Oxidative Stress Response Kinetics after 60 Minutes at Different (1.4 ATA and 2.5 ATA) Hyperbaric Hyperoxia Exposures
Abstract
1. Introduction
2. Results
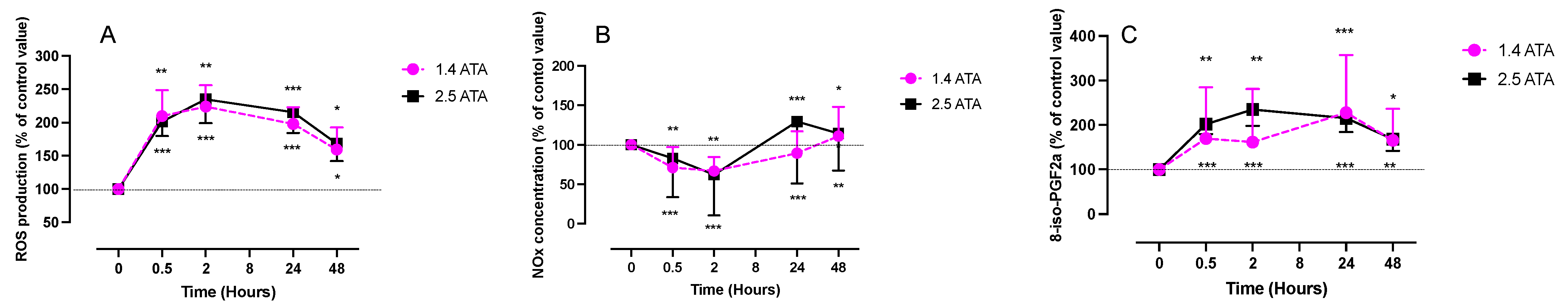
2.1. ROS, NOx, and 8-Isoprostane (8-Iso-PGF2α) Levels after One Hour of Oxygen Exposure at 1.4 and 2.5 ATA
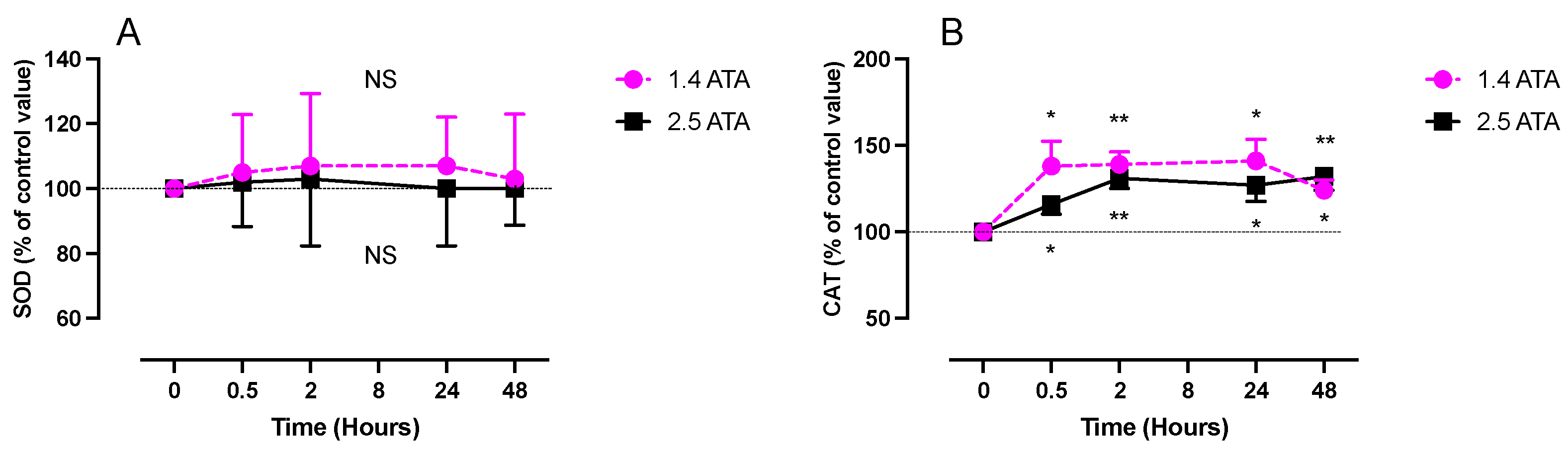
2.2. Inflammatory Response (IL-6, Neopterin and Creatinine) after One Hour of Oxygen Exposure at 1.4 and 2.5 ATA
3. Discussion
Limitations
- -
- This study is, to our knowledge, one of the first to investigate the kinetics of responses to a single short hyperbaric oxygen exposure at 1.4 ATA and 2.5 ATA.
- -
- The measurements were conducted until 48 h post-exposure and putatively open the avenue to new possible applications for hyperbaric oxygen breathing protocols.
- -
- The subject numbers are limited, but the sample can be considered as homogenous since all were healthy participants.
- -
- The analysis was not made in the nucleus of the cells but in the plasma, red blood cells, or urine; this could be considered a weakness for some, but it would need a thoroughly different experimental setting.
4. Materials and Methods
4.1. Experimental Protocol
4.2. Blood Sample Analysis
4.2.1. Determination of ROS by Electron Paramagnetic Resonance (EPR)
4.2.2. Superoxide Dismutase (SOD) and Catalase (CAT)
4.2.3. Total Aminothiols (CYS: Cysteine; CYSGLY: Cysteinylglycine and GSH: Glutathione)
4.3. Urine Sample Analysis
4.3.1. Nitric Oxide Metabolites (NO2 + NO3)
4.3.2. 8-Isoprostane (8-Iso-PGF2α)
4.3.3. Interleukin-6
4.3.4. Creatinine and Neopterin Concentrations
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 8-iso-PGF2a | 8-isoprostane |
| ATA | Atmosphere absolute |
| CAT | Catalase |
| CYSGLY | Cysteinylglycine |
| EPR | Electron Paramagnetic Resonance |
| FiO2 | Inspired Fraction of Oxygen |
| GSH | Glutathione |
| H2O2 | Hydrogen peroxide |
| HBOT | Hyperbaric oxygen therapy |
| IL-6 | Interleukine-6 |
| NO | Nitric oxide |
| NOx | Nitric oxide metabolites |
| NRF2 | Nuclear Factor Erythroid 2 Related—Factor 2 |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide dismutase |
References
- Lee, J.S.; Cha, Y.S.; Lim, J. Association between number of hyperbaric oxygen therapy sessions and neurocognitive outcomes of acute carbon monoxide poisoning. Front. Med. 2023, 10, 1127978. [Google Scholar] [CrossRef]
- Biggs, A.T.; Littlejohn, L.F.; Dainer, H.M. Alternative Uses of Hyperbaric Oxygen Therapy in Military Medicine: Current Positions and Future Directions. Mil. Med. 2022, 187, e40–e46. [Google Scholar] [CrossRef] [PubMed]
- Harch, P.G. Systematic Review and Dosage Analysis: Hyperbaric Oxygen Therapy Efficacy in Mild Traumatic Brain Injury Persistent Postconcussion Syndrome. Front. Neurol. 2022, 13, 815056. [Google Scholar] [CrossRef]
- MacLaughlin, K.J.; Barton, G.P.; Braun, R.K.; MacLaughlin, J.E.; Lamers, J.J.; Marcou, M.D.; Eldridge, M.W. Hyperbaric air mobilizes stem cells in humans; a new perspective on the hormetic dose curve. Front. Neurol. 2023, 14, 1192793. [Google Scholar] [CrossRef] [PubMed]
- Perdrizet, G.A. Principles and practice of hyperbaric medicine: A medical practitioner’s primer, part I. Conn. Med. 2014, 78, 325–332. [Google Scholar] [PubMed]
- de Wolde, S.D.; Hulskes, R.H.; de Jonge, S.W.; Hollmann, M.W.; van Hulst, R.A.; Weenink, R.P.; Kox, M. The Effect of Hyperbaric Oxygen Therapy on Markers of Oxidative Stress and the Immune Response in Healthy Volunteers. Front. Physiol. 2022, 13, 826163. [Google Scholar] [CrossRef]
- Balestra, C.; Arya, A.K.; Leveque, C.; Virgili, F.; Germonpre, P.; Lambrechts, K.; Lafere, P.; Thom, S.R. Varying Oxygen Partial Pressure Elicits Blood-Borne Microparticles Expressing Different Cell-Specific Proteins-Toward a Targeted Use of Oxygen? Int. J. Mol. Sci. 2022, 23, 7888. [Google Scholar] [CrossRef]
- Fratantonio, D.; Virgili, F.; Zucchi, A.; Lambrechts, K.; Latronico, T.; Lafere, P.; Germonpre, P.; Balestra, C. Increasing Oxygen Partial Pressures Induce a Distinct Transcriptional Response in Human PBMC: A Pilot Study on the “Normobaric Oxygen Paradox”. Int. J. Mol. Sci. 2021, 22, 458. [Google Scholar] [CrossRef]
- De Bels, D.; Tillmans, F.; Corazza, F.; Bizzari, M.; Germonpre, P.; Radermacher, P.; Orman, K.G.; Balestra, C. Hyperoxia Alters Ultrastructure and Induces Apoptosis in Leukemia Cell Lines. Biomolecules 2020, 10, 282. [Google Scholar] [CrossRef]
- Cimino, F.; Balestra, C.; Germonpre, P.; De Bels, D.; Tillmans, F.; Saija, A.; Speciale, A.; Virgili, F. Pulsed high oxygen induces a hypoxic-like response in Human Umbilical Endothelial Cells (HUVECs) and in humans. J. Appl. Physiol. 2012, 113, 1684–1689. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Marzorati, M.; Porcelli, S.; Bosco, G.; Balestra, C.; Montorsi, M.; Lafortuna, C.; Vezzoli, A. The “ON-OFF” Switching Response of Reactive Oxygen Species in Acute Normobaric Hypoxia: Preliminary Outcome. Int. J. Mol. Sci. 2023, 24, 4012. [Google Scholar] [CrossRef]
- Leveque, C.; Mrakic Sposta, S.; Theunissen, S.; Germonpré, P.; Lambrechts, K.; Vezzoli, A.; Gussoni, M.; Levenez, M.; Lafère, P.; Guerrero, F.; et al. Oxidative Stress Response Kinetics after 60 Minutes at Different Levels (10% or 15%) of Normobaric Hypoxia Exposure. Int. J. Mol. Sci. 2023, 24, 10188. [Google Scholar] [CrossRef] [PubMed]
- Balestra, C.; Lambrechts, K.; Mrakic-Sposta, S.; Vezzoli, A.; Levenez, M.; Germonpre, P.; Virgili, F.; Bosco, G.; Lafere, P. Hypoxic and Hyperoxic Breathing as a Complement to Low-Intensity Physical Exercise Programs: A Proof-of-Principle Study. Int. J. Mol. Sci. 2021, 22, 9600. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, S.; Balestra, C.; Bolognesi, S.; Borgers, G.; Vissenaeken, D.; Obeid, G.; Germonpre, P.; Honore, P.M.; De Bels, D. Effects of Acute Hypobaric Hypoxia Exposure on Cardiovascular Function in Unacclimatized Healthy Subjects: A “Rapid Ascent” Hypobaric Chamber Study. Int. J. Environ. Res. Public Health 2022, 19, 5394. [Google Scholar] [CrossRef]
- Arya, A.K.; Balestra, C.; Bhopale, V.M.; Tuominen, L.J.; Raisanen-Sokolowski, A.; Dugrenot, E.; L’Her, E.; Bhat, A.R.; Thom, S.R. Elevations of Extracellular Vesicles and Inflammatory Biomarkers in Closed Circuit SCUBA Divers. Int. J. Mol. Sci. 2023, 24, 5969. [Google Scholar] [CrossRef]
- Salvagno, M.; Coppalini, G.; Taccone, F.S.; Strapazzon, G.; Mrakic-Sposta, S.; Rocco, M.; Khalife, M.; Balestra, C. The Normobaric Oxygen Paradox-Hyperoxic Hypoxic Paradox: A Novel Expedient Strategy in Hematopoiesis Clinical Issues. Int. J. Mol. Sci. 2022, 24, 82. [Google Scholar] [CrossRef] [PubMed]
- Leveque, C.; Mrakic-Sposta, S.; Lafere, P.; Vezzoli, A.; Germonpre, P.; Beer, A.; Mievis, S.; Virgili, F.; Lambrechts, K.; Theunissen, S.; et al. Oxidative Stress Response’s Kinetics after 60 Minutes at Different (30% or 100%) Normobaric Hyperoxia Exposures. Int. J. Mol. Sci. 2022, 24, 664. [Google Scholar] [CrossRef]
- Louge, P.; Pignel, R.; Serratrice, J.; Stirnemann, J. Validation of sham treatment in hyperbaric medicine: A randomised trial. Diving Hyperb. Med. 2023, 53, 51–54. [Google Scholar] [CrossRef]
- Balestra, C.; Kot, J. Oxygen: A Stimulus, Not “Only” a Drug. Medicina 2021, 57, 1161. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Akhigbe, R.; Ajayi, A. The impact of reactive oxygen species in the development of cardiometabolic disorders: A review. Lipids Health Dis. 2021, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Maroon, J.C. The effect of hyperbaric oxygen therapy on cognition, performance, proteomics, and telomere length—The difference between zero and one: A case report. Front. Neurol. 2022, 13, 949536. [Google Scholar] [CrossRef]
- Zhilyaev, S.Y.; Moskvin, A.N.; Platonova, T.F.; Gutsaeva, D.R.; Churilina, I.V.; Demchenko, I.T. Hyperoxic vasoconstriction in the brain is mediated by inactivation of nitric oxide by superoxide anions. Neurosci. Behav. Physiol. 2003, 33, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Schildknecht, S.; Ullrich, V. Peroxynitrite as regulator of vascular prostanoid synthesis. Arch. Biochem. Biophys. 2009, 484, 183–189. [Google Scholar] [CrossRef]
- Muskat, J.C.; Babbs, C.F.; Goergen, C.J.; Rayz, V.L. Transport of nitrite from large arteries modulates regional blood flow during stress and exercise. Front. Cardiovasc. Med. 2023, 10, 1146717. [Google Scholar] [CrossRef] [PubMed]
- Schottlender, N.; Gottfried, I.; Ashery, U. Hyperbaric Oxygen Treatment: Effects on Mitochondrial Function and Oxidative Stress. Biomolecules 2021, 11, 1827. [Google Scholar] [CrossRef]
- Eventoff, W.; Tanaka, N.; Rossmann, M.G. Crystalline bovine liver catalase. J. Mol. Biol. 1976, 103, 799–801. [Google Scholar] [CrossRef]
- Fita, I.; Silva, A.; Murthy, M.; Rossmann, M. The refined structure of beef liver catalase at 2·5 Å resolution. Acta Crystallogr. Sect. B Struct. Sci. 1986, 42, 497–515. [Google Scholar] [CrossRef]
- Benedetti, S.; Lamorgese, A.; Piersantelli, M.; Pagliarani, S.; Benvenuti, F.; Canestrari, F. Oxidative stress and antioxidant status in patients undergoing prolonged exposure to hyperbaric oxygen. Clin. Biochem. 2004, 37, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Eken, A.; Aydin, A.; Sayal, A.; Ustündağ, A.; Duydu, Y.; Dündar, K. The effects of hyperbaric oxygen treatment on oxidative stress and SCE frequencies in humans. Clin. Biochem. 2005, 38, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, C.A.; Mendis-Handagama, C.; Kalmar, J.R.; Arnold, R.R.; Kinkade, J.M., Jr. Changes in the localization of catalase during differentiation of neutrophilic granulocytes. Blood 1994, 83, 2654–2668. [Google Scholar] [CrossRef]
- Sureda, A.; Ferrer, M.D.; Tauler, P.; Maestre, I.; Aguiló, A.; Córdova, A.; Tur, J.A.; Roche, E.; Pons, A. Intense physical activity enhances neutrophil antioxidant enzyme gene expression. Immunocytochemistry evidence for catalase secretion. Free. Radic. Res. 2007, 41, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and sequential cell death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef]
- Han, G.; Liu, K.; Li, L.; Li, X.; Zhao, P. The effects of hyperbaric oxygen therapy on neuropathic pain via mitophagy in microglia. Mol. Pain 2017, 13, 1744806917710862. [Google Scholar] [CrossRef]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.K.; Yan, M. Dissecting the Crosstalk between Nrf2 and NF-κB Response Pathways in Drug-Induced Toxicity. Front. Cell Dev. Biol. 2021, 9, 809952. [Google Scholar] [CrossRef]
- Burtscher, J.; Mallet, R.T.; Pialoux, V.; Millet, G.P.; Burtscher, M. Adaptive Responses to Hypoxia and/or Hyperoxia in Humans. Antioxid. Redox Signal. 2022, 37, 887–912. [Google Scholar] [CrossRef]
- Michalak, Ł.; Bulska, M.; Strząbała, K.; Szcześniak, P. Neopterin as a marker of cellular immunological response. Adv. Hyg. Exp. Med. 2017, 71, 727–736. [Google Scholar] [CrossRef]
- Huber, C.; Fuchs, D.; Hausen, A.; Margreiter, R.; Reibnegger, G.; Spielberger, M.; Wachter, H. Pteridines as a new marker to detect human T cells activated by allogeneic or modified self major histocompatibility complex (MHC) determinants. J. Immunol. 1983, 130, 1047–1050. [Google Scholar] [CrossRef]
- Huber, C.; Batchelor, J.R.; Fuchs, D.; Hausen, A.; Lang, A.; Niederwieser, D.; Reibnegger, G.; Swetly, P.; Troppmair, J.; Wachter, H. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J. Exp. Med. 1984, 160, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Ghisoni, K.; Martins Rde, P.; Barbeito, L.; Latini, A. Neopterin as a potential cytoprotective brain molecule. J. Psychiatr. Res. 2015, 71, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, I.; Schottlender, N.; Ashery, U. Hyperbaric Oxygen Treatment-From Mechanisms to Cognitive Improvement. Biomolecules 2021, 11, 1520. [Google Scholar] [CrossRef] [PubMed]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Bosco, G.; Paganini, M.; Giacon, T.A.; Oppio, A.; Vezzoli, A.; Dellanoce, C.; Moro, T.; Paoli, A.; Zanotti, F.; Zavan, B.; et al. Oxidative Stress and Inflammation, MicroRNA, and Hemoglobin Variations after Administration of Oxygen at Different Pressures and Concentrations: A Randomized Trial. Int. J. Environ. Res. Public Health 2021, 18, 9755. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; D’Alessandro, F.; Paganini, M.; Dellanoce, C.; Cialoni, D.; Bosco, G. Change in Oxidative Stress Biomarkers During 30 Days in Saturation Dive: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 7118. [Google Scholar] [CrossRef]
- Moretti, S.; Mrakic-Sposta, S.; Roncoroni, L.; Vezzoli, A.; Dellanoce, C.; Monguzzi, E.; Branchi, F.; Ferretti, F.; Lombardo, V.; Doneda, L.; et al. Oxidative stress as a biomarker for monitoring treated celiac disease. Clin. Transl. Gastroenterol. 2018, 9, 157. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Vezzoli, A.; Rizzato, A.; Della Noce, C.; Malacrida, S.; Montorsi, M.; Paganini, M.; Cancellara, P.; Bosco, G. Oxidative stress assessment in breath-hold diving. Eur. J. Appl. Physiol. 2019, 119, 2449–2456. [Google Scholar] [CrossRef]
- Bosco, G.; Rizzato, A.; Quartesan, S.; Camporesi, E.; Mrakic-Sposta, S.; Moretti, S.; Balestra, C.; Rubini, A. Spirometry and oxidative stress after rebreather diving in warm water. Undersea Hyperb. Med. 2018, 45, 191–198. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. A quantitative method to monitor reactive oxygen species production by electron paramagnetic resonance in physiological and pathological conditions. Oxidative Med. Cell. Longev. 2014, 2014, 306179. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Vezzoli, A. Comment on Menzel et al. Common and Novel Markers for Measuring Inflammation and Oxidative Stress Ex Vivo in Research and Clinical Practice—Which to Use Regarding Disease Outcomes? Antioxidants 2021, 10, 414. Antioxidants 2021, 10, 836. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. Assessment of a standardized ROS production profile in humans by electron paramagnetic resonance. Oxidative Med. Cell. Longev. 2012, 2012, 973927. [Google Scholar] [CrossRef]
- Dellanoce, C.; Cozzi, L.; Zuddas, S.; Pratali, L.; Accinni, R. Determination of different forms of aminothiols in red blood cells without washing erythrocytes. Biomed. Chromatogr. 2014, 28, 327–331. [Google Scholar] [CrossRef]
- Vezzoli, A.; Dellanoce, C.; Mrakic-Sposta, S.; Montorsi, M.; Moretti, S.; Tonini, A.; Pratali, L.; Accinni, R. Oxidative Stress Assessment in Response to Ultraendurance Exercise: Thiols Redox Status and ROS Production according to Duration of a Competitive Race. Oxidative Med. Cell. Longev. 2016, 2016, 6439037. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Bilianou, E.; Balbarini, A.; Gesualdo, M.; Ghiadoni, L.; Metra, M.; Palmiero, P.; Pedrinelli, R.; Salvetti, M.; Scicchitano, P.; et al. Task force on: ‘Early markers of atherosclerosis: Influence of age and sex’. J. Cardiovasc. Med. 2013, 14, 757–766. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leveque, C.; Mrakic Sposta, S.; Theunissen, S.; Germonpré, P.; Lambrechts, K.; Vezzoli, A.; Bosco, G.; Lévénez, M.; Lafère, P.; Guerrero, F.; et al. Oxidative Stress Response Kinetics after 60 Minutes at Different (1.4 ATA and 2.5 ATA) Hyperbaric Hyperoxia Exposures. Int. J. Mol. Sci. 2023, 24, 12361. https://doi.org/10.3390/ijms241512361
Leveque C, Mrakic Sposta S, Theunissen S, Germonpré P, Lambrechts K, Vezzoli A, Bosco G, Lévénez M, Lafère P, Guerrero F, et al. Oxidative Stress Response Kinetics after 60 Minutes at Different (1.4 ATA and 2.5 ATA) Hyperbaric Hyperoxia Exposures. International Journal of Molecular Sciences. 2023; 24(15):12361. https://doi.org/10.3390/ijms241512361
Chicago/Turabian StyleLeveque, Clément, Simona Mrakic Sposta, Sigrid Theunissen, Peter Germonpré, Kate Lambrechts, Alessandra Vezzoli, Gerardo Bosco, Morgan Lévénez, Pierre Lafère, François Guerrero, and et al. 2023. "Oxidative Stress Response Kinetics after 60 Minutes at Different (1.4 ATA and 2.5 ATA) Hyperbaric Hyperoxia Exposures" International Journal of Molecular Sciences 24, no. 15: 12361. https://doi.org/10.3390/ijms241512361
APA StyleLeveque, C., Mrakic Sposta, S., Theunissen, S., Germonpré, P., Lambrechts, K., Vezzoli, A., Bosco, G., Lévénez, M., Lafère, P., Guerrero, F., & Balestra, C. (2023). Oxidative Stress Response Kinetics after 60 Minutes at Different (1.4 ATA and 2.5 ATA) Hyperbaric Hyperoxia Exposures. International Journal of Molecular Sciences, 24(15), 12361. https://doi.org/10.3390/ijms241512361








