Oxygen Variations—Insights into Hypoxia, Hyperoxia and Hyperbaric Hyperoxia—Is the Dose the Clue?
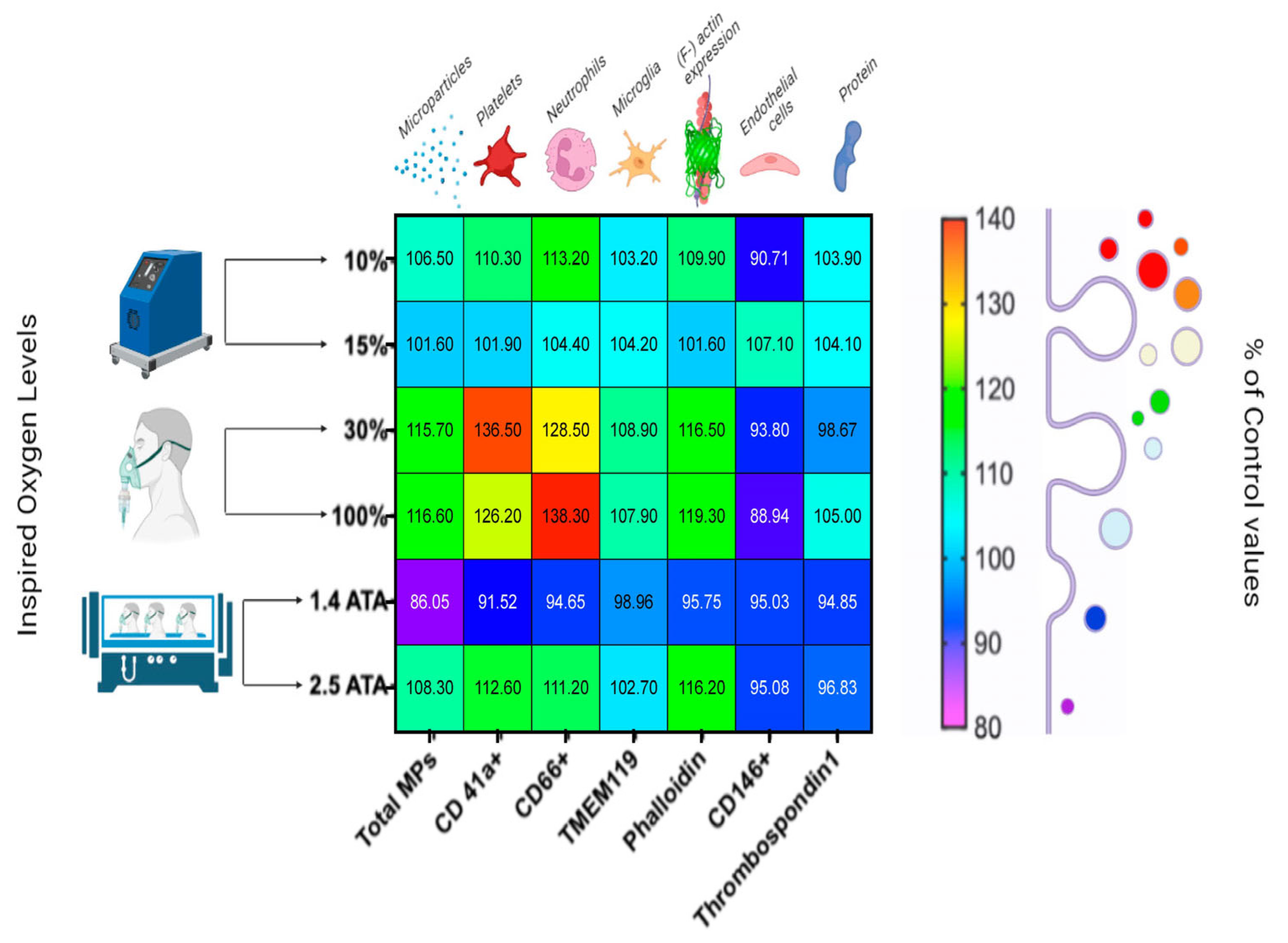
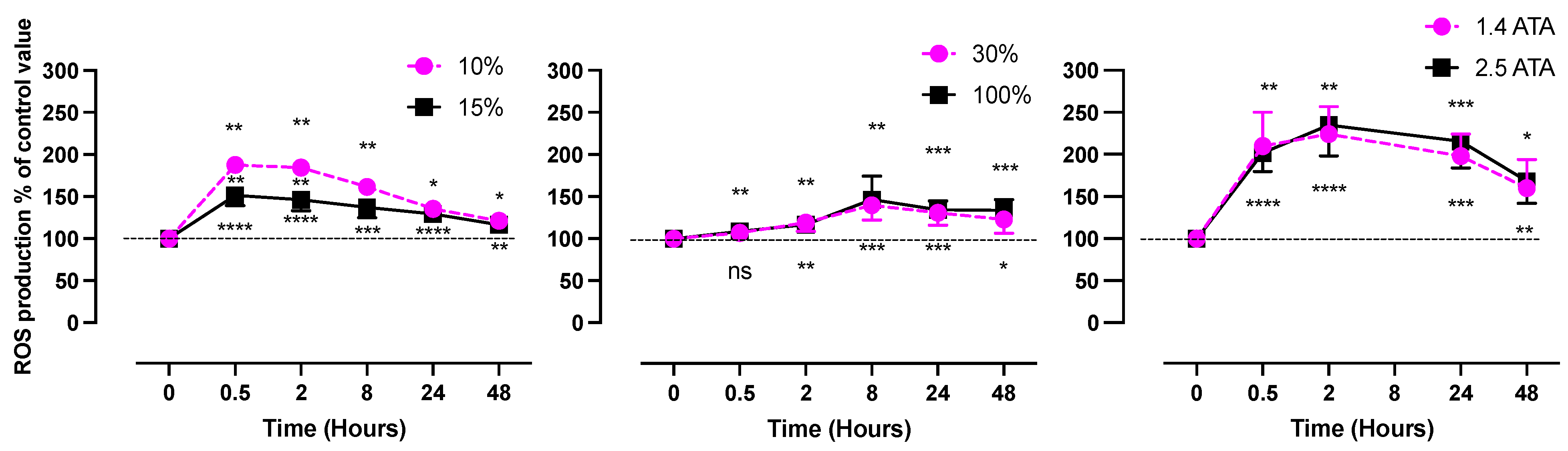
- Hypoxic Oxygen levels
- 2.
- Normobaric Hyperoxic Oxygen levels
- 3.
- Hyperbaric Hyperoxic Oxygen levels
Conflicts of Interest
References
- Brugniaux, J.V.; Coombs, G.B.; Barak, O.F.; Dujic, Z.; Sekhon, M.S.; Ainslie, P.N. Highs and lows of hyperoxia: Physiological, performance, and clinical aspects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1–R27. [Google Scholar] [CrossRef] [PubMed]
- Samaja, M.; Chiumello, D. Oxygen administration during general anaesthesia for surgery. BMJ 2022, 379, o2823. [Google Scholar] [CrossRef]
- Hadanny, A.; Efrati, S. The Hyperoxic-Hypoxic Paradox. Biomolecules 2020, 10, 958. [Google Scholar] [CrossRef]
- Salvagno, M.; Coppalini, G.; Taccone, F.S.; Strapazzon, G.; Mrakic-Sposta, S.; Rocco, M.; Khalife, M.; Balestra, C. The Normobaric Oxygen Paradox-Hyperoxic Hypoxic Paradox: A Novel Expedient Strategy in Hematopoiesis Clinical Issues. Int. J. Mol. Sci. 2022, 24, 82. [Google Scholar] [CrossRef] [PubMed]
- Cimino, F.; Balestra, C.; Germonpre, P.; De Bels, D.; Tillmans, F.; Saija, A.; Speciale, A.; Virgili, F. Pulsed high oxygen induces a hypoxic-like response in Human Umbilical Endothelial Cells (HUVECs) and in humans. J. Appl. Physiol. 2012, 113, 1684–1689. [Google Scholar] [CrossRef]
- Arieli, R.; Yalov, A.; Goldenshluger, A. Modeling pulmonary and CNS O2 toxicity and estimation of parameters for humans. J. Appl. Physiol. 2002, 92, 248–256. [Google Scholar] [CrossRef]
- Samaja, M.; Ottolenghi, S. The Oxygen Cascade from Atmosphere to Mitochondria as a Tool to Understand the (Mal)adaptation to Hypoxia. Int. J. Mol. Sci. 2023, 24, 3670. [Google Scholar] [CrossRef] [PubMed]
- Mancardi, D.; Ottolenghi, S.; Attanasio, U.; Tocchetti, C.G.; Paroni, R.; Pagliaro, P.; Samaja, M. Janus, or the Inevitable Battle Between Too Much and Too Little Oxygen. Antioxid. Redox Signal. 2022, 37, 972–989. [Google Scholar] [CrossRef] [PubMed]
- Balestra, C.; Arya, A.K.; Leveque, C.; Virgili, F.; Germonpre, P.; Lambrechts, K.; Lafere, P.; Thom, S.R. Varying Oxygen Partial Pressure Elicits Blood-Borne Microparticles Expressing Different Cell-Specific Proteins-Toward a Targeted Use of Oxygen? Int. J. Mol. Sci. 2022, 23, 7888. [Google Scholar] [CrossRef]
- Thom, S.R.; Bhopale, V.M.; Arya, A.K.; Ruhela, D.; Bhat, A.R.; Mitra, N.; Hoffstad, O.; Malay, D.S.; Mirza, Z.K.; Lantis, J.C.; et al. Blood-Borne Microparticles Are an Inflammatory Stimulus in Type 2 Diabetes Mellitus. Immunohorizons 2023, 7, 71–80. [Google Scholar] [CrossRef]
- Bhopale, V.M.; Ruhela, D.; Brett, K.D.; Nugent, N.Z.; Fraser, N.K.; Levinson, S.L.; DiNubile, M.J.; Thom, S.R. Plasma gelsolin modulates the production and fate of IL-1β-containing microparticles following high-pressure exposure and decompression. J. Appl. Physiol. 2021, 130, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Bhopale, V.M.; Yu, K.; Huang, W.; Kane, M.A.; Margolis, D.J. Neutrophil microparticle production and inflammasome activation by hyperglycemia due to cytoskeletal instability. J. Biol. Chem. 2017, 292, 18312–18324. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Hampton, M.; Troiano, M.A.; Mirza, Z.; Malay, D.S.; Shannon, S.; Jennato, N.B.; Donohue, C.M.; Hoffstad, O.; Woltereck, D.; et al. Measurements of CD34+/CD45-dim Stem Cells Predict Healing of Diabetic Neuropathic Wounds. Diabetes 2016, 65, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Fratantonio, D.; Virgili, F.; Zucchi, A.; Lambrechts, K.; Latronico, T.; Lafere, P.; Germonpre, P.; Balestra, C. Increasing Oxygen Partial Pressures Induce a Distinct Transcriptional Response in Human PBMC: A Pilot Study on the “Normobaric Oxygen Paradox”. Int. J. Mol. Sci. 2021, 22, 458. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Cardenosa, A.; Burtscher, J.; Burtscher, M.; Camacho-Cardenosa, M. Editorial: Hypoxia as a therapeutic tool in search of healthy aging. Front. Physiol. 2023, 13, 1112129. [Google Scholar] [CrossRef]
- Leveque, C.; Mrakic Sposta, S.; Theunissen, S.; Germonpré, P.; Lambrechts, K.; Vezzoli, A.; Gussoni, M.; Levenez, M.; Lafère, P.; Guerrero, F.; et al. Oxidative Stress Response Kinetics after 60 Minutes at Different Levels (10% or 15%) of Normobaric Hypoxia Exposure. Int. J. Mol. Sci. 2023, 24, 10188. [Google Scholar] [CrossRef]
- Peacock, A.J. ABC of oxygen: Oxygen at high altitude. BMJ 1998, 317, 1063–1066. [Google Scholar] [CrossRef]
- Bavunoglu, I.; Genc, H.; Konukoglu, D.; Cicekci, H.; Sozer, V.; Gelisgen, R.; Uzun, H. Oxidative stress parameters and inflammatory and immune mediators as markers of the severity of sepsis. J. Infect. Dev. Ctries. 2016, 10, 1045–1052. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Marzorati, M.; Porcelli, S.; Bosco, G.; Balestra, C.; Montorsi, M.; Lafortuna, C.; Vezzoli, A. The “ON-OFF” Switching Response of Reactive Oxygen Species in Acute Normobaric Hypoxia: Preliminary Outcome. Int. J. Mol. Sci. 2023, 24, 4012. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Mathew, T.; Sarada, S.K.S. Intonation of Nrf2 and Hif1-α pathway by curcumin prophylaxis: A potential strategy to augment survival signaling under hypoxia. Respir. Physiol. Neurobiol. 2018, 258, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Joanny, P.; Steinberg, J.; Robach, P.; Richalet, J.P.; Gortan, C.; Gardette, B.; Jammes, Y. Operation Everest III (Comex’97): The effect of simulated sever hypobaric hypoxia on lipid peroxidation and antioxidant defence systems in human blood at rest and after maximal exercise. Resuscitation 2001, 49, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.C.; Askew, E.W.; Roberts, D.E.; Prior, R.L.; Ensign, W.Y., Jr.; Hesslink, R.E., Jr. Oxidative stress in humans training in a cold, moderate altitude environment and their response to a phytochemical antioxidant supplement. Wilderness Environ. Med. 2002, 13, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Araneda, O.F.; García, C.; Lagos, N.; Quiroga, G.; Cajigal, J.; Salazar, M.P.; Behn, C. Lung oxidative stress as related to exercise and altitude. Lipid peroxidation evidence in exhaled breath condensate: A possible predictor of acute mountain sickness. Eur. J. Appl. Physiol. 2005, 95, 383–390. [Google Scholar] [CrossRef]
- Møller, P.; Loft, S.; Lundby, C.; Olsen, N.V. Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. FASEB J. 2001, 15, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Liu, J.; Li, G.; Gu, Y.; Guo, M.; Guan, Y.; Tian, Z.; Ma, W.; Wang, C.; Ji, X. Proteomic Analysis Reveals That Mitochondria Dominate the Hippocampal Hypoxic Response in Mice. Int. J. Mol. Sci. 2022, 23, 14094. [Google Scholar] [CrossRef]
- Li, A.L.; Lian, L.; Chen, X.N.; Cai, W.H.; Fan, X.B.; Fan, Y.J.; Li, T.T.; Xie, Y.Y.; Zhang, J.P. The role of mitochondria in myocardial damage caused by energy metabolism disorders: From mechanisms to therapeutics. Free Radic. Biol. Med. 2023, 208, 236–251. [Google Scholar] [CrossRef]
- Baude, J.; Cooper, J.S. Hyperbaric Contraindicated Chemotherapeutic Agents. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yehiam, S.Z.; Simkin, S.K.; Al-Taie, R.; Wong, M.; Battin, M.; Dai, S. Incomplete peripheral retinal vascularisation in retinopathy of prematurity: Is it the consequence of changing oxygen saturation? Front. Pediatr. 2023, 11, 1203068. [Google Scholar] [CrossRef]
- Shetty, S.; Tolentino, D.; Kulkarni, A.; Duffy, D.; Greenough, A. Comparison of Outcomes of Less Invasive Surfactant Administration in Prematurely Born Infants in the Delivery Suite and the Neonatal Unit. Am. J. Perinatol. 2023. [Google Scholar] [CrossRef]
- Kim, E.S.; Calkins, K.L.; Chu, A. Retinopathy of Prematurity: The Role of Nutrition. Pediatr. Ann. 2023, 52, e303–e308. [Google Scholar] [CrossRef]
- Dormishian, A.; Schott, A.; Aguilar, A.C.; Jimenez, V.; Bancalari, E.; Tolosa, J.; Claure, N. Etiology and Mechanism of Intermittent Hypoxemia Episodes in Spontaneously Breathing Extremely Premature Infants. J. Pediatr. 2023, 262, 113623. [Google Scholar] [CrossRef] [PubMed]
- Georgeson, G.D.; Szony, B.J.; Streitman, K.; Varga, I.S.; Kovács, A.; Kovács, L.; László, A. Antioxidant enzyme activities are decreased in preterm infants and in neonates born via caesarean section. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 103, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.T.; Chen, B.H.; Chen, H.H.; Lee, J.C.; Kuo, T.J.; Chiu, H.C.; Lu, W.H. Hypoxia-Induced Kidney Injury in Newborn Rats. Toxics 2023, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Mussap, M.; Longini, M.; Fanos, V.; Bellieni, C.V.; Proietti, F.; Cataldi, L.; Buonocore, G. Oxidative kidney damage in preterm newborns during perinatal period. Clin. Biochem. 2007, 40, 656–660. [Google Scholar] [CrossRef]
- Torbati, D.; Tan, G.H.; Smith, S.; Frazier, K.S.; Gelvez, J.; Fakioglu, H.; Totapally, B.R. Multiple-organ effect of normobaric hyperoxia in neonatal rats. J. Crit. Care 2006, 21, 85–93; discussion 93–84. [Google Scholar] [CrossRef]
- Huang, L.-T.; Chen, C.-M. Kidney Injuries and Evolution of Chronic Kidney Diseases Due to Neonatal Hyperoxia Exposure Based on Animal Studies. Int. J. Mol. Sci. 2022, 23, 8492. [Google Scholar] [CrossRef]
- Leveque, C.; Mrakic-Sposta, S.; Lafere, P.; Vezzoli, A.; Germonpre, P.; Beer, A.; Mievis, S.; Virgili, F.; Lambrechts, K.; Theunissen, S.; et al. Oxidative Stress Response’s Kinetics after 60 Minutes at Different (30% or 100%) Normobaric Hyperoxia Exposures. Int. J. Mol. Sci. 2022, 24, 664. [Google Scholar] [CrossRef]
- Vinkel, J.; Arenkiel, B.; Hyldegaard, O. The Mechanisms of Action of Hyperbaric Oxygen in Restoring Host Homeostasis during Sepsis. Biomolecules 2023, 13, 1228. [Google Scholar] [CrossRef]
- Gottfried, I.; Schottlender, N.; Ashery, U. Hyperbaric Oxygen Treatment-From Mechanisms to Cognitive Improvement. Biomolecules 2021, 11, 1520. [Google Scholar] [CrossRef]
- Robba, C.; Battaglini, D.; Cinotti, R.; Asehnoune, K.; Stevens, R.; Taccone, F.S.; Badenes, R.; Pelosi, P. Individualized Thresholds of Hypoxemia and Hyperoxemia and their Effect on Outcome in Acute Brain Injured Patients: A Secondary Analysis of the ENIO Study. In Neurocritical Care; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar] [CrossRef]
- Yılmaz, A.; Kaya, N.; Meriç, R.; Bayramli, Z.; Öroğlu, B.; Celkan, T.T.; Vural, M.; Perk, Y. Use of hyperbaric oxygen therapy of purpura fulminans in an extremely low birth weight preterm: A case report. J. Neonatal. Perinatal. Med. 2023, 16, 339–342. [Google Scholar] [CrossRef]
- Jeremic, R.; Pekovic, S.; Lavrnja, I.; Bjelobaba, I.; Djelic, M.; Dacic, S.; Brkic, P. Hyperbaric Oxygenation Prevents Loss of Immature Neurons in the Adult Hippocampal Dentate Gyrus Following Brain Injury. Int. J. Mol. Sci. 2023, 24, 4261. [Google Scholar] [PubMed]
- Lavrnja, I.; Parabucki, A.; Brkic, P.; Jovanovic, T.; Dacic, S.; Savic, D.; Pantic, I.; Stojiljkovic, M.; Pekovic, S. Repetitive hyperbaric oxygenation attenuates reactive astrogliosis and suppresses expression of inflammatory mediators in the rat model of brain injury. Mediat. Inflamm. 2015, 2015, 498405. [Google Scholar] [CrossRef]
- Parabucki, A.B.; Bozić, I.D.; Bjelobaba, I.M.; Lavrnja, I.C.; Brkić, P.D.; Jovanović, T.S.; Savić, D.Z.; Stojiljković, M.B.; Peković, S.M. Hyperbaric oxygenation alters temporal expression pattern of superoxide dismutase 2 after cortical stab injury in rats. Croat. Med. J. 2012, 53, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Brkic, P.; Stojiljkovic, M.; Jovanovic, T.; Dacic, S.; Lavrnja, I.; Savic, D.; Parabucki, A.; Bjelobaba, I.; Rakic, L.; Pekovic, S. Hyperbaric oxygenation improves locomotor ability by enhancing neuroplastic responses after cortical ablation in rats. Brain Inj. 2012, 26, 1273–1284. [Google Scholar] [CrossRef]
- Arya, A.K.; Balestra, C.; Bhopale, V.M.; Tuominen, L.J.; Raisanen-Sokolowski, A.; Dugrenot, E.; L’Her, E.; Bhat, A.R.; Thom, S.R. Elevations of Extracellular Vesicles and Inflammatory Biomarkers in Closed Circuit SCUBA Divers. Int. J. Mol. Sci. 2023, 24, 5969. [Google Scholar] [CrossRef] [PubMed]
- De Wolde, S.D.; Hulskes, R.H.; de Jonge, S.W.; Hollmann, M.W.; van Hulst, R.A.; Weenink, R.P.; Kox, M. The Effect of Hyperbaric Oxygen Therapy on Markers of Oxidative Stress and the Immune Response in Healthy Volunteers. Front. Physiol. 2022, 13, 826163. [Google Scholar] [CrossRef]
- Leveque, C.; Mrakic Sposta, S.; Theunissen, S.; Germonpré, P.; Lambrechts, K.; Vezzoli, A.; Bosco, G.; Lévénez, M.; Lafère, P.; Guerrero, F.; et al. Oxidative Stress Response Kinetics after 60 Minutes at Different (1.4 ATA and 2.5 ATA) Hyperbaric Hyperoxia Exposures. Int. J. Mol. Sci. 2023, 24, 12361. [Google Scholar] [CrossRef]
- Louge, P.; Pignel, R.; Serratrice, J.; Stirnemann, J. Validation of sham treatment in hyperbaric medicine: A randomised trial. Diving Hyperb. Med. 2023, 53, 51–54. [Google Scholar] [CrossRef]
- Mozayeni, B.R.; Duncan, W.; Zant, E.; Love, T.L.; Beckman, R.L.; Stoller, K.P. The National Brain Injury Rescue and Rehabilitation Study—A multicenter observational study of hyperbaric oxygen for mild traumatic brain injury with post-concussive symptoms. Med. Gas Res. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- MacLaughlin, K.J.; Barton, G.P.; Braun, R.K.; MacLaughlin, J.E.; Lamers, J.J.; Marcou, M.D.; Eldridge, M.W. Hyperbaric air mobilizes stem cells in humans; a new perspective on the hormetic dose curve. Front. Neurol. 2023, 14, 1192793. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balestra, C.; Mrakic-Sposta, S.; Virgili, F. Oxygen Variations—Insights into Hypoxia, Hyperoxia and Hyperbaric Hyperoxia—Is the Dose the Clue? Int. J. Mol. Sci. 2023, 24, 13472. https://doi.org/10.3390/ijms241713472
Balestra C, Mrakic-Sposta S, Virgili F. Oxygen Variations—Insights into Hypoxia, Hyperoxia and Hyperbaric Hyperoxia—Is the Dose the Clue? International Journal of Molecular Sciences. 2023; 24(17):13472. https://doi.org/10.3390/ijms241713472
Chicago/Turabian StyleBalestra, Costantino, Simona Mrakic-Sposta, and Fabio Virgili. 2023. "Oxygen Variations—Insights into Hypoxia, Hyperoxia and Hyperbaric Hyperoxia—Is the Dose the Clue?" International Journal of Molecular Sciences 24, no. 17: 13472. https://doi.org/10.3390/ijms241713472
APA StyleBalestra, C., Mrakic-Sposta, S., & Virgili, F. (2023). Oxygen Variations—Insights into Hypoxia, Hyperoxia and Hyperbaric Hyperoxia—Is the Dose the Clue? International Journal of Molecular Sciences, 24(17), 13472. https://doi.org/10.3390/ijms241713472






