Raman Spectroscopy Profiling of Splenic T-Cells in Sepsis and Endotoxemia in Mice
Abstract
1. Introduction
2. Results and Discussion
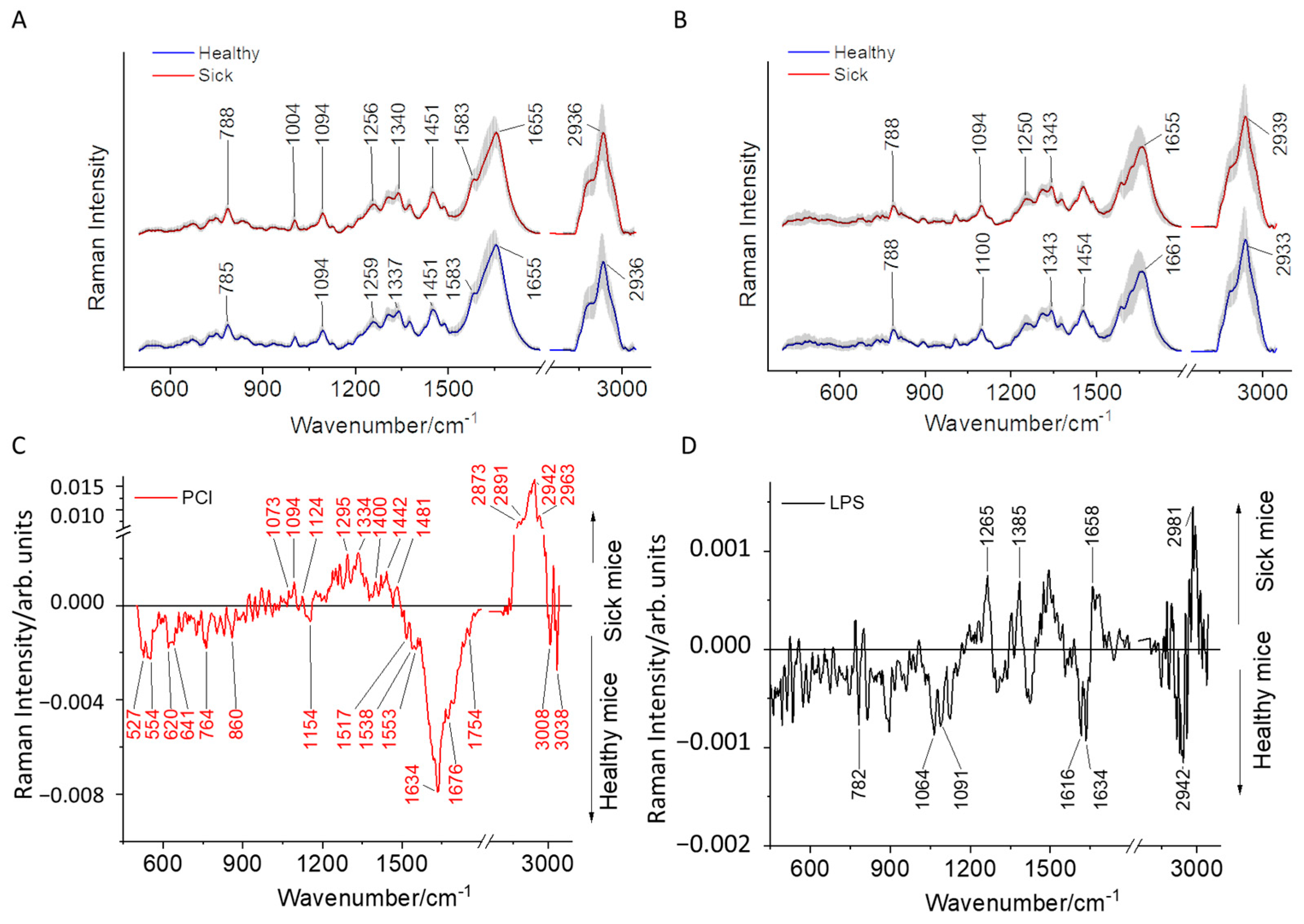
2.1. Comparison of Raman Difference Spectrum between Mice with Sepsis/Inflammation and Sham
| Tentative Assignments of the Raman Peaks (cm−1) | ||||
|---|---|---|---|---|
| Nucleic Acids | Proteins | Lipids | Lipids and Proteins | Amino Acids |
| 755 764 776 779 782 788 893 982 1085–1106 1175–1196 1304–1334 1517–1589 | 620 764 1004–1043 1127 1229–1295 1337–1349 1409–1463 1604–1676 2942–2984 | 1064 1130–1166 1367–1376 1463 1532–1538 1742–1766 2849 2873–2900 3011 3023 | 2918 2915 | 527 554 596 641 647 659 695 743 815–839 |
2.2. Comparison of Raman Models Built for T-Lymphocytes from Endotoxemic and Septic Mice
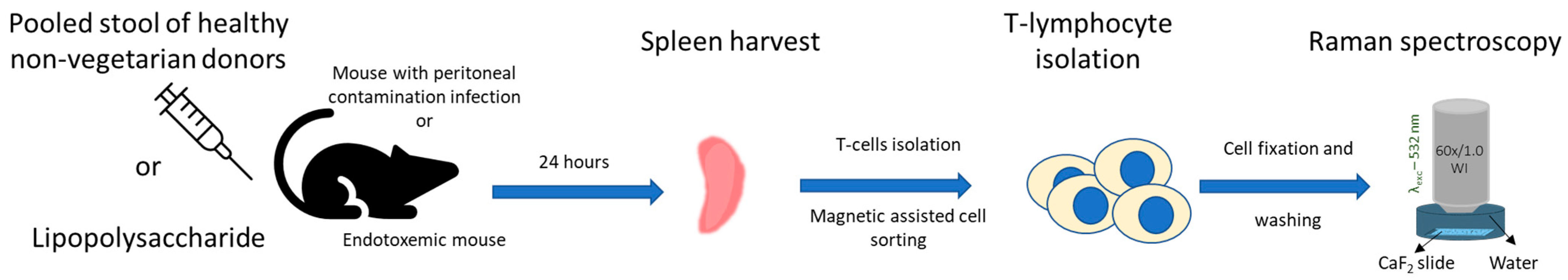
3. Material and Methods
3.1. Animal Ethic Statement
3.2. Peritoneal Contamination Infection (PCI) Mouse Model
3.3. LPS-Induced Inflammation Mouse Model
3.4. Splenocyte Isolation
3.5. Raman Spectroscopy Investigation of the Splenocytes
3.6. Raman Spectral Data Analysis
3.7. Raman Spectral Model for PCI Mice
3.8. Raman Spectral Model for LPS Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Signore, A. About inflammation and infection. EJNMMI Res. 2013, 3, 8. [Google Scholar] [PubMed]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [PubMed]
- Nicholson, L.B. The immune system. Essays Biochem. 2016, 60, 275–301. [Google Scholar] [CrossRef] [PubMed]
- Foerster, M.; Haefner, D.; Kroegel, C. Bcl-2-Mediated Regulation of CD69-Induced Apoptosis of Human Eosinophils: Identification and Characterization of a Novel Receptor-Induced Mechanism and Relationship to CD95-Transduced Signalling. Scand. J. Immunol. 2002, 56, 417–428. [Google Scholar]
- Li, Y.; Tan, R.; Chen, Y.; Liu, Z.; Chen, E.; Pan, T.; Qu, H. SC2sepsis: Sepsis single-cell whole gene expression database. Database 2022, 2022, baac061. [Google Scholar] [CrossRef]
- Reyes, M.; Filbin, M.R.; Bhattacharyya, R.P.; Billman, K.; Eisenhaure, T.; Hung, D.T.; Levy, B.D.; Baron, R.M.; Blainey, P.C.; Goldberg, M.B.; et al. An immune-cell signature of bacterial sepsis. Nat. Med. 2020, 26, 333–340. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, C.; Cai, P.; Shen, Z.; Sun, W.; Xu, H.; Fang, M.; Yao, X.; Zhu, L.; Gao, X.; et al. Single-cell analysis of COVID-19, sepsis, and HIV infection reveals hyperinflammatory and immunosuppressive signatures in monocytes. Cell Rep. 2021, 37, 109793. [Google Scholar] [CrossRef]
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef]
- Luperto, M.; Zafrani, L. T cell dysregulation in inflammatory diseases in ICU. Intensive Care Med. Exp. 2022, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Yang, W.-L.; Matsuo, S.; Wang, P. Differential alterations of tissue T-cell subsets after sepsis. Immunol. Lett. 2015, 168, 41–50. [Google Scholar] [CrossRef]
- Ramoji, A.; Neugebauer, U.; Bocklitz, T.; Foerster, M.; Kiehntopf, M.; Bauer, M.; Popp, J. Toward a spectroscopic hemogram: Raman spectroscopic differentiation of the two most abundant leukocytes from peripheral blood. Anal. Chem. 2012, 84, 5335–5342. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Xu, J.; Brinkhof, B.; Wang, H.; Cui, Z.; Huang, W.E.; Ye, H. A single-cell Raman-based platform to identify developmental stages of human pluripotent stem cell-derived neurons. Proc. Natl. Acad. Sci. USA 2020, 117, 18412–18423. [Google Scholar] [CrossRef] [PubMed]
- Morrish, R.; Yim, K.H.W.; Pagliara, S.; Palombo, F.; Chahwan, R.; Stone, N. Single Cell Label-Free Probing of Chromatin Dynamics during B Lymphocyte Maturation. Front. Cell Dev. Biol. 2021, 9, 646616. [Google Scholar] [CrossRef] [PubMed]
- Mannie, M.D.; McConnell, T.J.; Xie, C.; Li, Y. Activation-dependent phases of T cells distinguished by use of optical tweezers and near infrared Raman spectroscopy. J. Immunol. Methods 2005, 297, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, U.; Clement, J.H.; Bocklitz, T.; Krafft, C.; Popp, J. Identification and differentiation of single cells from peripheral blood by Raman spectroscopic imaging. J. Biophotonics 2010, 3, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Schie, I.W.; Alber, L.; Gryshuk, A.L.; Chan, J.W. Investigating drug induced changes in single, living lymphocytes based on Raman micro-spectroscopy. Analyst 2014, 139, 2726–2733. [Google Scholar] [CrossRef]
- Brown, K.L.; Palyvoda, O.Y.; Thakur, J.S.; Nehlsen-Cannarella, S.L.; Fagoaga, O.R.; Gruber, S.A.; Auner, G.W. Raman spectroscopic differentiation of activated versus non-activated T lymphocytes: An in vitro study of an acute allograft rejection model. J. Immunol. Methods 2009, 340, 48–54. [Google Scholar] [CrossRef]
- McNerney, G.P.; Hubner, W.; Chen, B.K.; Huser, T. Manipulating CD4+ T cells by optical tweezers for the initiation of cell-cell transfer of HIV-1. J. Biophotonics 2010, 3, 216–223. [Google Scholar] [CrossRef]
- Hobro, A.J.; Kumagai, Y.; Akira, S.; Smith, N.I. Raman spectroscopy as a tool for label-free lymphocyte cell line discrimination. Analyst 2016, 141, 3756–3764. [Google Scholar] [CrossRef]
- Schie, I.W.; Rüger, J.; Mondol, A.S.; Ramoji, A.; Neugebauer, U.; Krafft, C.; Popp, J. High-Throughput Screening Raman Spectroscopy Platform for Label-Free Cellomics. Anal. Chem. 2018, 90, 2023–2030. [Google Scholar] [CrossRef]
- Pistiki, A.; Ramoji, A.; Ryabchykov, O.; Thomas-Rüddel, D.; Press, A.T.; Makarewicz, O.; Giamarellos-Bourboulis, E.J.; Bauer, M.; Bocklitz, T.; Popp, J.; et al. Biochemical Analysis of Leukocytes after In Vitro and In Vivo Activation with Bacterial and Fungal Pathogens Using Raman Spectroscopy. Int. J. Mol. Sci. 2021, 22, 10481. [Google Scholar] [CrossRef]
- Ramoji, A.; Ryabchykov, O.; Galler, K.; Tannert, A.; Markwart, R.; Requardt, R.P.; Rubio, I.; Bauer, M.; Bocklitz, T.; Popp, J.; et al. Raman Spectroscopy Follows Time-Dependent Changes in T Lymphocytes Isolated from Spleen of Endotoxemic Mice. Immunohorizons 2019, 3, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Töpfer, N.; Müller, M.; Dahms, M.; Ramoji, A.; Popp, J.; Slevogt, H.; Neugebauer, U. Raman spectroscopy reveals LPS-induced changes of biomolecular composition in monocytic THP-1 cells in a label-free manner. Integr. Biol. 2019, 11, 87–98. [Google Scholar] [CrossRef]
- Ramoji, A.; Thomas-Rüddel, D.; Ryabchykov, O.; Bauer, M.; Arend, N.M.; Giamarellos-Bourboulis, E.J.; Eugen-Olsen, J.; Kiehntopf, M.; Bocklitz, T.; Popp, J.; et al. Leukocyte Activation Profile Assessed by Raman Spectroscopy Helps Diagnosing Infection and Sepsis. Crit. Care Explor. 2021, 3, e0394. [Google Scholar]
- Chaudhary, N.; Nguyen, T.N.Q.; Cullen, D.; Meade, A.D.; Wynne, C. Discrimination of immune cell activation using Raman micro-spectroscopy in an in-vitro & ex-vivo model. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 248, 119118. [Google Scholar] [CrossRef]
- Borek-Dorosz, A.; Nowakowska, A.M.; Leszczenko, P.; Adamczyk, A.; Pieczara, A.; Jakubowska, J.; Pastorczak, A.; Ostrowska, K.; Ząbczyńska, M.; Sowinski, K.; et al. Raman-based spectrophenotyping of the most important cells of the immune system. J. Adv. Res. 2022, 41, 191–203. [Google Scholar] [CrossRef]
- Ichimura, T.; Chiu, L.-D.; Fujita, K.; Machiyama, H.; Yamaguchi, T.; Watanabe, T.M.; Fujita, H. Non-label immune cell state prediction using Raman spectroscopy. Sci. Rep. 2016, 6, 37562. [Google Scholar] [CrossRef]
- Feuerer, N.; Carvajal Berrio, D.A.; Billing, F.; Segan, S.; Weiss, M.; Rothbauer, U.; Marzi, J.; Schenke-Layland, K. Raman Microspectroscopy Identifies Biochemical Activation Fingerprints in THP-1- and PBMC-Derived Macrophages. Biomedicines 2022, 10, 989. [Google Scholar] [CrossRef]
- Markwart, R.; Condotta, S.A.; Requardt, R.P.; Borken, F.; Schubert, K.; Weigel, C.; Bauer, M.; Griffith, T.S.; Förster, M.; Brunkhorst, F.M.; et al. Immunosuppression after sepsis: Systemic inflammation and sepsis induce a loss of naive T-cells but no enduring cell-autonomous defects in T-cell function. PLoS ONE 2014, 9, e115094. [Google Scholar] [CrossRef]
- Gonnert, F.A.; Recknagel, P.; Seidel, M.; Jbeily, N.; Dahlke, K.; Bockmeyer, C.L.; Winning, J.; Lösche, W.; Claus, R.A.; Bauer, M. Characteristics of Clinical Sepsis Reflected in a Reliable and Reproducible Rodent Sepsis Model. J. Surg. Res. 2011, 170, e123–e134. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Ayala, A.; Bahrami, S.; Bauer, M.; Boros, M.; Cavaillon, J.-M.; Chaudry, I.H.; Coopersmith, C.M.; Deutschman, C.S.; Drechsler, S.; et al. Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS): An International Expert Consensus Initiative for Improvement of Animal Modeling in Sepsis. Shock 2018, 50, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.; Choi, A.M.; Nakahira, K. Mitochondrial DNA in Sepsis. Curr. Opin. Crit. Care 2017, 23, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.I.; Didari, T.; Khan, F.; Mojtahedzadeh, M.; Abdollahi, M. The Role of Epigenetic Alterations Involved in Sepsis: An Overview. Curr. Pharm. Des. 2018, 24, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Seemann, S.; Zohles, F.; Lupp, A. Comprehensive comparison of three different animal models for systemic inflammation. J. Biomed. Sci. 2017, 24, 60–77. [Google Scholar] [CrossRef] [PubMed]
- Singer, M. Cellular Dysfunction in Sepsis. Clin. Chest Med. 2008, 29, 655–660. [Google Scholar] [CrossRef]
- van der Slikke, E.C.; Star, B.S.; Quinten, V.M.; ter Maaten, J.C.; Ligtenberg, J.J.; van Meurs, M.; Gansevoort, R.T.; Bakker, S.J.; Chao, M.-R.; Henning, R.H.; et al. Association between oxidized nucleobases and mitochondrial DNA damage with long-term mortality in patients with sepsis. Free Radic. Biol. Med. 2022, 179, 156–163. [Google Scholar] [CrossRef]
- Desdín-Micó, G.; Soto-Heredero, G.; Mittelbrunn, M. Mitochondrial activity in T cells. Mitochondrion 2018, 41, 51–57. [Google Scholar] [CrossRef]
- D’Souza, A.D.; Parikh NFau-Kaech Susan, M.; Kaech Sm Fau-Shadel Gerald, S.; Shadel, G.S. Convergence of multiple signaling pathways is required to coordinately up-regulate mtDNA and mitochondrial biogenesis during T cell activation. Mitochondrion 2007, 7, 374–385. [Google Scholar]
- Lasalvia, M.; Scrima, R.; Perna, G.; Piccoli, C.; Capitanio, N.; Biagi, P.F.; Schiavulli, L.; Ligonzo, T.; Centra, M.; Casamassima, G.; et al. Exposure to 1.8 GHz electromagnetic fields affects morphology, DNA-related Raman spectra and mitochondrial functions in human lympho-monocytes. PLoS ONE 2018, 13, e0192894. [Google Scholar] [CrossRef]
- Fernandez, J.A.; Abbas, P.O.; Dardenner, P.; Baeten, V. Discrimination of Corsican honey by FT-Raman Spectroscopy and chemometrics. Biotechnol. Agron. Soc. Environ. 2019, 15, 75–84. [Google Scholar]
- Salehi, H.; Ramoji, A.; Mougari, S.; Merida, P.; Neyret, A.; Popp, J.; Horvat, B.; Muriaux, D.; Cuisinier, F. Specific intracellular signature of SARS-CoV-2 infection using confocal Raman microscopy. Commun. Chem. 2022, 5, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Deobagkar-Lele, M.; Majumdar, S.; Chandrasekar, B.; Victor, E.; Ahmed, S.M.; Wadhwa, N.; Verma, T.; Kumar, S.; Sundaresan, N.R.; et al. Molecular profiling of sepsis in mice using Fourier Transform Infrared Microspectroscopy. J. Biophotonics 2016, 9, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Castellano, F.; Molinier-Frenkel, V. Control of T-Cell Activation and Signaling by Amino-Acid Catabolizing Enzymes. Front. Cell Dev. Biol. 2020, 8, 613416. [Google Scholar] [CrossRef] [PubMed]
- Lamerton, R.E.; Lightfoot, A.; Nieves, D.J.; Owen, D.M. The Role of Protein and Lipid Clustering in Lymphocyte Activation. Front. Immunol. 2021, 12, 600961. [Google Scholar] [CrossRef]
- Mierzchala-Pasierb, M.; Lipinska-Gediga, M.; Fleszar, M.G.; Lesnik, P.; Placzkowska, S.; Serek, P.; Wisniewski, J.; Gamian, A.; Krzystek-Korpacka, M. Altered profiles of serum amino acids in patients with sepsis and septic shock—Preliminary findings. Arch. Biochem. Biophys. 2020, 691, 108508. [Google Scholar]
- Miao, H.; Chen, S.; Ding, R. Evaluation of the Molecular Mechanisms of Sepsis Using Proteomics. Front. Immunol. 2021, 12, 733537. [Google Scholar] [CrossRef]
- Su, L.; Li, H.; Xie, A.; Liu, D.; Rao, W.; Lan, L.; Li, X.; Li, F.; Xiao, K.; Wang, H.; et al. Dynamic changes in amino acid concentration profiles in patients with sepsis. PLoS ONE 2015, 10, e0121933. [Google Scholar]
- Howie, D.; Bokum, A.T.; Necula, A.S.; Cobbold, S.P.; Waldmann, H. The Role of Lipid Metabolism in T Lymphocyte Differentiation and Survival. Front. Immunol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Wu, W.; Shi, X.; Xu, C. Regulation of T cell signalling by membrane lipids. Nat. Rev. Immunol. 2016, 16, 690–701. [Google Scholar] [CrossRef]
- Barker, G.; Leeuwenburgh, C.; Brusko, T.A.-O.; Moldawer, L.; Reddy, S.T.; Guirgis, F.A.-O. Lipid and Lipoprotein Dysregulation in Sepsis: Clinical and Mechanistic Insights into Chronic Critical Illness. J. Clin. Med. 2021, 10, 1693. [Google Scholar]
- Wasyluk, W.; Zwolak, A. Metabolic Alterations in Sepsis. J. Clin. Med. 2021, 10, 2412. [Google Scholar] [CrossRef] [PubMed]
- Press, A.T.; Babic, P.; Hoffmann, B.; Müller, T.; Foo, W.; Hauswald, W.; Benecke, J.; Beretta, M.; Cseresnyés, Z.; Hoeppener, S.; et al. Targeted delivery of a phosphoinositide 3-kinase γ inhibitor to restore organ function in sepsis. EMBO Mol. Med. 2021, 13, e14436. [Google Scholar]
- Sommerfeld, O.; Medyukhina, A.; Neugebauer, S.; Ghait, M.; Ulferts, S.; Lupp, A.; König, R.; Wetzker, R.; Schulz, S.; Figge, M.T.; et al. Targeting Complement C5a Receptor 1 for the Treatment of Immunosuppression in Sepsis. Mol. Ther. 2021, 29, 338–346. [Google Scholar] [CrossRef]
- Leibniz Institute of Photonic Technology. RAMANMETRIX; Leibniz Institute of Photonic Technology: Jena, Germany, 2021. [Google Scholar]
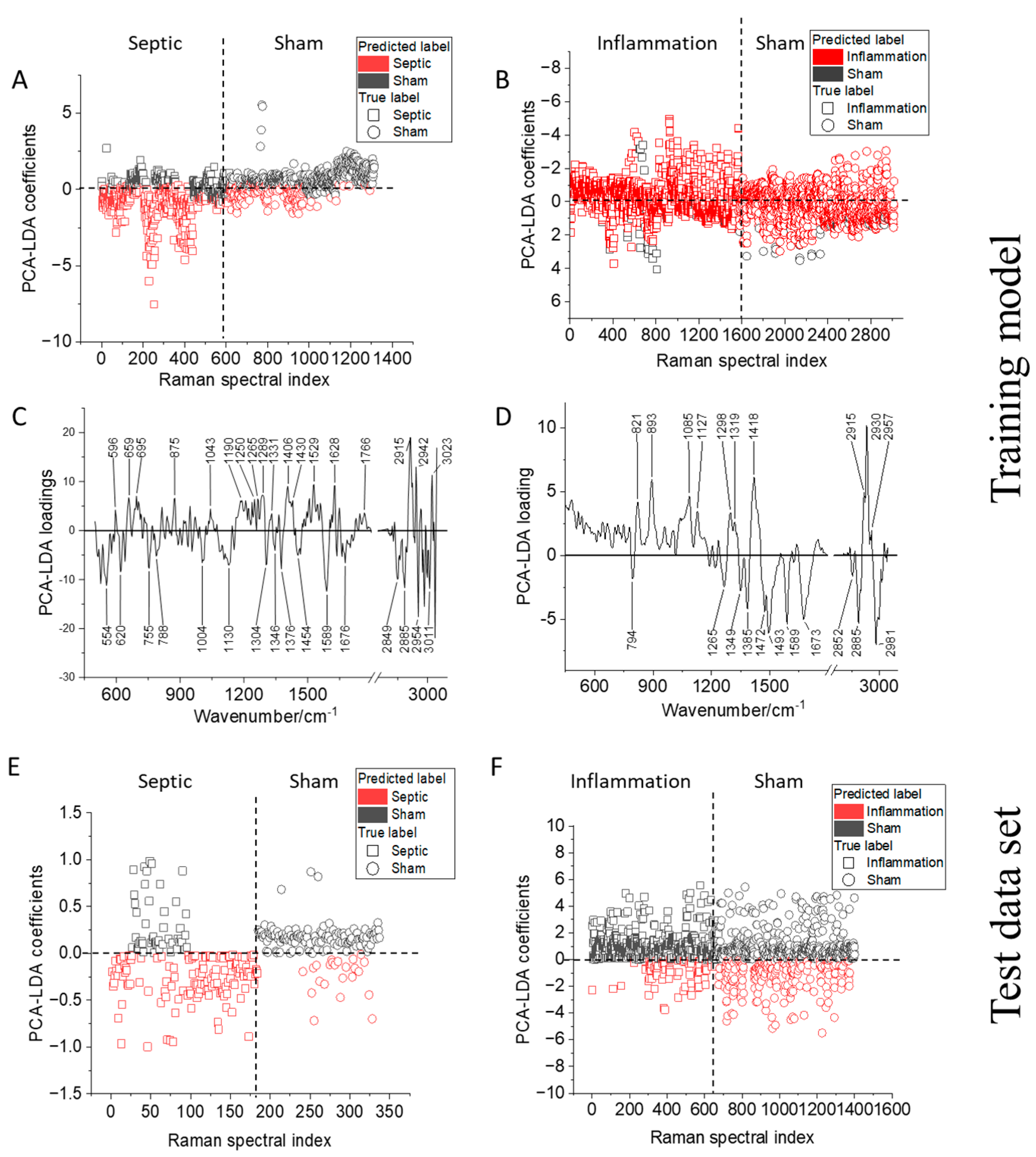
| Mice Models | Balanced Accuracy */Cohen’s Kappa | |||
|---|---|---|---|---|
| PCA-LDA Model for Classification Using the Training Data Set | PCA-LDA Model for Prediction Using the Test Data Set | |||
| Individual Spectra | Majority Vote | Individual Spectra | Majority Vote | |
| PCI | 0.67/0.33 | 1/1 | 0.79/0.57 | 1/1 |
| LPS | 0.51/0.02 | 0.5/0 | 0.40/−0.21 | 0.5/0 |
| Clinical Feature 24 h Post-Infection | FVB/N Septic Mice | C57BL/6 Septic Mice (Previously Published Data in Ref. [29]) |
|---|---|---|
| Clinical severity score | 2.2 | 2.0 |
| Mortality 10 days post-infection (%) | 78% | 56% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osadare, I.E.; Xiong, L.; Rubio, I.; Neugebauer, U.; Press, A.T.; Ramoji, A.; Popp, J. Raman Spectroscopy Profiling of Splenic T-Cells in Sepsis and Endotoxemia in Mice. Int. J. Mol. Sci. 2023, 24, 12027. https://doi.org/10.3390/ijms241512027
Osadare IE, Xiong L, Rubio I, Neugebauer U, Press AT, Ramoji A, Popp J. Raman Spectroscopy Profiling of Splenic T-Cells in Sepsis and Endotoxemia in Mice. International Journal of Molecular Sciences. 2023; 24(15):12027. https://doi.org/10.3390/ijms241512027
Chicago/Turabian StyleOsadare, Ibukun Elizabeth, Ling Xiong, Ignacio Rubio, Ute Neugebauer, Adrian T. Press, Anuradha Ramoji, and Juergen Popp. 2023. "Raman Spectroscopy Profiling of Splenic T-Cells in Sepsis and Endotoxemia in Mice" International Journal of Molecular Sciences 24, no. 15: 12027. https://doi.org/10.3390/ijms241512027
APA StyleOsadare, I. E., Xiong, L., Rubio, I., Neugebauer, U., Press, A. T., Ramoji, A., & Popp, J. (2023). Raman Spectroscopy Profiling of Splenic T-Cells in Sepsis and Endotoxemia in Mice. International Journal of Molecular Sciences, 24(15), 12027. https://doi.org/10.3390/ijms241512027







