Pentacyclic Triterpenes from Olive Leaves Formulated in Microemulsion: Characterization and Role in De Novo Lipogenesis in HepG2 Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of ME-EXT
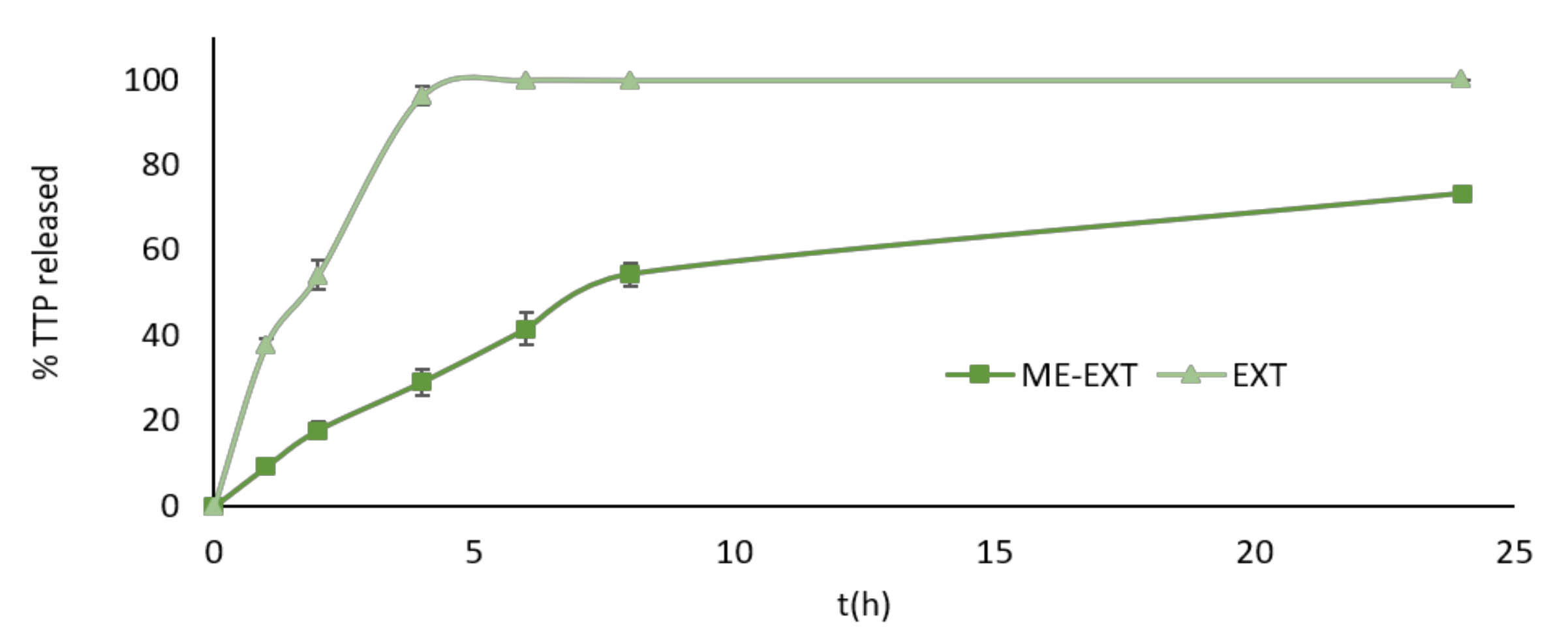
2.2. In Vitro Release Study
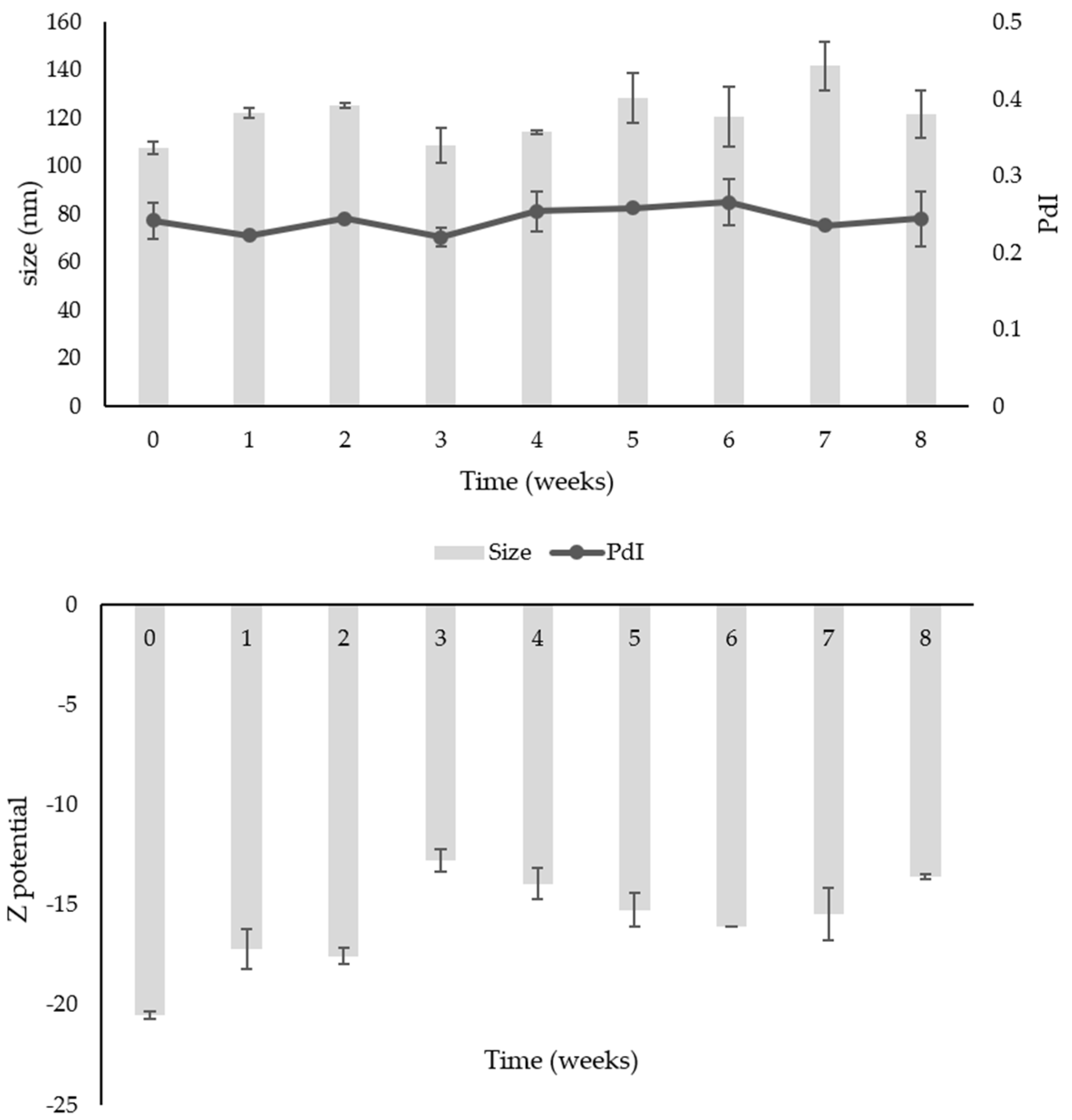
2.3. Stability Study over Time
2.4. Parallel Artificial Membrane Permeability Assay (PAMPA)
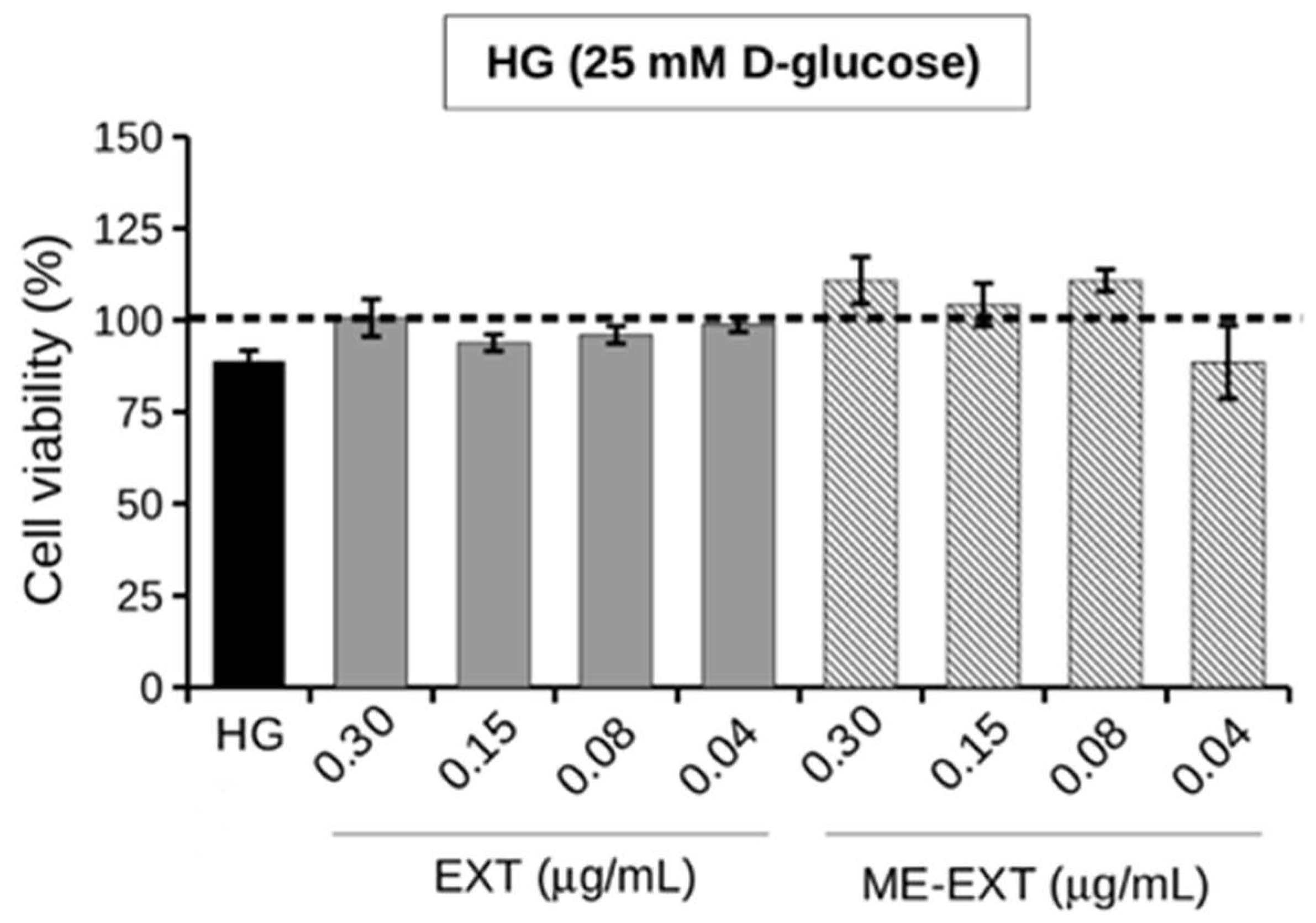
2.5. Effect of EXT and ME-EXT on HepG2 Cell Viability under HG Conditions
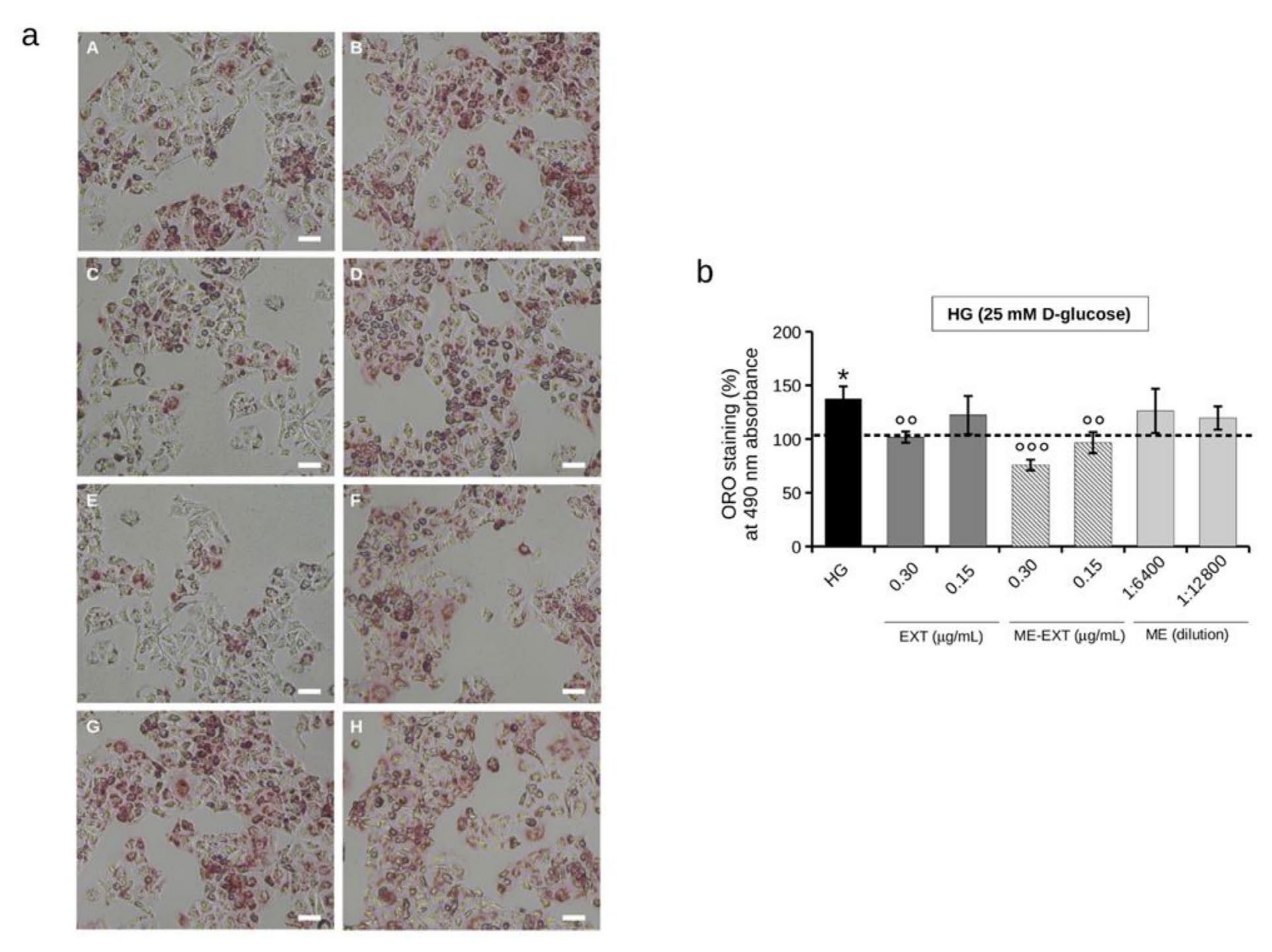
2.6. Effect of EXT and ME-EXT on Intracellular Lipid Accumulation in HepG2 Cells under HG Conditions
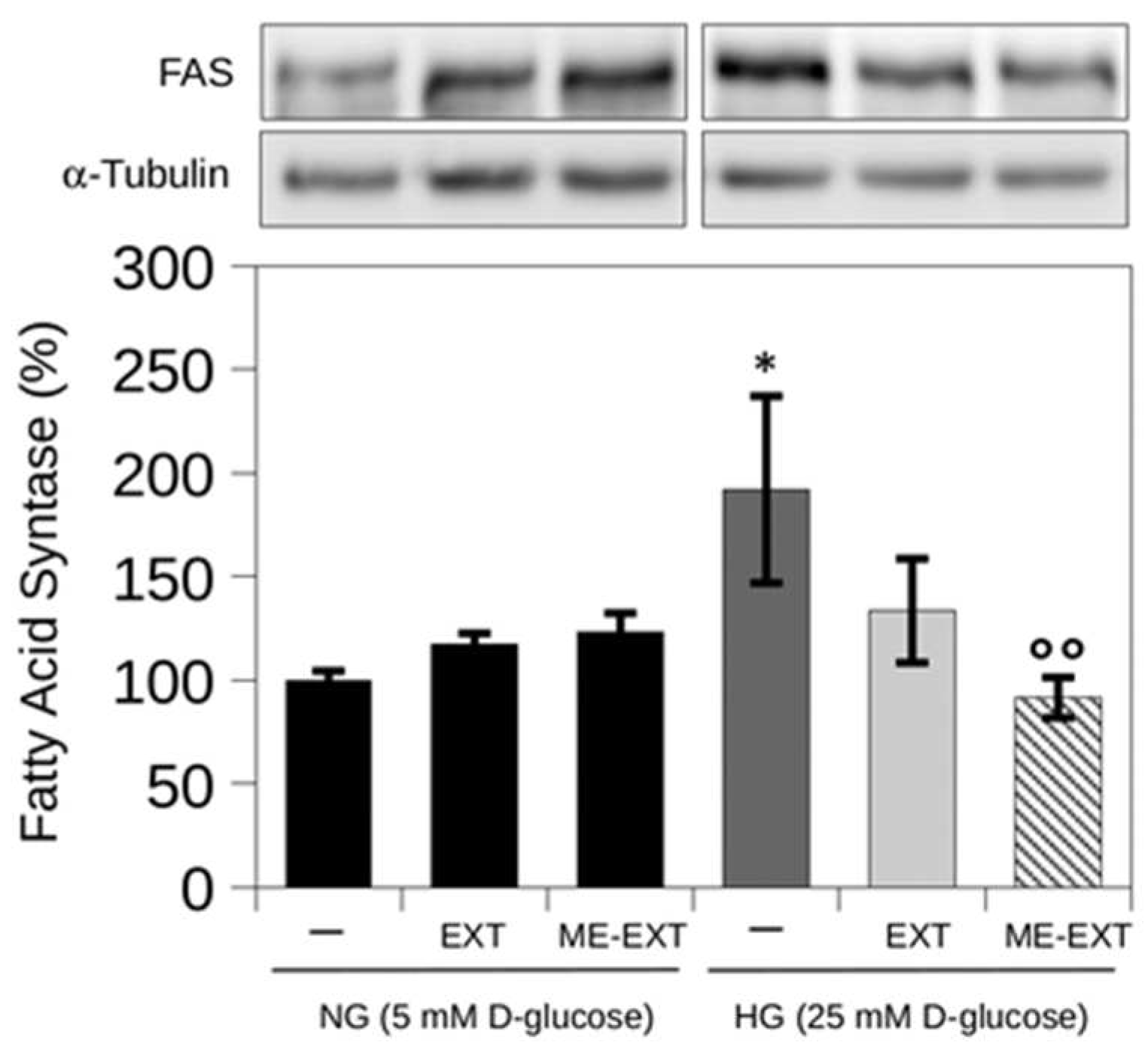
2.7. The Effect of EXT and ME-EXT on High Glucose-Induced FASN Expression in HepG2 Cells
3. Materials and Methods
3.1. Materials
3.2. HPLC-DAD Analysis
3.3. Pseudoternary Phase Diagram
3.4. Preparation of Microemulsion (ME)
3.5. Particle Size and ζ-Potential Measurements
3.6. Morphological Characterization
3.7. In Vitro Release Studies
3.8. Evaluation of Stability over Time
3.9. PAMPA Assay
3.10. Cell Line and Culture Conditions
3.11. Cell Viability Assay
3.12. Intracellular Neutral Lipids Detection (Oil Red O Staining)
3.13. Fatty Acid Synthase (FASN) Detection Using Western Blot Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markhali, F.S.; Teixeira, J.A.; Rocha, C.M.R. Olive Tree Leaves—A Source of Valuable Active Compounds. Processes 2020, 8, 1177. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Gegechkori, V.; Mohammed, E.U.R.; Ku, H.; Morton, D.W. Isolation of Bioactive Pentacyclic Triterpenoid Acids from Olive Tree Leaves with Flash Chromatography. Appl. Sci. 2022, 12, 996. [Google Scholar] [CrossRef]
- Stiti, N.; Hartmann, M.A. Nonsterol Triterpenoids as Major Constituents of Olea europaea. J. Lipids 2012, 2012, 476595. [Google Scholar] [CrossRef] [PubMed]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Palomares, E.E.; Pérez-Jiménez, A.; García-Salguero, L.; Mokhtari, K.; Reyes-Zurita, F.J.; Peragón-Sánchez, J.; Lupiáñez, J.A. Nutraceutical Role of Polyphenols and Triterpenes Present in the Extracts of Fruits and Leaves of Olea europaea as Antioxidants, Anti-Infectives and Anticancer Agents on Healthy Growth. Molecules 2022, 27, 2341. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Palomares, E.E.; Perez-Jimenez, A.; Reyes-Zurita, F.J.; Salguero, L.G.; Mokhtari, K.; Herrera-Merchan, A.; Medina, P.P.; Peragon, J.; Lupianez, J.A. Anti-cancer and Anti-angiogenic Properties of Various Natural Pentacyclic Tri-terpenoids and Some of their Chemical Derivatives. Curr. Org. Chem. 2015, 19, 919–947. [Google Scholar] [CrossRef]
- Guinda, Á.; Rada, M.; Delgado, T.; Gutiérrez-Adánez, P.; Castellano, J.M. Pentacyclic Triterpenoids from Olive Fruit and Leaf. J. Agric. Food Chem. 2010, 58, 9685–9691. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Kim, Y.H.; Kim, H.H.; Ji, H.Y.; Yoo, S.D.; Choi, W.R.; Lee, S.M.; Han, C.K.; Lee, H.S. Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharm. Drug Dispos. 2007, 28, 51–57. [Google Scholar] [CrossRef]
- Song, M.; Hang, T.J.; Wang, Y.; Jiang, L.; Wu, X.L.; Zhang, Z.; Shen, J.; Zhang, Y. Determination of oleanolic acid in human plasma and study of its pharmacokinetics in Chinese healthy male volunteers by HPLC tandem mass spectrometry. J. Pharm. Biomed. Anal. 2006, 40, 190–196. [Google Scholar] [CrossRef]
- De la Torre, R.; Carbó, M.; Pujadas, M.; Biel, S.; Mesa, M.D.; Covas, M.I.; Expósito, M.; Espejo, J.A.; Sanchez-Rodriguez, E.; Díaz-Pellicer, P.; et al. Pharmacokinetics of maslinic and oleanolic acids from olive oil—Effects on endothelial function in healthy adults. A randomized, controlled, dose-response study. Food Chem. 2020, 322, 126676. [Google Scholar] [CrossRef]
- Sánchez-González, M.; Colom, H.; Lozano-Mena, G.; Juan, M.E.; Planas, J.M. Population pharmacokinetics of maslinic acid, a triterpene from olives, after intravenous and oral administration in rats. Mol. Nutr. Food Res. 2014, 58, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Jinhua, W. Ursolic acid: Pharmacokinetics process in vitro and in vivo, a mini review. Arch. Pharm. 2019, 352, e1800222. [Google Scholar] [CrossRef] [PubMed]
- Rada, M.; Ruiz-Gutiérrez, V.; Guinda, Á. Determination of triterpenic acids in human serum by high-performance liquid chromatography: Triterpenoid interaction with serum protein. J. Agric. Food Chem. 2011, 59, 2308–2313. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quesada, C.; López-Biedma, A.; Warleta, F.; Campos, M.; Beltrán, G.; Gaforio, J.J. Bioactive properties of the main triterpenes found in olives, virgin olive oil, and leaves of Olea europaea. J. Agric. Food Chem. 2013, 61, 12173–12182. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Song, J.; Kim, H.R.; Hwang, K.A. Oleanolic acid regulates NF-κB signaling by suppressing MafK expression in RAW 264.7 cells. BMB Rep. 2014, 47, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Yin, M.C. Antioxidative and anti-inflammatory protection of oleanolic acid and ursolic acid in PC12 cells. J. Food Sci. 2008, 73, H174–H178. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Castellano, J.M.; Guinda, A.; Delgado, T.; Rada, M.; Cayuela, J.A. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes 2013, 62, 1791–1799. [Google Scholar] [CrossRef]
- Bu, Y.; Shi, T.; Meng, M.; Kong, G.; Tian, Y.; Chen, Q.; Yao, X.; Feng, G.; Chen, H.; Lu, Z. A novel screening model for the molecular drug for diabetes and obesity based on tyrosine phosphatase Shp2. Bioorg. Med. Chem. Lett. 2011, 21, 1902. [Google Scholar] [CrossRef]
- Buus, N.H.; Hansson, N.C.; Rodriguez-Rodriguez, R.; Stankevicius, E.; Andersen, M.R.; Simonsen, U. Antiatherogenic effects of oleanolic acid in apolipoprotein E knockout mice. Eur. J. Pharmacol. 2011, 670, 519–526. [Google Scholar] [CrossRef]
- Erdmann, J.; Kujaciński, M.; Wiciński, M. Beneficial Effects of Ursolic Acid and Its Derivatives-Focus on Potential Biochemical Mechanisms in Cardiovascular Conditions. Nutrients 2021, 13, 3900. [Google Scholar] [CrossRef]
- Kang, Y.M.; Kim, H.M.; Lee, M.; An, H.J. Oleanolic Acid Alleviates Atopic Dermatitis-like Responses In Vivo and In Vitro. Int. J. Mol. Sci. 2021, 22, 12000. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, X. Improved dissolution of oleanolic acid with ternary solid dispersions. AAPS PharmSciTech 2007, 8, E113. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Yang, H.; Long, C. Oleanolic Acid Improves the Symptom of Renal Ischemia Reperfusion Injury via the PI3K/AKT Pathway. Urol. Int. 2021, 105, 215–220. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Sayed, A.M.; Sharif, A.M.; Azhar, E.I.; Rateb, M.E. Olive-Derived Triterpenes Suppress SARS CoV-2 Main Protease: A Promising Scaffold for Future Therapeutics. Molecules 2021, 26, 2654. [Google Scholar] [CrossRef] [PubMed]
- Ghante, M.H.; Jamkhande, P.G. Role of Pentacyclic Triterpenoids in Chemoprevention and Anticancer Treatment: An Overview on Targets and Underling Mechanisms. J. Pharmacopunct. 2019, 22, 55–67. [Google Scholar] [CrossRef]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef]
- Banerjee, S.; Bose, S.; Mandal, S.C.; Dawn, S.; Sahoo, U.; Ramadan, M.A.; Mandal, S.K. Pharmacological Property of Pentacyclic Triterpenoids. Egypt. J. Chem. 2019, 62, 13–35. [Google Scholar] [CrossRef]
- Wang, X.; Bai, H.; Zhang, X.; Liu, J.; Cao, P.; Liao, N.; Zhang, W.; Wang, Z.; Hai, C. Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis. Carcinogenesis 2013, 34, 1323–1330. [Google Scholar] [CrossRef]
- Jannus, F.; Medina-O’Donnell, M.; Rivas, F.; Díaz-Ruiz, L.; Rufino-Palomares, E.E.; Lupiáñez, J.A.; Parra, A.; Reyes-Zurita, F.J. A Diamine-PEGylated Oleanolic Acid Derivative Induced Efficient Apoptosis through a Death Receptor and Mitochondrial Apoptotic Pathway in HepG2 Human Hepatoma Cells. Biomolecules 2020, 10, 1375. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Antwi, S.O.; Craver, E.C.; Nartey, Y.A.; Sartorius, K.; Patel, T. Metabolic Risk Factors for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease: A Prospective Study. Cancers 2022, 14, 6234. [Google Scholar] [CrossRef]
- Paul, B.; Lewinska, M.; Andersen, J.B. Lipid alterations in chronic liver disease and liver cancer. JHEP Rep. 2022, 4, 100479. [Google Scholar] [CrossRef] [PubMed]
- Currie, E.; Schulze, A.; Zechner, R.; Walther, T.C.; Farese, R.V., Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013, 18, 153–161. [Google Scholar] [CrossRef]
- Hao, Q.; Li, T.; Zhang, X.; Gao, P.; Qiao, P.; Li, S.; Geng, Z. Expression and roles of fatty acid synthase in hepatocellular carcinoma. Oncol. Rep. 2014, 32, 2471–2476. [Google Scholar] [CrossRef]
- Li, L.; Che, L.; Tharp, K.M.; Park, H.M.; Pilo, M.G.; Cao, D.; Cigliano, A.; Latte, G.; Xu, Z.; Ribback, S.; et al. Differential requirement for de novo lipogenesis in cholangiocarcinoma and hepatocellular carcinoma of mice and humans. Hepatology 2016, 63, 1900–1913. [Google Scholar] [CrossRef]
- Reveron-Thornton, R.F.; Teng, M.L.P.; Lee, E.Y.; Tran, A.; Vajanaphanich, S.; Tan, E.X.; Nerurkar, S.N.; Ng, R.X.; The, R.; Tripathy, D.P.; et al. Global and regional long-term survival following resection for HCC in the recent decade: A meta-analysis of 110 studies. Hepatol. Commun. 2022, 6, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Monteforte, E.; Luceri, C.; Bigagli, E.; Bilia, A.R.; Bergonzi, M.C. Nanoemulsion for improving solubility and permeability of Vitex agnus-castus extract: Formulation and in vitro evaluation using PAMPA and Caco-2 approaches. Drug Deliv. 2017, 24, 380–390. [Google Scholar] [CrossRef]
- De Stefani, C.; Vasarri, M.; Salvatici, M.C.; Grifoni, L.; Quintela, J.C.; Bilia, A.R.; Degl’Innocenti, D.; Bergonzi, M.C. Microemulsions Enhance the In Vitro Antioxidant Activity of Oleanolic Acid in RAW 264.7 Cells. Pharmaceutics 2022, 14, 2232. [Google Scholar] [CrossRef] [PubMed]
- Lodovichi, J.; Landucci, E.; Pitto, L.; Gisone, I.; D’Ambrosio, M.; Luceri, C.; Salvatici, M.C.; Bergonzi, M.C. Evaluation of the increase of the thymoquinone permeability formulated in polymeric micelles: In vitro test and in vivo toxicity assessment in Zebrafish embryos. Eur. J. Pharm. Sci. 2022, 169, 106090. [Google Scholar] [CrossRef] [PubMed]
- Vasarri, M.; Barletta, E.; Stio, M.; Bergonzi, M.C.; Galli, A.; Degl’Innocenti, D. Ameliorative Effect of Posidonia oceanica on High Glucose-Related Stress in Human Hepatoma HepG2 Cells. Int. J. Mol. Sci. 2023, 24, 5203. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.M. Synergy and other interactions in phytomedicins. Phytomedicins 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.H.; Lee, B.Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, L.; Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Rocco, F.; Innocenti, M.; Vanti, G.; Mulinacci, N.; Bergonzi, M.C. Formulation of a Phenol-Rich Extract from Unripe Olives (Olea europaea L.) in Microemulsion to Improve Its Solubility and Intestinal Permeability. Molecules 2020, 25, 3198. [Google Scholar] [CrossRef]
- Porter, C.J.; Pouton, C.W.; Cuine, J.F.; Charman, W.N. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.M.; Cui, F.D.; Mu, C.F.; Choi, M.K.; Kim, J.S.; Chung, S.J.; Shim, C.K.; Kim, D.D. Docetaxel microemulsion for enhanced oral bioavailability: Preparation and in vitro and in vivo evaluation. J. Control Release 2009, 140, 86–94. [Google Scholar] [CrossRef]
- Piazzini, V.; Rosseti, C.; Bigagli, E.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Prediction of Permeation and Cellular Transport of Silybum marianum Extract Formulated in a Nanoemulsion by Using PAMPA and Caco-2 Cell Models. Planta Med. 2017, 83, 1184–1193. [Google Scholar] [CrossRef]
- Lin, Y.L.; Shen, Q.; Katsumi, H.; Okada, N.; Fujita, T.; Jiang, X.H.; Yamamoto, A. Effects of Labrasol and other pharmaceutical excipients on the intestinal transport and absorption of rhodamine123, a P-glycoprotein substrate, in rats. Biol. Pharm. Bull. 2007, 30, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Campos, C. Chronic hyperglycemia and glucose toxicity: Pathology and clinical sequelae. Postgrad. Med. 2012, 124, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, M.; Su, J.; Li, Y.; Long, J.; Chu, J.; Wan, X.; Cao, Y.; Li, Q. Lipid Metabolism and Cancer. Life 2022, 12, 784. [Google Scholar] [CrossRef]
- Khan, W.; Augustine, D.; Rao, R.S.; Patil, S.; Awan, K.H.; Sowmya, S.V.; Haragannavar, V.C.; Prasad, K. Lipid metabolism in cancer: A systematic review. J. Carcinog. 2021, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Takemori, H.; Wang, C.; Fu, J.; Xu, M.; Xiong, L.; Li, N.; Wen, X. Role of salt inducible kinase 1 in high glucose-induced lipid accumulation in HepG2 cells and metformin intervention. Life Sci. 2017, 173, 107–115. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, X.; Bai, J.; Zhong, P.; Tan, S.; Zeng, W.; Chen, J.; Sun, Z.; Liu, Z.; Jin, J.; et al. Triterpenoids from the genus Ilex attenuate free fatty acid-induced lipid accumulation in HepG2 cells by regulating lipid metabolism disorder and the AMPK signalling pathway. J. Ethnopharmacol. 2023, 302, 115845. [Google Scholar] [CrossRef]
- Yang, H.M.; Yin, Z.Q.; Zhao, M.G.; Jiang, C.H.; Zhang, J.; Pan, K. Pentacyclic triterpenoids from Cyclocarya paliurus and their antioxidant activities in FFA-induced HepG2 steatosis cells. Phytochemistry 2018, 151, 119–127. [Google Scholar] [CrossRef]
- Sharma, H.; Kumar, P.; Deshmukh, R.R.; Bishayee, A.; Kumar, S. Pentacyclic triterpenes: New tools to fight metabolic syndrome. Phytomedicine 2018, 50, 166–177. [Google Scholar] [CrossRef]
- Balcazar, N.; Betancur, L.I.; Muñoz, D.L.; Cabrera, F.J.; Castaño, A.; Echeverri, L.F.; Acin, S. Ursolic Acid Lactone Obtained from Eucalyptus tereticornis Increases Glucose Uptake and Reduces Inflammatory Activity and Intracellular Neutral Fat: An In Vitro Study. Molecules 2021, 26, 2282. [Google Scholar] [CrossRef]
- Liu, G.; Cui, Z.; Gao, X.; Liu, H.; Wang, L.; Gong, J.; Wang, A.; Zhang, J.; Ma, Q.; Huang, Y.; et al. Corosolic acid ameliorates non-alcoholic steatohepatitis induced by high-fat diet and carbon tetrachloride by regulating TGF-β1/Smad2, NF-κB, and AMPK signaling pathways. Phytother. Res. 2021, 35, 5214–5226. [Google Scholar] [CrossRef]
- Azman, M.; Sabri, A.H.; Anjani, Q.K.; Mustaffa, M.F.; Hamid, K.A. Intestinal Absorption Study: Challenges and Absorption Enhancement Strategies in Improving Oral Drug Delivery. Pharmaceuticals 2022, 15, 975. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio, B.V.; Silva, P.B.; Ramos, M.A.; Negri, K.M.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar] [CrossRef]
- Dewi, M.K.; Chaerunisaa, A.Y.; Muhaimin, M.; Joni, I.M. Improved Activity of Herbal Medicines through Nanotechnology. Nanomaterials 2022, 12, 4073. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, P.P. Lipid microemulsions for improving drug dissolution and oral absorption: Physical and biopharmaceutical aspects. Pharm. Res. 1995, 12, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Solinas, G.; Borén, J.; Dulloo, A.G. De novo lipogenesis in metabolic homeostasis: More friend than foe? Mol. Metab. 2015, 4, 367–377. [Google Scholar] [CrossRef]
- Jensen-Urstad, A.P.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta 2012, 1821, 747–753. [Google Scholar] [CrossRef]
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef]
- Villanueva-Ortega, E.; Méndez-García, L.A.; Garibay-Nieto, G.N.; Laresgoiti-Servitje, E.; Medina-Bravo, P.; Olivos-García, A.; Muñoz-Ortega, M.H.; Ventura-Juárez, J.; Escobedo, G. Growth hormone ameliorates high glucose-induced steatosis on in vitro cultured human HepG2 hepatocytes by inhibiting de novo lipogenesis via ChREBP and FAS suppression. Growth Horm. IGF Res. 2020, 53–54, 101332. [Google Scholar] [CrossRef]
- Fhu, C.W.; Ali, A. Fatty Acid Synthase: An Emerging Target in Cancer. Molecules 2020, 25, 3935. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, G.; Ni, Q.; Wang, Y.; Gao, Q.; Zhang, Y. Microemulsion Delivery System Improves Cellular Uptake of Genipin and Its Protective Effect against Aβ1-42-Induced PC12 Cell Cytotoxicity. Pharmaceutics 2022, 14, 617. [Google Scholar] [CrossRef] [PubMed]
- Guinda, A.; Castellano, J.M.; Santos-Lozano, J.M.; Delgado-Hervás, T.; Gutiérrez-Adánez, P.; Rada, M. Determination of major bioactive compounds from olive leaf. LWT-Food Sci. Technol. 2015, 64, 431–438. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Murthy, R.S. Microemulsions: A potential drug delivery system. Curr. Drug Deliv. 2006, 3, 167–180. [Google Scholar] [CrossRef] [PubMed]
- USP 25 the United States Pharmacopeia. NF 20 The National Formulary, 20th ed.; Revision; Supplement; United States Pharmacopeial Convention: Rockville, MD, USA, 2002; print. [Google Scholar]
- Vasarri, M.; Barletta, E.; Degl’Innocenti, D. Posidonia oceanica (L.) Delile Extract Reduces Lipid Accumulation through Autophagy Activation in HepG2 Cells. Pharmaceuticals 2021, 14, 969. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasarri, M.; Degl’Innocenti, D.; Albonetti, L.; Bilia, A.R.; Bergonzi, M.C. Pentacyclic Triterpenes from Olive Leaves Formulated in Microemulsion: Characterization and Role in De Novo Lipogenesis in HepG2 Cells. Int. J. Mol. Sci. 2023, 24, 12113. https://doi.org/10.3390/ijms241512113
Vasarri M, Degl’Innocenti D, Albonetti L, Bilia AR, Bergonzi MC. Pentacyclic Triterpenes from Olive Leaves Formulated in Microemulsion: Characterization and Role in De Novo Lipogenesis in HepG2 Cells. International Journal of Molecular Sciences. 2023; 24(15):12113. https://doi.org/10.3390/ijms241512113
Chicago/Turabian StyleVasarri, Marzia, Donatella Degl’Innocenti, Laura Albonetti, Anna Rita Bilia, and Maria Camilla Bergonzi. 2023. "Pentacyclic Triterpenes from Olive Leaves Formulated in Microemulsion: Characterization and Role in De Novo Lipogenesis in HepG2 Cells" International Journal of Molecular Sciences 24, no. 15: 12113. https://doi.org/10.3390/ijms241512113
APA StyleVasarri, M., Degl’Innocenti, D., Albonetti, L., Bilia, A. R., & Bergonzi, M. C. (2023). Pentacyclic Triterpenes from Olive Leaves Formulated in Microemulsion: Characterization and Role in De Novo Lipogenesis in HepG2 Cells. International Journal of Molecular Sciences, 24(15), 12113. https://doi.org/10.3390/ijms241512113








