Developing New Diagnostic Tools Based on SERS Analysis of Filtered Salivary Samples for Oral Cancer Detection
Abstract
1. Introduction
2. Results
2.1. Subject Data and Classification
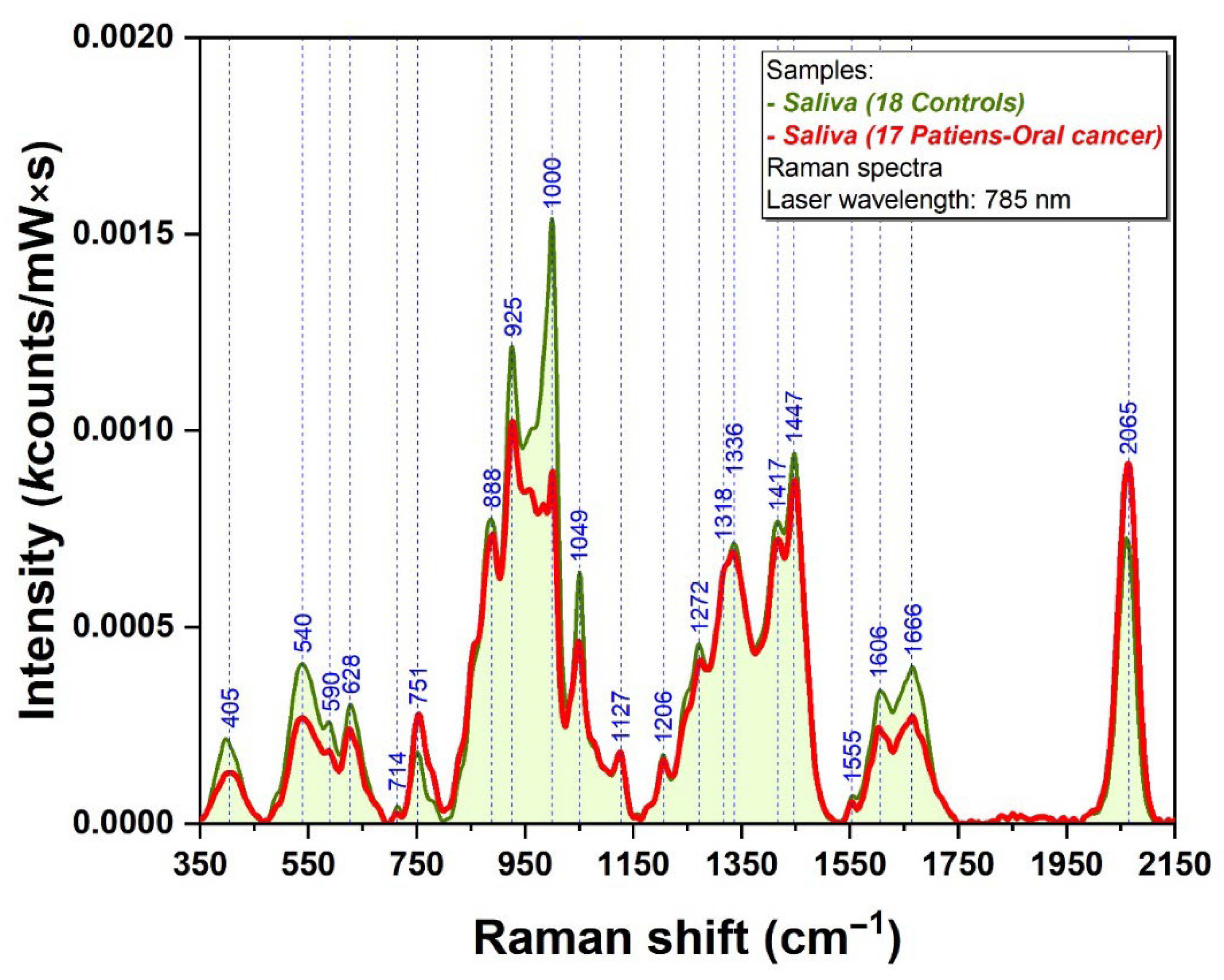
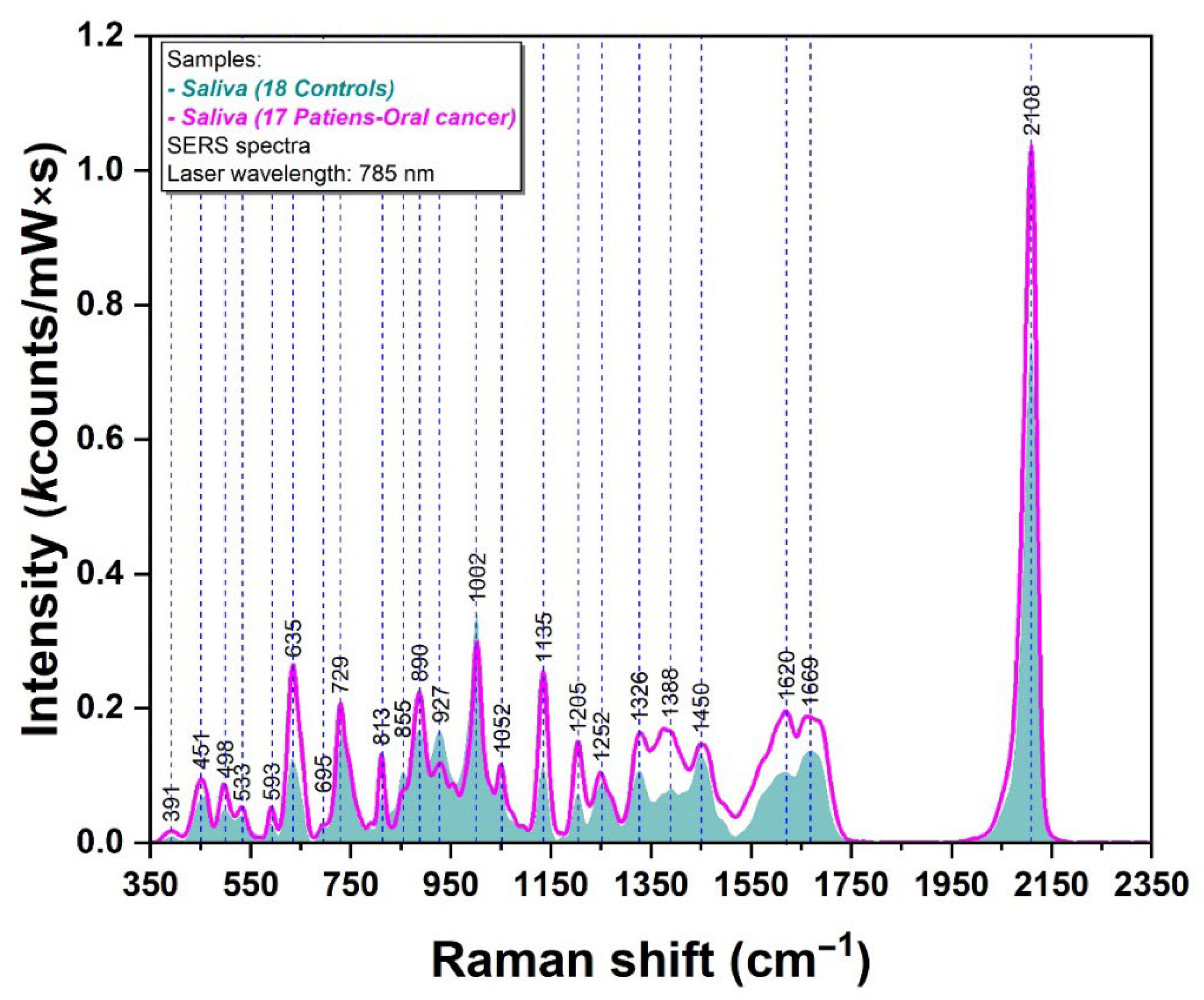
2.2. Raman/SERS Analysis of Saliva Samples
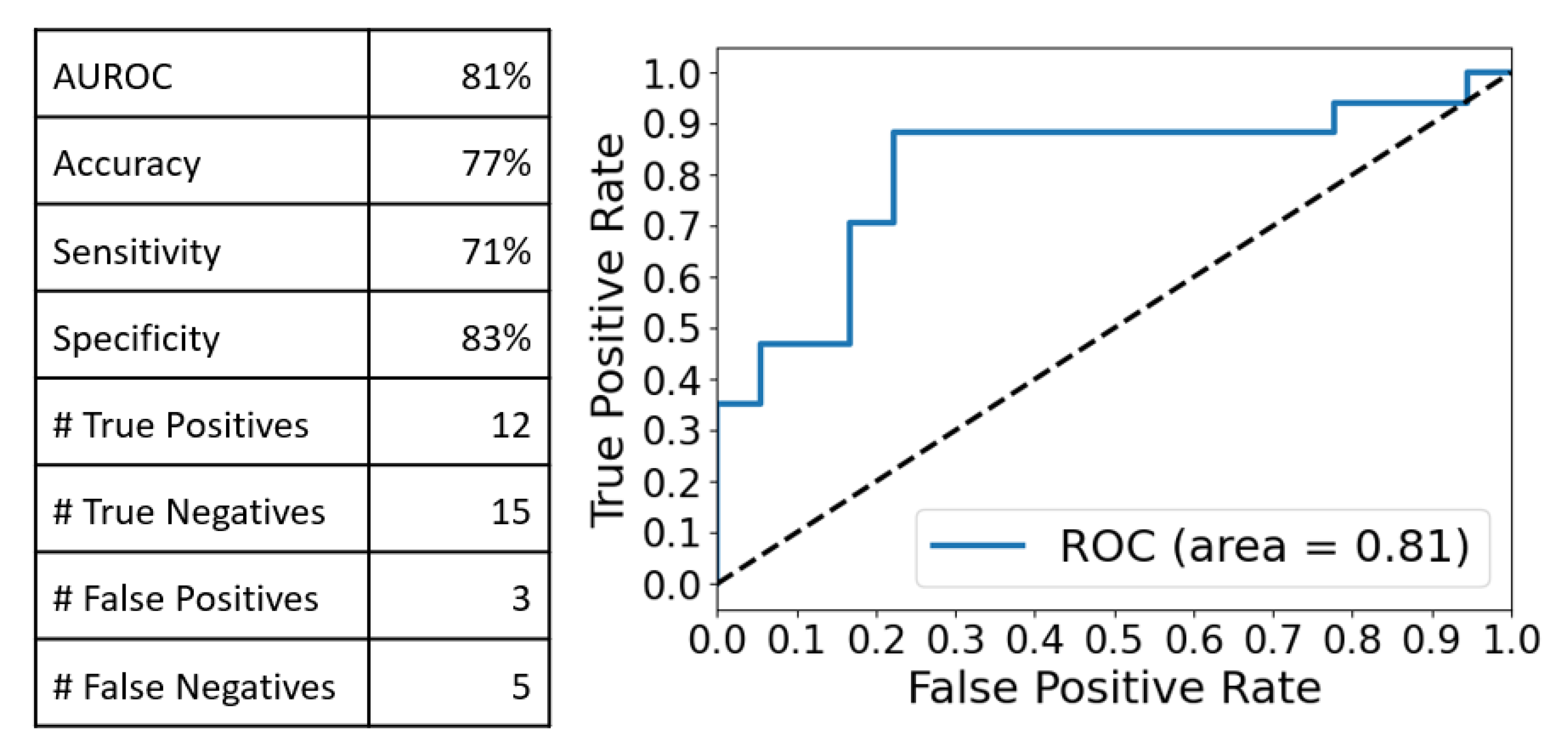
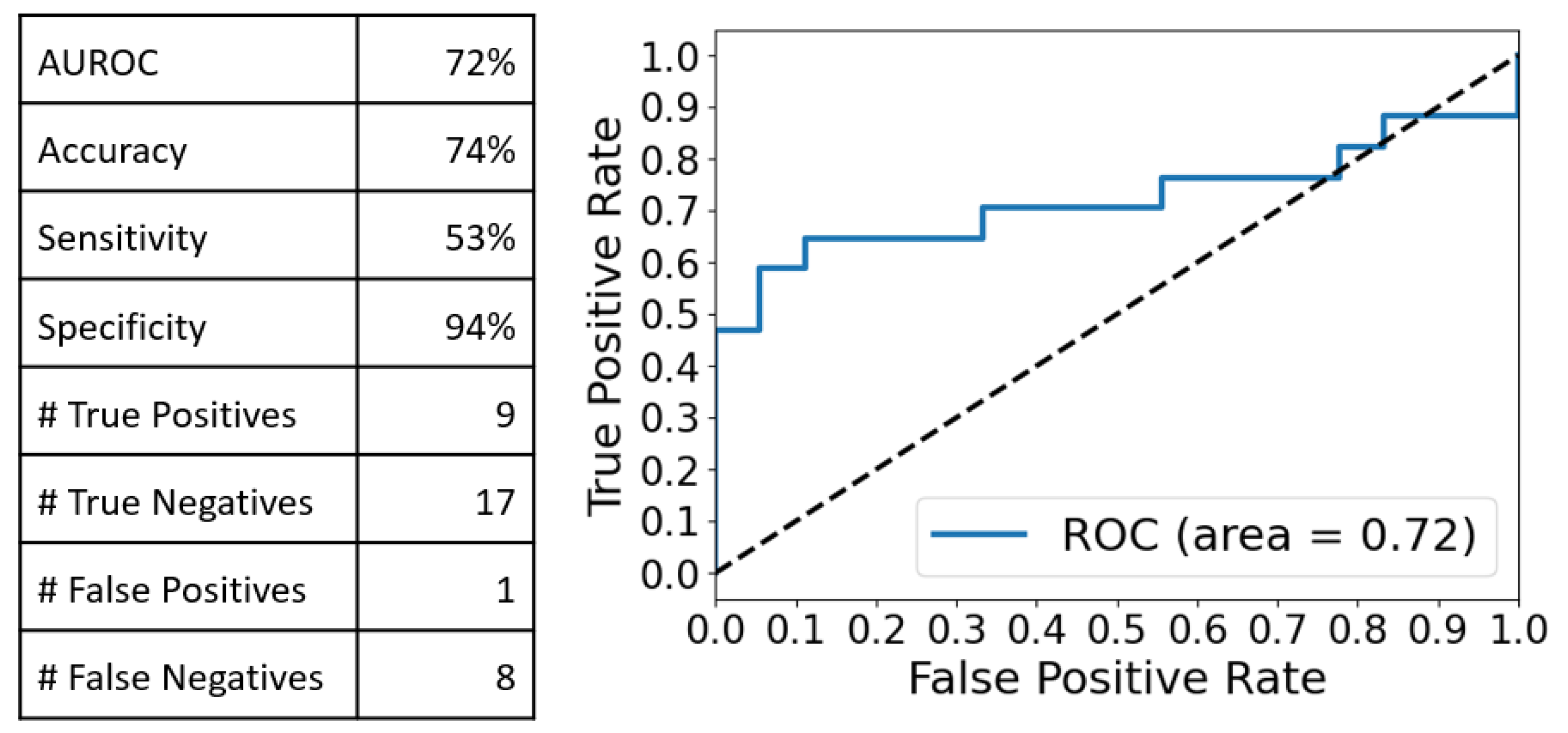
2.3. Multivariate Analysis of the SERS Spectra
3. Discussion
4. Materials and Methods
4.1. Sample Collection
- Adult patients with malignant pathologies in the oro-maxillofacial area.
- Healthy adult subjects (for the control group).
- Minor patients or adults without pathologies in the oro-maxillofacial area.
- Subjects who are not healthy (not eligible for the control group).
4.2. Sample Processing
4.3. SERS Substrate Preparation
4.4. Raman/SERS Measurements
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soames, J.V.; Southam, J.C. Oral Pathology, 4th ed.; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Zalewska, A.; Waszkiewicz, N.; López-Pintor, R.M. The Use of Saliva in the Diagnosis of Oral and Systemic Diseases. Dis. Markers 2019, 2019, 9149503. [Google Scholar] [CrossRef]
- Hardy, M.; Kelleher, L.; de Carvalho Gomes, P.; Buchan, E.; Chu, H.O.M.; Goldberg Oppenheimer, P. Methods in Raman spectroscopy for saliva studies—A review. Appl. Spectrosc. Rev. 2022, 57, 177–233. [Google Scholar] [CrossRef]
- Levine, M. Topics in Dental Biochemistry; Springer: Berlin, Heidelberg, 2011; ISBN 978-3-540-88115-5. [Google Scholar]
- Zhang, C.-Z.; Cheng, X.-Q.; Li, J.-Y.; Zhang, P.; Yi, P.; Xu, X.; Zhou, X.-D. Saliva in the diagnosis of diseases. Int. J. Oral Sci. 2016, 8, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Gaidhani, K.A.; Harwalkar, M.; Nirgude, P.S. World Journal of Pharmaceutical ReseaRch SEED EXTRACTS. World J. Pharm. Res. 2014, 3, 5041–5048. [Google Scholar]
- Ozdogan, M.S.; Gungormus, M.; Ince Yusufoglu, S.; Ertem, S.Y.; Sonmez, C.; Orhan, M. Salivary opiorphin in dental pain: A potential biomarker for dental disease. Arch. Oral Biol. 2019, 99, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Colceriu-Șimon, I.M.; Hedeșiu, M.; Toma, V.; Armencea, G.; Moldovan, A.; Știufiuc, G.; Culic, B.; Țuarmure, V.; Dinu, C.; Berindan-Neagoe, I.; et al. The Effects of Low-Dose Irradiation on Human Saliva: A Surface-Enhanced Raman Spectroscopy Study. Diagnostics 2019, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Magacz, M.; Kędziora, K.; Sapa, J.; Krzyściak, W. The significance of lactoperoxidase system in oral health: Application and efficacy in oral hygiene products. Int. J. Mol. Sci. 2019, 20, 1443. [Google Scholar] [CrossRef]
- Flieger, J.; Kawka, J.; Tatarczak-Michalewska, M. Levels of the thiocyanate in the saliva of tobacco smokers in comparison to e-cigarette smokers and nonsmokers measured by HPLC on a phosphatidylcholine column. Molecules 2019, 24, 3790. [Google Scholar] [CrossRef]
- Calado, G.; Behl, I.; Daniel, A.; Byrne, H.J.; Lyng, F.M. Raman spectroscopic analysis of saliva for the diagnosis of oral cancer: A systematic review. Transl. Biophotonics 2019, 1, e201900001. [Google Scholar] [CrossRef]
- Sevanian, A.; Davies, K.J.; Hochstein, P. Serum urate as an antioxidant for ascorbic acid. Am. Soc. Clin. Nutr. 1991, 54, 1129S–1134S. [Google Scholar] [CrossRef]
- Le Ru, E.; Etchegoin, P. Principles of Surface Enhanced Raman Spectroscopy and Related Plasmonic Effects, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Li, X.; Keshavarz, M.; Kassanos, P.; Kidy, Z.; Roddan, A.; Yeatman, E.; Thompson, A.J. SERS Detection of Breast Cancer-Derived Exosomes Using a Nanostructured Pt-Black Template. Adv. Sens. Res. 2023, 2, 2200039. [Google Scholar] [CrossRef]
- Kadam, U.S.; Chavhan, R.L.; Schulz, B.; Irudayaraj, J. Single molecule Raman spectroscopic assay to detect transgene from GM plants. Anal. Biochem. 2017, 532, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Kadam, U.S.; Schulz, B.; Irudayaraj, J.M.K. Multiplex single-cell quantification of rare RNA transcripts from protoplasts in a model plant system. Plant J. 2017, 90, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Kadam, U.; Moeller, C.A.; Irudayaraj, J.; Schulz, B. Effect of T-DNA insertions on mRNA transcript copy numbers upstream and downstream of the insertion site in Arabidopsis thaliana explored by surface enhanced Raman spectroscopy. Plant Biotechnol. J. 2014, 12, 568–577. [Google Scholar] [CrossRef]
- Kadam, U.S.; Schulz, B.; Lrudayaraj, J. Detection and quantification of alternative splice sites in Arabidopsis genes AtDCL2 and AtPTB2 with highly sensitive surface enhanced Raman spectroscopy (SERS) and gold nanoprobes. FEBS Lett. 2014, 588, 1637–1643. [Google Scholar] [CrossRef]
- Feng, S.; Huang, S.; Lin, D.; Chen, G.; Xu, Y.; Li, Y.; Huang, Z.; Pan, J.; Chen, R.; Zeng, H. Surface-enhanced Raman spectroscopy of saliva proteins for the noninvasive differentiation of benign and malignant breast tumors. Int. J. Nanomed. 2015, 10, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Știufiuc, G.F.; Toma, V.; Buse, M.; Mărginean, R.; Morar-Bolba, G.; Culic, B.; Tetean, R.; Leopold, N.; Pavel, I.; Lucaciu, C.M.C.M.; et al. Solid Plasmonic Substrates for Breast Cancer Detection by Means of SERS Analysis of Blood Plasma. Nanomaterials 2020, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Koster, H.J.; Guillen-Perez, A.; Gomez-Diaz, J.S.; Navas-Moreno, M.; Birkeland, A.C.; Carney, R.P. Fused Raman spectroscopic analysis of blood and saliva delivers high accuracy for head and neck cancer diagnostics. Sci. Rep. 2022, 12, 18464. [Google Scholar] [CrossRef]
- Leopold, N.; Lendl, B. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Onaciu, A.; Toma, V.; Moldovan, C.; Țigu, A.B.; Cenariu, D.; Culic, C.; Borșa, R.M.; David, L.; Știufiuc, G.F.; Tetean, R.; et al. Nanoscale Investigation of DNA Demethylation in Leukemia Cells by Means of Ultrasensitive Vibrational Spectroscopy. Sensors 2022, 23, 346. [Google Scholar] [CrossRef]
- Tefas, C.; Mărginean, R.; Toma, V.; Petrushev, B.; Fischer, P.; Tanțău, M.; Știufiuc, R. Surface-enhanced Raman scattering for the diagnosis of ulcerative colitis: Will it change the rules of the game? Anal. Bioanal. Chem. 2021, 413, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Moisoiu, V.; Stefancu, A.; Gulei, D.; Boitor, R.; Magdo, L.; Raduly, L.; Pasca, S.; Kubelac, P.; Mehterov, N.; Chis, V.; et al. SERS-based differential diagnosis between multiple solid malignancies: Breast, colorectal, lung, ovarian and oral cancer. Int. J. Nanomed. 2019, 14, 6165–6178. [Google Scholar] [CrossRef] [PubMed]
- Știufiuc, G.F.; Toma, V.; Onaciu, A.; Chiș, V.; Lucaciu, C.M.; Știufiuc, R.I. Proving Nanoscale Chiral Interactions of Cyclodextrins and Propranolol Enantiomers by Means of SERS Measurements Performed on a Solid Plasmonic Substrate. Pharmaceutics 2021, 13, 1594. [Google Scholar] [CrossRef]
- Munteanu, V.C.; Munteanu, R.A.; Gulei, D.; Mărginean, R.; Schițcu, V.H.; Onaciu, A.; Toma, V.; Știufiuc, G.F.; Coman, I.; Știufiuc, R.I. New Insights into the Multivariate Analysis of SER Spectra Collected on Blood Samples for Prostate Cancer Detection: Towards a Better Understanding of the Role Played by Different Biomolecules on Cancer Screening: A Preliminary Study. Cancers 2022, 14, 3227. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Weimer, W.A. High enhancement factor gold films for surface enhanced Raman spectroscopy. Chem. Phys. Lett. 2003, 374, 302–306. [Google Scholar] [CrossRef]
- Bankapur, A.; Zachariah, E.; Chidangil, S.; Valiathan, M.; Mathur, D. Raman Tweezers Spectroscopy of Live, Single Red and White Blood Cells. PLoS ONE 2010, 5, e10427. [Google Scholar] [CrossRef]
- Cao, X.; Wang, Z.; Bi, L.; Zheng, J. Label-Free Detection of Human Serum Using Surface-Enhanced Raman Spectroscopy Based on Highly Branched Gold Nanoparticle Substrates for Discrimination of Non-Small Cell Lung Cancer. J. Chem. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Maiti, N.C.; Apetri, M.M.; Zagorski, M.G.; Carey, P.R.; Anderson, V.E. Raman Spectroscopic Characterization of Secondary Structure in Natively Unfolded Proteins: α-Synuclein. J. Am. Chem. Soc. 2004, 126, 2399–2408. [Google Scholar] [CrossRef]
- Ryzhikova, E.; Ralbovsky, N.M.; Halámková, L.; Celmins, D.; Malone, P.; Molho, E.; Quinn, J.; Zimmerman, E.A.; Lednev, I.K. Multivariate Statistical Analysis of Surface Enhanced Raman Spectra of Human Serum for Alzheimer’s Disease Diagnosis. Appl. Sci. 2019, 9, 3256. [Google Scholar] [CrossRef]
- Buchan, E.; Kelleher, L.; Clancy, M.; Stanley Rickard, J.J.; Oppenheimer, P.G. Spectroscopic molecular-fingerprint profiling of saliva. Anal. Chim. Acta 2021, 1185, 339074. [Google Scholar] [CrossRef]
- Wu, Q.; Qiu, S.; Yu, Y.; Chen, W.; Lin, H.; Lin, D.; Feng, S.; Chen, R. Assessment of the radiotherapy effect for nasopharyngeal cancer using plasma surface-enhanced Raman spectroscopy technology. Biomed. Opt. Express 2018, 9, 3413. [Google Scholar] [CrossRef]
- Lin, D.; Pan, J.; Huang, H.; Chen, G.; Qiu, S.; Shi, H.; Chen, W.; Yu, Y.; Feng, S.; Chen, R. Label-free blood plasma test based on surface-enhanced Raman scattering for tumor stages detection in nasopharyngeal cancer. Sci. Rep. 2015, 4, 4751. [Google Scholar] [CrossRef]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Tian, F.; Carvalho, L.F.d.C.e.S.d.; Casey, A.; Nogueira, M.S.; Byrne, H.J. Surface-Enhanced Raman Analysis of Uric Acid and Hypoxanthine Analysis in Fractionated Bodily Fluids. Nanomaterials 2023, 13, 1216. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yan, B.; Xue, L.; Li, Y.; Luo, X.; Ji, P. Surface-enhanced Raman spectroscopy of blood serum based on gold nanoparticles for the diagnosis of the oral squamous cell carcinoma. Lipids Health Dis. 2017, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Carney, R.P.; Hazari, S.; Smith, Z.J.; Knudson, A.; Robertson, C.S.; Lam, K.S.; Wachsmann-Hogiu, S. 3D plasmonic nanobowl platform for the study of exosomes in solution. Nanoscale 2015, 7, 9290–9297. [Google Scholar] [CrossRef]
- Zhang, H.; Silva, A.C.; Zhang, W.; Rutigliano, H.; Zhou, A. Raman Spectroscopy characterization extracellular vesicles from bovine placenta and peripheral blood mononuclear cells. PLoS ONE 2020, 15, e0235214. [Google Scholar] [CrossRef]
- Dingari, N.C.; Horowitz, G.L.; Kang, J.W.; Dasari, R.R.; Barman, I. Raman Spectroscopy Provides a Powerful Diagnostic Tool for Accurate Determination of Albumin Glycation. PLoS ONE 2012, 7, e32406. [Google Scholar] [CrossRef]
- Faur, C.I.; Dinu, C.; Toma, V.; Jurj, A.; Mărginean, R.; Onaciu, A.; Roman, R.C.; Culic, C.; Chirilă, M.; Rotar, H.; et al. A New Detection Method of Oral and Oropharyngeal Squamous Cell Carcinoma Based on Multivariate Analysis of Surface Enhanced Raman Spectra of Salivary Exosomes. J. Pers. Med. 2023, 13, 762. [Google Scholar] [CrossRef]
- Tatischeff, I.; Larquet, E.; Falcón-Pérez, J.M.; Turpin, P.Y.; Kruglik, S.G. Fast characterisation of cell-derived extracellular vesicles by nanoparticles tracking analysis, cryo-electron microscopy, and Raman tweezers microspectroscopy. J. Extracell. Vesicles 2012, 1. [Google Scholar] [CrossRef]
- Stremersch, S.; Marro, M.; Pinchasik, B.E.; Baatsen, P.; Hendrix, A.; De Smedt, S.C.; Loza-Alvarez, P.; Skirtach, A.G.; Raemdonck, K.; Braeckmans, K. Identification of individual exosome-like vesicles by surface enhanced raman spectroscopy. Small 2016, 12, 3292–3301. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.; van den Tweel, T.J.J.; de Mul, F.F.M.; Greve, J. Surface-enhanced Raman spectroscopy of DNA bases. J. Raman Spectrosc. 1986, 17, 289–298. [Google Scholar] [CrossRef]
- Prescott, B.; Steinmetz, W.; Thomas, G.J. Characterization of DNA structures by laser Raman spectroscopy. Biopolymers 1984, 23, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Fălămaș, A.; Rotaru, H.; Hedeșiu, M. Surface-enhanced Raman spectroscopy (SERS) investigations of saliva for oral cancer diagnosis. Lasers Med. Sci. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Iancu, S.D.; Cozan, R.G.; Stefancu, A.; David, M.; Moisoiu, T.; Moroz-Dubenco, C.; Bajcsi, A.; Chira, C.; Andreica, A.; Leopold, L.F.; et al. SERS liquid biopsy in breast cancer. What can we learn from SERS on serum and urine? Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 273, 120992. [Google Scholar] [CrossRef]
- Phyo, J.B.; Woo, A.; Yu, H.J.; Lim, K.; Cho, B.H.; Jung, H.S.; Lee, M.-Y. Label-Free SERS Analysis of Urine Using a 3D-Stacked AgNW-Glass Fiber Filter Sensor for the Diagnosis of Pancreatic Cancer and Prostate Cancer. Anal. Chem. 2021, 93, 3778–3785. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borșa, R.-M.; Toma, V.; Onaciu, A.; Moldovan, C.-S.; Mărginean, R.; Cenariu, D.; Știufiuc, G.-F.; Dinu, C.-M.; Bran, S.; Opriș, H.-O.; et al. Developing New Diagnostic Tools Based on SERS Analysis of Filtered Salivary Samples for Oral Cancer Detection. Int. J. Mol. Sci. 2023, 24, 12125. https://doi.org/10.3390/ijms241512125
Borșa R-M, Toma V, Onaciu A, Moldovan C-S, Mărginean R, Cenariu D, Știufiuc G-F, Dinu C-M, Bran S, Opriș H-O, et al. Developing New Diagnostic Tools Based on SERS Analysis of Filtered Salivary Samples for Oral Cancer Detection. International Journal of Molecular Sciences. 2023; 24(15):12125. https://doi.org/10.3390/ijms241512125
Chicago/Turabian StyleBorșa, Rareș-Mario, Valentin Toma, Anca Onaciu, Cristian-Silviu Moldovan, Radu Mărginean, Diana Cenariu, Gabriela-Fabiola Știufiuc, Cristian-Mihail Dinu, Simion Bran, Horia-Octavian Opriș, and et al. 2023. "Developing New Diagnostic Tools Based on SERS Analysis of Filtered Salivary Samples for Oral Cancer Detection" International Journal of Molecular Sciences 24, no. 15: 12125. https://doi.org/10.3390/ijms241512125
APA StyleBorșa, R.-M., Toma, V., Onaciu, A., Moldovan, C.-S., Mărginean, R., Cenariu, D., Știufiuc, G.-F., Dinu, C.-M., Bran, S., Opriș, H.-O., Văcăraș, S., Onișor-Gligor, F., Sentea, D., Băciuț, M.-F., Iuga, C.-A., & Știufiuc, R.-I. (2023). Developing New Diagnostic Tools Based on SERS Analysis of Filtered Salivary Samples for Oral Cancer Detection. International Journal of Molecular Sciences, 24(15), 12125. https://doi.org/10.3390/ijms241512125













