Using Normalized Carcinoembryonic Antigen and Carbohydrate Antigen 19 to Predict and Monitor the Efficacy of Neoadjuvant Chemotherapy in Locally Advanced Gastric Cancer
Abstract
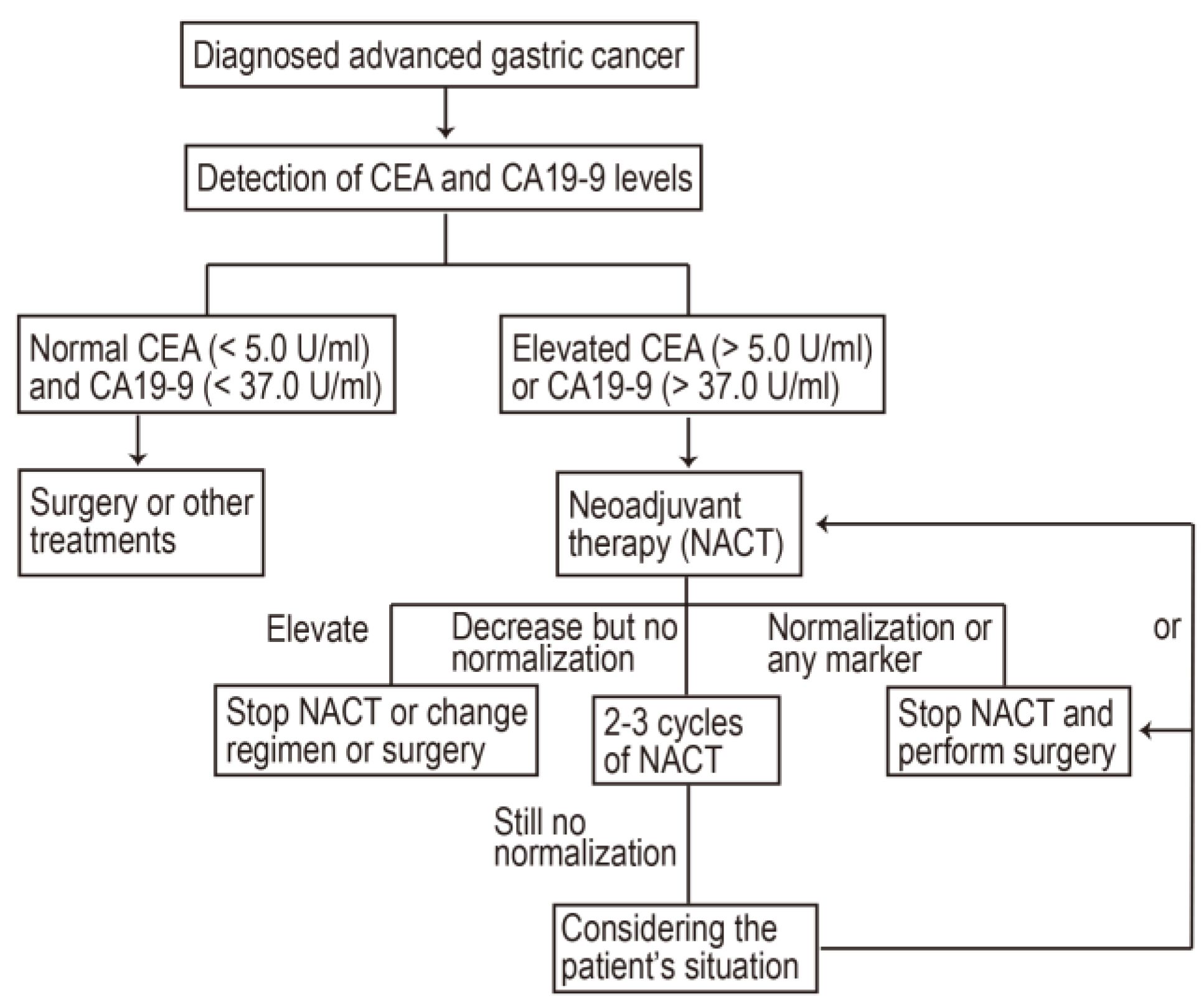
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Neoadjuvant Therapy
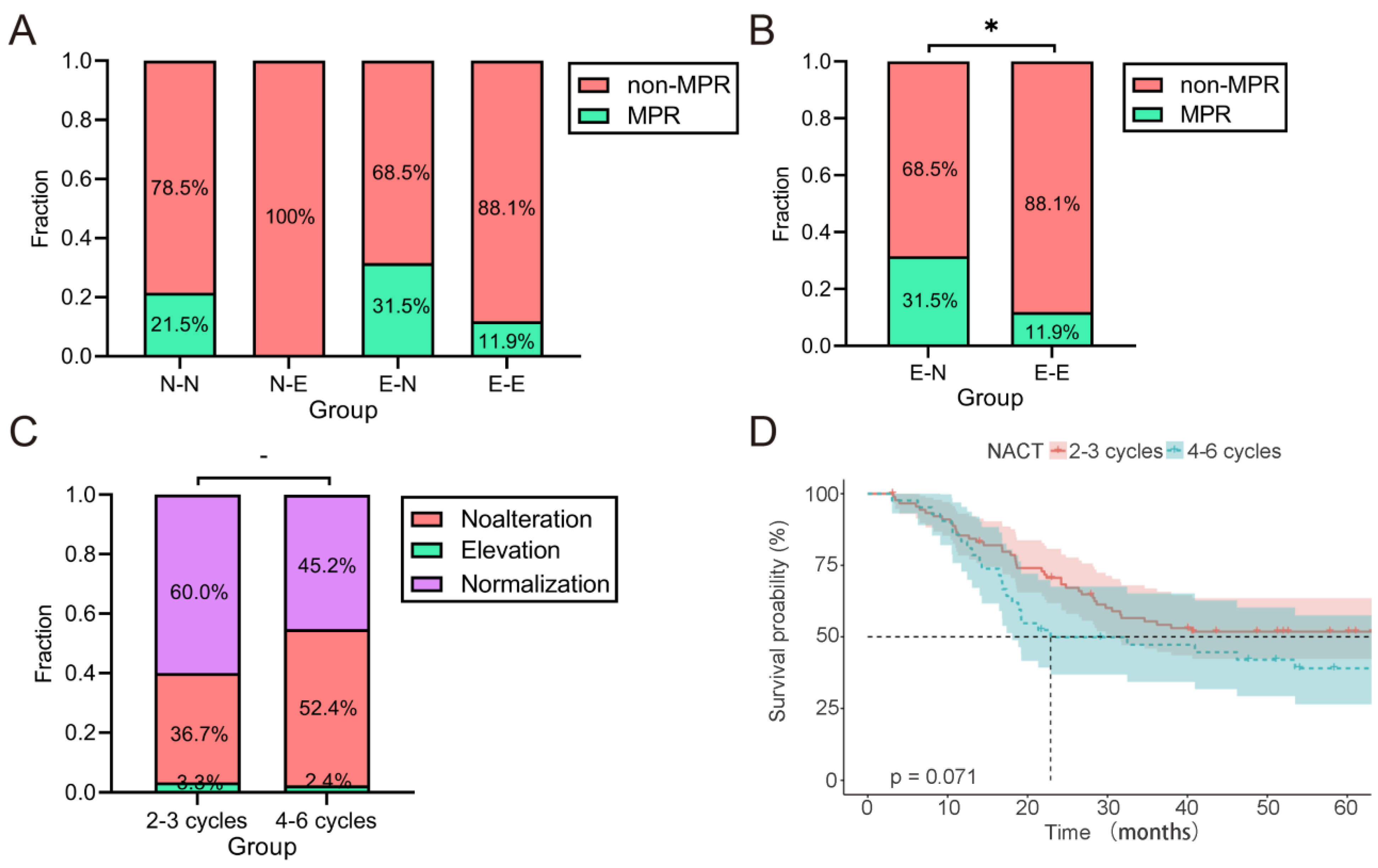
2.3. Treatment Outcomes
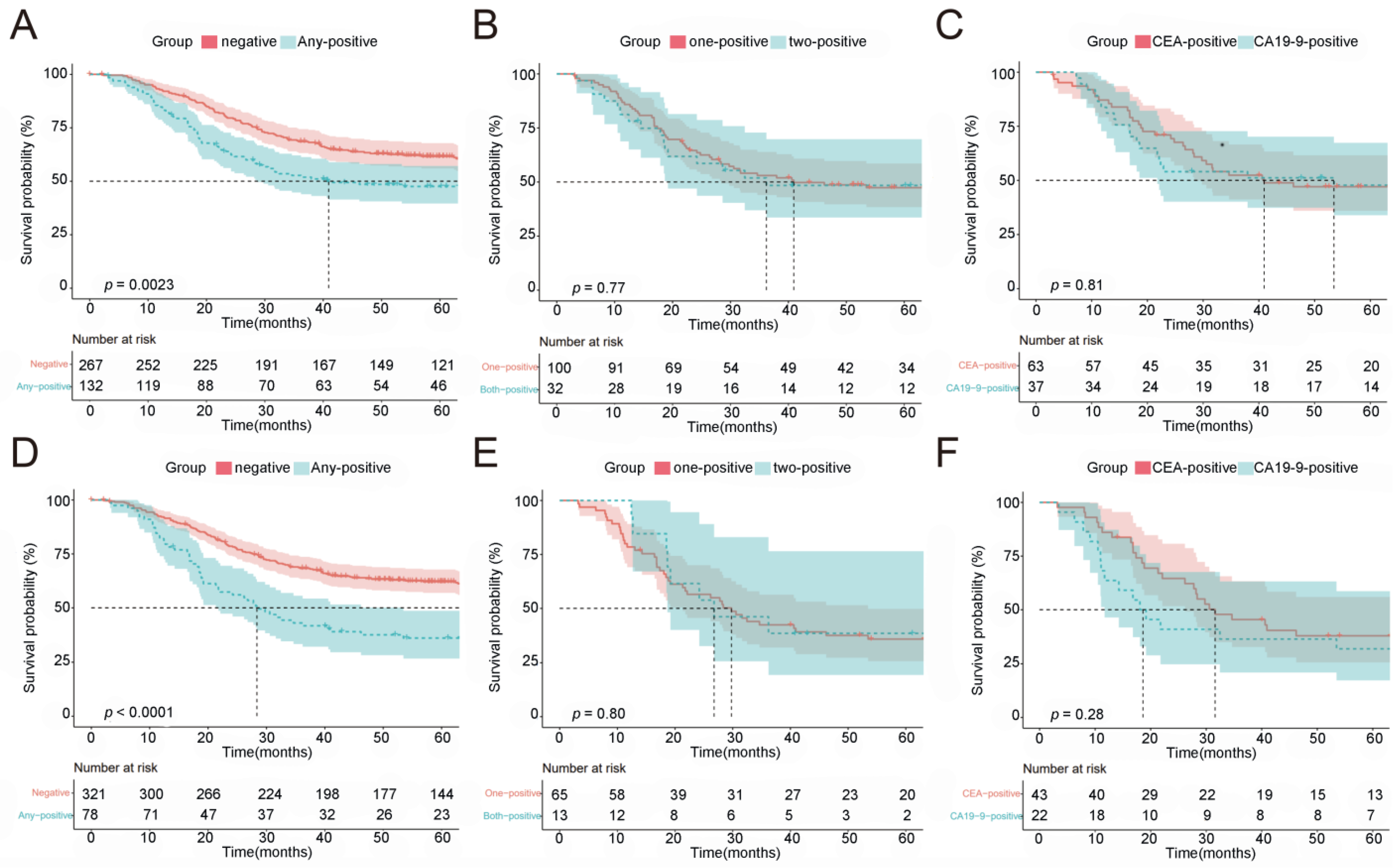
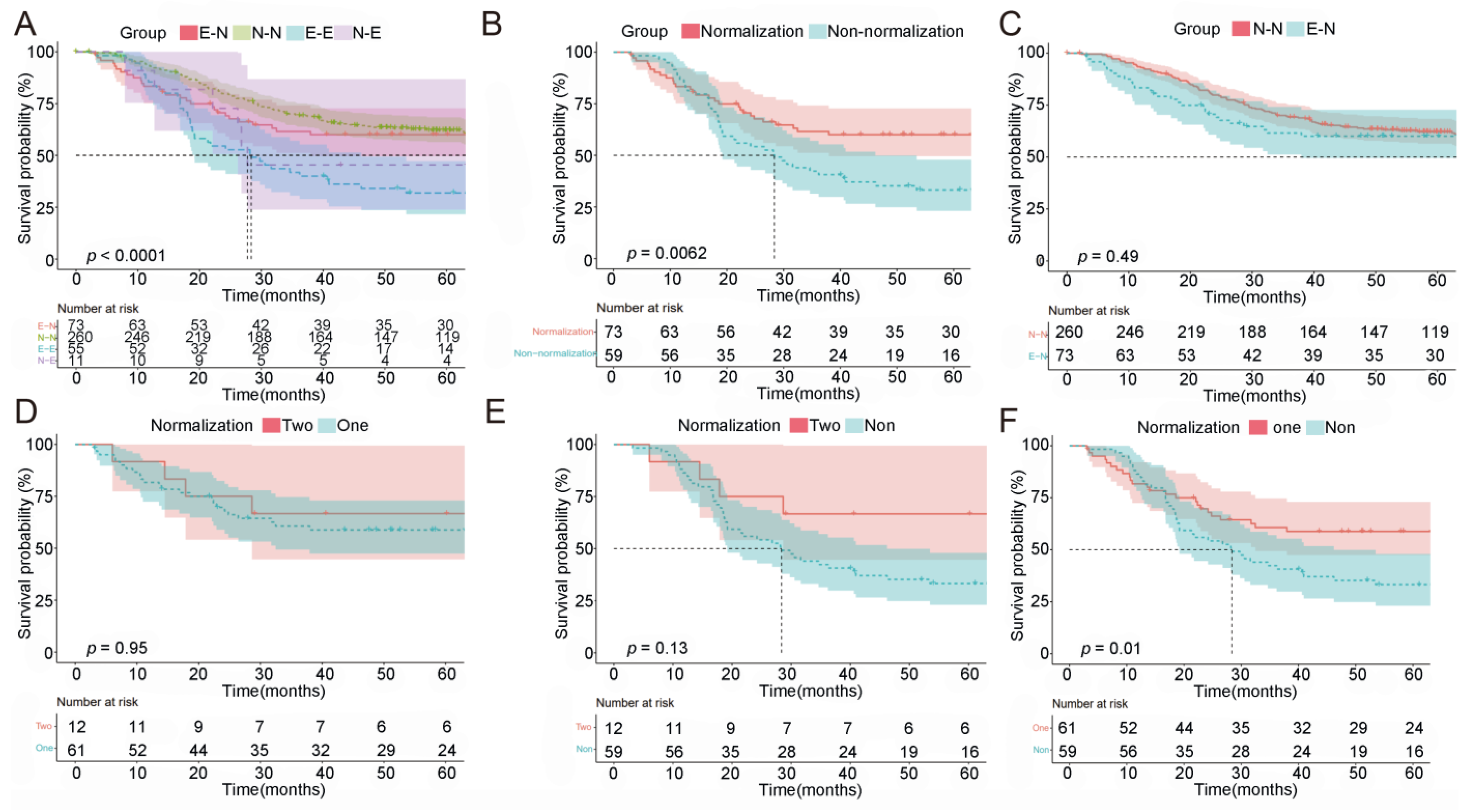
2.4. Prognostic Value of Normalization of Post-NACT CEA/CA19-9
2.5. Patients with Elevated CEA/CA19-9 Levels and Normalization following NACT Had a Survival Benefit
2.6. The Magnitude of Change in CEA/CA19-9 Levels and OS
2.7. Elevated CEA/CA19-9 Predicted a Higher Probability of Metastasis
3. Discussion
4. Materials and Methods
4.1. Study Design and Participant Recruitment
4.2. Treatment
4.3. CEA and CA19-9 Classification
4.4. Pathological Examination
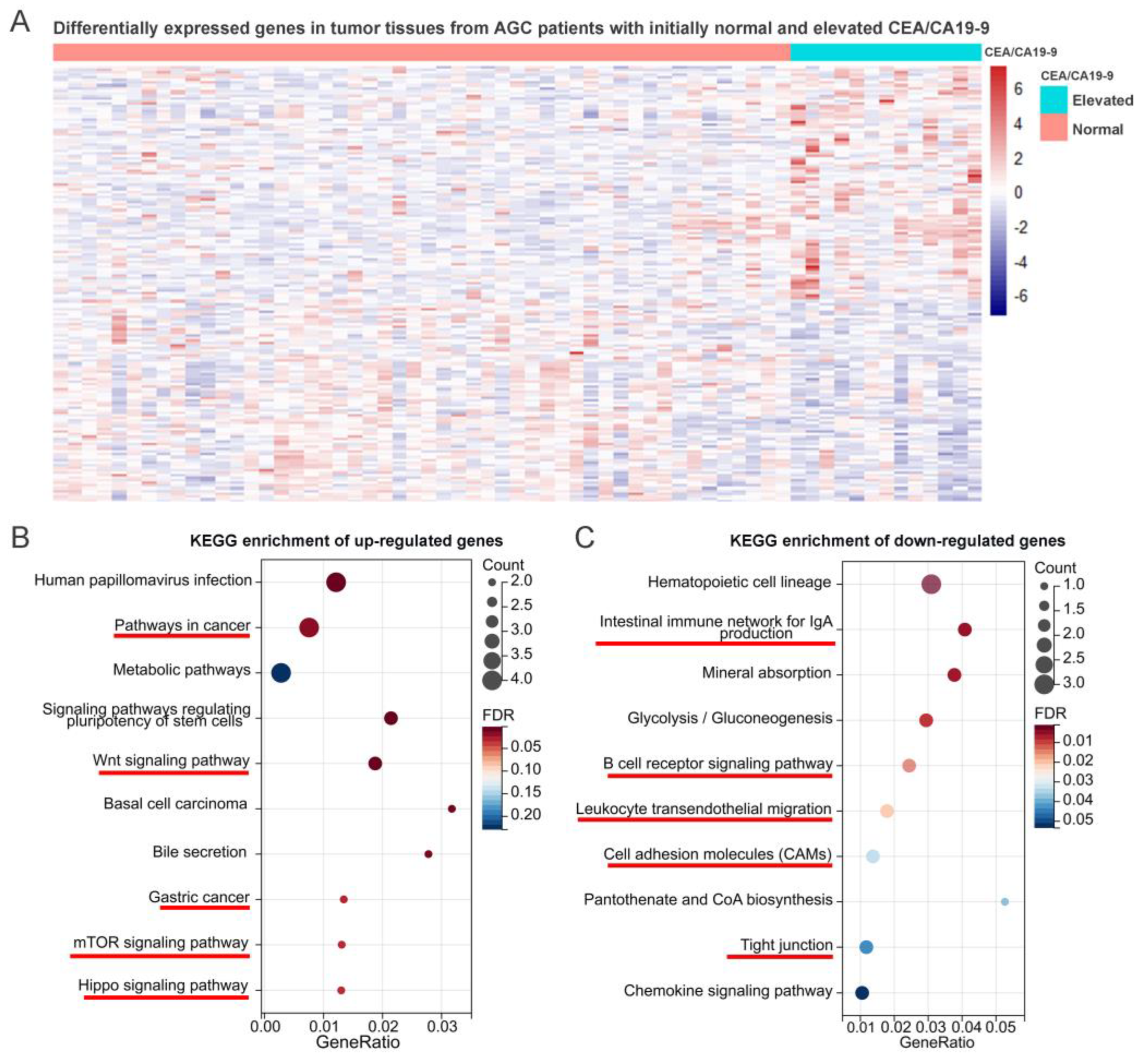
4.5. KEGG Enrichment Analyses
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Li, S.; Ying, X.; Zhang, L.; Shan, F.; Jia, Y.; Ji, J. The clinical value and usage of inflammatory and nutritional markers in survival prediction for gastric cancer patients with neoadjuvant chemotherapy and D2 lymphadenectomy. Gastric Cancer 2020, 23, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, H.; Li, Z.; Xue, Y.; Wang, Y.; Zhou, Z.; Yu, J.; Bu, Z.; Chen, L.; Du, Y.; et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): An open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021, 22, 1081–1092. [Google Scholar]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouche, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [PubMed]
- Kang, Y.K.; Yook, J.H.; Park, Y.K.; Lee, J.S.; Kim, Y.W.; Kim, J.Y.; Ryu, M.H.; Rha, S.Y.; Chung, I.J.; Kim, I.H.; et al. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J. Clin. Oncol. 2021, 39, 2903–2913. [Google Scholar] [CrossRef]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D.; Committee, E.G. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27 (Suppl. S5), v38–v49. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Almhanna, K.; Bentrem, D.J.; Chao, J.; Das, P.; Denlinger, C.S.; Fanta, P.; Farjah, F.; Fuchs, C.S.; et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 1286–1312. [Google Scholar] [CrossRef]
- Schuhmacher, C.; Gretschel, S.; Lordick, F.; Reichardt, P.; Hohenberger, W.; Eisenberger, C.F.; Haag, C.; Mauer, M.E.; Hasan, B.; Welch, J.; et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J. Clin. Oncol. 2010, 28, 5210–5218. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, X.; Peng, X.; Chen, M.J.M.; Li, Z.; Wei, B.; Wen, X.; Wei, B.; Dong, Y.; Bu, Z.; et al. Multi-omics characterization of molecular features of gastric cancer correlated with response to neoadjuvant chemotherapy. Sci. Adv. 2020, 6, eaay4211. [Google Scholar] [CrossRef]
- Schneider, B.J.; Shah, M.A.; Klute, K.; Ocean, A.; Popa, E.; Altorki, N.; Lieberman, M.; Schreiner, A.; Yantiss, R.; Christos, P.J.; et al. Phase I Study of Epigenetic Priming with Azacitidine Prior to Standard Neoadjuvant Chemotherapy for Patients with Resectable Gastric and Esophageal Adenocarcinoma: Evidence of Tumor Hypomethylation as an Indicator of Major Histopathologic Response. Clin. Cancer Res. 2017, 23, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Ramos, M.; Dias, A.R.; Cardili, L.; Ribeiro, R.R.E.; Charruf, A.Z.; de Castria, T.B.; Zilberstein, B.; Ceconello, I.; Avancini Ferreira Alves, V.; et al. Lymph node regression after neoadjuvant chemotherapy: A predictor of survival in gastric cancer. J. Surg. Oncol. 2020, 121, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shan, F.; Wang, Y.; Zhang, Y.; Zhang, L.; Li, S.; Jia, Y.; Xue, K.; Miao, R.; Li, Z.; et al. Correlation of pathological complete response with survival after neoadjuvant chemotherapy in gastric or gastroesophageal junction cancer treated with radical surgery: A meta-analysis. PLoS ONE 2018, 13, e0189294. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ma, Q.; Wang, L.; He, C.; Lu, S.; Ni, Z.; Hua, Z.; Zhu, Z.; Yang, Z.; Zheng, Y.; et al. Prediction Model of Tumor Regression Grade for Advanced Gastric Cancer After Preoperative Chemotherapy. Front. Oncol. 2021, 11, 607640. [Google Scholar] [CrossRef]
- Chen, L.; Hao, Y.; Cong, X.; Zou, M.; Li, S.; Zhu, L.; Song, H.; Xue, Y. Peripheral Venous Blood Platelet-to-Lymphocyte Ratio (PLR) for Predicting the Survival of Patients with Gastric Cancer Treated with SOX or XELOX Regimen Neoadjuvant Chemotherapy. Technol. Cancer Res. Treat. 2019, 18, 1533033819829485. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, N. Clinical evaluation of CEA, CA19-9, CA72-4 and CA125 in gastric cancer patients with neoadjuvant chemotherapy. World J. Surg. Oncol. 2014, 12, 397. [Google Scholar] [CrossRef]
- Tsai, S.; George, B.; Wittmann, D.; Ritch, P.S.; Krepline, A.N.; Aldakkak, M.; Barnes, C.A.; Christians, K.K.; Dua, K.; Griffin, M.; et al. Importance of Normalization of CA19-9 Levels Following Neoadjuvant Therapy in Patients with Localized Pancreatic Cancer. Ann. Surg. 2020, 271, 740–747. [Google Scholar] [CrossRef]
- Lee, B.S.; Lee, S.H.; Son, J.H.; Jang, D.K.; Chung, K.H.; Paik, W.H.; Ryu, J.K.; Kim, Y.T. Prognostic value of CA 19-9 kinetics during gemcitabine-based chemotherapy in patients with advanced cholangiocarcinoma. J. Gastroenterol. Hepatol. 2016, 31, 493–500. [Google Scholar] [CrossRef]
- Buijsen, J.; van Stiphout, R.G.; Menheere, P.P.; Lammering, G.; Lambin, P. Blood biomarkers are helpful in the prediction of response to chemoradiation in rectal cancer: A prospective, hypothesis driven study on patients with locally advanced rectal cancer. Radiother. Oncol. 2014, 111, 237–242. [Google Scholar] [CrossRef]
- Coleman, R.L.; Gordon, A.; Barter, J.; Sun, S.; Rackoff, W.; Herzog, T.J. Early changes in CA125 after treatment with pegylated liposomal doxorubicin or topotecan do not always reflect best response in recurrent ovarian cancer patients. Oncologist 2007, 12, 72–78. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Miyamoto, Y.; Beppu, T.; Nitta, H.; Imai, K.; Hayashi, H.; Baba, Y.; Yoshida, N.; Chikamoto, A.; Baba, H. Post-chemotherapeutic CEA and CA19-9 are prognostic factors in patients with colorectal liver metastases treated with hepatic resection after oxaliplatin-based chemotherapy. Anticancer Res. 2015, 35, 2359–2368. [Google Scholar] [PubMed]
- Hanke, B.; Riedel, C.; Lampert, S.; Happich, K.; Martus, P.; Parsch, H.; Himmler, B.; Hohenberger, W.; Hahn, E.G.; Wein, A. CEA and CA 19-9 measurement as a monitoring parameter in metastatic colorectal cancer (CRC) under palliative first-line chemotherapy with weekly 24-hour infusion of high-dose 5-fluorouracil (5-FU) and folinic acid (FA). Ann. Oncol. 2001, 12, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Colloca, G.; Venturino, A.; Governato, I. CA125-related tumor cell kinetics variables after chemotherapy in advanced ovarian cancer: A systematic review. Clin. Transl. Oncol. 2016, 18, 813–824. [Google Scholar] [CrossRef]
- Pajuelo-Lozano, N.; Alcala, S.; Sainz, B., Jr.; Perona, R.; Sanchez-Perez, I. Targeting MAD2 modulates stemness and tumorigenesis in human Gastric Cancer cell lines. Theranostics 2020, 10, 9601–9618. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, F.; Xu, L.; Li, C.; Yang, X.; Guo, B.; Gu, J.; Wang, L. Wnt/beta-Catenin Signaling Axis Is Required for TFEB-Mediated Gastric Cancer Metastasis and Epithelial-Mesenchymal Transition. Mol. Cancer Res. 2020, 18, 1650–1659. [Google Scholar] [CrossRef]
- Xing, X.; Zhang, L.; Wen, X.; Wang, X.; Cheng, X.; Du, H.; Hu, Y.; Li, L.; Dong, B.; Li, Z.; et al. PP242 suppresses cell proliferation, metastasis, and angiogenesis of gastric cancer through inhibition of the PI3K/AKT/mTOR pathway. Anticancer Drugs 2014, 25, 1129–1140. [Google Scholar] [CrossRef]
- Xu, J.; Li, N.; Deng, W.; Luo, S. Long noncoding RNA FER1L4 suppresses proliferation, invasion, migration and lymphatic metastasis of gastric cancer cells through inhibiting the Hippo-YAP signaling pathway. Am. J. Transl. Res. 2020, 12, 5481–5495. [Google Scholar]
- Hartgrink, H.H.; van de Velde, C.J.; Putter, H.; Songun, I.; Tesselaar, M.E.; Kranenbarg, E.K.; de Vries, J.E.; Wils, J.A.; van der Bijl, J.; van Krieken, J.H.; et al. Neo-adjuvant chemotherapy for operable gastric cancer: Long term results of the Dutch randomised FAMTX trial. Eur. J. Surg. Oncol. 2004, 30, 643–649. [Google Scholar] [CrossRef]
- Kambara, Y.; Miyake, H.; Nagai, H.; Yoshioka, Y.; Shibata, K.; Asai, S.; Yuasa, N. CA19-9 is a significant prognostic marker of patients with stage III gastric cancer. Eur. J. Surg. Oncol. 2020, 46 Pt A, 1918–1924. [Google Scholar] [CrossRef]
- Yajima, H.; Omura, N.; Matai, K.; Mitsumori, N.; Yoshida, K.; Yanaga, K. Clinicopathological features of CA19-9-producing gastric cancer. Hepatogastroenterology 2014, 61, 221–225. [Google Scholar]
- Li, Y.; Wei, Y.; He, Q.; Wang, X.; Fan, C.; Li, G. Clinicopathological and prognostic significance of high circulating lymphocyte ratio in patients receiving neoadjuvant chemotherapy for advanced gastric cancer. Sci. Rep. 2018, 8, 6223. [Google Scholar] [CrossRef]
- Chen, Y.H.; Xiao, J.; Chen, X.J.; Wang, H.S.; Liu, D.; Xiang, J.; Peng, J.S. Nomogram for predicting pathological complete response to neoadjuvant chemotherapy in patients with advanced gastric cancer. World J. Gastroenterol. 2020, 26, 2427–2439. [Google Scholar] [CrossRef]
- Ohe, Y.; Fushida, S.; Yamaguchi, T.; Kinoshita, J.; Saito, H.; Okamoto, K.; Nakamura, K.; Tajima, H.; Ninomiya, I.; Ohta, T. Peripheral Blood Platelet-Lymphocyte Ratio Is Good Predictor of Chemosensitivity and Prognosis in Gastric Cancer Patients. Cancer Manag. Res. 2020, 12, 1303–1311. [Google Scholar] [CrossRef]
- Moriyama, J.; Oshima, Y.; Nanami, T.; Suzuki, T.; Yajima, S.; Shiratori, F.; Funahashi, K.; Shimada, H. Prognostic impact of CEA/CA19-9 at the time of recurrence in patients with gastric cancer. Surg. Today 2021, 51, 1638–1648. [Google Scholar] [CrossRef]
- Ohi, M.; Mori, K.; Toiyama, Y.; Mohri, Y.; Okigami, M.; Yasuda, H.; Saigusa, S.; Tanaka, K.; Inoue, Y.; Kusunoki, M. Preoperative prediction of peritoneal metastasis in gastric cancer as an indicator for neoadjuvant treatment. Anticancer Res. 2015, 35, 3511–3518. [Google Scholar]
- Xiao, J.; He, X.; Wang, Z.; Hu, J.; Sun, F.; Qi, F.; Yang, S.; Xiao, Z. Serum carbohydrate antigen 19-9 and prognosis of patients with gastric cancer. Tumor Biol. 2014, 35, 1331–1334. [Google Scholar] [CrossRef]
- Wang, J.H.; Mai, C.; Hong, J.; Zhang, Q.; Tang, H.S.; Tang, Y.Q.; Cui, S.Z. Predicting value of serum CEA and CA19-9 in neoadjuvant chemotherapy for advanced gastric carcinoma. Zhonghua Wei Chang Wai Ke Za Zhi 2012, 15, 1273–1276. [Google Scholar]
- Sano, T.; Coit, D.G.; Kim, H.H.; Roviello, F.; Kassab, P.; Wittekind, C.; Yamamoto, Y.; Ohashi, Y. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer 2017, 20, 217–225. [Google Scholar] [CrossRef]
- Gastric Cancer Association; China Anti-Cancer Association. Chinese expert consensus on perioperative treatment of locally advanced gastric cancer (2021 version). Zhonghua Wei Chang Wai Ke Za Zhi 2021, 24, 741–748. [Google Scholar]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]

| All Patients | Without Elevated CEA/CA19-9 | With Elevated CEA/CA19-9 | p-Value | |
|---|---|---|---|---|
| Number of patients | 399 | 267 | 132 | - |
| Range of CEA value: range | 0.2–420.4 U/mL | 0.2–4.96 U/mL | 0.98–420.4 U/mL | - |
| Range of CA19-9 value: range | 0.6–9867 U/mL | 0.6–36.9 U/mL | 0.6–9867 U/mL | - |
| Age in years, (range) | 61.0 (21.0~86.0) | 60.0 (21.0~79.0) | 64.0 (34.0~86.0) | 0.026 * |
| Gender | 0.190 | |||
| Male, n (%) | 301 (75.4) | 203 (76.0) | 98 (74.2) | |
| Female, n (%) | 98 (24.6) | 64 (24.0) | 34 (21.7) | |
| BMI (median) | 23.69 | 23.69 | 23.70 | 0.775 |
| Location | 0.378 | |||
| Upper, n (%) | 141 (35.3) | 90 (33.7) | 51 (38.6) | |
| Middle, n (%) | 67 (16.8) | 47 (17.6) | 20 (15.2) | |
| Lower, n (%) | 183 (45.9) | 123 (46.1) | 60 (45.5) | |
| Total, n (%) | 8 (2.0) | 7 (2.6) | 1 (0.8) | |
| Diameter | 0.083 | |||
| <Median, n (%) | 203 (50.9) | 144 (53.9) | 59 (44.7) | |
| >Median, n (%) | 196 (49.1) | 123 (46.1) | 73 (55.3) | |
| Differentiation | 0.108 | |||
| Differential, n (%) | 212 (53.1) | 134 (50.2) | 78 (59.1) | |
| Undifferential, n (%) | 187 (46.9) | 133 (49.8) | 54 (40.9) | |
| cT stage | 0.148 | |||
| T1-T2, n (%) | 6 (1.5) | 3 (1.1) | 3 (2.3) | |
| T3, n (%) | 158 (39.6) | 114 (42.7) | 44 (33.3) | |
| T4, n (%) | 235 (58.9) | 150 (56.2) | 85 (64.4) | |
| cN stage | 0.002 ** | |||
| N0, n (%) | 65 (16.3) | 53 (19.9) | 12 (9.1) | |
| N1, n (%) | 123 (30.8) | 85 (31.8) | 38 (28.8) | |
| N2, n (%) | 158 (39.6) | 103 (38.6) | 55 (41.7) | |
| N3, n (%) | 53 (13.3) | 26 (9.7) | 27 (20.5) | |
| cTNM stage | 0.002 ** | |||
| II, n (%) | 72 (18.0) | 57 (21.3) | 15 (11.4) | |
| III, n (%) | 316 (79.2) | 207 (77.5) | 109 (82.6) | |
| IV, n (%) | 11 (2.8) | 3 (1.1) | 8 (6.1) | |
| TRG in primary lesion | 0.175 | |||
| Grade 0 | 29 (7.3) | 19 (7.1) | 10 (7.6) | |
| Grade 1 | 57 (14.3) | 37 (13.9) | 20 (15.2) | |
| Grade 2 | 106 (26.6) | 82 (30.7) | 24 (18.2) | |
| Grade 3 | 207 (51.9) | 129 (48.3) | 78 (59.1) | |
| NACT | 0.959 | |||
| SOX, n (%) | 191 (47.9) | 127 (47.6) | 64 (48.5) | |
| XELOX, n (%) | 72 (18.0) | 49 (18.4) | 23 (17.4) | |
| mFOLFOX7, n (%) | 84 (21.1) | 57 (21.3) | 27 (20.1) | |
| Others, n (%) | 52 (13.0) | 34 (12.7) | 18 (13.6) | |
| Cycles (median) | 2.85 | 2.75 | 2.95 | 0.055 |
| Surgical setting | 0.634 | |||
| Total gastrectomy, n (%) | 183 (45.9) | 120 (44.9) | 63 (47.7) | |
| Distal gastrectomy, n (%) | 176 (44.1) | 120 (44.9) | 56 (42.4) | |
| Proximal gastrectomy, n (%) | 40 (10.0) | 27 (10.1) | 13 (8.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.-H.; Wu, X.-L.; Gan, X.-J.; Wang, Y.-D.; Jia, F.-Z.; Wang, Y.-X.; Zhang, Y.; Gao, X.-Y.; Li, Z.-Y. Using Normalized Carcinoembryonic Antigen and Carbohydrate Antigen 19 to Predict and Monitor the Efficacy of Neoadjuvant Chemotherapy in Locally Advanced Gastric Cancer. Int. J. Mol. Sci. 2023, 24, 12192. https://doi.org/10.3390/ijms241512192
Tang X-H, Wu X-L, Gan X-J, Wang Y-D, Jia F-Z, Wang Y-X, Zhang Y, Gao X-Y, Li Z-Y. Using Normalized Carcinoembryonic Antigen and Carbohydrate Antigen 19 to Predict and Monitor the Efficacy of Neoadjuvant Chemotherapy in Locally Advanced Gastric Cancer. International Journal of Molecular Sciences. 2023; 24(15):12192. https://doi.org/10.3390/ijms241512192
Chicago/Turabian StyleTang, Xiao-Huan, Xiao-Long Wu, Xue-Jun Gan, Yi-Ding Wang, Fang-Zhou Jia, Yi-Xue Wang, Yan Zhang, Xiang-Yu Gao, and Zi-Yu Li. 2023. "Using Normalized Carcinoembryonic Antigen and Carbohydrate Antigen 19 to Predict and Monitor the Efficacy of Neoadjuvant Chemotherapy in Locally Advanced Gastric Cancer" International Journal of Molecular Sciences 24, no. 15: 12192. https://doi.org/10.3390/ijms241512192
APA StyleTang, X.-H., Wu, X.-L., Gan, X.-J., Wang, Y.-D., Jia, F.-Z., Wang, Y.-X., Zhang, Y., Gao, X.-Y., & Li, Z.-Y. (2023). Using Normalized Carcinoembryonic Antigen and Carbohydrate Antigen 19 to Predict and Monitor the Efficacy of Neoadjuvant Chemotherapy in Locally Advanced Gastric Cancer. International Journal of Molecular Sciences, 24(15), 12192. https://doi.org/10.3390/ijms241512192






