Recent Advances in Molecular Mechanism and Breeding Utilization of Brown Planthopper Resistance Genes in Rice: An Integrated Review
Abstract
1. Introduction
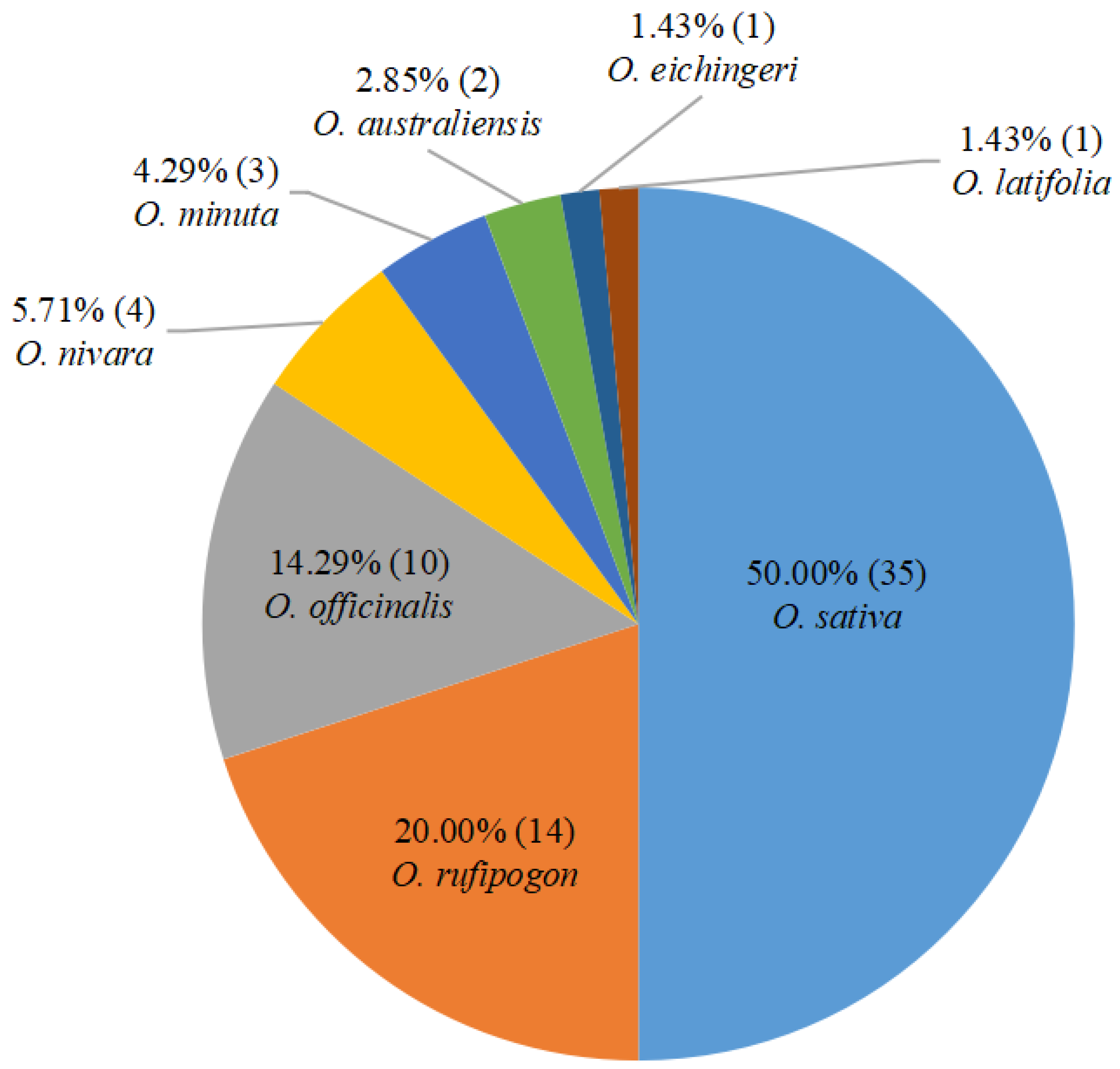
2. BPH-Resistant Rice Germplasm Resources
3. Mapping of BPH-Resistant Genes/QTLs in Rice
4. Cloning of BPH-Resistant Genes in Rice
4.1. Genes Associated with LecRKs
4.2. Genes Associated with CC-NB-LRR Proteins
4.3. LRD Protein-Related Genes
4.4. B3-DNA Domain Protein Gene
4.5. SCR Domain Protein-Related Gene
5. Molecular Mechanism of Rice Resistance to BPH
5.1. Recognition of Feeding Signals of Rice to BPH
5.2. Signaling of Rice Defense against BPH
5.2.1. Plant Hormones
5.2.2. Calcium Signaling
5.2.3. MAPK Cascade Reaction
5.2.4. Transcription Factors
5.3. miRNA-Mediated Rice BPH Resistance
6. Marker-Assisted Breeding for BPH Resistance
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khush, G.S. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol. Biol. 2005, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tuyen, L.Q.; Liu, Y.; Jiang, L.; Wang, B.; Wang, Q.; Hanh, T.T.T.; Wan, J. Identification of quantitative trait loci associated with small brown planthopper (Laodelphax striatellus Fallén) resistance in rice (Oryza sativa L.). Hereditas 2012, 149, 16–23. [Google Scholar] [CrossRef]
- Chandler, R.F. Improving the rice plant and its culture. Nature 1969, 221, 1007–1010. [Google Scholar] [CrossRef]
- Anonymous. Planthopphers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia; Heong, K.L., Hardy, B., Eds.; IRRI Books; International Rice Research Institute: Los Baños, Philippines, 2009; p. 164471. [Google Scholar]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Chang, X.; Wang, F.; Fang, Q.; Chen, F.; Yao, H.; Gatehouse, A.; Ye, G. Virus-induced plant volatiles mediate the olfactory behaviour of its insect vectors. Plant Cell Environ. 2021, 44, 2700–2715. [Google Scholar] [CrossRef] [PubMed]
- Sarao, P.S.; Sahi, G.K.; Neelam, K.; Mangat, G.S.; Patra, B.C.; Singh, K. Donors for resistance to brown planthopper Nilaparvata lugens Stålfrom wild rice species. Rice Sci. 2016, 23, 219–224. [Google Scholar] [CrossRef]
- Feng, C.; Wan, Z.; Lan, L.; Le, K. Rice responses and resistance to planthopper-borne viruses at transcriptomic and proteomic levels. Curr. Issues Mol. Biol. 2015, 19, 43–52. [Google Scholar]
- Lu, K.; Chen, X.; Liu, W.; Zhang, Z.; Wang, Y.; You, K.; Li, Y.; Zhang, R.; Zhou, Q. Characterization of heat shock protein 70 transcript from Nilaparvata lugens Stål: Its response to temperature and insecticide stresses. Pestic. Biochem. Phys. 2017, 142, 102–110. [Google Scholar] [CrossRef]
- Way, M.J.; Heong, K.L. The role of biodiversity in the dynamics and management of insect pests of tropical irrigated rice—A review. Bull. Entomol. Res. 1994, 84, 567–587. [Google Scholar] [CrossRef]
- Wu, S.F.; Zeng, B.; Zheng, C.; Mu, X.C.; Zhang, Y.; Hu, J.; Zhang, S.; Gao, C.F.; Shen, J.L. The evolution of insecticide resistance in the brown planthopper (Nilaparvata lugens Stål) of China in the period 2012-2016. Sci. Rep. 2018, 8, 4586. [Google Scholar] [CrossRef]
- Wu, J.; Ge, L.; Liu, F.; Song, Q.; Stanley, D. Pesticide-induced planthopper population resurgence in rice cropping systems. Annu. Rev. Entomol. 2020, 65, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.Q.; Huang, L.J.; Yang, G.Q.; Song, Q.S.; Stanley, D.; Gurr, G.M.; Wu, J.C. Molecular basis for insecticide-enhanced thermotolerance in the brown planthopper Nilaparvata lugens Stål (Hemiptera: Delphacidae). Mol. Ecol. 2013, 22, 5624–5634. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.C. Host plant resistance to insects: Modern approaches and limitations. Plant Prot. Assoc. India 2007, 35, 179–184. [Google Scholar]
- Gurr, G.M.; Liu, J.; Read, D.; Catindig, J.; Cheng, J.A.; Lan, L.P.; Heong, K.L. Parasitoids of asian rice planthopper (hemiptera: Delphacidae) pests and prospects for enhancing biological control by ecological engineering. Ann. Appl. Biol. 2015, 158, 149–176. [Google Scholar] [CrossRef]
- Khush, G.S. Green revolution: The way forward. Nat. Rev. Genet. 2001, 2, 815–822. [Google Scholar] [CrossRef]
- Zhang, Q. Strategies for developing green super rice. Proc. Natl. Acad. Sci. USA 2007, 104, 16402–16409. [Google Scholar] [CrossRef] [PubMed]
- Brar, D.S.; Khush, G.S. Alien introgression in rice. Plant Mol. Biol. 1997, 35, 35–47. [Google Scholar] [CrossRef]
- Jena, K.K. The species of the genus Oryza and transfer of useful genes from wild species into cultivated rice, O. sativa. Breed. Sci. 2010, 60, 518–523. [Google Scholar] [CrossRef]
- Cha, Y.S.; Ji, H.; Yun, D.W.; Ahn, B.O.; Lee, M.C.; Suh, S.C.; Lee, C.S.; Ahn, E.K.; Jeon, Y.H.; Jin, I.D.; et al. Fine mapping of the rice Bph1 gene, which confers resistance to the brown planthopper (Nilaparvata lugens Stål), and development of sts markers for marker-assisted selection. Mol. Cells 2008, 26, 146–151. [Google Scholar]
- Pathak, M.D.; Cheng, C.H.; Fortuno, M.E. Resistance to nephotettix impicticeps and Nilaparvata lugens in varieties of rice. Nature 1969, 223, 502–504. [Google Scholar] [CrossRef]
- Athwal, D. Genetics of resistance to brown planthoppers and green leafhoppers in Oryza sativa L. Crop Sci. 1971, 11, 747–750. [Google Scholar] [CrossRef]
- Sun, L.H.; Wang, C.M.; Su, C.C.; Liu, Y.Q.; Zhai, H.Q.; Wan, J.M. Mapping and marker-assisted selection of a brown planthopper resistance gene bph2 in rice (Oryza sativa L.). Yi Chuan Xue Bao 2006, 33, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Jairin, J.; Phengrat, K.; Teangdeerith, S.; Vanavichit, A.; Toojinda, T. Mapping of a broad-spectrum brown planthopper resistance gene, Bph3, on rice chromosome 6. Mol. Breeding 2007, 19, 35–44. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.; Chen, H.; Liu, Y.; He, J.; Kang, H.; Sun, Z.; Pan, G.; Wang, Q.; Hu, J.; et al. A gene cluster encoding lectin receptor kinases confers broad-spectrum and durable insect resistance in rice. Nat. Biotechnol. 2015, 33, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Jairin, J.U.R.R.; Sansen, K.; Wongboon, W.; Kothcharerk, J. Detection of a brown planthopper resistance gene bph4 at the same chromosomal position of Bph3 using two different genetic backgrounds of rice [Oryza sativa]. Breeding Sci. 2010, 60, 71–75. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Khush, G.S. Genetic analysis of brown planthopper resistance in twenty varieties of rice, Oryza sativa L. Theor. Appl. Genet. 1978, 53, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Khush, G.S.; Karim, A.N.M.R.; Angeles, E.R. Genetics of resistance of rice cultivar ARC 10550 to Bangladesh brown planthopper biotype. J. Genet. 1985, 64, 121–125. [Google Scholar] [CrossRef]
- Guo, J.; Xu, C.; Wu, D.; Zhao, Y.; Qiu, Y.; Wang, X.; Ouyang, Y.; Cai, B.; Liu, X.; Jing, S.; et al. Bph6 encodes an exocyst-localized protein and confers broad resistance to planthoppers in rice. Nat. Genet. 2018, 50, 297–306. [Google Scholar] [CrossRef]
- Qiu, Y.; Guo, J.; Jing, S.; Zhu, L.; He, G. Fine mapping of the rice brown planthopper resistance gene Bph7 and characterization of its resistance in the 93-11 background. Euphytica 2014, 198, 369–379. [Google Scholar] [CrossRef]
- Nemoto, H.; Ikeda, R.; Kaneda, C. New genes for resistance to brown planthopper, Nilaparvata lugens Stål, in rice. Jpn. J. Breed. 1989, 39, 23–28. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, J.; Nie, L.; Hu, Y.; Zhang, N.; Guo, Q.; Guo, J.; Du, B.; Zhu, L.; He, G.; et al. Molecular and functional analysis of a brown planthopper resistance protein with two nucleotide-binding site domains. J. Exp. Bot. 2021, 72, 2657–2671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, J.; Wang, Z.; Jing, S.; Wang, Y.; Ouyang, Y.; Cai, B.; Xin, X.; Liu, X.; Zhang, C.; et al. Allelic diversity in an NLR gene Bph9 enables rice to combat planthopper variation. Proc. Nati. Acad. Sci. USA 2016, 113, 12850–12855. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Brar, D.S.; Multani, D.S.; Khush, G.S. Molecular tagging of genes for brown planthopper resistance and earliness introgressed from Oryza australiensis into cultivated rice, O. sativa. Genome 1994, 37, 217–221. [Google Scholar] [CrossRef]
- Renganayaki, K.; Fritz, A.K.; Sadasivam, S.; Pammi, S.; Harrington, S.E.; McCouch, S.R.; Kumar, S.M.; Reddy, A.S. Mapping and progress toward map-based cloning of brown planthopper biotype-4 resistance gene introgressed from Oryza officinalis into cultivated rice, O. sativa. Crop Sci. 2002, 42, 2112–2117. [Google Scholar] [CrossRef]
- Hirabayashi, H. RFLP analysis of a new gene for resistance to brown planthopper derived from O. officinalis on rice chromosome 4. Breed. Res. 1999, 1, 48. [Google Scholar]
- Qiu, Y.; Guo, J.; Jing, S.; Zhu, L.; He, G. Development and characterization of japonica rice lines carrying the brown planthopper-resistance genes Bph12 and Bph6. Theor. Appl. Genet. 2012, 124, 485–494. [Google Scholar] [CrossRef]
- Hirabayashi, H. Identification of brown planthopper resistance gene derived from O. officinalis using molecular markers in rice. Breed Sci 1998, 48, 82. [Google Scholar]
- Yang, H.; Ren, X.; Weng, Q.; Zhu, L.; He, G. Molecular mapping and genetic analysis of a rice brown planthopper (Nilaparvata lugens Stål) resistance gene. Hereditas 2002, 136, 39–43. [Google Scholar] [CrossRef]
- Du, B.; Zhang, W.; Liu, B.; Hu, J.; Wei, Z.; Shi, Z.; He, R.; Zhu, L.; Chen, R.; Han, B.; et al. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc. Nati. Acad. Sci. USA 2009, 106, 22163–22168. [Google Scholar] [CrossRef]
- Yang, H.; You, A.; Yang, Z.; Zhang, F.; He, R.; Zhu, L.; He, G. High-resolution genetic mapping at the Bph15 locus for brown planthopper resistance in rice (Oryza sativa L.). Theor. Appl. Genet. 2004, 110, 182–191. [Google Scholar] [CrossRef]
- Sun, L.N.A.U.; Su, C.; Wang, C.; Zhai, H.; Wan, J. Mapping of a major resistance gene to the brown planthopper in the rice [Oryza sativa] cultivar Rathu Heenati. Breeding Sci. 2005, 55, 391–396. [Google Scholar] [CrossRef]
- Rongbai, L. The evaluation and utilization of new genes for brown planthopper resistance in common wild rice (Oryza rufipogon griff.). Mol. Entomol. 2010, 1, 361–371. [Google Scholar] [CrossRef]
- Ji, H.; Kim, S.; Kim, Y.; Suh, J.; Park, H.; Sreenivasulu, N.; Misra, G.; Kim, S.; Hechanova, S.L.; Kim, H.; et al. Map-based cloning and characterization of the Bph18 gene from wild rice conferring resistance to brown planthopper insect pest. Sci. Rep. 2016, 6, 34376. [Google Scholar] [CrossRef]
- Jena, K.K.; Jeung, J.U.; Lee, J.H.; Choi, H.C.; Brar, D.S. High-resolution mapping of a new brown planthopper (BPH) resistance gene, Bph18(t), and marker-assisted selection for BPH resistance in rice (Oryza sativa L.). Theor. Appl. Genet. 2006, 112, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Wang, L.; Pang, X.F.; Pan, Q.H. Genetic analysis and fine mapping of a rice brown planthopper (Nilaparvata lugens Stål) resistance gene Bph19(t). Mol. Genet. Genom. 2006, 275, 321–329. [Google Scholar] [CrossRef]
- Rahman, M.L.; Jiang, W.; Chu, S.H.; Qiao, Y.; Ham, T.; Woo, M.; Lee, J.; Khanam, M.S.; Chin, J.; Jeung, J.; et al. High-resolution mapping of two rice brown planthopper resistance genes, Bph20(t) and Bph21(t), originating from Oryza minuta. Theor. Appl. Genet. 2009, 119, 1237–1246. [Google Scholar] [CrossRef]
- Yang, L.; Li, R.B.; Li, Y.R.; Huang, F.K.; Chen, Y.Z.; Huang, S.S.; Huang, L.F.; Liu, C.; Ma, Z.F.; Huang, D.H.; et al. Genetic mapping of bph20(t) and bph21(t) loci conferring brown planthopper resistance to Nilaparvata lugens Stål in rice (Oryza sativa L.). Euphytica 2012, 183, 161–171. [Google Scholar] [CrossRef]
- Deen, R.; Ramesh, K.; Gautam, S.K.; Rao, Y.K.; Ram, T. Identification of new gene for BPH resistance introgressed O. rufipogon. Rice Genet. Newsl. 2010, 25, 70–71. [Google Scholar]
- Hou, L.Y.; Peng, S.T.; Xing-Hua, W.; Yu, P.; Qun, X.U.; Yuan, X.P.; Yu, H.Y.; Wang, Y.P.; Wang, C.H.; Wan, G. Genetic analysis and preliminary mapping of two recessive resistance genes to brown planthopper, Nilaparvata lugens Stål in rice. Rice Sci. 2011, 18, 238–242. [Google Scholar] [CrossRef]
- Myint, K.K.M.; Fujita, D.; Matsumura, M.; Sonoda, T.; Yoshimura, A.; Yasui, H. Mapping and pyramiding of two major genes for resistance to the brown planthopper (Nilaparvata lugens Stål) in the rice cultivar ADR52. Theor. Appl. Genet. 2012, 124, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Hattori, M.; Yoshioka, H.; Yoshioka, M.; Takahashi, A.; Wu, J.; Sentoku, N.; Yasui, H. Map-based cloning and characterization of a brown planthopper resistance gene Bph26 from Oryza sativa L. Indica cultivar ADR52. Sci. Rep. 2014, 4, 5872. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Qiu, Y.; Zhang, Y.; Huang, F.; Meng, J.; Wei, S.; Li, R.; Chen, B. Fine mapping and characterization of Bph27, a brown planthopper resistance gene from wild rice (Oryza rufipogon griff.). Theor. Appl. Genet. 2013, 126, 219–229. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, Y.; Liu, Y.; Jiang, L.; Wu, H.; Kang, H.; Liu, S.; Chen, L.; Liu, X.; Cheng, X.; et al. High-resolution mapping of brown planthopper (BPH) resistance gene Bph27(t) in rice (Oryza sativa L.). Mol. Breed. 2013, 31, 549–557. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; He, J.; Liu, Y.; Jiang, L.; Liu, L.; Wang, C.; Cheng, X.; Wan, J. Fine mapping of brown planthopper (Nilaparvata lugens Stål) resistance gene Bph28(t) in rice (Oryza sativa L.). Mol. Breed. 2014, 33, 909–918. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, L.; Zhang, Y.; Cao, C.; Liu, F.; Huang, F.; Qiu, Y.; Li, R.; Lou, X. Map-based cloning and characterization of bph29, a b3 domain-containing recessive gene conferring brown planthopper resistance in rice. J. Exp. Bot. 2015, 66, 6035–6045. [Google Scholar] [CrossRef]
- Shi, S.; Wang, H.; Nie, L.; Tan, D.; Zhou, C.; Zhang, Q.; Li, Y.; Du, B.; Guo, J.; Huang, J.; et al. Bph30 confers resistance to brown planthopper by fortifying sclerenchyma in rice leaf sheaths. Mol. Plant 2021, 14, 1714–1732. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, S.; Guo, Q.; Nie, L.; Du, B.; Chen, R.; Zhu, L.; He, G. High-resolution mapping of a gene conferring strong antibiosis to brown planthopper and developing resistant near-isogenic lines in 9311 background. Mol. Breed. 2018, 38, 107. [Google Scholar] [CrossRef]
- Prahalada, G.D.; Shivakumar, N.; Lohithaswa, H.C.; Sidde Gowda, D.K.; Ramkumar, G.; Kim, S.; Ramachandra, C.; Hittalmani, S.; Mohapatra, T.; Jena, K.K. Identification and fine mapping of a new gene, Bph31 conferring resistance to brown planthopper biotype 4 of india to improve rice, Oryza sativa L. Rice 2017, 10, 41. [Google Scholar] [CrossRef]
- Ren, J.; Gao, F.; Wu, X.; Lu, X.; Zeng, L.; Lv, J.; Su, X.; Luo, H.; Ren, G. Bph32, a novel gene encoding an unknown SCR domain-containing protein, confers resistance against the brown planthopper in rice. Sci. Rep. 2016, 6, 37645. [Google Scholar] [CrossRef]
- Hu, J.; Chang, X.; Zou, L.; Tang, W.; Wu, W. Identification and fine mapping of Bph33, a new brown planthopper resistance gene in rice (Oryza sativa L.). Rice 2018, 11, 55. [Google Scholar] [CrossRef]
- Kumar, K.; Sarao, P.S.; Bhatia, D.; Neelam, K.; Kaur, A.; Mangat, G.S.; Brar, D.S.; Singh, K. High-resolution genetic mapping of a novel brown planthopper resistance locus, Bph34 in Oryza sativa L. X Oryza nivara (Sharma & Shastry) derived interspecific F2 population. Theor. Appl. Genet. 2018, 131, 1163–1171. [Google Scholar] [PubMed]
- Zhang, Y.; Qin, G.; Ma, Q.; Wei, M.; Li, R. Identification of major locus Bph35 resistance to brown planthopper in rice. Rice Sci. 2020, 27, 237–245. [Google Scholar]
- Li, Z.; Xue, Y.; Zhou, H.; Li, Y.; Usman, B.; Jiao, X.; Wang, X.; Liu, F.; Qin, B.; Li, R.; et al. High-resolution mapping and breeding application of a novel brown planthopper resistance gene derived from wild rice (Oryza. rufipogon griff). Rice 2019, 12, 41. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, L.; Yan, L.; Shu, W.; Wang, X.; Qiu, Y. Mapping and characterization of a quantitative trait locus resistance to the brown planthopper in the rice variety IR64. Hereditas 2019, 156, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, Q.; Chen, Y.; Huang, J.; Guo, Q.; Li, Y.; Wang, W.; Qiu, Y.; Guan, W.; Zhang, J.; et al. Balancing selection and wild gene pool contribute to resistance in global rice germplasm against planthopper. J. Integr. Plant Biol. 2021, 63, 1695–1711. [Google Scholar] [CrossRef]
- Balachiranjeevi, C.H.; Prahalada, G.D.; Mahender, A.; Jamaloddin, M.; Sevilla, M.A.L.; Marfori-Nazarea, C.M.; Vinarao, R.; Sushanto, U.; Baehaki, S.E.; Li, Z.K.; et al. Identification of a novel locus, Bph38(t), conferring resistance to brown planthopper (Nilaparvata lugens Stål.) Using early backcross population in rice (Oryza sativa L.). Euphytica 2019, 215, 185. [Google Scholar] [CrossRef]
- Yang, M.; Lin, J.; Cheng, L.; Zhou, H.; Chen, S.; Liu, F.; Li, R.; Qiu, Y. Identification of a novel planthopper resistance gene from wild rice (Oryza rufipogon griff.). Crop J. 2020, 8, 1057–1070. [Google Scholar] [CrossRef]
- Tan, H.Q.; Palyam, S.; Gouda, J.; Kumar, P.P.; Chellian, S.K. Identification of two QTLs, Bph41 and Bph42, and their respective gene candidates for brown planthopper resistance in rice. Sci. Rep. 2022, 12, 18538. [Google Scholar] [CrossRef]
- Wang, X.; Han, Y.; Zhang, Y.; Deng, B.; Wu, B.; Guo, X.; Qin, Y.; Fang, Y.; Liu, F.; Qin, B.; et al. QTL mapping integrated with BSA-seq analysis identifies a novel gene conferring resistance to brown planthopper from common wild rice (Oryza rufipogon griff.). Euphytica 2022, 218, 34. [Google Scholar] [CrossRef]
- Kaur, P.; Neelam, K.; Sarao, P.S.; Babbar, A.; Kumar, K.; Vikal, Y.; Khanna, R.; Kaur, R.; Mangat, G.S.; Singh, K. Molecular mapping and transfer of a novel brown planthopper resistance gene bph42 from Oryza rufipogon (griff.) to cultivated rice (Oryza sativa L.). Mol. Biol. Rep. 2022, 49, 8597–8606. [Google Scholar] [CrossRef]
- Kim, J.; An, X.; Yang, K.; Miao, S.; Qin, Y.; Hu, Y.; Du, B.; Zhu, L.; He, G.; Chen, R. Molecular mapping of a new brown planthopper resistance gene Bph43 in rice (Oryza sativa L.). Agronomy 2022, 12, 808. [Google Scholar] [CrossRef]
- Kiswanto, I.; Soetopo, L.; Adiredjo, A.L. Identification of novel candidate of brown planthopper resistance gene Bph44 in rice (Oryza sativa L.). Genome 2022, 65, 505–511. [Google Scholar] [CrossRef]
- Li, C.; Wu, D.; Huang, S.; Meng, M.; Shih, H.; Lai, M.; Chen, L.; Jena, K.K.; Hechanova, S.L.; Ke, T.; et al. The Bph45 gene confers resistance against brown planthopper in rice by reducing the production of limonene. Int. J. Mol. Sci. 2023, 24, 1798. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xiao, C.; Cheng, M.; Gao, G.; Zhang, Q.; He, Y. Fine mapping and pyramiding of brown planthopper resistance genes qBph3 and qBph4 in an introgression line from wild rice O. officinalis. Mol. Breed. 2015, 35, 3. [Google Scholar] [CrossRef]
- Van Mai, T.; Fujita, D.; Matsumura, M.; Yoshimura, A.; Yasui, H. Genetic basis of multiple resistance to the brown planthopper (Nilaparvata lugens Stål) and the green rice leafhopper (Nephotettix cincticeps Uhler) in the rice cultivar ‘ASD7’ (Oryza sativa L. ssp Indica). Breed. Sci. 2015, 65, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.F.; Cheng, L.; Liu, F.; Li, R.B. Identification of a new locus conferring antixenosis to the brown planthopper in rice cultivar swarnalata (Oryza sativa L.). Genet. Mol. Res. 2013, 12, 3201–3211. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Wan, J.; Zhai, H.Q.; Wang, C.M.; Yoshimura, A. A new locus for resistance to brown planthopper identified in the indica rice variety DV85. Plant Breed. 2010, 124, 93–95. [Google Scholar] [CrossRef]
- Tao Rong, P.G.J.L. Genetic analysis and mapping of the gene conferring brown planthopper resistance in indica rice cultivar ‘BP360e’. J. Nanjing Agric. Univ. 2019, 42, 14–20. [Google Scholar]
- Hu, J.; Xiao, C.; Cheng, M.; Gao, G.; Zhang, Q.; He, Y. A new finely mapped Oryza australiensis-derived QTL in rice confers resistance to brown planthopper. Gene 2015, 561, 132–137. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Panda, R.S.; Mohapatra, S.L.; Nanda, A.; Behera, L.; Jena, M.; Sahu, R.K.; Sahu, S.C.; Mohapatra, T. Identification of novel quantitative trait loci associated with brown planthopper resistance in the rice landrace Salkathi. Euphytica 2017, 213, 38. [Google Scholar] [CrossRef]
- Akanksha, S.; Lakshmi, V.J.; Singh, A.K.; Deepthi, Y.; Chirutkar, P.M.; Ramdeen; Balakrishnan, D.; Sarla, N.; Mangrauthia, S.K.; Ram, T. Genetics of novel brown planthopper Nilaparvata lugens Stål resistance genes in derived introgression lines from the interspecific cross O. sativa var. Swarna x O. nivara. J. Genet. 2019, 98, 113. [Google Scholar] [CrossRef]
- Cheng, X.; Wu, Y.; Guo, J.; Du, B.; Chen, R.; Zhu, L.; He, G. A rice lectin receptor-like kinase that is involved in innate immune responses also contributes to seed germination. Plant J. 2013, 76, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Du, B.; Shangguan, X.; Zhao, Y.; Pan, Y.; Zhu, L.; He, Y.; He, G. BAC and RNA sequencing reveal the brown planthopper resistance gene Bph15 in a recombination cold spot that mediates a unique defense mechanism. BMC Genom. 2014, 15, 674. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Hu, J.; Ao, Y.; Cheng, M.; Gao, G.; Zhang, Q.; He, G.; He, Y. Development and evaluation of near-isogenic lines for brown planthopper resistance in rice. 9311. Sci. Rep. 2016, 6, 38159. [Google Scholar] [CrossRef]
- Hu, L.; Wu, Y.; Wu, D.; Rao, W.; Guo, J.; Ma, Y.; Wang, Z.; Shangguan, X.; Wang, H.; Xu, C.; et al. The coiled-coil and nucleotide binding domains of brown planthopper resistance14 function in signaling and resistance against planthopper in rice. Plant Cell 2018, 29, 3157–3185. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Guo, J.; Jing, S.; Zhu, L.; He, G. High-resolution mapping of the brown planthopper resistance gene Bph6 in rice and characterizing its resistance in the 9311 and Nipponbare near isogenic backgrounds. Theor. Appl. Genet. 2010, 121, 1601–1611. [Google Scholar] [CrossRef]
- Wu, D.; Guo, J.; Zhang, Q.; Shi, S.; Guan, W.; Zhou, C.; Chen, R.; Du, B.; Zhu, L.; He, G. Necessity of rice resistance to planthoppers for OsEXO70H3 regulating SAMSL excretion and lignin deposition in cell walls. New Phytol. 2022, 234, 1031–1046. [Google Scholar] [CrossRef]
- Zheng, X.; Xin, Y.; Peng, Y.; Shan, J.; Zhang, N.; Wu, D.; Guo, J.; Huang, J.; Guan, W.; Shi, S.; et al. Lipidomic analyses reveal enhanced lipolysis in planthoppers feeding on resistant host plants. Sci. China Life Sci. 2021, 64, 1502–1521. [Google Scholar] [CrossRef]
- Pannak, S.; Wanchana, S.; Aesomnuk, W.; Pitaloka, M.K.; Jamboonsri, W.; Siangliw, M.; Meyers, B.C.; Toojinda, T.; Arikit, S. Functional Bph14 from Rathu Heenati promotes resistance to BPH at the early seedling stage of rice (Oryza sativa L.) as revealed by QTL-seq. Theor. Appl. Genet. 2023, 136, 25. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, L.; He, G. Genetic and molecular understanding of host rice resistance and Nilaparvata lugens Stål adaptation. Curr. Opin. Insect. Sci 2021, 45, 14–20. [Google Scholar] [CrossRef]
- Huang, H.; Liu, C.; Cai, Y.; Zhang, M.; Bao, Y.; Zhang, C. A salivary sheath protein essential for the interaction of the brown planthopper with rice plants. Insect Biochem. Molec. 2015, 66, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Yu, H.; Jian, Y.; Zeng, J.; Ji, R.; Chen, H.; Lou, Y. A salivary EF-hand calcium-binding protein of the brown planthopper Nilaparvata lugens functions as an effector for defense responses in rice. Sci. Rep. 2017, 7, 40498. [Google Scholar] [CrossRef]
- Shangguan, X.; Zhang, J.; Liu, B.; Zhao, Y.; Wang, H.; Wang, Z.; Guo, J.; Rao, W.; Jing, S.; Guan, W.; et al. A mucin-like protein of planthopper is required for feeding and induces immunity response in plants. Plant Physiol. 2018, 176, 552–565. [Google Scholar] [CrossRef]
- Guo, J.; Wang, H.; Guan, W.; Guo, Q.; Wang, J.; Yang, J.; Peng, Y.; Shan, J.; Gao, M.; Shi, S.; et al. A tripartite rheostat controls self-regulated host plant resistance to insects. Nature 2023, 618, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Yamamoto, K.; Suetsugu, Y.; Kuwazaki, S.; Hattori, M.; Jairin, J.; Sanada-Morimura, S.; Matsumura, M. Genetic mapping of the rice resistance-breaking gene of the brown planthopper Nilaparvata lugens Stål. Proc. Bio. Sci. 2014, 281, 296–306. [Google Scholar]
- Wang, M.; Yang, D.; Ma, F.; Zhu, M.; Shi, Z.; Miao, X. OsHLH61-OsbHLH96 influences rice defense to brown planthopper through regulating the pathogen-related genes. Rice 2019, 12, 9. [Google Scholar] [CrossRef]
- Chen, L.; Cao, T.; Zhang, J.; Lou, Y. Overexpression of OsGID1 enhances the resistance of rice to the brown planthopper Nilaparvata lugens Stål. Int. J. Mol. Sci. 2018, 19, 2744. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, T.; Wang, W.; Cao, T.; Lou, Y. Silencing OsSLR1 enhances the resistance of rice to the brown planthopper Nilaparvata lugens Stål. Plant Cell Envi. 2017, 40, 2147–2159. [Google Scholar] [CrossRef]
- Lecourieux, D.; Ranjeva, R.; Pugin, A. Calcium in plant defence-signalling pathways. New Phytol. 2006, 171, 249–269. [Google Scholar] [CrossRef]
- King, R.W.; Zeevaart, J.A. Enhancement of phloem exudation from cut petioles by chelating agents. Plant Physiol. 1974, 53, 96–103. [Google Scholar] [CrossRef]
- Hõrak, H. Defense, fast and slow: Activation of different MAPK pathways in response to wounding. Plant Cell 2020, 32, 1788–1789. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhou, J.; Peng, X.; Xu, H.; Liu, C.; Du, B.; Yuan, H.; Zhu, L.; He, G. The Bphi008a gene interacts with the ethylene pathway and transcriptionally regulates MAPK genes in the response of rice to brown planthopper feeding. Plant Physiol. 2011, 156, 856–872. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Dadalto, S.; Gonçalves, A.; de Souza, G.; Barros, V.; Fietto, L. Transcription factor functional protein-protein interactions in plant defense responses. Proteomes 2014, 2, 85–106. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, P.; Riaño-Pachón, D.M.; Corrêa, L.G.G.; Rensing, S.A.; Kersten, B.; Mueller-Roeber, B. Plntfdb: Updated content and new features of the plant transcription factor database. Nucleic Acids Res. 2010, 38, D822–D827. [Google Scholar] [CrossRef]
- Shimono, M.; Sugano, S.; Nakayama, A.; Jiang, C.; Ono, K.; Toki, S.; Takatsuji, H. Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell 2007, 19, 2064–2076. [Google Scholar] [CrossRef]
- Huangfu, J.; Li, J.; Li, R.; Ye, M.; Kuai, P.; Zhang, T.; Lou, Y. The transcription factor OsWRKY45 negatively modulates the resistance of rice to the brown planthopper Nilaparvata lugens Stål. Int. J. Mol. Sci. 2016, 17, 697. [Google Scholar] [CrossRef]
- Hu, L.; Ye, M.; Li, R.; Lou, Y. OsWRKY53, a versatile switch in regulating herbivore-induced defense responses inrice. Plant Signal. Behav. 2016, 11, e1169357. [Google Scholar] [CrossRef]
- Sun, B.; Shen, Y.; Chen, S.; Shi, Z.; Li, H.; Miao, X. A novel transcriptional repressor complex MYB22-TOPLESS-HDAC1 promotes rice resistance to brown planthopper by repressing F3′H expression. New Phytol. 2023, 239, 720–738. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, Y.; Yuan, D.; Duan, M.; Liu, Y.; Shen, Z.; Yang, C.; Qiu, Z.; Liu, D.; Wen, P.; et al. An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. USA 2020, 117, 271–277. [Google Scholar] [CrossRef]
- Lu, J.; Ju, H.; Zhou, G.; Zhu, C.; Erb, M.; Wang, X.; Wang, P.; Lou, Y. An EAR-motif-containing ERF transcription factor affects herbivore-induced signaling, defense and resistance in rice. Plant J. 2011, 68, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Gielen, H.; Remans, T.; Vangronsveld, J.; Cuypers, A. MicroRNAs in metal stress: Specific roles or secondary responses? Int. J. Mol. Sci. 2012, 13, 15826–15847. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lv, W.; Hu, L.; Rao, W.; Zeng, Y.; Zhu, L.; He, Y.; He, G. Identification and analysis of brown planthopper-responsive micrornas in resistant and susceptible rice plants. Sci. Rep. 2017, 7, 8712. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Tan, J.; Zhou, C.; Yang, X.; Yang, F.; Zhang, S.; Sun, S.; Miao, X.; Shi, Z. The OsmiR396 OsGRF8–FOsF3H flavonoid pathway mediates resistance to the brown planthopper in rice (Oryza sativa L.). Plant Biotechnol. J. 2019, 17, 1657–1669. [Google Scholar] [CrossRef]
- Ge, Y.; Han, J.; Zhou, G.; Xu, Y.; Ding, Y.; Shi, M.; Guo, C.; Wu, G. Silencing of miR156 confers enhanced resistance to brown planthopper in rice. Planta 2018, 248, 813–826. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Khush, G.S. Linkage relationships of some genes for disease and insect resistance and semidwarf stature in rice. Euphytica 1979, 28, 233–237. [Google Scholar] [CrossRef]
- Khush, G.S.; Brar, D.S. Genetics of resistance to insects in crop plants. Adv. Agron. 1991, 45, 223–274. [Google Scholar]
- Cruz, A.P.; Arida, A.; Heong, K.L.; Horgan, F.G. Aspects of brown planthopper adaptation to resistant rice varieties with the Bph3 gene. Entomol. Exp. Appl. 2011, 141, 245–257. [Google Scholar] [CrossRef]
- Alam, S.N.; Cohen, M.B. Durability of brown planthopper, Nilaparvata lugens Stål, resistance in rice variety IR64 in greenhouse selection studies. Entomol. Exp. Appl. 1998, 89, 71–78. [Google Scholar] [CrossRef]
- Pathak, P.K.; Heinrichs, E.A. Selection of biotype populations 2 and 3 of Nilaparvata lugens Stål by exposure to resistant rice varieties. Environ. Entomol. 1982, 11, 347–371. [Google Scholar] [CrossRef]
- Ribaut, J.M.; Hoisington, D. Marker-assisted selection: New tools and strategies. Trends Plant Sci. 1998, 3, 239. [Google Scholar] [CrossRef]
- Sharma, P.N.; Torii, A.; Takumi, S.; Mori, N.; Nakamura, C. Marker-assisted pyramiding of brown planthopper (Nilaparvata lugens Stål) resistance genes Bph1 and Bph2 on rice chromosome 12. Hereditas 2004, 140, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, W.; Liu, H.; Zeng, Y.; Du, B.; Zhu, L.; He, G.; Chen, R. Marker assisted pyramiding of Bph6 and Bph9 into elite restorer line 93–11 and development of functional marker for Bph9. Rice 2017, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Chen, R.; Guo, J.; He, G. Current understanding of the genomic, genetic, and molecular control of insect resistance in rice. Mol. Breed. 2020, 40, 24. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Liu, Y.; Dai, H.; He, J.; Kang, H.; Pan, G.; Huang, J.; Qiu, Z.; Wang, Q.; et al. Marker assisted pyramiding of two brown planthopper resistance genes, Bph3 and Bph27 (t), into elite rice cultivars. Rice 2016, 9, 27. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Wang, L.; Liu, J.; Shang, K.; Hua, H. Biological effects of rice harbouring Bph14 and Bph15 on brown planthopper, Nilaparvata lugens. Pest Manag. Sci. 2011, 67, 528–534. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Wu, C.; Yang, C.; Hua, H.; Gao, G.; Xiao, J.; He, Y. Pyramiding and evaluation of the brown planthopper resistance genes Bph14 and Bph15 in hybrid rice. Mol. Breed. 2012, 29, 61–69. [Google Scholar] [CrossRef]
- Han, Y.; Wu, C.; Yang, L.; Zhang, D.; Xiao, Y. Resistance to Nilaparvata lugens in rice lines introgressed with the resistance genes Bph14 and Bph15 and related resistance types. PLoS ONE 2018, 13, e0198630. [Google Scholar] [CrossRef]
- Wang, H.; Ye, S.; Mou, T. Molecular breeding of rice restorer lines and hybrids for brown planthopper (BPH) resistance using the Bph14 and Bph15 genes. Rice 2016, 9, 53. [Google Scholar] [CrossRef]
- Wei, H.; Xiao, H.; Kan, H.; Jiang, Y.; Yang, Z. Application of marker-assisted backcross to introgress Bph3, Bph14 and Bph15 into an elite indica rice variety for improving its resistance to brown planthopper. Plant Breed. 2016, 135, 291–300. [Google Scholar]
- Hu, J.; Cheng, M.; Gao, G.; Zhang, Q.; Xiao, J.; He, Y. Pyramiding and evaluation of three dominant brown planthopper resistance genes in the eliteindica rice 9311 and its hybrids. Pest Manag. Sci. 2013, 69, 802–808. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Xiao, Y.; Yu, J.; Li, J.; Meng, Q.; Qing, X.; Xiao, G. Pyramiding Xa21, Bph14, and Bph15 genes into the elite restorer line yuehui9113 increases resistance to bacterial blight and the brown planthopper in rice. Crop Prot. 2019, 115, 31–39. [Google Scholar] [CrossRef]
- Ji, Z.; Yang, S.; Zeng, Y.; Liang, Y.; Yang, C.; Qian, Q. Pyramiding blast, bacterial blight and brown planthopper resistance genes in rice restorer lines. J. Integr. Agr. 2016, 15, 1432–1440. [Google Scholar] [CrossRef]
- Korinsak, S.; Siangliw, M.; Kotcharerk, J.; Jairin, J.; Siangliw, J.L.; Jongdee, B.; Pantuwan, G.; Sidthiwong, N.; Toojinda, T. Improvement of the submergence tolerance and the brown planthopper resistance of the Thai jasmine rice cultivar KDML105 by pyramiding Sub1 and Qbph12. Field Crop. Res. 2016, 188, 105–112. [Google Scholar] [CrossRef]
- Xu, J. Pyramiding of two BPH resistance genes and Stv-bi gene using marker-assisted selection in japonica rice. Crop Breed. Appl. Biot. 2013, 13, 99–106. [Google Scholar] [CrossRef]
- Fan, F.; Li, N.; Chen, Y.; Liu, X.; Sun, H.; Wang, J.; He, G.; Zhu, Y.; Li, S. Development of elite BPH-resistant wide-spectrum restorer lines for three and two line hybrid rice. Front. Plant Sci. 2017, 8, 986. [Google Scholar] [CrossRef]
- Reinke, R.; Kim, S.; Kim, B. Developing japonica rice introgression lines with multiple resistance genes for brown planthopper, bacterial blight, rice blast, and rice stripe virus using molecular breeding. Mol. Genet. Genom. 2018, 293, 1565–1575. [Google Scholar] [CrossRef]
- Jena, K.K.; Hechanova, S.L.; Verdeprado, H.; Prahalada, G.D.; Kim, S. Development of 25 near-isogenic lines (NILs) with ten BPH resistance genes in rice (Oryza sativa L.): Production, resistance spectrum, and molecular analysis. Theor. Appl. Genet. 2017, 130, 2345–2360. [Google Scholar] [CrossRef]
- Dixit, S.; Singh, U.M.; Singh, A.K.; Alam, S.; Venkateshwarlu, C.; Nachimuthu, V.V.; Yadav, S.; Abbai, R.; Selvaraj, R.; Devi, M.N.; et al. Marker assisted forward breeding to combine multiple biotic-abiotic stress resistance/tolerance in rice. Rice 2020, 13, 29. [Google Scholar] [CrossRef]
- McCouch, S.R. Gene nomenclature system for rice. Rice 2008, 1, 72–84. [Google Scholar] [CrossRef]
- Liu, M.; Fan, F.; He, S.; Guo, Y.; Chen, G.; Li, N.; Li, N.; Yuan, H.; Si, F.; Yang, F.; et al. Creation of elite rice with high-yield, superior-quality and high resistance to brown planthopper based on molecular design. Rice 2022, 15, 17. [Google Scholar] [CrossRef] [PubMed]
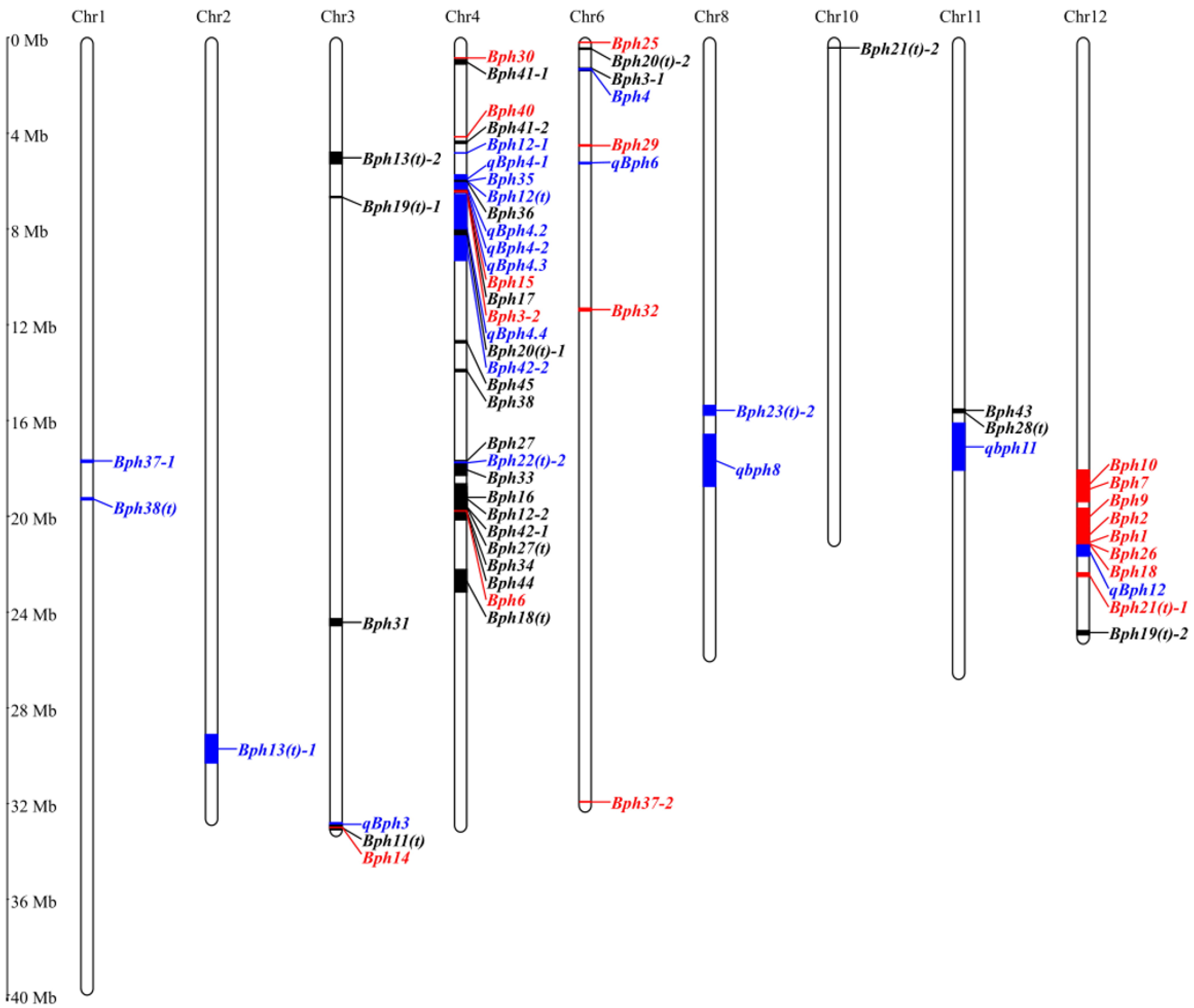

| Genes/QTLs | Chromosome | Germplasm | Linked Markers | Position (Mbp) | PEV | Reference |
|---|---|---|---|---|---|---|
| Bph1 | 12L | Mudgo | pBPH4-pBPH14 | 22.86 | Major gene | [20,21] |
| Bph2 (bph2) | 12L | ASD7 | RM463-RM7102 | 22.87~22.89 | Major gene | [22,23] |
| Bph3-1 (Bph3) # | 6S | Ptb33, Rathu Heenati | RM589-RM588 | 1.38~1.48 | Major gene | [24] |
| Bph3-2 (Bph3) *# | 4S | Rathu Heenatis | RHD9-RHC10 | 6.20~6.97 | Major gene | [25] |
| Bph4 (bph4) | 6S | Babawee | RM589-RM586 | 1.38~1.47 | 58.8–70.1% | [26,27] |
| Bph5 (bph5) | — | ARC10550 | — | — | Major gene | [28] |
| Bph6 * | 4L | Swarnalata | H-Y9 | 21.40 | Major gene | [29] |
| Bph7 | 12L | T12 | RM3448-RM313 | 19.95~20.87 | 38.3% | [30] |
| Bph8 (bph8) | — | Chin Saba | — | — | Major gene | [31] |
| Bph9 * | 12L | Pokkali | InD2-RsaI | 22.85~22.97 | Major gene | [32,33] |
| Bph10 (bph10) | 12L | O. australiensis | RG457 | 19.55~26.98 | Major gene | [34] |
| Bph11(t) (bph11) | 3L | O. officinalis | G1318 | 35.60~35.80 | Major gene | [35,36] |
| Bph12-1 (Bph12) # | 4S | B14 (O. officinalis) | RM16459-RM1305 | 5.21~5.56 | 73.8% | [37] |
| Bph12-2 (bph12) # | 4L | O. officinalis | G271-R93 | 20.34~21.31 | Major gene | [38] |
| Bph12(t) | 4S | O. latifolia | RM261-RM8213 | 4.44~6.57 | 70.6 % | [39] |
| Bph13(t)-1 [Bph13(t)] # | 2L | O. eichingeri | RM240-RM250 | 31.50~32.78 | 90% | |
| Bph13(t)-2 [Bph13(t)] # | 3S | IR54745-2-21 (O. officinalis) | RG100-RG191 | 5.18~5.70 | Major gene | |
| Bph14 * | 3L | B5 (O. officinalis) | SM1-G1318 | 35.68~35.70 | Major gene | [40] |
| Bph15 * | 4S | B5 (O. officinalis) | RG1-RG2 | 6.68~6.90 | Major gene | [41] |
| Bph16 | 4L | O. officinalis | G271-R93 | 20.17~21.14 | Major gene | [38] |
| Bph17 | 4S | Rathu Heenati | RM8213-RM5953 | 4.44~9.38 | Major gene | [42] |
| Bph18(t) [bph18(t)] | 4L | O. rufipogon | RM273-RM6506 | 24.05~25.05 | Major gene | [43] |
| Bph18 * | 12L | IR65482-7-216-1-2 (O. australiensis) | BIM3-BN162 | 22.88 | Major gene | [44,45] |
| Bph19(t)-1 [bph19(t)] # | 3S | AS20-1 | RM6308-RM3134 | 7.18~7.24 | Major gene | [46] |
| Bph19(t)-2 [bph19(t)] # | 12L | O. rufipogon | RM17 | 26.98 | Major gene | [43] |
| Bph20(t)-1 [Bph20(t)] # | 4S | IR71033-121-15 (O. minuta) | B42-B44 | 8.76 | Major gene | [47] |
| Bph20(t)-2 [bph20(t)] # | 6S | O. rufipogon | BYL7-BYL8 | 0.47~0.53 | Major gene | [48] |
| Bph21(t)-1 [Bph21(t)] # | 12L | IR71033-121-15 (O. minuta) | S12094A-B122 | 24.20~24.36 | Major gene | [47] |
| Bph21(t)-2 [bph21(t)] # | 10S | O. rufipogon | RM222-RM244 | 2.62~5.00 | Major gene | [48] |
| Bph22(t)-1[Bph22(t)] # | 4L | O. glaberrima | RM471-RM5742 | 18.99~21.56 | Major gene | |
| Bph23(t)-1 [Bph23(t)] # | — | O. minuta | — | — | Major gene | [49] |
| Bph22(t)-2 [bph22(t)] # | 4L | O. rufipogon | RM8212-RM261 | 19.11~19.57 | 11.3% | [50] |
| Bph23(t)-2 [bph23(t)] # | 8L | O. rufipogon | RM2655-RM3572 | 16.63~17.07 | 14.9% | [50] |
| Bph24(t) [bph24(t)] | — | IR73678-6-9-B (O. rufipogon) | — | — | Major gene | [49] |
| Bph25 | 6S | ADR52 | S00310-RM8101 | 0.21 | Major gene | [51] |
| Bph26 * | 12L | ADR52 | DS72B-DS173B | 22.87~22.89 | Major gene | [52] |
| Bph27 | 4L | GX2183 (O. rufipogon) | RM16846-RM16888 | 19.12~19.50 | Major gene | [53] |
| Bph27(t) | 4L | Balamawee | Q52-Q20 | 20.79~21.33 | Major gene | [54] |
| Bph28(t) | 11L | DV85 | Indel55-Indel66 | 16.90~16.96 | Major gene | [55] |
| Bph29 (bph29) * | 6S | RBPH54 (O. rufipogon) | BYL8-BID2 | 0.48~0.49 | Major gene | [56] |
| Bph30 * | 4S | AC-1613 | SSR28-SSR69 | 0.92~0.94 | Major gene | [57,58] |
| Bph31 | 3L | CR2711-76 | PA25-RM2334 | 26.25~26.57 | Major gene | [59] |
| Bph32 * | 6S | Ptb33 | RM19291-RM8072 | 12.23~12.36 | Major gene | [60] |
| Bph33 | 4S | KOLAYAL, PPLIYAL | H99-H101 | 19.29~19.79 | Major gene | [61] |
| Bph34 | 4L | IRGC104646 (O. nivara) | RM17007-RM1699 | 21.32~21.47 | Major gene | [62] |
| Bph35 | 4S | RBPH660 (O. rufipogon) | RM3471-PSM20 | 6.28~6.94 | 51.27% | [63] |
| Bph36 | 4S | GX2183 (O. rufipogon) | S13-X48 | 6.46~6.50 | Major gene | [64] |
| Bph37-1 (Bph37) # | 1L | IR64 | RM302-YM35 | 19.10~19.20 | 36.9% | [65] |
| Bph37-2 (Bph37) *# | 6S | SE382 | — | 3.45 | Major gene | [66] |
| Bph38(t) | 1L | Khazar | SNP693369-id 10112165 | 20.80~20.90 | 35.91% | [67] |
| Bph38 | 4L | GX2183 (O. rufipogon) | YM112-YM190 | 15.00~15.10 | Major gene | [68] |
| Bph40 * | 4S | SE232, SE67, C334, | — | 4.48~4.49 | Major gene | [57] |
| Bph41-1 (Bph41) # | 4S | SWD10 | SWRm_01617-SWRm_01522 | 0.90~1.10 | Major gene | [69] |
| Bph41-2 (Bph41) # | 4S | GXU202 (O. rufipogon) | W4_4_3-W1_6_3 | 4.68~4.78 | Major gene | [70] |
| Bph42-1 (Bph42) # | 4S | SWD10 | SWRm_01695- SWRm_00328 | 20.60~21.80 | Major gene | [69] |
| Bph42-2 (bph42) # | 4S | O. rufipogon | RM16282-RM16335 | 9.07~9.58 | 29% | [71] |
| Bph43 | 11S | IRGC 8678 | InDel16_22-InDel16-30 | 16.79~16.90 | Major gene | [72] |
| Bph44 | 4L | Balamawee | Q31-RM17007 | 21.38~21.47 | Major gene | [73] |
| Bph45 | 4L | Tainung71 (O. nivara) | — | 13.70~13.80 | Major gene | [74] |
| qBph3 | 3L | IR02W101 (O. officinalis) | t6-f3 | 35.63~35.47 | 28% | [75] |
| qBph6 | 6S | IR71033-121-15 | RM469-RM568 | 5.64~5.71 | 19.6% | [76] |
| qbph8 | 8L | Swarnalata | RM339-RM515 | 17.94~20.28 | 6.6% | [77] |
| qbph11 | 11L | DV85 | XNpb202-C1172 | 17.43~19.56 | 68.4% | [78] |
| qBph4-1 (qBph4) # | 4S | IR02W101 (O. officinalis) | P17-xc4_27 | 6.70~6.90 | 35% | [75] |
| qBph4-2 (qBph4) # | 4S | BP360e | RM16382-INDEL4-5 | 6.20~6.70 | 56% | [79] |
| qBph4.2 | 4S | IR65482-17-511 (O. officinalis) | RM261-XC4-27 | 6.58~6.89 | 36–44% | [80] |
| qBph4.3 | 4S | Salkathi | RM551-RM335 | 0.177~0.688 | 37.02% | [81] |
| qBph4.4 | 4S | Salkathi | RM335-RM5633 | 0.688~13.07 | 7.1% | [81] |
| qBph12 | 12L | ASD7 | RM28466-RM7376 | 22.94~23.44 | 28.8% | [76] |
| Bph39(t) [bph39(t)] | — | O. nivara | — | — | Major gene | [82] |
| Bph40(t)-1 [bph40(t)] | — | O. nivara | — | — | Major gene | [82] |
| Gene | Chr. | Donor Parent | Subcellular Localization | Encoded Protein | Year | Reference |
|---|---|---|---|---|---|---|
| Bph1 | 12L | Mudgo | Endomembrane system | CC-NB-NB-LRR | 2016 | [33] |
| Bph2 | 12L | ASD7 | Endomembrane system | CC-NB-NB-LRR | 2016 | [33,52] |
| Bph3 | 4S | Rathu Heenati | Plasma membrane | LRK | 2015 | [25] |
| Bph6 | 4L | Swarnalata | Exocyst | LRD | 2018 | [29] |
| Bph7 | 12L | T12 | Endomembrane system | CC-NB-NB-LRR | 2016 | [33] |
| Bph9 | 12L | Pokkali | Endomembrane system | CC-NB-NB-LRR | 2016 | [33] |
| Bph10 | 12L | IR65482-4-136-2-2 | Endomembrane system | CC-NB-NB-LRR | 2016 | [33] |
| Bph14 | 3L | B5 | Nucleus andcytoplasm | CC-NB-LRR | 2009 | [40] |
| Bph15 | 4S | B5 | Plasma membrane | LRK | 2013 | [83] |
| Bph18 | 12L | IR65482-7-216-1-2(O. australiensis) | Endomembrane system | CC-NB-NB-LRR | 2016 | [44] |
| Bph21 | 12L | IR71033-121-15 (O. minuta) | Endomembrane system | CC-NB-NB-LRR | 2016 | [33] |
| Bph26 | 6S | ADR52 | Endomembrane system | CC-NB-NB-LRR | 2014 | [33,52] |
| Bph29 | 6S | RBPH54 | Nucleus | B3 DNA | 2015 | [56] |
| Bph30 | 4S | AC-1613 | Endomembrane system | LRD | 2021 | [57] |
| Bph32 | 6S | Ptb33 | Plasma membrane | SCR | 2016 | [60] |
| Bph37 | 6L | SE382 | — | CC-NB | 2021 | [66] |
| Bph40 | 4S | SE232, SE67, C334 | — | LRD | 2021 | [57] |
| Pyramided Genes/QTLs | Trait | Resistant Lines/Varieties | References |
|---|---|---|---|
| Bph1 + Bph2 | BPH resistance | Tsukushibare | [123] |
| Bph6 + Bph9 | BPH resistance | 9311 | [124] |
| Bph6 + Bph9 | BPH resistance | Wei-Liang-You 7713 | [125] |
| Bph6 + Bph12 | BPH resistance | 93-11, Nipponbare | [37] |
| Bph3 + Bph27 | BPH resistance | 93-11, Ningjing3 | [126] |
| Bph14 + Bph15 | BPH resistance | Minghui 63; Huahui938; Huang-Hua-Zhan; Hua-Liang-You2171; Yi-Liang-You 311 | [125,127,128,129,130] |
| Bph3 + Bph14 + Bph15 | BPH resistance | Hemeizhan | [131] |
| Bph14 + Bph15 + Bph18 | BPH resistance | 9311 | [132] |
| Bph14 + Bph15 +Xa21 | BPH and bacterial blight resistance | Yuehui9113 | [133] |
| Bph3 + Pita + Pi1 + Pi2 + xa5 + Xa23 | BPH, bacterial blight and blast resistance | HN88 | [134] |
| Qbph12 + Sub1 | BPH resistance and submergence tolerance | KDML105 | [135] |
| Bph14 + Bph15 + Stv-bi | BPH and rice stripe disease resistance | Shengdao15, Shengdao16, Xudao3 | [136] |
| Bph6 + Bph9 + Gn8.1 + Rf3 + Rf4 + Rf5 + Rf6 | BPH resistance, big panicle and fertility restoration gene | 9311 | [137] |
| Bph18 + Xa40 + qSTV11SG + Pib + Pik | BPH resistance, bacterial blight, rice stripe virus, and blast | Junam | [138] |
| Bph25 + Bph26 | BPH resistance | Taichung 65 | [51] |
| Bph17 + Bph21 + Bph32 | BPH resistance | IR24 | [139] |
| Bph3 + Bph17 + Pi9 + Xa4 + xa5 + Xa21 + Gm4 + Gm8 + qDTY1.1 + qDTY3.1 | BPH resistance, gall midge, blast, bacterial leaf blight and drought tolerance | Swarna + drought | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, L.; Luo, T.; Huang, D.; Wei, M.; Ma, Z.; Liu, C.; Qin, Y.; Zhou, X.; Lu, Y.; Li, R.; et al. Recent Advances in Molecular Mechanism and Breeding Utilization of Brown Planthopper Resistance Genes in Rice: An Integrated Review. Int. J. Mol. Sci. 2023, 24, 12061. https://doi.org/10.3390/ijms241512061
Yan L, Luo T, Huang D, Wei M, Ma Z, Liu C, Qin Y, Zhou X, Lu Y, Li R, et al. Recent Advances in Molecular Mechanism and Breeding Utilization of Brown Planthopper Resistance Genes in Rice: An Integrated Review. International Journal of Molecular Sciences. 2023; 24(15):12061. https://doi.org/10.3390/ijms241512061
Chicago/Turabian StyleYan, Liuhui, Tongping Luo, Dahui Huang, Minyi Wei, Zengfeng Ma, Chi Liu, Yuanyuan Qin, Xiaolong Zhou, Yingping Lu, Rongbai Li, and et al. 2023. "Recent Advances in Molecular Mechanism and Breeding Utilization of Brown Planthopper Resistance Genes in Rice: An Integrated Review" International Journal of Molecular Sciences 24, no. 15: 12061. https://doi.org/10.3390/ijms241512061
APA StyleYan, L., Luo, T., Huang, D., Wei, M., Ma, Z., Liu, C., Qin, Y., Zhou, X., Lu, Y., Li, R., Qin, G., & Zhang, Y. (2023). Recent Advances in Molecular Mechanism and Breeding Utilization of Brown Planthopper Resistance Genes in Rice: An Integrated Review. International Journal of Molecular Sciences, 24(15), 12061. https://doi.org/10.3390/ijms241512061







