Impact of Somatic DNA Repair Mutations on the Clinical Outcomes of Bone Metastases from Castration-Resistant Prostate Cancer
Abstract
1. Introduction
2. Results
2.1. Patients: Sample Disposition and Genomic Profile
2.2. Clinical Outcomes
3. Discussion
4. Materials and Methods
4.1. Study Design and Aims
4.2. Patients
4.3. Study Procedures and Somatic Variants Analyses
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abida, W.; Armenia, J.; Gopalan, A.; Brennan, R.; Walsh, M.; Barron, D.; Danila, D.; Rathkopf, D.; Morris, M.; Slovin, S.; et al. Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precis. Oncol. 2017, 1, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Carreira, S.; Sandhu, S.; Miranda, S.; Mossop, H.; Perez-Lopez, R.; Nava Rodrigues, D.; Robinson, D.; Omlin, A.; Tunariu, N.; et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N. Engl. J. Med. 2015, 373, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- MJonsson, P.; Bandlamudi, C.; Cheng, M.L.; Srinivasan, P.; Chavan, S.S.; Friedman, N.D.; Rosen, E.Y.; Richards, A.L.; Bouvier, N.; Selcuklu, S.D.; et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature 2019, 571, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Porta, N.; Bianchini, D.; McGovern, U.; Elliott, T.; Jones, R.; Syndikus, I.; Ralph, C.; Jain, S.; Varughese, M.; et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2019, 21, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Abida, W.; Patnaik, A.; Campbell, D.; Shapiro, J.; Bryce, A.H.; McDermott, R.; Sautois, B.; Vogelzang, N.J.; Bambury, R.M.; Voog, E.; et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring aBRCA1orBRCA2gene alteration. J. Clin. Oncol. 2020, 38, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 383, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Fan, Q.; Ren, K.; Andreassen, P.R. PALB2 functionally connects the breast cancer susceptibility proteins BRCA1 and BRCA2. Mol. Cancer. Res. 2009, 7, 1110–1118. [Google Scholar] [CrossRef]
- Folias, A.; Matkovic, M.; Bruun, D.; Reid, S.; Hejna, J.; Grompe, M.; D’Andrea, A.; Moses, R. BRCA1 interacts directly with the Fanconi anemia protein FANCA. Hum. Mol. Genet. 2002, 11, 2591–2597. [Google Scholar] [CrossRef][Green Version]
- Zannini, L.; Delia, D.; Buscemi, G. CHK2 kinase in the DNA damage response and beyond. J. Mol. Cell Biol. 2014, 6, 442–457. [Google Scholar] [CrossRef]
- Weigelt, B.; Bi, R.; Kumar, R.; Blecua, P.; Mandelker, D.L.; Geyer, F.C.; Pareja, F.; James, P.A.; Couch, F.J.; Eccles, D.M.; et al. The Landscape of Somatic Genetic Alterations in Breast Cancers from ATM Germline Mutation Carriers. Gynecol. Oncol. 2018, 110, 1030–1034. [Google Scholar] [CrossRef]
- Wu, Y.M.; Cieslik, M.; Lonigro, R.J.; Vats, P.; Reimers, M.A.; Cao, X.; Ning, Y.; Wang, L.; Kunju, L.P.; De Sarkar, N.; et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell 2018, 173, 1770–1782. [Google Scholar] [CrossRef]
- Armenia, J.; Wankowicz, S.A.M.; Liu, D.; Gao, J.; Kundra, R.; Reznik, E.; Chatila, W.K.; Chakravarty, D.; Han, G.C.; Coleman, I.; et al. The long tail of oncogenic drivers in prostate cancer. Nat. Genet. 2018, 50, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; David Liu, D.; Gao, J.; Kundra, R.; Reznik, E.; Chatila, W.K.; Chakravarty, D.; Han, G.C.; Coleman, I.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Annala, M.; Vandekerkhove, G.; Khalaf, D.; Taavitsainen, S.; Beja, K.; Warner, E.W.; Sunderland, K.; Kollmannsberger, C.; Eigl, B.J.; Finch, D.; et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2018, 8, 444–457. [Google Scholar] [CrossRef]
- Torga, G.; Pienta, K.J. Patient-Paired Sample Congruence Between 2 Commercial Liquid Biopsy Tests. JAMA Oncol. 2018, 4, 868–870. [Google Scholar] [CrossRef] [PubMed]
- Hatano, K.; Nonomura, N. Genomic Profiling of Prostate Cancer: An Updated Review. World J. Men’s Health 2022, 40, 368–379. [Google Scholar] [CrossRef]
- Castro, E.; Romero-Laorden, N.; del Pozo, A.; Lozano, R.; Medina, A.; Puente, J.; Piulats, J.M.; Lorente, D.; Saez, M.I.; Morales-Barrera, R.; et al. PROREPAIR-B: A Prospective Cohort Study of the Impact of Germline DNA Repair Mutations on the Outcomes of Patients With Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2019, 37, 490–503. [Google Scholar] [CrossRef]
- Weinfurt, K.P.; Li, Y.; Castel, L.D.; Saad, F.; Timbie, J.W.; Glendenning, G.A.; Schulman, K.A. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann. Oncol. 2005, 16, 579–584. [Google Scholar] [CrossRef] [PubMed]
- De Puy, V.; Anstrom, K.J.; Castel, L.D.; Schulman, K.A.; Weinfurt, K.P.; Saad, F. Effects of skeletal morbidities on longitudinal patient-reported outcomes and survival in patients with metastatic prostate cancer. Support Care Cancer 2007, 15, 869–876. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER). Clinical Trials Endpoints for the Approval of Cancer Drugs and Biologics; U.S. Department of Health and Human Services, Food and Drug Administration: Washington, DC, USA, 2007.
- Kyriakopoulos, C.E.; Chen, Y.-H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef] [PubMed]
- ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-Cancer Analysis of Whole Genomes Consortium. Pan-cancer analysis of whole genomes. Nature 2020, 578, 82–93. [Google Scholar] [CrossRef]
- Eeles, R.; Goh, C.; Castro, E.; Bancroft, E.; Guy, M.; Al Olama, A.A.; Easton, D.; Kote-Jarai, Z. The genetic epidemiology of prostate cancer and its clinical implications. Nat. Rev. Urol. 2013, 11, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.; Omlin, A.; Higano, C.; Sweeney, C.; Chanza, N.M.; Mehra, N.; Kuppen, M.C.P.; Beltran, H.; Conteduca, V.; De Almeida, D.V.P.; et al. Activity of Platinum-Based Chemotherapy in Patients With Advanced Prostate Cancer With and Without DNA Repair Gene Aberrations. JAMA Netw. Open 2020, 3, e2021692. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Goh, C.; Olmos, D.; Saunders, E.; Leongamornlert, D.; Tymrakiewicz, M.; Mahmud, N.; Dadaev, T.; Govindasami, K. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J. Clin. Oncol. 2013, 31, 1748–1757. [Google Scholar] [CrossRef]
- De Giorgi, U.; Sansovini, M.; Severi, S.; Nicolini, S.; Monti, M.; Gurioli, G.; Foca, F.; Casadei, C.; Conteduca, V.; Celli, M.; et al. Circulating androgen receptor gene amplification and resistance to177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: Results of a Phase 2 trial. Br. J. Cancer 2021, 125, 1226–1232. [Google Scholar] [CrossRef]
- Castro, E.; Lozano Mejorada, R.; Medina, A.; De Giorgi, U.; Romero Laorden, N.; Conteduca, V.; De Velasco, G.; Alameda, D.; Sanz, A.; Puente, J.; et al. Prospective analysis of the impact of germline mutations in homologous recombination (HR) genes on the response to radium-223 for metastatic castration resistant prostate cancer (mCRPC). Ann. Oncol. 2021, 32 (Suppl. S2), S626–S677. [Google Scholar] [CrossRef]
- Burgio, S.L.; Conteduca, V.; Rudnas, B.; Carrozza, F.; Campadelli, E.; Bianchi, E.; Fabbri, P.; Montanari, M.; Carretta, E.; Menna, C.; et al. PSA Flare With Abiraterone in Patients With Metastatic Castration-Resistant Prostate Cancer. Clin. Genitourin. Cancer 2015, 13, 39–43. [Google Scholar] [CrossRef]
- Burgio, S.L.; Conteduca, V.; Menna, C.; Carretta, E.; Rossi, L.; Bianchi, E.; Kopf, B.; Fabbri, F.; Amadori, D.; De Giorgi, U. Chromogranin A predicts outcome in prostate cancer patients treated with abiraterone. Endocr. Relat. Cancer 2014, 21, 487–493. [Google Scholar] [CrossRef]
- Martignano, F.; Gurioli, G.; Salvi, S.; Calistri, D.; Costantini, M.; Gunelli, R.; De Giorgi, U.; Foca, F.; Casadio, V. GSTP1 Methylation and Protein Expression in Prostate Cancer: Diagnostic Implications. Dis. Markers 2016, 2016, 4358292. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, V.; Jayaram, A.; Romero-Laorden, N.; Wetterskog, D.; Salvi, S.; Gurioli, G.; Scarpi, E.; Castro, E.; Marin-Aguilera, M.; Lolli, C.; et al. Plasma Androgen Receptor and Docetaxel for Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2018, 75, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Badia, X.; Chow, E.; Lipton, A.; Wardley, A. Impact of skeletal complications on patients’ quality of life, mobility, and functional independence. Support Care Cancer 2008, 16, 879–889. [Google Scholar] [CrossRef]
- Coleman, R. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef]
- Guise, T. Examining the metastatic niche: Targeting the microenvironment Semin. Oncology 2010, 37, S2–S14. [Google Scholar] [CrossRef] [PubMed]
- Cursano, M.; Iuliani, M.; Casadei, C.; Stellato, M.; Tonini, G.; Paganelli, G.; Santini, D.; De Giorgi, U. Combination radium-223 therapies in patients with bone metastases from castration-resistant prostate cancer: A review. Crit. Rev. Oncol. 2020, 146, 102864. [Google Scholar] [CrossRef]
- Saad, F.; Lipton, A.; Cook, R.; Chen, Y.M.; Smith, M.; Coleman, R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 2007, 110, 1860–1867. [Google Scholar] [CrossRef]
- Aass, N.; Fosså, S.D. Pre- and post-treatment daily life function in patients with hormone resistant prostate carcinoma treated with radiotherapy for spinal cord compression. Radiother. Oncol. 2005, 74, 259–265. [Google Scholar] [CrossRef]
- Rades, D.; Stalpers, L.J.; Veninga, T.; Rudat, V.; Schulte, R.; Hoskin, P.J. Evaluation of Functional Outcome and Local Control After Radiotherapy for Metastatic Spinal Cord Compression in Patients With Prostate Cancer. J. Urol. 2006, 175, 552–556. [Google Scholar] [CrossRef]
- Miles, B.; Tadi, P. Genetics, Somatic Mutation; Europe PMC: London, UK, 2023. [Google Scholar]
- Galletti, G.; Leach, B.I.; Sibilla, C.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Mateo, J.; Olmos, D.; Mehra, N.; et al. Mechanisms of resistance to systemic therapy in metastatic castration-resistant prostate cancer. Cancer Treat. Rev. 2017, 57, 16–27. [Google Scholar] [CrossRef]
- Hussain, M.; Corcoran, C.; Sibilla, C.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Mateo, J.; Olmos, D.; Mehra, N.; et al. Tumor Genomic Testing for >4000 Men with Metastatic Castration-resistant Prostate Cancer in the Phase III Trial PROfound (Olaparib). Clin. Cancer Res. 2022, 28, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.; Casadio, V.; Conteduca, V.; Lolli, C.; Gurioli, G.; Martignano, F.; Schepisi, G.; Testoni, S.; Scarpi, E.; Amadori, D.; et al. Circulating AR copy number and outcome to enzalutamide in docetaxel-treated metastatic castration-resistant prostate cancer. Oncotarget 2016, 7, 37839–37845. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, U.; Caroli, P.; Scarpi, E.; Conteduca, V.; Burgio, S.L.; Menna, C.; Moretti, A.; Galassi, R.; Rossi, L.; Amadori, D.; et al. 18F-Fluorocholine PET/CT for early response assessment in patients with metastatic castration-resistant prostate cancer treated with enzalutamide. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Caroli, P.; Sandler, I.; Matteucci, F.; De Giorgi, U.; Uccelli, L.; Celli, M.; Foca, F.; Barone, D.; Romeo, A.; Sarnelli, A.; et al. 68Ga-PSMA PET/CT in patients with recurrent prostate cancer after radical treatment: Prospective results in 314 patients. Eur. J. Nucl. Med. 2018, 45, 2035–2044. [Google Scholar] [CrossRef]
- Landis, J.R.; Kock, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]

| Variable | Number (%) Total = 150 |
|---|---|
| Age at diagnosis, years Median value (range): | 65.5 (42–86) |
| Gleason score | |
| <8 | 47 (31.3%) |
| ≥8 | 92 (61.4%) |
| Unknown | 11 (7.3%) |
| Stage at diagnosis | |
| Localized PCa | 73 (48.8%) |
| mHSPC | 64 (51.2%) |
| Type of mHSPC | |
| High risk and/or high volume | 59 (39.3%) |
| No high risk/volume | 66 (44%) |
| No mHSPC | 25 (16.7%) |
| Sites of metastases in mCRPC | |
| Bone only | 53 (35.4%) |
| Bone and visceral | 11 (7.3%) |
| Bone and nodes | 55 (36.6%) |
| Bone, visceral and nodes | 2 (1.4%) |
| Visceral or nodes only | 18 (12%) |
| No mCRPC | 11 (7.3%) |
| Treatment for mHSPC | |
| LhRH analogue | 69 (46%) |
| LhRH analogue + docetaxel | 31 (20.7%) |
| LhRH analogue + ARSI | 25 (16.6%) |
| No mHSPC | 25 (16.7%) |
| Tissue biopsy | |
| No | 46 (30.6%) |
| Yes | 104 (69.3%) |
| Liquid biopsy | |
| No | 45 (30%) |
| Yes | 105 (70%) |
| First line treatment for mCRPC | |
| Chemotherapy | 13 (8.7%) |
| ARSI | 126 (91.3%) |
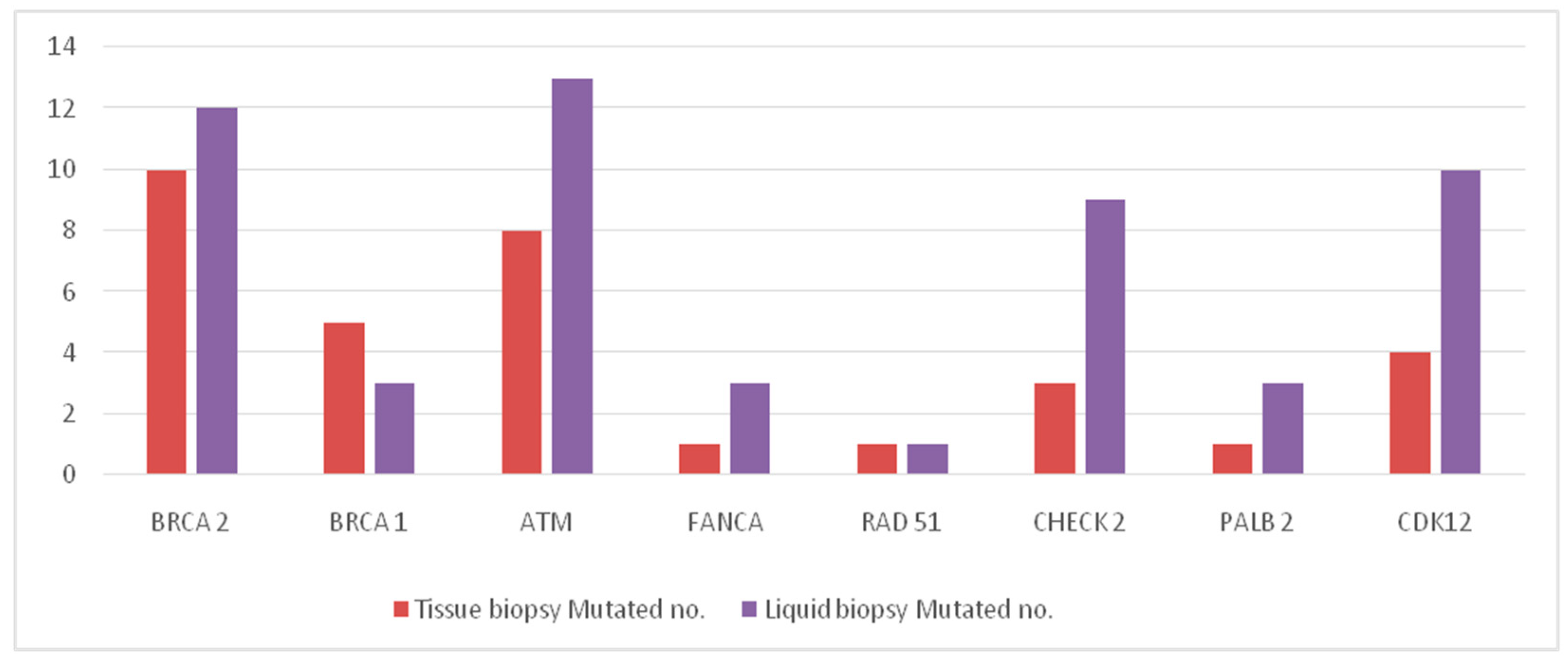
| Gene | Tissue Biopsy (n = 104) | Liquid Biopsy (n = 105) | ||
|---|---|---|---|---|
| Wild Type No. | Mutated No. | Wild Type No. | Mutated No. | |
| BRCA 2 | 94 | 10 | 93 | 12 |
| BRCA 1 | 99 | 5 | 102 | 3 |
| ATM | 96 | 8 | 92 | 13 |
| FANCA | 103 | 1 | 102 | 3 |
| RAD 51 | 103 | 1 | 104 | 1 |
| CHECK 2 | 100 | 3 | 96 | 9 |
| PALB 2 | 103 | 1 | 102 | 3 |
| CDK12 | 100 | 4 | 95 | 10 |
| Total mutations | - | 33 | - | 54 |
| Liquid Biopsy | ||||
|---|---|---|---|---|
| Tissue Biopsy | WT | Mutated | Total | K Value (95% CI) |
| N (%) | N (%) | N (%) | ||
| BRCA2 | ||||
| WT | 52 (98.1) | 1 (1.9) | 53 (100) | |
| Mutated | 0 | 8 (100) | 8 (100) | 0.94 (0.80 to 1.00) |
| Total | 52 | 9 | 61 | |
| DR (95% CI) | 1.64% (0–4.83) | |||
| ATM | ||||
| WT | 51 (96.2) | 2 (3.8) | 53 | |
| Mutated | 1 (12.5) | 7 (87.5) | 8 | 0.80 (0.57 to 1.00) |
| Total | 52 | 9 | 61 | |
| DR (95% CI) | 4.92% (0–10.34) | |||
| FANCA | ||||
| WT | 60 (100) | 0 | 60 (100) | |
| Mutated | 0 | 1 (100) | 1 (100) | 1.00 (1.00 to 1.00) |
| Total | 60 | 1 | 61 | |
| DR (95% CI) | 0% | |||
| RAD 51 | ||||
| WT | 60 (100) | 0 | 60 (100) | |
| Mutated | 1 (100) | 0 | 1 (100) | - |
| Total | 60 | 0 | 61 | |
| DR (95% CI) | 1.64% (0–4.83) | |||
| BRCA1 | ||||
| WT | 56 (100) | 0 | 56 (100) | |
| Mutated | 2 (40.0) | 3 (60.0) | 5 (100) | 0.73 (0.38 to 1.00) |
| Total | 58 | 3 | 61 | |
| DR (95% CI) | 3.28% (0–7.97) | |||
| CHECK 2 | ||||
| WT | 56 (96.7) | 2 (3.3) | 58 (100) | |
| Mutated | 0 | 3 (100) | 3 (100) | 0.73 (0.38 to 1.00) |
| Total | 56 | 5 | 61 | |
| DR (95% CI) | 3.28% (0–7.97) | |||
| PALB2 | ||||
| WT | 60 (100) | 0 | 60 (100) | |
| Mutated | 0 | 1 (100) | 1 (100) | - |
| Total | 60 | 1 | 61 | |
| DR (95% CI) | 0% | |||
| CDK12 | ||||
| WT | 56 (98.3) | 1 (1.7) | 57 (100) | |
| Mutated | 1 (25.0) | 3 (75.0) | 4 (100) | - |
| Total | 57 | 4 | 61 | |
| DR (95% CI) | 3.28% (0–7.97) | |||
| Variable | Total (No. 121) | Group A DDR Mutated (No. 38) | Group B DDR Normal (No. 83) | p Value |
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | ||
| Age at diagnosis (years) | ||||
| Median value (range) | 65 (42–85) | 65 (52–85) | 65 (42–82) | 0.773 |
| Gleason score | ||||
| <8 | 41 (33.9%) | 14 (36.8%) | 27 (32.5%) | 0.642 |
| ≥8 | 80 (66.1%) | 24 (63.2%) | 56 (67.5%) | |
| Stage at diagnosis | ||||
| LocalizedPCa | 59 (48.8%) | 19 (50%) | 40 (48.2%) | 0.853 |
| mHSPC | 62 (51.2%) | 19 (50%) | 43 (51.8%) | |
| mHSPC type | ||||
| High risk and/or volume | 59 (48.8%) | 17 (44.7%) | 42 (50.6%) | 0.549 |
| No high risk and/or volume | 62 (51.2%) | 21 (55.3%) | 41 (49.4%) | |
| Sites of metastases (mCRPC) | ||||
| Bone only | 53 | 16 (42.1%) | 37 (44.6%) | 0.772 |
| Bone and visceral | 11 | 4 (10.5%) | 7 (8.4%) | |
| Bone and nodes | 55 | 18 (47.4%) | 37 (44.6%) | |
| Bone, visceral and nodes | 2 | 0 | 2 (2.4%) | |
| Type of first line treatment mCRPC | ||||
| Chemotherapy | 13 | 4 (10.5%) | 9 (10.8%) | 0.958 |
| ARSI | 108 | 34 (89.5%) | 74 (89.2%) | |
| Denosumab or bisphosphonates | ||||
| Yes | 38 | 10 (26.3%) | 28 (33.7%) | 0.414 |
| No | 83 | 28 (73.7%) | 55 (66.3%) | |
| Variable | Group A (=38) | Group B (=83) | p Value |
|---|---|---|---|
| Age at mCRPC diagnosis (range) | 71 (53–86) | 69 (44–85) | 0.196 |
| Bone sites | |||
| 10 (26.3%) | 33 (39.8%) | |
| 28 (73.7%) | 50 (60.2%) | 0.152 |
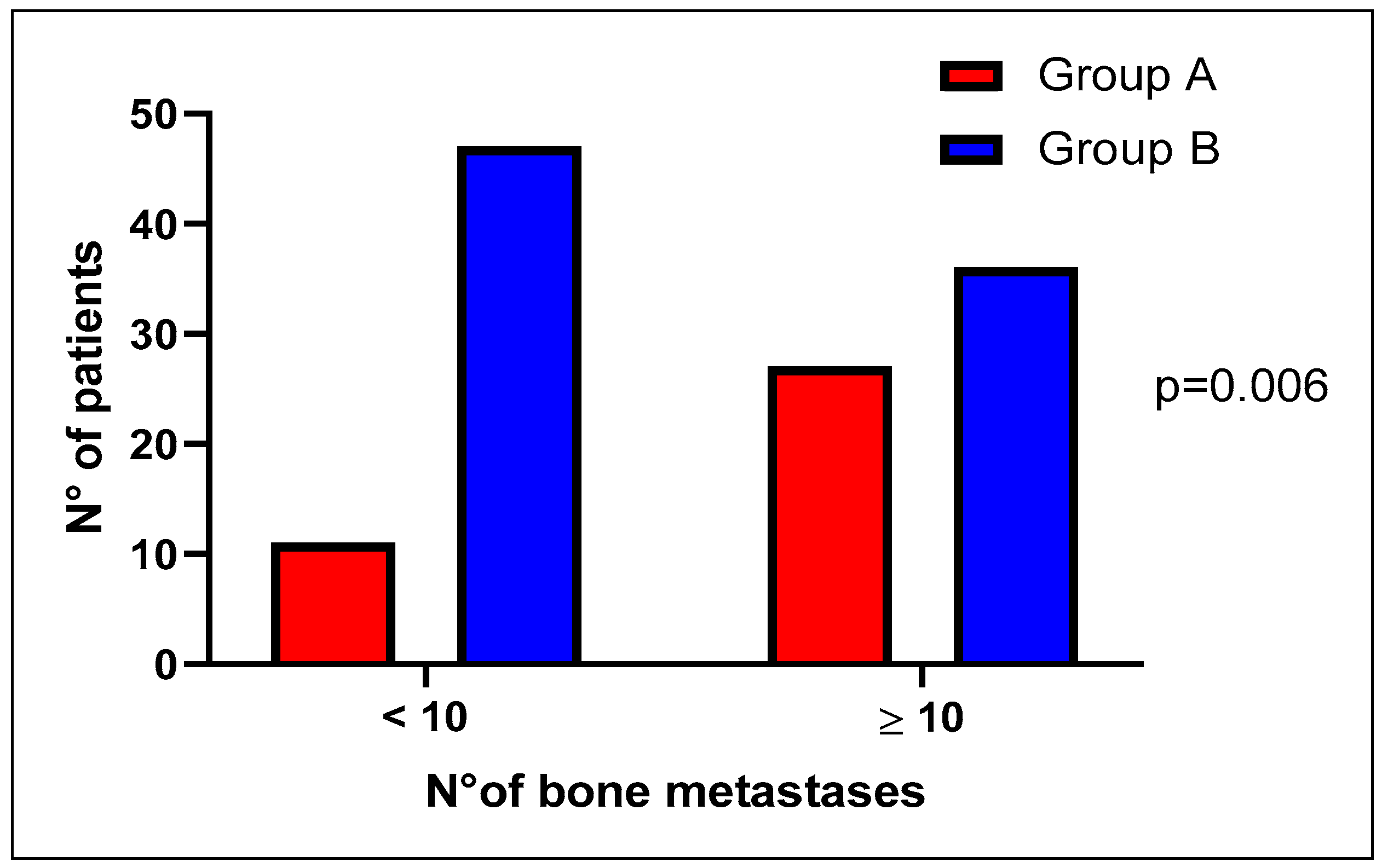
| Number of bone metastases | |||
| 7 (18.4%) | 29 (34.9%) | |
| 31 (81.6%) | 54 (65.1%) | 0.065 |
| Number of bone metastases | |||
| 11 (28.9%) | 47 (56.6%) | |
| 27 (71.1%) | 36 (43.4%) | 0.006 |
| Incidence of SREs | 16/38 (42.1%) | 38/83 (45.7%) | 0.706 |
| Median time to SRE onset (mo.) | 48 (13–not reached) | 21 (11–not reached) | 0.312 |
| Median time from bone metastases onset to death (mo.) | Not reached | 57.6 (44.6–not reached) | 0.763 |
| Bone pain | |||
| 17 (46%) | 45 (57.7%) | 0.238 |
| 20 (54%) | 33 (42.3%) | |
| 1 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cursano, M.C.; Giunta, E.F.; Scarpi, E.; Casadei, C.; Virga, A.; Ulivi, P.; Bleve, S.; Brighi, N.; Ravaglia, G.; Pantano, F.; et al. Impact of Somatic DNA Repair Mutations on the Clinical Outcomes of Bone Metastases from Castration-Resistant Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 12436. https://doi.org/10.3390/ijms241512436
Cursano MC, Giunta EF, Scarpi E, Casadei C, Virga A, Ulivi P, Bleve S, Brighi N, Ravaglia G, Pantano F, et al. Impact of Somatic DNA Repair Mutations on the Clinical Outcomes of Bone Metastases from Castration-Resistant Prostate Cancer. International Journal of Molecular Sciences. 2023; 24(15):12436. https://doi.org/10.3390/ijms241512436
Chicago/Turabian StyleCursano, Maria Concetta, Emilio Francesco Giunta, Emanuela Scarpi, Chiara Casadei, Alessandra Virga, Paola Ulivi, Sara Bleve, Nicole Brighi, Giorgia Ravaglia, Francesco Pantano, and et al. 2023. "Impact of Somatic DNA Repair Mutations on the Clinical Outcomes of Bone Metastases from Castration-Resistant Prostate Cancer" International Journal of Molecular Sciences 24, no. 15: 12436. https://doi.org/10.3390/ijms241512436
APA StyleCursano, M. C., Giunta, E. F., Scarpi, E., Casadei, C., Virga, A., Ulivi, P., Bleve, S., Brighi, N., Ravaglia, G., Pantano, F., Conteduca, V., Santini, D., & De Giorgi, U. (2023). Impact of Somatic DNA Repair Mutations on the Clinical Outcomes of Bone Metastases from Castration-Resistant Prostate Cancer. International Journal of Molecular Sciences, 24(15), 12436. https://doi.org/10.3390/ijms241512436









