Tako-Tsubo Syndrome in Amyotrophic Lateral Sclerosis: Single-Center Case Series and Brief Literature Review
Abstract
1. Introduction
2. Case Presentation
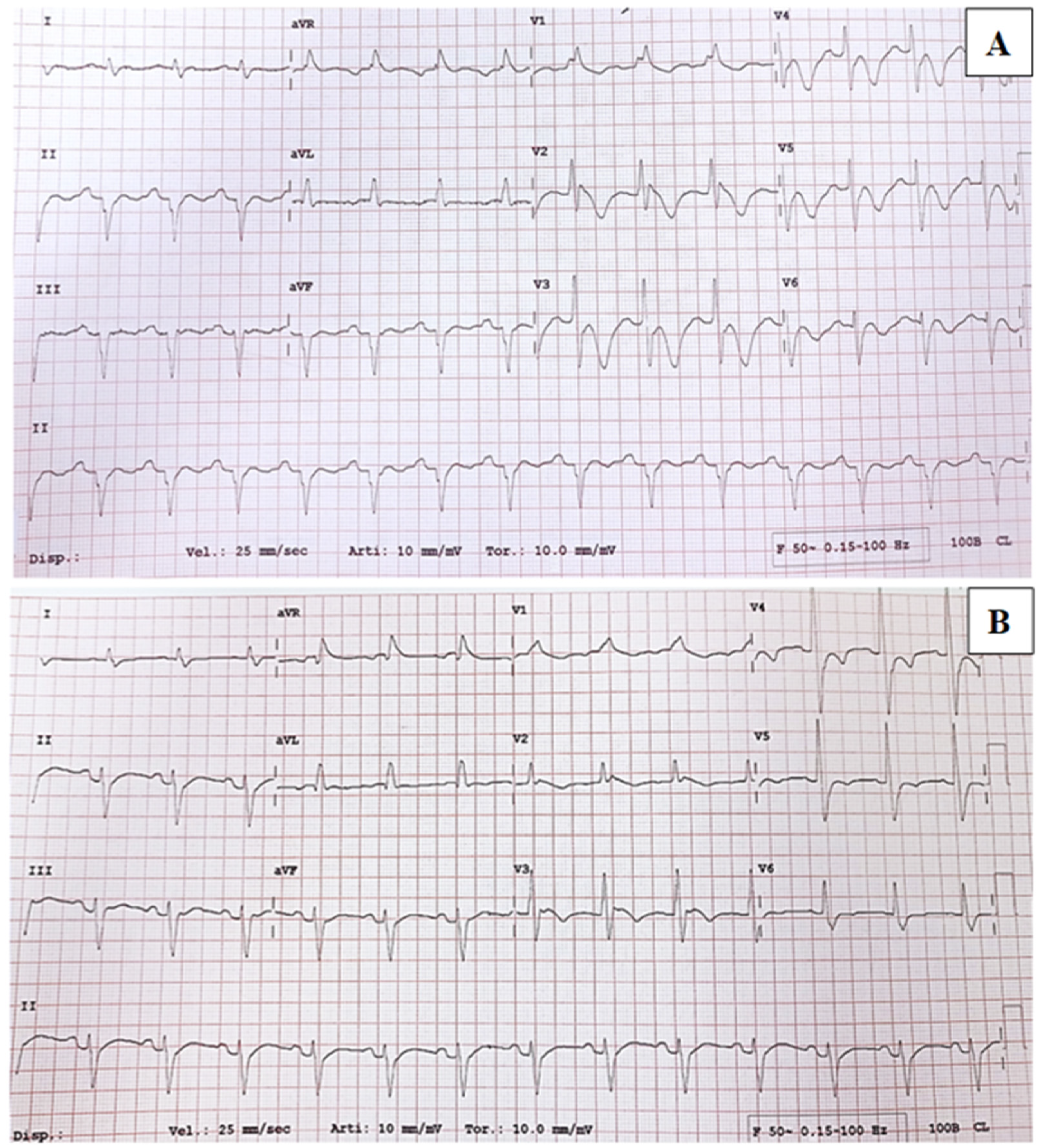
2.1. Case Series
2.2. Literature Review
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swinnen, B.; Robberecht, W. The phenotypic variability of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2014, 10, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Calvo, A.; Moglia, C.; Mazzini, L.; Mora, G.; PARALS Study Group. Phenotypic heterogeneity of amyotrophic lateral sclerosis: A population based study. J. Neurol. Neurosurg. Psychiatry 2011, 82, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Piccione, E.A.; Sletten, D.M.; Staff, N.P.; Low, P.A. Autonomic system and amyotrophic lateral sclerosis. Muscle Nerve 2015, 51, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Merico, A.; Cavinato, M. Autonomic dysfunction in the early stage of ALS with bulbar involvement. Amyotroph. Lateral Scler. 2011, 12, 363–367. [Google Scholar] [CrossRef]
- Vecchia, L.D.; De Maria, B.; Marinou, K.; Sideri, R.; Lucini, A.; Porta, A.; Mora, G. Cardiovascular neural regulation is impaired in amyotrophic lateral sclerosis patients. A study by spectral and complexity analysis of cardiovascular oscillations. Physiol. Meas. 2015, 36, 659–670. [Google Scholar] [CrossRef]
- Chida, K.; Sakamaki, S.; Takasu, T. Alteration in autonomic function and cardiovascular regulation in amyotrophic lateral sclerosis. J. Neurol. 1989, 236, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Congiu, P.; Mariani, S.; Milioli, G.; Parrino, L.; Tamburrino, L.; Borghero, G.; Defazio, G.; Pereira, B.; Fantini, M.L.; Puligheddu, M. Sleep cardiac dysautonomia and EEG oscillations in amyotrophic lateral sclerosis. Sleep 2019, 42, zsz164. [Google Scholar] [CrossRef]
- Drusehky, A.; Spitzer, A.; Platseh, G.; Claus, D.; Feistel, H.; Druschky, K.; Hilz, M.-J.; Neundörfer, B. Cardiac sympathetic denervation in early stages of amyotrophic lateral sclerosis demonstrated by 123I-MIBG-SPECT. Acta Neurol. Scand. 1999, 99, 308–314. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Sasagasako, N.; Koike, A.; Matsuura, M.; Koga, T.; Kawajiri, M.; Ohyagi, Y.; Iwaki, T.; Kira, J.-I. An autopsy case of amyotrophic lateral sclerosis with ampulla cardiomyopathy. Clin. Neurol. 2008, 48, 249–254. [Google Scholar]
- Mitani, M.; Funakawa, I.; Jinnai, K. Transient left ventricular apical ballooning, ‘Takotsubo’ cardiomyopathy, in an amyotrophic lateral sclerosis patient on long-term respiratory support. Rinsho Shinkeigaku 2005, 45, 740–743. [Google Scholar] [PubMed]
- Takayama, N.; Iwase, Y.; Ohtsu, S.; Sakio, H. “Takotsubo” cardiomyopathy developed in the postoperative period in a patient with amyotrophic lateral sclerosis. Masui. Jpn. J. Anesthesiol. 2004, 53, 403–406. [Google Scholar]
- Izumi, Y.; Miyamoto, R.; Fujita, K.; Yamamoto, Y.; Yamada, H.; Matsubara, T.; Unai, Y.; Tsukamoto, A.; Takamatsu, N.; Nodera, H.; et al. Distinct incidence of takotsubo syndrome between amyotrophic lateral sclerosis and synucleinopathies: A cohort study. Front. Neurol. 2018, 9, 1099. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Scantlebury, D.C.; Prasad, A. Diagnosis of takotsubo cardiomyopathy—Mayo Clinic criteria. Circ. J. 2014, 78, 2129–2139. [Google Scholar] [CrossRef]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- de Carvalho, M.; Dengler, R.; Eisen, A.; England, J.D.; Kaji, R.; Kimura, J.; Mills, K.; Mitsumoto, H.; Nodera, H.; Shefner, J.; et al. Electrodiagnostic criteria for diagnosis of ALS. Clin. Neurophysiol. 2008, 119, 497–503. [Google Scholar] [CrossRef]
- Riva, N.; Pozzi, L.; Russo, T.; Pipitone, G.B.; Schito, P.; Domi, T.; Agosta, F.; Quattrini, A.; Carrera, P.; Filippi, M. NEK1 Variants in a Cohort of Italian Patients with Amyotrophic Lateral Sclerosis. Front. Neurosci. 2022, 16, 833051. [Google Scholar] [CrossRef]
- Massari, F.M.; Tonella, T.; Tarsia, P.; Kirani, S.; Blasi, F.; Magrini, F. Sindrome tako-tsubo in giovane uomo affetto da sclerosi laterale amiotrofica. Descrizione di un caso clinico. G. Ital. Cardiol. 2011, 12, 388–391. [Google Scholar]
- Choi, S.-J.; Hong, Y.-H.; Shin, J.-Y.; Yoon, B.-N.; Sohn, S.-Y.; Park, C.S.; Sung, J.-J. Takotsubo cardiomyopathy in amyotrophic lateral sclerosis. J. Neurol. Sci. 2017, 375, 289–293. [Google Scholar] [CrossRef]
- Işcan, D.; Karaaslan, M.B.; Deveci, O.S.; Eker, R.A.; Koç, F. The importance of heart rate variability in predicting cardiac autonomic dysfunction in patients with amyotrophic lateral sclerosis. Int. J. Clin. Pract. 2021, 75, e14536. [Google Scholar] [CrossRef] [PubMed]
- Peters, S. Tako tsubo cardiomyopathy in respiratory stress syndrome in amyotrophic lateral sclerosis. Int. J. Cardiol. 2014, 177, 187. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Ieva, R.; Ferraretti, A.; Carapelle, E.; De Gennaro, L.; Specchio, L.M.; Di Biase, M.; Brunetti, N.D. Early recurrence of Tako-Tsubo cardiomyopathy in an elderly woman with amyotrophic lateral sclerosis: Different triggers inducing different apical ballooning patterns. J. Cardiovasc. Med. 2016, 17, e266–e268. [Google Scholar] [CrossRef]
- Gdynia, H.J.; Kurt, A.; Endruhn, S.; Ludolph, A.C.; Sperfeld, A.D. Cardiomyopathy in motor neuron diseases. J. Neurol. Neurosurg. Psychiatry 2006, 77, 671–673. [Google Scholar] [CrossRef]
- Asai, H.; Hirano, M.; Udaka, F.; Shimada, K.; Oda, M.; Kubori, T.; Nishinaka, K.; Tsujimura, T.; Izumi, Y.; Konishi, N.; et al. Sympathetic disturbances increase risk of sudden cardiac arrest in sporadic ALS. J. Neurol. Sci. 2007, 254, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Brettschneider, J.; Del Tredici, K.; Toledo, J.B.; Robinson, J.L.; Irwin, D.J.; Grossman, M.; Suh, E.R.; Van Deerlin, V.M.; Wood, E.M.; Baek, Y.; et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013, 74, 20–38. [Google Scholar] [CrossRef]
- Mendoza-Velásquez, J.J.; Flores-Vázquez, J.F.; Barrón-Velázquez, E.; Sosa-Ortiz, A.L.; Illigens, B.M.W.; Siepmann, T. Autonomic Dysfunction in α-Synucleinopathies. Front. Neurol. 2019, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Muse, J.; Taussky, P. Takotsubo Syndrome in Neurologic Disease. World Neurosurg. 2021, 149, 26–31. [Google Scholar] [CrossRef]
- Ziegelstein, R.C. Depression and tako-tsubo cardiomyopathy. Am. J. Cardiol. 2010, 105, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Rosenbohm, A.; Schmid, B.; Buckert, D.; Rottbauer, W.; Kassubek, J.; Ludolph, A.C.; Bernhardt, P. Cardiac Findings in Amyotrophic Lateral Sclerosis: A Magnetic Resonance Imaging Study. Front. Neurol. 2017, 8, 479. [Google Scholar] [CrossRef]
- Hammond, H.K.; Roth, D.A.; Ford, C.E.; Stamnas, G.W.; Ziegler, M.G.; Ennis, C. Myocardial adrenergic denervation supersensitivity depends on a postreceptor mechanism not linked with increased cAMP production. Circulation 1992, 85, 666–679. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Patient (Sex) | 1 (Case Report, M) | 2 (F) | 3 (M) | 4 (F) |
|---|---|---|---|---|
| ALS age of onset (years) | 54 | 74 | 60 | 71 |
| Site of onset | Upper limbs | Bulbar | Lower limbs | Lower limbs |
| ALS phenotype (according to Chiò, et al. 2011 [2]) | Classic | Bulbar | LMN | Classic |
| ALS disease duration (from clinical onset) | 73 months | 19 months (†) | 103 months (†) | 38 months (†) |
| TTS (months after ALS onset) | 73 months | 11 months | 86 months | 38 months |
| Awaji at ALS diagnosis | Probable | Probable | Possible | Possible |
| ALSFRS-R * | 15 | 45 | 19 | 18 |
| KSS * | 4 | 1 | 4 | 4 |
| MiToS * | 3 | 1 | 3 | 3 |
| ALS genetics | / | C9orf72 | / | / |
| Concomitant Medication | Riluzole, Acetyl-L-carnitine | Riluzole, Acetyl-L-carnitine, Baclofen | Riluzole | Riluzole |
| Time to Ventilation | 73 months | N/A | 86 months | 32 months |
| Time to Tracheostomy | N/A | N/A | 91 months | 38 months |
| Time to PEG | 75 months | N/A | N/A | 38 months |
| Elevated ST/negative T at ECG | Prolonged QT, unspecific T wave alterations | Sinus tachycardia, VEBs | Negative antero-septal T | Aspecific conduction alterations |
| Troponin T (ng/dL) | 231 | 421 | 459 | 316 |
| pro-BNP (pg/mL) | 939 | 34.309 | 3.074 | / |
| CPK (U/L) | N/A | 44 | 71 | 49 |
| Ejection Fraction | 45% | 30% | 35% | 35% |
| Apical dyskinesia/ballooning at echocardiography | Yes | Yes | Yes | Yes |
| Coronary CT/coronarography | Heart angio-CT: negative | Coronarography: negative | N/A | Heart CT: negative |
| Pulmonary arterial Pressure | Normal | 75 mmHg | Normal | Normal |
| Cardiovascular comorbidities | PSVT | HT | HT | T2DM, CAD |
| Intervening condition | Respiratory failure (adapted to NIV) | PE | PE + lower UTI | TS + PEG |
| Reference | Number of Cases | Type of Onset | Ventilatory Support | Bulbar Signs | Intervening Conditions |
|---|---|---|---|---|---|
| Takayama et al., 2004 [12] | 1 | Classic | N/A | N/A | Surgical gastrostomy; repair of incisional hernia (with tracheal intubation) |
| Mitani et al., 2005 [11] | 1 | Bulbar | NIV | Dysphagia, dysarthria. | Long-term NIV |
| Matsuyama et al., 2008 [10] | 1 | Classic | TIV | N/A | Tracheostomy |
| Massari et al., 2011 [19] | 1 | Classic | NIV | Dysphagia, dysarthria | Pneumonia |
| Peters, 2014 [22] | 1 | N/A | NIV | N/A | Respiratory distress syndrome, pneumonia |
| Santoro et al., 2016 [23] | 1 | N/A | N/A | N/A | Emotional distress (first episode); Femoral artery thrombosis (second episode) |
| Gdynia et al., 2006 [24] | 1 | Classic | N/A | N/A | Major surgery |
| Choi et al., 2017 [20] | 9 | Bulbar (pt.2,5,6) Cervical (pt.1,3,4,7,8) Respiratory (pt.9) | NIV (pt.2,5,7), TIV (pt.6,8) | N/A | N/A |
| Izumi et al., 2018 [13] | 4 | Bulbar (pt.1,4), Classic (pt.2,3) | TIV (pt.1,2) | N/A | UTI (pt.1); acute cholangitis (pt.2); pneumonia (pt.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napoli, G.; Rubin, M.; Cutillo, G.; Schito, P.; Russo, T.; Quattrini, A.; Filippi, M.; Riva, N. Tako-Tsubo Syndrome in Amyotrophic Lateral Sclerosis: Single-Center Case Series and Brief Literature Review. Int. J. Mol. Sci. 2023, 24, 12096. https://doi.org/10.3390/ijms241512096
Napoli G, Rubin M, Cutillo G, Schito P, Russo T, Quattrini A, Filippi M, Riva N. Tako-Tsubo Syndrome in Amyotrophic Lateral Sclerosis: Single-Center Case Series and Brief Literature Review. International Journal of Molecular Sciences. 2023; 24(15):12096. https://doi.org/10.3390/ijms241512096
Chicago/Turabian StyleNapoli, Giovanni, Martina Rubin, Gianni Cutillo, Paride Schito, Tommaso Russo, Angelo Quattrini, Massimo Filippi, and Nilo Riva. 2023. "Tako-Tsubo Syndrome in Amyotrophic Lateral Sclerosis: Single-Center Case Series and Brief Literature Review" International Journal of Molecular Sciences 24, no. 15: 12096. https://doi.org/10.3390/ijms241512096
APA StyleNapoli, G., Rubin, M., Cutillo, G., Schito, P., Russo, T., Quattrini, A., Filippi, M., & Riva, N. (2023). Tako-Tsubo Syndrome in Amyotrophic Lateral Sclerosis: Single-Center Case Series and Brief Literature Review. International Journal of Molecular Sciences, 24(15), 12096. https://doi.org/10.3390/ijms241512096







