Water-Deficit Stress in the Epiphytic Elkhorn Fern: Insight into Photosynthetic Response
Abstract
1. Introduction
2. Results
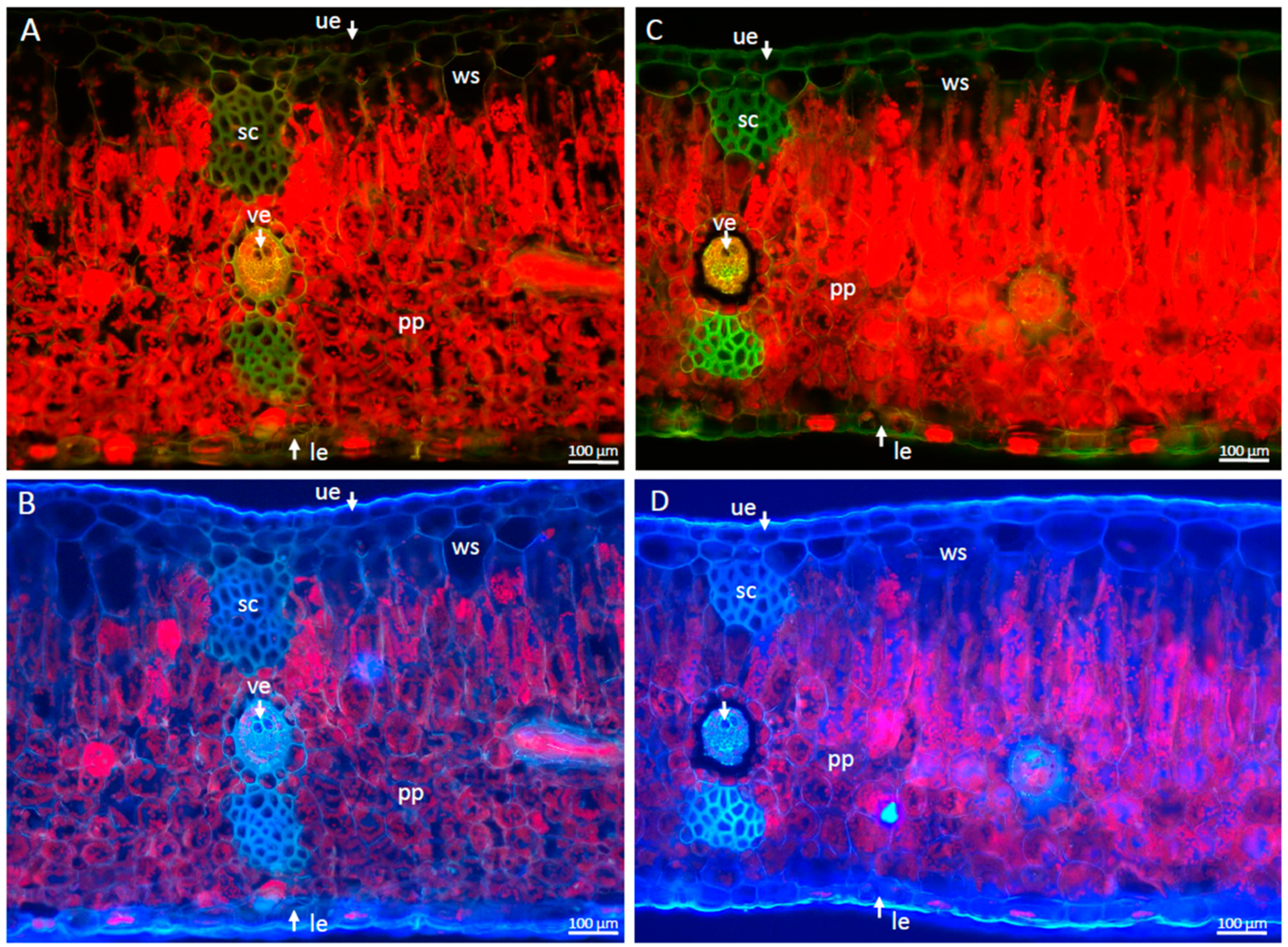
2.1. Morphological and Anatomical Changes
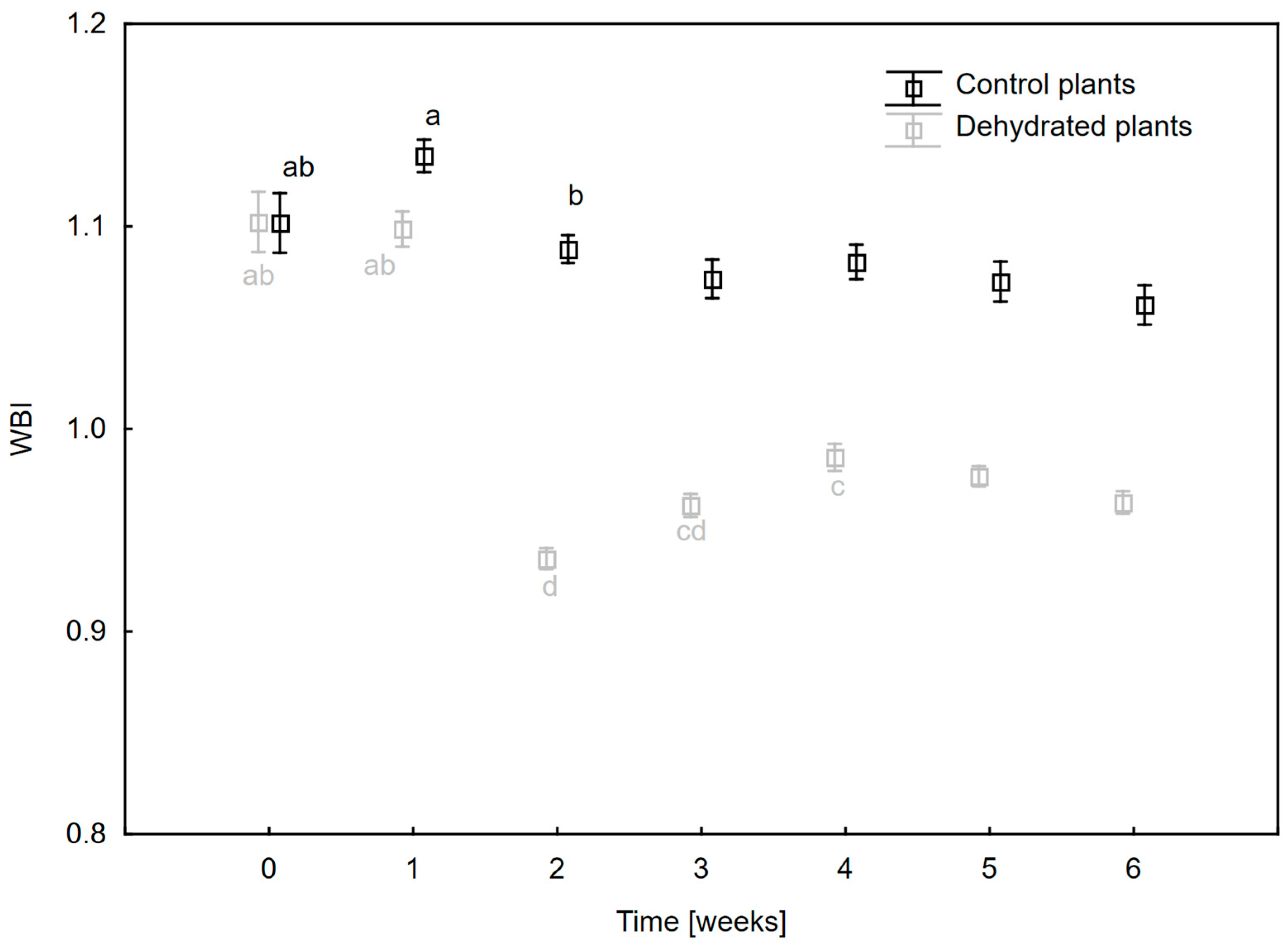
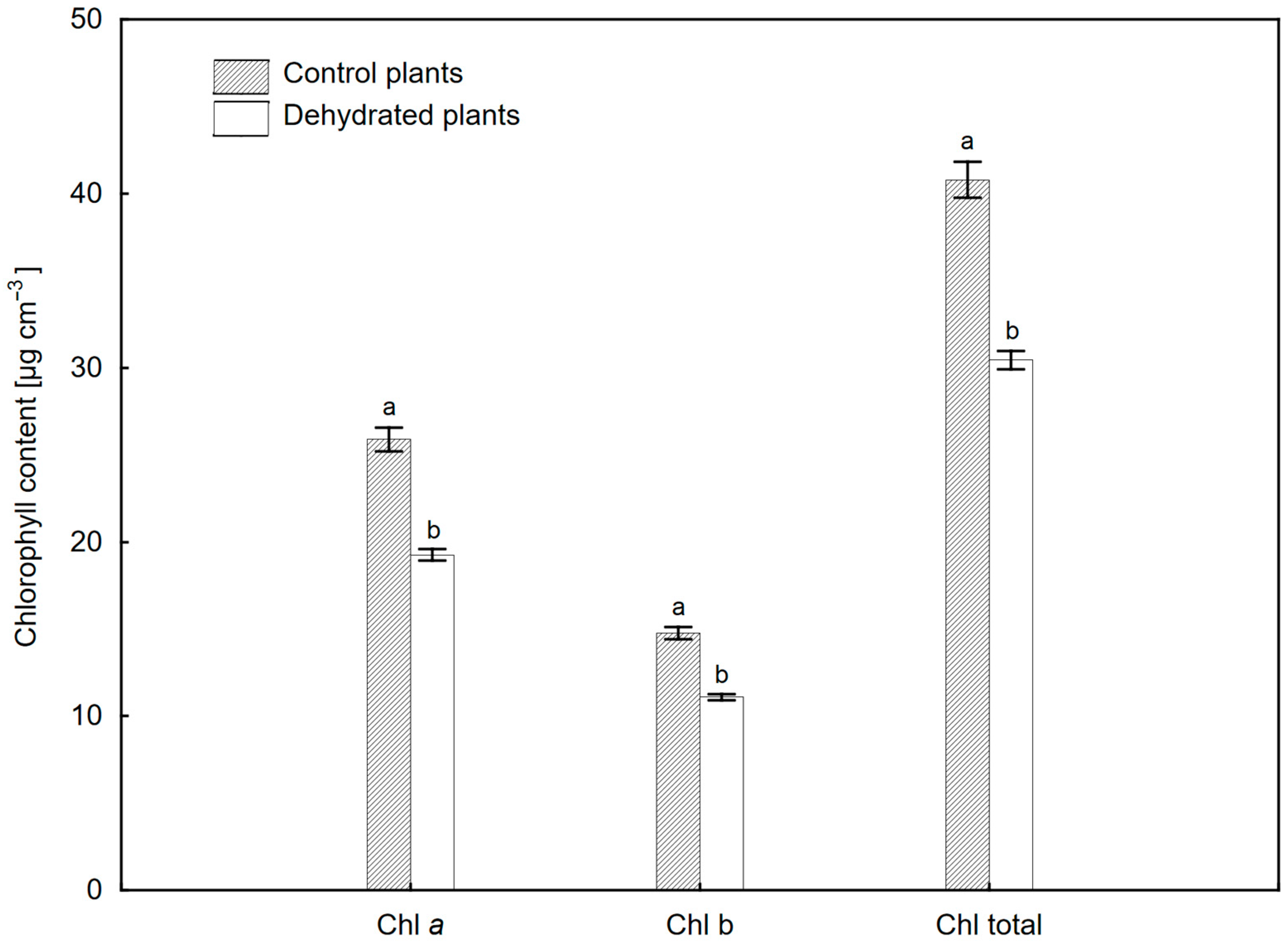
2.2. Changes in Hydration and Pigment Composition of Leaves
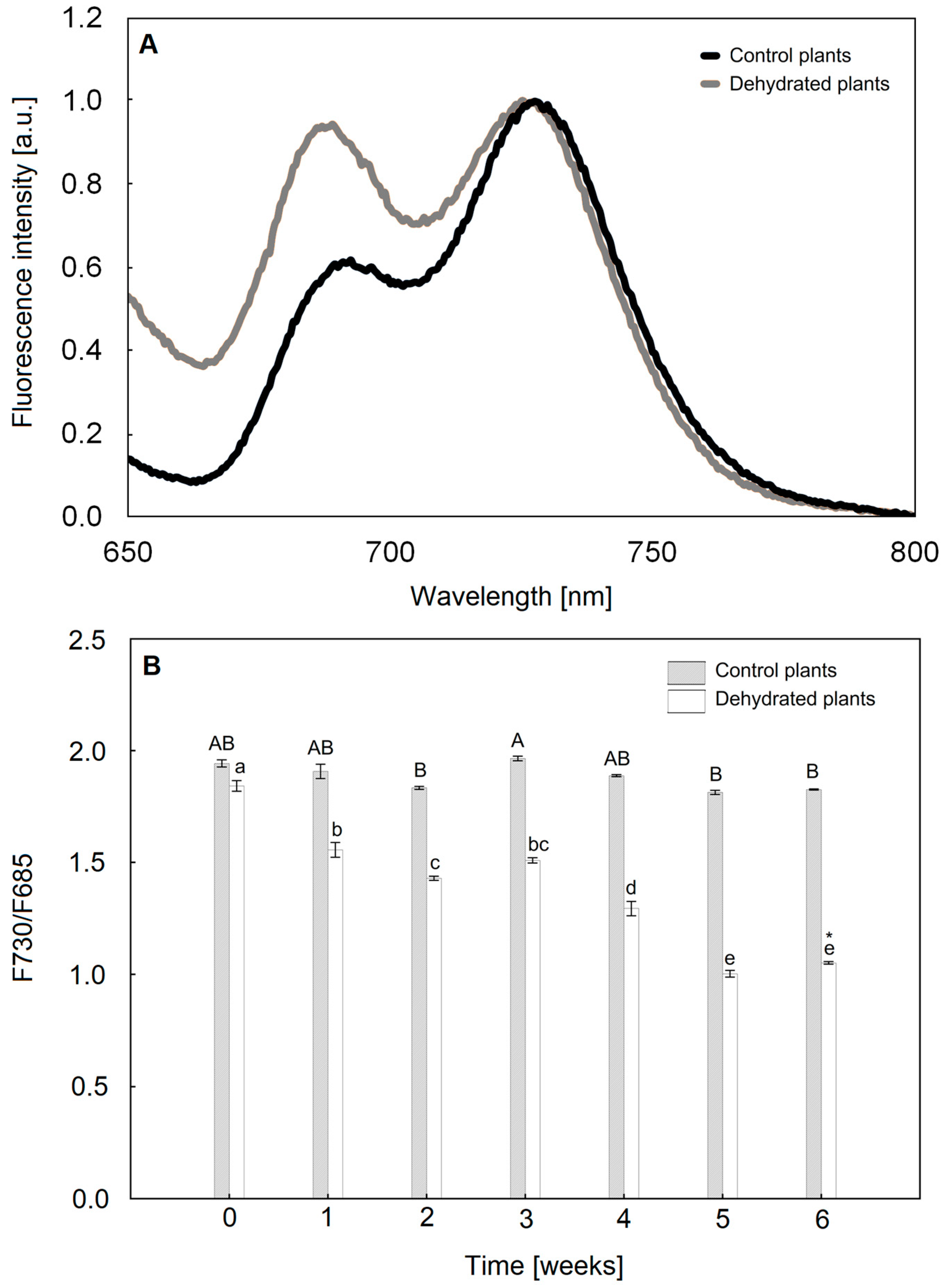
2.3. Chlorophyll a Fluorescence Analysis
2.4. The Size of the PSI and PSII Antenna Complexes
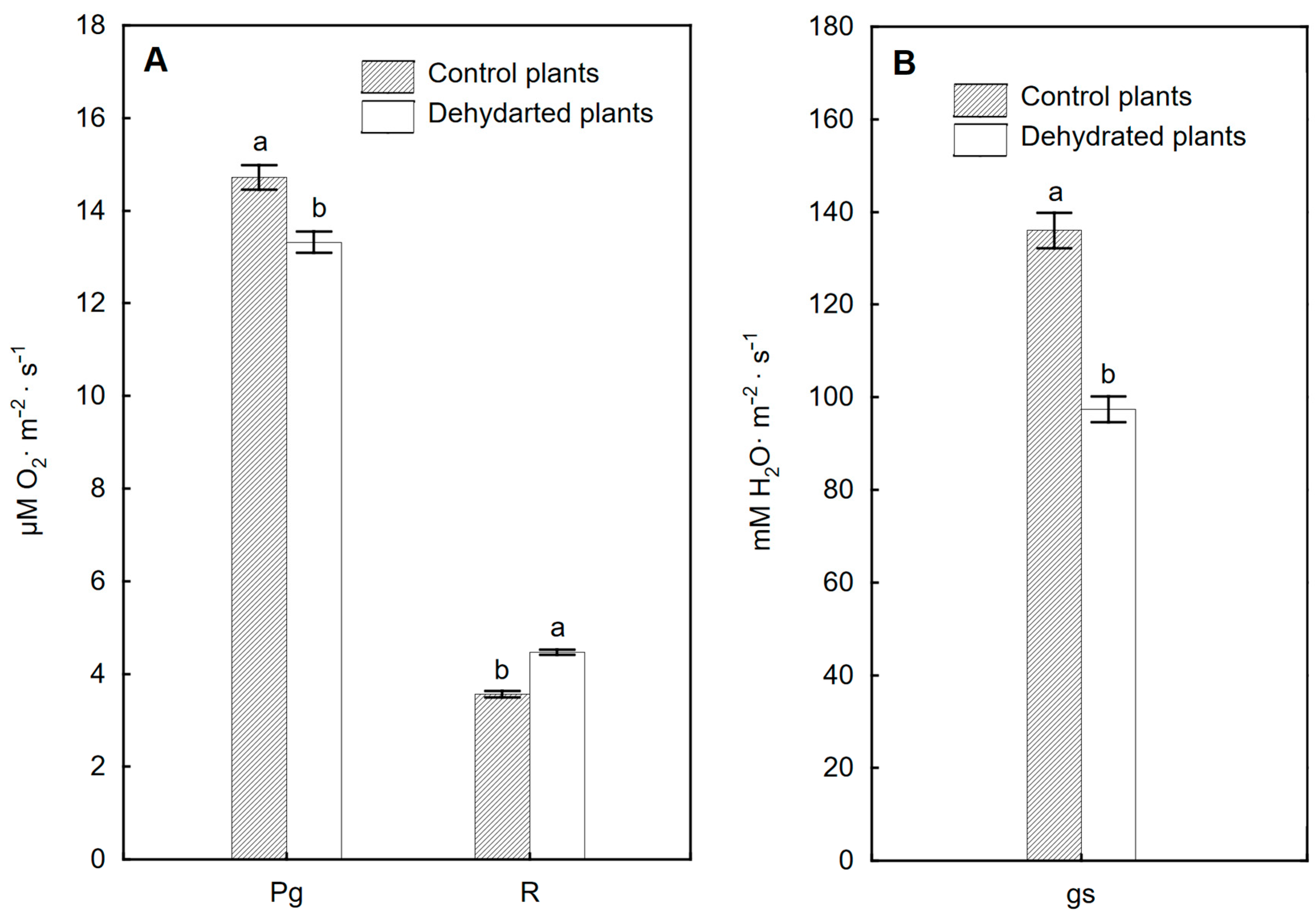
2.5. Efficiency of Gas Exchange of Leaves
2.6. Proline Content
3. Discussion
3.1. Morphological and Anatomical Features and Changes in the Content of Water and Pigments in Leaves
3.2. Light-Dependent Reactions of Photosynthesis under Drought Stress
3.3. Gas Exchange Regulation and Drought Adaptation Strategies
4. Materials and Methods
4.1. Plant Material
4.2. Plant Anatomy
4.3. Leaf Reflectance
4.4. Chlorophyll a Fluorescence
4.5. Low-Temperature Fluorescence at 77 K
4.6. Gas Exchange of Leaves
4.7. Chlorophyll Content
4.8. Proline Content
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lüttge, U. Vascular Plants as Epiphytes: Evolution and Ecophysiology; Springer: Berlin, Germany, 1989. [Google Scholar]
- Nishida, K.; Hanba, Y.T. Photosynthetic response of four fern species from different habitats to drought stress: Relationship between morpho-anatomical and physiological traits. Photosynthetica 2017, 55, 689–697. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, M.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Hura, T.; Grzesiak, S.; Hura, K.; Thiemt, E.; Tokarz, K.; Wedzony, M. Physiological and biochemical tools useful in drought-tolerance detection in genotypes of winter triticale: Accumulation of ferulic acid correlates with drought tolerance. Ann. Bot. 2007, 100, 767–775. [Google Scholar] [CrossRef]
- Peltzer, D.; Dreyer, E.; Polle, A. Differential temperature dependencies of antioxidative enzymes in two contrasting species: Fagus sylvatica and Coleus blumei. Plant Physiol. Biochem. 2002, 40, 141–150. [Google Scholar] [CrossRef]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Fujita, K. Effects of drought stress on photosynthetic gas exchange, chlorophyll fluorescence and stem diameter of soybean plants. Biol. Plant. 2006, 50, 138–141. [Google Scholar] [CrossRef]
- Wright, H.; De Longa, J.; Ladab, R.; Prangea, R. The relationship between water status and chlorophyll a fluorescence in grapes (Vitis spp.). Postharvest Biol. Tec. 2009, 51, 193–199. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, D.; Liu, F.; Dai, T.; Jing, Q.; Cao, W. Effects of salt and waterlogging stresses and their combination on leaf photosynthesis, chloroplast ATP synthesis, and antioxidant capacity in wheat. Plant Sci. 2009, 176, 575–582. [Google Scholar] [CrossRef]
- Sang, J.; Jiang, M.; Lin, F.; Xu, S.; Zhang, A.; Tan, M. Nitric oxide reduces hydrogen peroxide accumulation involved in water stress-induced subcellular anti-oxidant defense in maize plants. J. Integr. Plant Biol. 2008, 50, 231–243. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 4, 178–182. [Google Scholar] [CrossRef]
- Chaves, M.M.; Oliveira, M.M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef]
- McAdam, S.A.M.; Brodribb, T.J. Ancestral stomatal control results in a canalization of fern and lycophyte adaptation to drought. New Phytol. 2013, 198, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.E., Jr.; Rundel, P.W.; Cardelús, C.L. The influence of life form on carbon and nitrogen relationships in tropical rainforest ferns. Oecologia 2007, 153, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Minardi, B.D.; Voytena, A.P.; Santos, M.; Randi, A.M. The epiphytic fern Elaphoglossum luridum (Fée) Christ. (Dryopteridaceae) from Central and South America: Morphological and physiological responses to water stress. Sci. World J. 2014, 2014, 817892. [Google Scholar] [CrossRef]
- Hietz, P.; Briones, O. Correlation between water relations and within-canopy distribution of epiphytic ferns in a Mexican cloud forest. Oecologia 1998, 114, 305–316. [Google Scholar] [CrossRef]
- Tosens, T.; Nishida, K.; Gago, J.; Coopman, R.E.; Cabrera, H.M.; Carriquí, M.; Laanisto, L.; Morales, L.; Nadal, M.; Rojas, R.; et al. The photosynthetic capacity in 35 ferns and fern allies: Mesophyll CO2 diffusion as a key trait. New Phytol. 2016, 209, 1576–1590. [Google Scholar] [CrossRef] [PubMed]
- McAdam, S.A.; Brodribb, T.J. Stomatal innovation and the rise of seed plants. Ecol. Lett. 2012, 15, 1–8. [Google Scholar] [CrossRef]
- Zhang, S.B.; Sun, M.; Cao, K.F.; Hu, H.; Zhang, J.L. Leaf photosynthetic rate of tropical ferns is evolutionarily linked to water transport capacity. PLoS ONE 2014, 9, e84682. [Google Scholar] [CrossRef]
- Benzing, D.H. Vascular Epiphytes; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Sanusi, R.-A.M.; Nuruddin, A.A.; Hamid, H.A. Leaf chlorophyll fluorescence and gas exchange response to different light levels in Platycerium bifurcatum. Am. J. Agric. Biol. Sci. 2011, 6, 214–220. [Google Scholar] [CrossRef]
- Oliwa, J.; Skoczowski, A. Different response of photosynthetic apparatus to high-light stress in sporotrophophyll and nest leaves of Platycerium bifurcatum. Photosynthetica 2019, 57, 147–159. [Google Scholar] [CrossRef]
- Oliwa, J.; Stawoska, I.; Janeczko, A.; Oklestkova, J.; Skoczowski, A. Response of the photosynthetic apparatus in the tropical fern Platycerium bifurcatum to increased ozone concentration. Photosynthetica 2019, 57, 1119–1129. [Google Scholar] [CrossRef]
- Rut, G.; Krupa, J.; Rzepka, A. The influence of simulated osmotic drought on functioning of the photosynthetic apparatus in gametophytes of the epiphytic fern Platycerium bifurcatum. Pol. J. Nat. Sci. 2003, 1 (Suppl. 1), 114–115. [Google Scholar]
- Farrant, J.M.; Lehner, A.; Cooper, K.; Wiswedel, S. Desiccation tolerance in the vegetative tissues of the fern Mohria caffrorum is seasonally regulated. Plant J. 2009, 57, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Suhaimi, N.; Cicuzza, D. The effects of drought and the recovery phase of two tropical epiphytic ferns. J. Trop. Plant Physiol. 2020, 12, 33–41. [Google Scholar]
- Zhang, Q.; Chen, J.W.; Li, B.G.; Cao, K.F. Effect of drought on photosynthesis in two epiphytic and two terrestrial tropical fern species. Photosynthetica 2009, 47, 128–132. [Google Scholar] [CrossRef]
- Hietz, P. Fern Adaptations to Xeric Environments. In Fern Ecology; Mehltreter, K., Walker, L.R., Sharpe, J.M., Eds.; Cambridge University Press: Cambridge, MA, USA, 2010; pp. 140–176. [Google Scholar] [CrossRef]
- Oliwa, J.; Kornas, A.; Skoczowski, A. A low ratio of red/far-red in the light spectrum accelerates senescence in nest leaves of Platycerium bifurcatum. Acta Biol. Cracov. Bot. 2017, 59, 17–30. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Savé, R. The reflectance at the 950-970 nm region as an indicator of plant water status, Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Pu, R.; Ge, S.; Kelly, N.M.; Gong, P. Spectral absorption features as indicators of water status in coast live oak (Quercus agrifolia) leaves. Int. J. Remote Sens. 2003, 24, 1799–1810. [Google Scholar] [CrossRef]
- Penuelas, J.; Pinol, J.; Ogaya, R.; Filella, I. Estimation of plant water concentration by the reflectance water index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Estimation of vegetation water content and photosynthetic tissue area from spectral reflectance: A comparison of indices based on liquid water and chlorophyll absorption features. Remote Sens. Environ. 2003, 84, 526–537. [Google Scholar] [CrossRef]
- Tausz, M.; Hietz, P.; Briones, O. The significance of carotenoids and tocopherols in photoprotection of seven epiphytic fern species of a Mexican cloud forest. Funct. Plant Biol. 2001, 28, 775–783. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Liu, L. Wheat: Water deficit-induced senescence and its relationship to the remobilization of pre-stored carbon in wheat during grain filling. Agron. J. 2001, 93, 196–206. [Google Scholar] [CrossRef]
- Fathi, A.; Tari, D. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef]
- Flexas, J.; Hendrickson, L.; Chow, W.S. Photoinactivation of photosystem II in high light-acclimated grapevines. Aust. J. Plant. Physiol. 2001, 28, 755–764. [Google Scholar] [CrossRef]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Solovchenko, A. Quantification of screening pigments and their efficiency in situ. In Photoprotection in Plants; Springer Series in Biophysics; Springer: Berlin/Heidelberg, Germany, 2010; Volume 14. [Google Scholar] [CrossRef]
- Gitelson, A.; Solovchenko, A. Non-invasive quantification of foliar pigments: Possibilities and limitations of reflectance- and absorbance-based approaches. J. Photochem. Photobiol. B 2018, 178, 537–544. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzylak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 71, 38–45. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Filella, I.; Amaro, T.; Araus, J.L.; Penuelas, J. Relationship between photosynthetic radiation-use efficiency of barley canopies and the photochemical reflectance index (PRI). Physiol. Plant. 1996, 96, 211–216. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. BBA-Bioenergetics 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Živčák, M.; Brestič, M.; Olšovská, K.; Slamka, P. Performance index as a sensitive indicator of water stress in Triticum aestivum L. Plant Soil Environ. 2008, 54, 133–139. [Google Scholar] [CrossRef]
- Kalaji, M.H.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A. The use of chlorophyll fluorescence kinetics analysis to study the performance of photosynthetic machinery in plants. Emerg. Technol. Manag. Crop Stress Toler. 2014, 2, 347–384. [Google Scholar] [CrossRef]
- Oukarroum, A.; El Madidi, S.; Schansker, G.; Strasser, R.J. Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environ. Exp. Bot. 2007, 60, 438–446. [Google Scholar] [CrossRef]
- Liu, W.J.; Chen, Y.E.; Tian, W.J.; Du, J.B.; Zhang, Z.W.; Xu, F.; Zhang, F.; Yuan, S.; Lin, H.-H. Dephosphorylation of photosystem II proteins and phosphorylation of CP29 in barley photosynthetic membranes as a response to water stress. Biochim. Biophys. Acta 2009, 1787, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Tsimilli-Michael, M.; Strasser, R.J. Biophysical phenomics: Evaluation of the impact of mycorrhization with Piriformospora indica. In Piriformospora Indica. Sebacinales and Their Biotechnological Applications; Varma, A., Kost, G., Oelmüller., R., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 173–190. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, Germany, 2004; pp. 321–362. [Google Scholar] [CrossRef]
- Stirbet, A. Excitonic connectivity between photosystem II units: What is it, and how to measure it? Photosynth. Res. 2013, 116, 189–214. [Google Scholar] [CrossRef]
- Bąba, W.; Kalaji, H.M.; Kompała-Bąba, A.; Goltsev, V. Acclimatization of photosynthetic apparatus of tor grass (Brachypodium pinnatum) during expansion. PLoS ONE 2016, 11, e0156201. [Google Scholar] [CrossRef]
- Skoczowski, A.; Odrzywolska-Hasiec, M.; Oliwa, J.; Ciereszko, I.; Kornaś, A. Ecophysiological Variability of Alnus viridis (Chaix) DC. Green Alder Leaves in the Bieszczady Mountains (Poland). Plants 2021, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Bacarin, M.A.; Deuner, S.; da Silva, F.S.; Cassol, D.; Silva, D.M. Chlorophyll a fluorescence as indicative of the salt stress on Brassica napus L. Braz. J. Plant Physiol. 2011, 23, 245–253. [Google Scholar] [CrossRef]
- Hietz, P.; Briones, O. Photosynthesis, chlorophyll fluorescence and within canopy distribution of epiphytic ferns in a mexican cloud forest. Plant Biol. 2001, 3, 279–287. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hori, H.; Kai, S.; Ishikawa, T.; Ohnishi, A.; Tsumura, N.; Morita, N. Quality control of Photosystem II: Reversible and irreversible protein aggregation decides the fate of Photosystem II under excessive illumination. Front. Plant Sci. 2013, 29, 433. [Google Scholar] [CrossRef]
- Wiciarz, M.; Gubernator, B.; Kruk, J.; Niewiadomska, E. Enhanced chloroplastic generation of H2O2 in stress-resistant Thellungiella salsuginea in comparison to Arabidopsis thaliana. Physiol. Plant. 2015, 153, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, A.M.; Itoh, S.; Govindjee. Global spectral-kinetic analysis of room temperature chlorophyll a fluorescence from light-harvesting antenna mutants of barley. Philos. Trans. R. Soc. 2000, 355, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.M.; Gupta, N.; Nazir, M.; Mahajan, R.; Malik, F.A.; Sofi, N.R.; Shikari, A.B.; Salgotra, R.K. Impact of drought on photosynthesis: Molecular perspective. Plant Gene 2017, 11, 154–159. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, S.; He, X.; Li, Y.; Li, P.; Zhang, Y.; Chen, W. Growth. Secondary metabolites and enzyme activity responses of two edible fern species to drought stress and rehydration in northeast China. Agronomy 2019, 9, 137. [Google Scholar] [CrossRef]
- Anamul Hoque, M.; Okuma, E.; Nasrin Akhter Banu, M.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. J. Plant Physiol. 2007, 164, 553–561. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Alterations in photosynthetic pigments, protein, and carbohydrate metabolism in a wild plant Coronopus didymus L. (Brassicaceae) under lead stress. Acta Physiol. Plant 2017, 39, 176. [Google Scholar] [CrossRef]
- Ju, Y.-L.; Yue, X.-F.; Zhao, X.-f.; Zhao, H.; Fang, Y.-L. Physiological, micro-morphological and metabolomic analysis of grapevine (Vitis vinifera L.) leaf of plants under water stress. Plant Physiol. Biochem. 2018, 130, 501–510. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Penuelas, J.; Filella, I.; Baret, F. Semiempirical indices to assess carotenoids/chlorophyll a ratio from leaf spectra reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Merzlyak, M.N.; Solovchenko, A.E.; Smagin, A.I.; Gitelson, A.A. Apple flavonols during fruit adaptation to solar radiation spectral features and technique for non-destructive assessment. J. Plant. Physiol. 2005, 16, 151–160. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Grzesiak, M.; Filek, M.; Barbasz, A.; Kreczmer, B.; Hartikainen, H. Relationships between polyamines, ethylene, osmoprotectants and antioxidant enzymes activities in wheat seedlings after short-term PEG- and NaCl-induced stresses. Plant Growth Regul. 2013, 69, 177–189. [Google Scholar] [CrossRef]


| Parameters | W0 | W1 | W2 | W3 | W4 | W5 | W6 |
|---|---|---|---|---|---|---|---|
| ARI1 | 0.006 a | 0.009 a | −0.004 b | −0.003 b | −0.002 b | 0.001 ab | 0.002 ab |
| ±0.002 | ±0.002 | ±0.002 | ±0.001 | ±0.002 | ±0.002 | ±0.002 | |
| CRI1 | 0.161 a | 0.155 a | 0.072 b | 0.084 b | 0.095 b | 0.088 b | 0.111 ab |
| ±0.025 | ±0.012 | ±0.005 | ±0.007 | ±0.007 | ±0.008 | ±0.008 | |
| SIPI | 0.868 ab | 0.887 a | 0.843 b | 0.854 b | 0.857 ab | 0.850 b | 0.861 ab |
| ±0.010 | ±0.007 | ±0.005 | ±0.006 | ±0.006 | ±0.006 | ±0.006 | |
| FRI | −1.888 a | −2.947 b | −2.665 ab | −2.535 ab | −2.731 ab | −2.394 ab | −2.845 b |
| ±0.218 | ±0.342 | ±0.135 | ±0.177 | ±0.173 | ±0.187 | ±0.190 | |
| PRI | 0.090 a | 0.037 d | 0.076 ab | 0.070 bc | 0.077 ab | 0.079 ab | 0.055 c |
| ±0.004 | ±0.005 | ±0.004 | ±0.002 | ±0.004 | ±0.003 | ±0.003 |
| Parameters | W0 | W1 | W2 | W3 | W4 | W5 | W6 |
|---|---|---|---|---|---|---|---|
| Measured parameters and basic JIP test parameters | |||||||
| PI total | 9.619 a | 5.678 b | 4.808 bc | 4.748 bc | 3.483 c | 0.771 d | 0.321 d |
| ±0.780 | ±0.777 | ±0.488 | ±0.609 | ±0.411 | ±0.056 | ±0.029 | |
| Fv/F0 | 4.802 a | 4.656 a | 4.003 b | 3.940 b | 3.811 b | 3.912 b | 2.454 c |
| ±0.091 | ±0.132 | ±0.121 | ±0.171 | ±0.188 | ±0.099 | ±0.106 | |
| Fv/Fm | 0.827 a | 0.822 a | 0.799 b | 0.795 b | 0.789 b | 0.796 b | 0.708 c |
| ±0.003 | ±0.004 | ±0.005 | ±0.007 | ±0.008 | ±0.004 | ±0.009 | |
| AM | 50,216 a | 36,024 bc | 33,178 c | 43,033 ab | 31,311 c | 34,245 c | 28,701 c |
| ±3550 | ±1787 | ±1886 | ±3734 | ±1783 | ±3380 | ±2607 | |
| Specific Energy fluxes expressed per active RC of PSII | |||||||
| ABS/RC | 1.199 d | 1.279 d | 1.488 c | 1.570 c | 1.617 c | 1.957 b | 2.404 a |
| ±0.021 | ±0.046 | ±0.056 | ±0.082 | ±0.084 | ±0.042 | ±0.045 | |
| DI0/RC | 0.208 d | 0.230 d | 0.301 c | 0.326 c | 0.346 bc | 0.401 b | 0.706 a |
| ±0.006 | ±0.013 | ±0.018 | ±0.025 | ±0.030 | ±0.015 | ±0.033 | |
| TR0/RC | 0.992 d | 1.050 d | 1.186 c | 1.244 c | 1.271 c | 1.556 b | 1.698 a |
| ±0.015 | ±0.034 | ±0.040 | ±0.059 | ±0.058 | ±0.029 | ±0.016 | |
| ET0/RC | 0.735 bc | 0.752 b | 0.870 a | 0.881 a | 0.884 a | 0.739 bc | 0.655 c |
| ±0.017 | ±0.031 | ±0.028 | ±0.039 | ±0.036 | ±0.037 | ±0.013 | |
| RE0/RC | 0.326 a | 0.271 b | 0.320 a | 0.354 a | 0.323 a | 0.218 c | 0.215 c |
| ±0.009 | ±0.014 | ±0.011 | ±0.014 | ±0.018 | ±0.012 | ±0.011 | |
| Quantum yield parameters | |||||||
| φPo | 0.827 a | 0.822 a | 0.799 b | 0.795 b | 0.789 b | 0.796 b | 0.708 c |
| ±0.003 | ±0.004 | ±0.005 | ±0.007 | ±0.008 | ±0.004 | ±0.009 | |
| φEo | 0.613 a | 0.588 ab | 0.588 ab | 0.565 b | 0.551 b | 0.377 c | 0.274 d |
| ±0.011 | ±0.012 | ±0.015 | ±0.012 | ±0.013 | ±0.014 | ±0.009 | |
| φRo | 0.272 a | 0.212 bc | 0.217 bc | 0.229 b | 0.202 c | 0.111 d | 0.090 d |
| ±0.008 | ±0.008 | ±0.007 | ±0.011 | ±0.011 | ±0.005 | ±0.005 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliwa, J.; Skoczowski, A.; Rut, G.; Kornaś, A. Water-Deficit Stress in the Epiphytic Elkhorn Fern: Insight into Photosynthetic Response. Int. J. Mol. Sci. 2023, 24, 12064. https://doi.org/10.3390/ijms241512064
Oliwa J, Skoczowski A, Rut G, Kornaś A. Water-Deficit Stress in the Epiphytic Elkhorn Fern: Insight into Photosynthetic Response. International Journal of Molecular Sciences. 2023; 24(15):12064. https://doi.org/10.3390/ijms241512064
Chicago/Turabian StyleOliwa, Jakub, Andrzej Skoczowski, Grzegorz Rut, and Andrzej Kornaś. 2023. "Water-Deficit Stress in the Epiphytic Elkhorn Fern: Insight into Photosynthetic Response" International Journal of Molecular Sciences 24, no. 15: 12064. https://doi.org/10.3390/ijms241512064
APA StyleOliwa, J., Skoczowski, A., Rut, G., & Kornaś, A. (2023). Water-Deficit Stress in the Epiphytic Elkhorn Fern: Insight into Photosynthetic Response. International Journal of Molecular Sciences, 24(15), 12064. https://doi.org/10.3390/ijms241512064







