymorphisms Are Associated with Susceptibility to Bronchial Asthma. Am. J. Respir. Cell Mol. Biol. 2011, 44, 787–793. [Google Scholar] [CrossRef]
- Liang, S.; Zhou, Z.; Zhou, Z.; Liu, J.; Huang, W.; Dong, H.; Zou, F.; Zhao, H.; Yu, C.; Cai, S. CBX4 Regulates Long-Form Thymic Stromal Lymphopoietin–mediated Airway Inflammation through SUMOylation in House Dust Mite–induced Asthma. Am. J. Respir. Cell Mol. Biol. 2022, 66, 648–660. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Tang, S.; Wang, J.; Liu, T.; Zeng, R.; Zhu, W.; Zhang, K.; Wu, J. The different functions of short and long thymic stromal lymphopoietin isoforms in autophagy-mediated asthmatic airway inflammation and remodeling. Immunobiology 2021, 226, 152124. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiri, K.; Fornasa, G.; Rescigno, M. Thymic Stromal Lymphopoietin: To Cut a Long Story Short. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 174–182. [Google Scholar] [CrossRef]
- Bjerkan, L.; Sonesson, A.; Schenck, K. Multiple Functions of the New Cytokine-Based Antimicrobial Peptide Thymic Stromal Lymphopoietin (TSLP). Pharmaceuticals 2016, 9, 41. [Google Scholar] [CrossRef]
- Alkashgari, H.R.; Ruiz-Jimenez, C.; Stoian, C.; Coats, J.S.; Baez, I.; Chirshev, E.; Martinez, S.R.; Dovat, S.; Francis-Boyle, O.L.; Casiano, C.A.; et al. TSLP as a Potential Therapy in the Treatment of CRLF2 B Cell Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2022, 24, 474. [Google Scholar] [CrossRef]
- Braile, M.; Fiorelli, A.; Sorriento, D.; Di Crescenzo, R.M.; Galdiero, M.R.; Marone, G.; Santini, M.; Varricchi, G.; Loffredo, S. Human Lung-Resident Macrophages Express and Are Targets of Thymic Stromal Lymphopoietin in the Tumor Microenvironment. Cells 2021, 10, 2012. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.K.Y.; Lau, T.S.; Chung, K.Y.; Tam, C.; Cheung, T.H.; Yim, S.F.; Lee, J.H.S.; Leung, R.W.T.; Qin, J.; Or, Y.Y.Y.; et al. Short-Form Thymic Stromal Lymphopoietin (sfTSLP) Is the Predominant Isoform Expressed by Gynaecologic Cancers and Promotes Tumour Growth. Cancers 2021, 13, 980. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, M.; Okoye, I.; Lacy, P. Epithelial cell alarmin cytokines: Frontline mediators of the asthma inflammatory response. Front. Immunol. 2022, 13, 975914. [Google Scholar] [CrossRef]
- Zoltowska, A.M.; Lei, Y.; Fuchs, B.; Rask, C.; Adner, M.; Nilsson, G.P. The interleukin-33 receptor ST2 is important for the development of peripheral airway hyperresponsiveness and inflammation in a house dust mite mouse model of asthma. Clin. Exp. Allergy 2016, 46, 479–490. [Google Scholar] [CrossRef]
- Moussion, C.; Ortega, N.; Girard, J.-P. The IL-1-Like Cytokine IL-33 Is Constitutively Expressed in the Nucleus of Endothelial Cells and Epithelial Cells In Vivo: A Novel ‘Alarmin’? PLoS ONE 2008, 3, e3331. [Google Scholar] [CrossRef] [PubMed]
- Dziki, J.L.; Hussey, G.; Badylak, S.F. Alarmins of the extracellular space. Semin. Immunol. 2018, 38, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Lv, Z.; Chen, Y.; Li, Y.; Huang, K.; Corrigan, C.J.; Ying, S. Bronchial Allergen Challenge of Patients with Atopic Asthma Triggers an Alarmin (IL-33, TSLP, and IL-25) Response in the Airways Epithelium and Submucosa. J. Immunol. 2018, 201, 2221–2231. [Google Scholar] [CrossRef]
- Paplinska-Goryca, M.; Misiukiewicz-Stepien, P.; Nejman-Gryz, P.; Proboszcz, M.; Mlacki, M.; Gorska, K.; Krenke, R. Epithelial-macrophage-dendritic cell interactions impact alarmins expression in asthma and COPD. Clin. Immunol. 2020, 215, 108421. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2006, 81, 1–5. [Google Scholar] [CrossRef]
- Marone, G.; Spadaro, G.; Braile, M.; Poto, R.; Criscuolo, G.; Pahima, H.; Loffredo, S.; Levi-Schaffer, F.; Varricchi, G. Tezepelumab: A novel biological therapy for the treatment of severe uncontrolled asthma. Expert Opin. Investig. Drugs 2019, 28, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, M.M.; Coimbra, M.M.; Parker, S.F.; Vaz, P.D.; Ribeiro-Claro, P.J.A. Structural Dynamics of Chloromethanes through Computational Spectroscopy: Combining INS and DFT. Molecules 2022, 27, 7661. [Google Scholar] [CrossRef]
- Han, J.; Dakhama, A.; Jia, Y.; Wang, M.; Zeng, W.; Takeda, K.; Shiraishi, Y.; Okamoto, M.; Ziegler, S.F.; Gelfand, E.W. Responsiveness to respiratory syncytial virus in neonates is mediated through thymic stromal lymphopoietin and OX40 ligand. J. Allergy Clin. Immunol. 2012, 130, 1175–1186.e9. [Google Scholar] [CrossRef]
- Tourdot, S.; Mathie, S.; Hussell, T.; Edwards, L.; Wang, H.; Openshaw, P.J.M.; Schwarze, J.; Lloyd, C.M. Respiratory syncytial virus infection provokes airway remodelling in allergen-exposed mice in absence of prior allergen sensitization. Clin. Exp. Allergy 2008, 38, 1016–1024. [Google Scholar] [CrossRef]
- Pattarini, L.; Trichot, C.; Bogiatzi, S.; Grandclaudon, M.; Meller, S.; Keuylian, Z.; Durand, M.; Volpe, E.; Madonna, S.; Cavani, A.; et al. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J. Exp. Med. 2017, 214, 1529–1546. [Google Scholar] [CrossRef]
- Astrakhan, A.; Omori, M.; Nguyen, T.; Becker-Herman, S.; Iseki, M.; Aye, T.; Hudkins, K.; Dooley, J.; Farr, A.; Alpers, C.E.; et al. Local increase in thymic stromal lymphopoietin induces systemic alterations in B cell development. Nat. Immunol. 2007, 8, 522–531. [Google Scholar] [CrossRef]
- Kim, B.S.; Siracusa, M.C.; Saenz, S.A.; Noti, M.; Monticelli, L.A.; Sonnenberg, G.F.; Hepworth, M.R.; Van Voorhees, A.S.; Comeau, M.R.; Artis, D. TSLP Elicits IL-33–Independent Innate Lymphoid Cell Responses to Promote Skin Inflammation. Sci. Transl. Med. 2013, 5, 170ra16. [Google Scholar] [CrossRef] [PubMed]
- Hams, E.; Fallon, P.G. Innate type 2 cells and asthma. Curr. Opin. Pharmacol. 2012, 12, 503–509. [Google Scholar] [CrossRef]
- Leyva-Castillo, J.M.; Hener, P.; Michea, P.; Karasuyama, H.; Chan, S.; Soumelis, V.; Li, M. Skin thymic stromal lymphopoietin initiates Th2 responses through an orchestrated immune cascade. Nat. Commun. 2013, 4, 2847. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.; Rochman, Y.; Spolski, R.; Samsel, L.; Leonard, W.J. Thymic Stromal Lymphopoietin Is Produced by Dendritic Cells. J. Immunol. 2011, 187, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.D.; Beasley, R.; Agusti, A.; Anderson, G.P.; Bel, E.; Brusselle, G.; Cullinan, P.; Custovic, A.; Ducharme, F.M.; Fahy, J.V.; et al. After asthma: Redefining airways diseases. Lancet 2018, 391, 350–400. [Google Scholar] [CrossRef]
- Nagata, Y.; Kamijuku, H.; Taniguchi, M.; Ziegler, S.; Seino, K.-I. Differential Role of Thymic Stromal Lymphopoietin in the Induction of Airway Hyperreactivity and Th2 Immune Response in Antigen-Induced Asthma with Respect to Natural Killer T Cell Function. Int. Arch. Allergy Immunol. 2007, 144, 305–314. [Google Scholar] [CrossRef]
- Wong, C.K.; Hu, S.; Cheung, P.F.Y.; Lam, C.W.K. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: Implications in allergic inflammation. Am. J. Respir. Cell Mol. Biol. 2010, 43, 305–315. [Google Scholar] [CrossRef]
- Soh, W.T.; Zhang, J.; Hollenberg, M.D.; Vliagoftis, H.; Rothenberg, M.E.; Sokol, C.L.; Robinson, C.; Jacquet, A. Protease allergens as initiators–regulators of allergic inflammation. Allergy 2023, 78, 1148–1168. [Google Scholar] [CrossRef]
- Schiffers, C.; Hristova, M.; Habibovic, A.; Dustin, C.M.; Danyal, K.; Reynaert, N.L.; Wouters, E.F.M.; Van Der Vliet, A. The Transient Receptor Potential Channel Vanilloid 1 Is Critical in Innate Airway Epithelial Responses to Protease Allergens. Am. J. Respir. Cell Mol. Biol. 2020, 63, 198–208. [Google Scholar] [CrossRef]
- Miller, M.H.; Swaby, L.G.; Vailoces, V.S.; LaFratta, M.; Zhang, Y.; Zhu, X.; Hitchcock, D.J.; Jewett, T.J.; Zhang, B.; Tigno-Aranjuez, J.T. LMAN1 is a receptor for house dust mite allergens. Cell Rep. 2023, 42, 112208. [Google Scholar] [CrossRef] [PubMed]
- Murrison, L.B.; Ren, X.; Preusse, K.; He, H.; Kroner, J.; Chen, X.; Jenkins, S.; Johansson, E.; Biagini, J.M.; Weirauch, M.T.; et al. TSLP disease-associated genetic variants combined with airway TSLP expression influence asthma risk. J. Allergy Clin. Immunol. 2022, 149, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Reche, P.A.; Soumelis, V.; Gorman, D.M.; Clifford, T.; Liu, M.-R.; Travis, M.; Zurawski, S.M.; Johnston, J.; Liu, Y.-J.; Spits, H.; et al. Human Thymic Stromal Lymphopoietin Preferentially Stimulates Myeloid Cells. J. Immunol. 2001, 167, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; O’connor, B.; Ratoff, J.; Meng, Q.; Mallett, K.; Cousins, D.; Robinson, D.; Zhang, G.; Zhao, J.; Lee, T.H.; et al. Thymic Stromal Lymphopoietin Expression Is Increased in Asthmatic Airways and Correlates with Expression of Th2-Attracting Chemokines and Disease Severity. J. Immunol. 2005, 174, 8183–8190. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Lee, H.-C.; Nakayama, T.; Ziegler, S.F. TSLP enhances the function of helper type 2 cells. Eur. J. Immunol. 2011, 41, 1862–1871. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, B. Functions of thymic stromal lymphopoietin in immunity and disease. Immunol. Res. 2012, 52, 211–223. [Google Scholar] [CrossRef]
- Corren, J. New Targeted Therapies for Uncontrolled Asthma. J. Allergy Clin. Immunol. Pract. 2019, 7, 1394–1403. [Google Scholar] [CrossRef]
- Zoumot, Z.; Al Busaidi, N.; Tashkandi, W.; Aljohaney, A.A.; Isse, S.; Vidyasagar, K.; Ukwaja, K.N. Tezepelumab for Patients with Severe Uncontrolled Asthma: A Systematic Review and Meta-Analysis. J. Asthma Allergy 2022, 15, 1665–1679. [Google Scholar] [CrossRef]
- Kabata, H.; Moro, K.; Fukunaga, K.; Suzuki, Y.; Miyata, J.; Masaki, K.; Betsuyaku, T.; Koyasu, S.; Asano, K. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat. Commun. 2013, 4, 2675. [Google Scholar] [CrossRef]
- Liu, S.; Verma, M.; Michalec, L.; Liu, W.; Sripada, A.; Rollins, D.; Good, J.; Ito, Y.; Chu, H.; Gorska, M.M.; et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: The role of thymic stromal lymphopoietin. J. Allergy Clin. Immunol. 2018, 141, 257–268.e6. [Google Scholar] [CrossRef]
- Esnault, S.; Rosenthal, L.A.; Wang, D.-S.; Malter, J.S. Thymic stromal lymphopoietin (TSLP) as a bridge between infection and atopy. Int. J. Clin. Exp. Pathol. 2008, 1, 325–330. [Google Scholar] [PubMed]
- Nakagome, K.; Nagata, M. Innate Immune Responses by Respiratory Viruses, Including Rhinovirus, During Asthma Exacerbation. Front. Immunol. 2022, 13, 865973. [Google Scholar] [CrossRef]
- Ibrahim, B.; Achour, D.; Zerimech, F.; de Nadai, P.; Siroux, V.; Tsicopoulos, A.; Matran, R.; Granger, V.; Nadif, R. Plasma thymic stromal lymphopoietin (TSLP) in adults with non-severe asthma: The EGEA study. Thorax 2023, 78, 207–210. [Google Scholar] [CrossRef]
- Klimek, L.; Hagemann, J.; Welkoborsky, H.-J.; Cuevas, M.; Casper, I.; Förster-Ruhrmann, U.; Klimek, F.; Hintschich, C.A.; Huppertz, T.; Bergmann, C.; et al. Epithelial immune regulation of inflammatory airway diseases: Chronic rhinosinusitis with nasal polyps (CRSwNP). Allergol. Sel. 2022, 6, 148–166. [Google Scholar] [CrossRef]
- Buchheit, K.M.; Cahill, K.N.; Katz, H.R.; Murphy, K.C.; Feng, C.; Lee-Sarwar, K.; Lai, J.; Bhattacharyya, N.; Israel, E.; Boyce, J.A.; et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 2016, 137, 1566–1576.e5. [Google Scholar] [CrossRef]
- Schaper-Gerhardt, K.; Rossbach, K.; Nikolouli, E.; Werfel, T.; Gutzmer, R.; Mommert, S. The role of the histamine H 4 receptor in atopic dermatitis and psoriasis. Br. J. Pharmacol. 2020, 177, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Schaper, K.; Rossbach, K.; Köther, B.; Stark, H.; Kietzmann, M.; Werfel, T.; Gutzmer, R. Stimulation of the histamine 4 receptor upregulates thymic stromal lymphopoietin (TSLP) in human and murine keratinocytes. Pharmacol. Res. 2016, 113, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Takabayashi, T.; Sakashita, M.; Imoto, Y.; Tokunaga, T.; Ninomiya, T.; Morikawa, T.; Yoshida, K.; Noguchi, E.; Fujieda, S. Expression and Functional Analysis of CST1 in Intractable Nasal Polyps. Am. J. Respir. Cell Mol. Biol. 2018, 59, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Favier, V.; Charriot, J.; Crampette, L.; Bourdin, A.; Ahmed, E. What place will tezepelumab hold in the treatment paradigm in chronic rhinosinusitis? Expert Rev. Clin. Immunol. 2023, 19, 821–825. [Google Scholar] [CrossRef]
- Chang, W.; Lv, H.; Tan, L.; Gao, Z.; Liu, P.; Qin, D.; Zhang, W.; Xu, Y. Downregulation of deubiquitinating enzyme USP25 promotes the development of allergic rhinitis by enhancing TSLP signaling in the nasal epithelium. Mol. Med. Rep. 2022, 26, 347. [Google Scholar] [CrossRef]
- Corren, J.; Larson, D.; Altman, M.C.; Segnitz, R.M.; Avila, P.C.; Greenberger, P.A.; Baroody, F.; Moss, M.H.; Nelson, H.; Burbank, A.J.; et al. Effects of combination treatment with tezepelumab and allergen immunotherapy on nasal responses to allergen: A randomized controlled trial. J. Allergy Clin. Immunol. 2023, 151, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-D.; Deng, Y.-X.; Ma, H.-X.; Chen, X.-G.; Chen, L.-H.; Qu, J. Thymic Stromal Lymphopoietin-Related Allergic Pathway in Patients With Vernal Keratoconjunctivitis. Cornea 2019, 38, 344–351. [Google Scholar] [CrossRef]
- Maurer, M.; Khan, D.A.; Komi, D.E.A.; Kaplan, A.P. Biologics for the Use in Chronic Spontaneous Urticaria: When and Which. J. Allergy Clin. Immunol. Pract. 2021, 9, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhou, B.; Zhao, M.; Tang, J.; Lu, Q. Promoter demethylation contributes to TSLP overexpression in skin lesions of patients with atopic dermatitis. Clin. Exp. Dermatol. 2013, 39, 48–53. [Google Scholar] [CrossRef]
- Wallmeyer, L.; Dietert, K.; Sochorová, M.; Gruber, A.D.; Kleuser, B.; Vávrová, K.; Hedtrich, S. TSLP is a direct trigger for T cell migration in filaggrin-deficient skin equivalents. Sci. Rep. 2017, 7, 774. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Mitra, N.; Hoffstad, O.; Margolis, D.J. Association of Filaggrin Loss of Function and Thymic Stromal Lymphopoietin Variation With Treatment Use in Pediatric Atopic Dermatitis. JAMA Dermatol. 2017, 153, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.-J.; Wu, L.S.-H.; Lockett, G.A.; Karmaus, W.J. TSLP polymorphisms, allergen exposures, and the risk of atopic disorders in children. Ann. Allergy, Asthma Immunol. 2016, 116, 139–145.e1. [Google Scholar] [CrossRef]
- Luo, J.; Zhu, Z.; Zhai, Y.; Zeng, J.; Li, L.; Wang, D.; Deng, F.; Chang, B.; Zhou, J.; Sun, L. The Role of TSLP in Atopic Dermatitis: From Pathogenetic Molecule to Therapeutical Target. Mediat. Inflamm. 2023, 2023, 7697699. [Google Scholar] [CrossRef]
- Nygaard, U.; Hvid, M.; Johansen, C.; Buchner, M.; Fölster-Holst, R.; Deleuran, M.; Vestergaard, C. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1930–1938. [Google Scholar] [CrossRef]
- Simpson, E.L.; Parnes, J.R.; She, D.; Crouch, S.; Rees, W.; Mo, M.; van der Merwe, R. Tezepelumab, an anti–thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J. Am. Acad. Dermatol. 2019, 80, 1013–1021. [Google Scholar] [CrossRef]
- Noti, M.; Wojno, E.D.T.; Kim, B.S.; Siracusa, M.C.; Giacomin, P.R.; Nair, M.G.; Benitez, A.J.; Ruymann, K.R.; Muir, A.B.; Hill, D.A.; et al. Thymic stromal lymphopoietin–elicited basophil responses promote eosinophilic esophagitis. Nat. Med. 2013, 19, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Vickery, B.P.; Wood, R.A. The use of biologics in food allergy. Clin. Exp. Allergy 2021, 51, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- de Silva, D.; Singh, C.; Arasi, S.; Muraro, A.; Zuberbier, T.; Ebisawa, M.; Lozano, M.A.; Roberts, G. Systematic review of monotherapy with biologicals for children and adults with IgE-mediated food allergy. Clin. Transl. Allergy 2022, 12, e12123. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, A.B.; Mayer, L.; Berin, M.C. Thymic Stromal Lymphopoietin Is Required for Gastrointestinal Allergy but Not Oral Tolerance. Gastroenterology 2010, 139, 1301–1309.e4. [Google Scholar] [CrossRef]
- Frossard, C.P.; Zimmerli, S.C.; Garriz, J.M.R.; Eigenmann, P.A. Food allergy in mice is modulated through the thymic stromal lymphopoietin pathway. Clin. Transl. Allergy 2015, 6, 2. [Google Scholar] [CrossRef]
- Chu, D.K.; Llop-Guevara, A.; Walker, T.D.; Flader, K.; Goncharova, S.; Boudreau, J.E.; Moore, C.L.; In, T.S.; Waserman, S.; Coyle, A.J.; et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J. Allergy Clin. Immunol. 2013, 131, 187–200.e8. [Google Scholar] [CrossRef]
- Rizzi, A.; Presti, E.L.; Chini, R.; Gammeri, L.; Inchingolo, R.; Lohmeyer, F.M.; Nucera, E.; Gangemi, S. Emerging Role of Alarmins in Food Allergy: An Update on Pathophysiological Insights, Potential Use as Disease Biomarkers, and Therapeutic Implications. J. Clin. Med. 2023, 12, 2699. [Google Scholar] [CrossRef]
- Ukleja-Sokołowska, N.; Żbikowska-Gotz, M.; Lis, K.; Adamczak, R.; Bartuzi, Z. Assessment of TSLP, IL 25 and IL 33 in patients with shrimp allergy. Allergy Asthma Clin. Immunol. 2021, 17, 76. [Google Scholar] [CrossRef]
- Paparo, L.; Picariello, G.; Bruno, C.; Pisapia, L.; Canale, V.; Sarracino, A.; Nocerino, R.; Carucci, L.; Cosenza, L.; Cozzolino, T.; et al. Tolerogenic Effect Elicited by Protein Fraction Derived From Different Formulas for Dietary Treatment of Cow’s Milk Allergy in Human Cells. Front. Immunol. 2020, 11, 604075. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Berin, M.C. Food allergy and the microbiome: Current understandings and future directions. J. Allergy Clin. Immunol. 2019, 144, 1468–1477. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, X.; Chen, F.; Rolnik, B.M.; Chleilat, F.; Ling, Z.; Snyder, M.P.; Zhou, X. The Roles and Mechanisms of Gut Microbiota in Food Allergy. Adv. Gut Microbiome Res. 2023, 2023, 9575410. [Google Scholar] [CrossRef]
- Messerschmidt, J.L.; Azin, M.; Dempsey, K.E.; Demehri, S. TSLP/dendritic cell axis promotes CD4+ T cell tolerance to the gut microbiome. J. Clin. Investig. 2023, 8, e160690. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.-E.; Chun, Y.; Jeong, S.; Jumreornvong, O.; Sicherer, S.H.; Bunyavanich, S. Multidimensional study of the oral microbiome, metabolite, and immunologic environment in peanut allergy. J. Allergy Clin. Immunol. 2021, 148, 627–632.e3. [Google Scholar] [CrossRef]
- Redhu, N.S.; Gounni, A.S. Function and mechanisms of TSLP/TSLPR complex in asthma and COPD. Clin. Exp. Allergy 2012, 42, 994–1005. [Google Scholar] [CrossRef]
- Connor, L.M.; Tang, S.-C.; Cognard, E.; Ochiai, S.; Hilligan, K.L.; Old, S.I.; Pellefigues, C.; White, R.F.; Patel, D.; Smith, A.A.T.; et al. Th2 responses are primed by skin dendritic cells with distinct transcriptional profiles. J. Exp. Med. 2017, 214, 125–142. [Google Scholar] [CrossRef]
- Sacks, D.; Lee, S.H.; Kang, B.; Kamenyeva, O.; Ferreira, T.; Cho, K.; Khillan, J.; Kabat, J.; Kelsall, B. Dermis resident macrophages orchestrate localized ILC2-eosinophil circuitries to maintain their M2-like properties and promote non-healing cutaneous leishmaniasis. Res. Sq. 2023, rs.3.rs-2644705. [Google Scholar] [CrossRef]
- Lu, H.; Wu, X.; Peng, Y.; Sun, R.; Nie, Y.; Li, J.; Wang, M.; Luo, Y.; Peng, L.; Fei, Y.; et al. TSLP promoting B cell proliferation and polarizing follicular helper T cell as a therapeutic target in IgG4-related disease. J. Transl. Med. 2022, 20, 414. [Google Scholar] [CrossRef]
- Langwiński, W.; Szczepankiewicz, D.; Narożna, B.; Stegmayr, J.; Wagner, D.; Alsafadi, H.; Lindstedt, S.; Stachowiak, Z.; Nowakowska, J.; Skrzypski, M.; et al. Allergic inflammation in lungs and nasal epithelium of rat model is regulated by tissue-specific miRNA expression. Mol. Immunol. 2022, 147, 115–125. [Google Scholar] [CrossRef]
- Yoo, J.; Omori, M.; Gyarmati, D.; Zhou, B.; Aye, T.; Brewer, A.; Comeau, M.R.; Campbell, D.J.; Ziegler, S.F. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J. Exp. Med. 2005, 202, 541–549.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Comeau, M.R.; De Smedt, T.; Liggitt, H.D.; Dahl, M.E.; Lewis, D.B.; Gyarmati, D.; Aye, T.; Campbell, D.J.; Ziegler, S.F. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 2005, 6, 1047–1053. [Google Scholar] [CrossRef]
- Al-Shami, A.; Spolski, R.; Kelly, J.; Keane-Myers, A.; Leonard, W.J. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 2005, 202, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Leu, S.-W.; Xu, F.; Zhou, X.; Yin, H.; Cai, L.; Zhang, L. Local blockade of TSLP receptor alleviated allergic disease by regulating airway dendritic cells. Clin. Immunol. 2008, 129, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Christmann, R.B.; Mathes, A.; Affandi, A.J.; Padilla, C.; Nazari, B.; Bujor, A.M.; Stifano, G.; Lafyatis, R. Thymic stromal lymphopoietin is up-regulated in the skin of patients with systemic sclerosis and induces profibrotic genes and intracellular signaling that overlap with those induced by interleukin-13 and transforming growth factor β. Arthritis Rheum. 2013, 65, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Han, N.-R.; Oh, H.-A.; Nam, S.-Y.; Moon, P.-D.; Kim, D.-W.; Kim, H.-M.; Jeong, H.-J. TSLP Induces Mast Cell Development and Aggravates Allergic Reactions through the Activation of MDM2 and STAT6. J. Investig. Dermatol. 2014, 134, 2521–2530. [Google Scholar] [CrossRef]
- Shubin, N.J.; Clauson, M.; Niino, K.; Kasprzak, V.; Tsuha, A.; Guga, E.; Bhise, G.; Acharya, M.; Snyder, J.M.; Debley, J.S.; et al. Thymic stromal lymphopoietin protects in a model of airway damage and inflammation via regulation of caspase-1 activity and apoptosis inhibition. Mucosal Immunol. 2020, 13, 584–594. [Google Scholar] [CrossRef]
- Shikotra, A.; Choy, D.F.; Ohri, C.M.; Doran, E.; Butler, C.; Hargadon, B.; Shelley, M.; Abbas, A.R.; Austin, C.D.; Jackman, J.; et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J. Allergy Clin. Immunol. 2012, 129, 104–111.e9. [Google Scholar] [CrossRef]
- Park, S.; Park, Y.; Son, S.-H.; Lee, K.; Jung, Y.W.; Lee, K.Y.; Jeon, Y.H.; Byun, Y. Synthesis and biological evaluation of peptide-derived TSLP inhibitors. Bioorganic Med. Chem. Lett. 2017, 27, 4710–4713. [Google Scholar] [CrossRef]
- Calzetta, L.; Aiello, M.; Frizzelli, A.; Pistocchini, E.; Ritondo, B.L.; Rogliani, P.; Chetta, A. Investigational Treatments in Phase I and II Clinical Trials: A Systematic Review in Asthma. Biomedicines 2022, 10, 2330. [Google Scholar] [CrossRef]
- Numazaki, M.; Abe, M.; Hanaoka, K.; Imamura, E.; Maeda, M.; Kimura, A.; Miyanohara, J.; Saito, T.; Arai, K.; Suzuki, H.; et al. ASP7266, a Novel Antibody against Human Thymic Stromal Lymphopoietin Receptor for the Treatment of Allergic Diseases. J. Pharmacol. Exp. Ther. 2022, 380, 26–33. [Google Scholar] [CrossRef]
- Venkataramani, S.; Low, S.; Weigle, B.; Dutcher, D.; Jerath, K.; Menzenski, M.; Frego, L.; Truncali, K.; Gupta, P.; Kroe-Barrett, R.; et al. Design and characterization of Zweimab and Doppelmab, high affinity dual antagonistic anti-TSLP/IL13 bispecific antibodies. Biochem. Biophys. Res. Commun. 2018, 504, 19–24. [Google Scholar] [CrossRef]
- Marković, I.; Savvides, S.N. Modulation of Signaling Mediated by TSLP and IL-7 in Inflammation, Autoimmune Diseases, and Cancer. Front. Immunol. 2020, 11, 1557. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, P.M.; Panettieri, R.A.; Taube, C.; Brindicci, C.; Fleming, M.; Altman, P. Development of an inhaled anti-TSLP therapy for asthma. Pulm. Pharmacol. Ther. 2023, 78, 102184. [Google Scholar] [CrossRef]
- Kardas, G.; Panek, M.; Kuna, P.; Damiański, P.; Kupczyk, M. Monoclonal antibodies in the management of asthma: Dead ends, current status and future perspectives. Front. Immunol. 2022, 13, 983852. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Tezepelumab: First Approval. Drugs 2022, 82, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Pelaia, G.; Crimi, C.; Maglio, A.; Stanziola, A.A.; Calabrese, C.; Terracciano, R.; Longhini, F.; Vatrella, A. Novel Biological Therapies for Severe Asthma Endotypes. Biomedicines 2022, 10, 1064. [Google Scholar] [CrossRef]
- Nolasco, S.; Pelaia, C.; Scioscia, G.; Campisi, R.; Crimi, C. Tezepelumab for asthma. Drugs Today 2022, 58, 591–603. [Google Scholar] [CrossRef]
- Matera, M.G.; Ora, J.; Rogliani, P.; Cazzola, M. An overview of the preclinical discovery and development of tezepelumab for the treatment of asthma. Expert Opin. Drug Discov. 2023, 18, 951–963. [Google Scholar] [CrossRef]

| Barrier Surface Disorder | Long TSLP | Short TSLP |
|---|---|---|
| Asthma | ↑↑ upregulated | - unaffected |
| Atopic dermatitis | ↑ upregulated | ↓↓ downregulated |
| Ulcerative colitis | ↑ upregulated | - unaffected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolinska, S.; Antolín-Amérigo, D.; Popescu, F.-D.; Jutel, M. Thymic Stromal Lymphopoietin (TSLP), Its Isoforms and the Interplay with the Epithelium in Allergy and Asthma. Int. J. Mol. Sci. 2023, 24, 12725. https://doi.org/10.3390/ijms241612725
Smolinska S, Antolín-Amérigo D, Popescu F-D, Jutel M. Thymic Stromal Lymphopoietin (TSLP), Its Isoforms and the Interplay with the Epithelium in Allergy and Asthma. International Journal of Molecular Sciences. 2023; 24(16):12725. https://doi.org/10.3390/ijms241612725
Chicago/Turabian StyleSmolinska, Sylwia, Darío Antolín-Amérigo, Florin-Dan Popescu, and Marek Jutel. 2023. "Thymic Stromal Lymphopoietin (TSLP), Its Isoforms and the Interplay with the Epithelium in Allergy and Asthma" International Journal of Molecular Sciences 24, no. 16: 12725. https://doi.org/10.3390/ijms241612725
APA StyleSmolinska, S., Antolín-Amérigo, D., Popescu, F.-D., & Jutel, M. (2023). Thymic Stromal Lymphopoietin (TSLP), Its Isoforms and the Interplay with the Epithelium in Allergy and Asthma. International Journal of Molecular Sciences, 24(16), 12725. https://doi.org/10.3390/ijms241612725









