Phospho-DIGE Identified Phosphoproteins Involved in Pathways Related to Tumour Growth in Endometrial Cancer
Abstract
1. Introduction
2. Results
2.1. Proteomic Study
2.2. Western Blotting
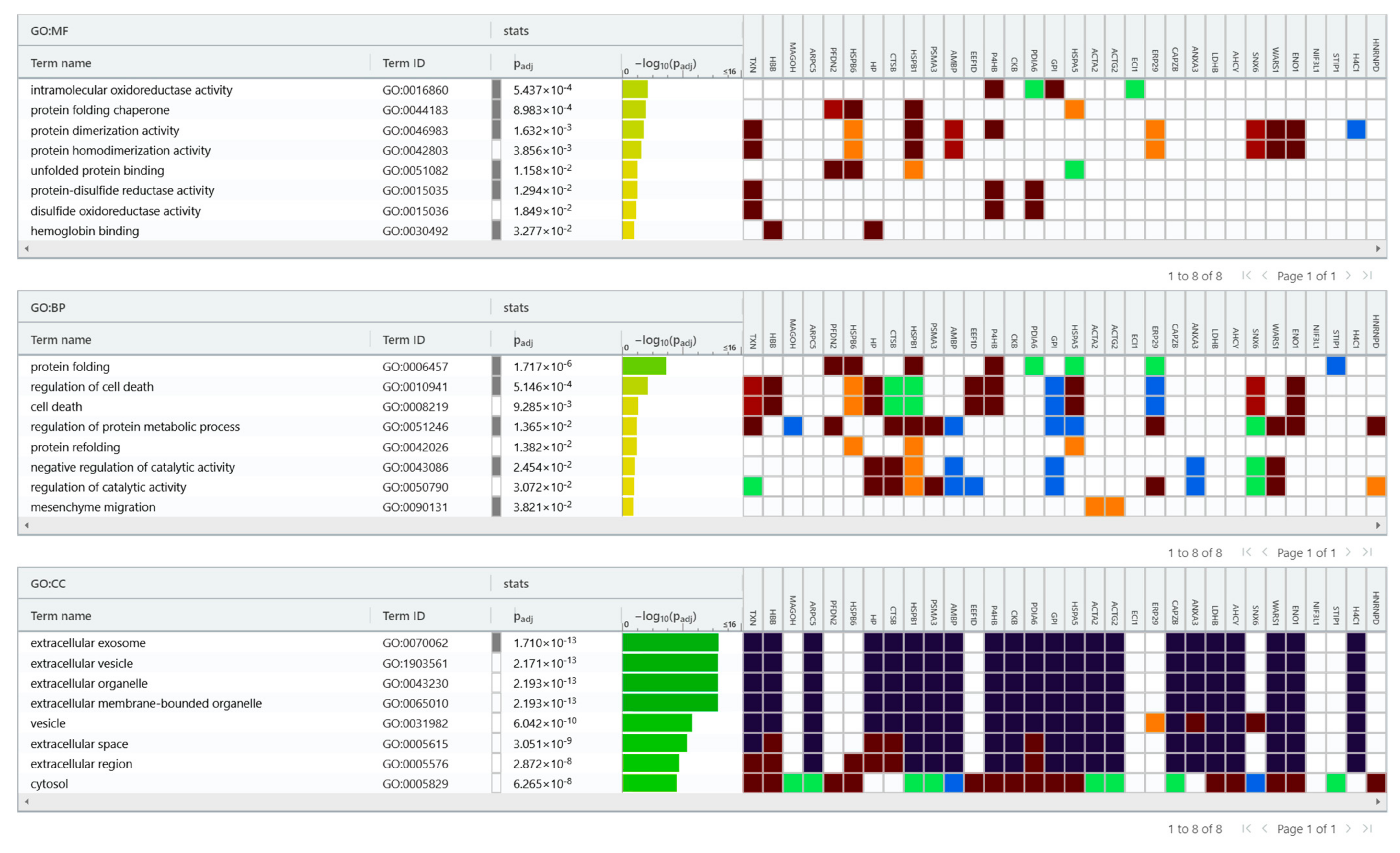
2.3. Bioinformatic Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Phosphoprotein and Phosphopeptide Isolation
4.3. Sample Preparation for 2D-DIGE and Gel Image Analysis
4.4. Western Blotting
4.5. Trypsin Digestion and MS Analysis
4.6. Bioinformatic Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial cancer. Nat. Rev. Dis. Prim. 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Cote, M.L.; Ruterbusch, J.J.; Olson, S.H.; Lu, K.; Ali-Fehmi, R. The Growing Burden of Endometrial Cancer: A Major Racial Disparity Affecting Black Women. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1407–1415. [Google Scholar] [CrossRef]
- Barry, J.A.; Azizia, M.M.; Hardiman, P.J. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syn-drome: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 748–758. [Google Scholar] [CrossRef]
- Saed, L.; Varse, F.; Baradaran, H.R.; Moradi, Y.; Khateri, S.; Friberg, E.; Khazaei, Z.; Gharahjeh, S.; Tehrani, S.; Sioofy-Khojineet, A.B.; et al. The effect of diabetes on the risk of endometrial Cancer: An updated systematic review and meta-analysis. BMC Cancer 2019, 19, 527. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirzaet, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Ryan, N.A.J.; Glaire, M.A.; Blake, D.; Cabrera-Dandy, M.; Evans, D.G.; Crosbie, E.J. The proportion of endometrial cancers associated with Lynch syndrome: A systematic review of the literature and meta-analysis. Genet. Med. 2019, 21, 2167–2180. [Google Scholar] [CrossRef]
- Wang, Y.; van der Zee, M.; Fodde, R.; Blok, L.J. Wnt/Β-catenin and sex hormone signaling in endometrial homeostasis and cancer. Oncotarget 2010, 1, 674–684. [Google Scholar] [CrossRef]
- Cheung, L.W.T.; Hennessy, B.T.; Li, J.; Yu, S.; Myers, A.P.; Djordjevic, B.; Lu, Y.; Stemke-Hale, K.; Dyer, M.D.; Zhang, F.; et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011, 1, 170–185. [Google Scholar] [CrossRef]
- Risinger, J.I.; Hayes, K.; Maxwell, G.L.; Carney, M.E.; Dodge, R.K.; Barrett, J.C.; Berchuck, A. PTEN mutation in endometrial cancers is associated with favorable clinical and pathologic characteristics. Clin. Cancer Res. 1998, 4, 3005–3010. [Google Scholar]
- Lacey, J.V.J.; Yang, H.; Gaudet, M.M.; Dunning, A.; Lissowska, J.; Sherman, M.E.; Peplonska, B.; Brinton, L.A.; Healey, C.S.; Ahmed, S.; et al. Endometrial cancer and genetic variation in PTEN, PIK3CA, AKT1, MLH1, and MSH2 within a population-based case-control study. Gynecol. Oncol. 2011, 120, 167–173. [Google Scholar] [CrossRef]
- Alexander-Sefre, F.; Salvesen, H.B.; Ryan, A.; Singh, N.; Akslen, L.A.; MacDonald, N.; Wilbanks, G.; Jacobset, J.I.J. Molecular as-sessment of depth of myometrial invasion in stage I endometrial cancer: A model based on K-ras mutation analysis. Gynecol. Oncol. 2003, 91, 218–225. [Google Scholar] [CrossRef]
- Holland, C.M.; Day, K.; Evans, A.; Smith, S.K. Expression of the VEGF and angiopoietin genes in endometrial atypical hyperplasia and endometrial cancer. Br. J. Cancer 2003, 89, 891–898. [Google Scholar] [CrossRef]
- Markowska, A.; Pawałowska, M.; Lubin, J.; Markowska, J. Signalling pathways in endometrial cancer. Wspolczesna Onkol. 2014, 18, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ma, C.X.; Wang, J. Endometrial Carcinogenesis and Molecular Signaling Pathways. Am. J. Mol. Biol. 2014, 04, 134–149. [Google Scholar] [CrossRef]
- Dou, Y.; Kawaler, E.A.; Cui Zhou, D.; Gritsenko, M.A.; Huang, C.; Blumenberg, L.; Karpova, A.; Petyuk, V.A.; Savage, S.R.; Satpathy, S.; et al. Proteogenomic Characterization of Endometrial Carcinoma. Cell 2020, 180, 729–748.e26. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Muzio, L.L. The crucial role of protein phosphorylation in cell signalingand its use as targeted therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, F.M.; Gray, N.S. Kinase inhibitors: The road ahead. Nat. Rev. Drug Discov. 2018, 17, 353–376. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Capaci, V.; Aloisio, M.; Salviati, E.; Campiglia, P.; Molinario, G.; Licastro, D.; Di Lorenzo, G.; Romano, F.; Ricci, G.; et al. A label-free proteomic approach for the identification of biomarkers in the exosome of endometrial cancer serum. Cancers 2022, 14, 6262. [Google Scholar] [CrossRef] [PubMed]
- Celsi, F.; Monasta, L.; Arrigoni, G.; Battisti, I.; Licastro, D.; Aloisio, M.; Di Lorenzo, G.; Romano, F.; Ricci, G.; Ura, B. Gel-Based Proteomic Identification of Suprabasin as a Potential New Candidate Biomarker in Endometrial Cancer. Int. J. Mol. Sci. 2022, 23, 2076. [Google Scholar] [CrossRef] [PubMed]
- Yeonggyeong, P.; Min Jeong, K.; Yoonhee, C.; Na Hyun, K.; Leeseul, K.; Seung Pyo Daniel, H.; Hyung-Gyo, C.; Emma, Y.; Young Kwang, C. Role of mass spectrometry-based serum proteomics signatures in predicting clinical outcomes and toxicity in patients with cancer treated with immunotherapy. J. Immunother. Cancer 2022, 10, e003566. [Google Scholar]
- Capdeville, R.; Buchdunger, E.; Zimmermann, J.; Matter, A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat. Rev. Drug Discov. 2002, 1, 493–502. [Google Scholar] [CrossRef]
- Steinberg, M. Dasatinib: A tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia. Clin. Ther. 2007, 29, 2289–2308. [Google Scholar] [CrossRef]
- Paez, J.G.; Jänne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J.; et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef]
- Goutsouliak, K.; Veeraraghavan, J.; Sethunath, V.; De Angelis, C.; Osborne, C.K.; Rimawi, M.F.; Schiff, R. Towards personalized treatment for early stage HER2-positive breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 233–250. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Yang, M.; Hong, F.; Yang, S. Protein Phosphorylation in Cancer: Role of Nitric Oxide Signaling Pathway. Biomolecules 2021, 11, 1009. [Google Scholar] [CrossRef]
- Singh, V.; Ram, M.; Kumar, R.; Prasad, R.; Roy, B.K.; Singh, K.K. Phosphorylation: Implications in Cancer. Protein J. 2017, 36, 1–6. [Google Scholar] [CrossRef]
- Kostenko, S.; Moens, U. Heat shock protein 27 phosphorylation: Kinases, phosphatases, functions and pathology. Cell Mol. Life Sci. 2009, 66, 3289–3307. [Google Scholar] [CrossRef]
- Lin, J.; Deng, Z.; Tanikawa, C.; Shuin, T.; Miki, T.; Matsuda, K.; Nakamura, Y. Downregulation of the tumor suppressor HSPB7, involved in the p53 pathway, in renal cell carcinoma by hypermethylation. Int. J. Oncol. 2014, 44, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Li, Y.; Tan, X.; Fu, L. Small Heat Shock Proteins in Cancers: Functions and Therapeutic Potential for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 6611. [Google Scholar] [CrossRef]
- Liu, S.; Yan, B.; Lai, W.; Chen, L.; Xiao, D.; Xi, S.; Jiang, Y.; Dong, X.; An, J.; Chen, X.; et al. As a novel p53 direct target, bidirectional gene HspB2/αB-crystallin regulates the ROS level and Warburg effect. Biochim. Biophys. Acta 2014, 1839, 592–603. [Google Scholar] [CrossRef]
- Manish Kumar, S.; Sharma, B.; Tiwari, P.K. The small heat shock protein Hsp27: Present understanding and future prospects. J. Therm. Biol. 2017, 69, 149–154. [Google Scholar]
- Grierson, P.M.; Dodhiawala, P.B.; Cheng, Y.; Hung-Po Chen, T.; Ali Khawar, I.; Wei, Q.; Zhang, D.; Li, L.; Herndon, J.; Monahan, J.B.; et al. The MK2/Hsp27 axis is a major survival mechanism for pancreatic ductal adenocarcinoma under genotoxic stress. Sci. Transl. Med. 2021, 13, eabb5445. [Google Scholar] [PubMed]
- Chunjie, T.; Yeon Ju, K.; Sai, H.; Oak-Sung, C.; Jin-Sol, L.; Seo-Kyung, J.; Youn-Uk, C.; Chan Bae, P.; Yun-Hoon, C. Suppressed expression of LDHB promotes age-related hearing loss via aerobic glycolysis. Cell Death Dis. 2020, 11, 375. [Google Scholar]
- Mishra, D.; Banerjee, D. Lactate dehydrogenases as metabolic links between tumor and stroma in the tumor microenvironment. Cancers 2019, 11, 750. [Google Scholar] [CrossRef]
- Cheng, A.; Zhang, P.; Wang, B.; Yang, D.; Duan, X.; Jiang, Y.; Xu, T.; Jiang, Y.; Shi, J.; Ding C, et.al. Aurora-A mediated phosphorylation of LDHB promotes glycolysis and tumor progression by relieving the substrate-inhibition effect. Nat. Commun. 2019, 10, 5566. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Li, K.Y.; Cai, L.; Hensley, C.T.; Kim, J.; Zacharias, L.G.; Yang, C.; Do, Q.N.; Doucette, S.l.; Burguete, D.; et al. Lactate metabolism in human lung tumors. Cell 2017, 171, 358–371. [Google Scholar] [CrossRef]
- Schweppe, D.K.; Rigas, J.R.; Gerber, S.A. Quantitative phosphoproteomic profiling of human non-small cell lung cancer tumors. J. Proteomics. 2013, 91, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Perryman, M.B.; Friedman, D.; Roberts, R.; Ma, T.S. Determination of the catalytic site of creatine kinase by site-directed mutagenesis. Biochim. Biophys. Acta 1994, 1206, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Xu-Hui, L.; Xiang-Jun, C.; Wen-Bin, O.; Qian, Z.; Zhi-Rong, L.; Yi, Z.; Long, M.; Tao, H.; Yong-Bin, Y.; Hai-Meng, Z. Knockdown of creatine kinase B inhibits ovarian cancer progression by decreasing glycolysis. Int. J. Biochem. Cell Biol. 2013, 45, 979–986. [Google Scholar]
- Mertins, P.; Mani, D.R.; Ruggles, K.V.; Gillette, M.A.; Clauser, K.R.; Wang, P.; Wang, X.; Qiao, J.W.; Cao, S.; Petralia, P. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 2016, 534, 55–62. [Google Scholar] [CrossRef]
- Zheng, Y.; Miyamoto, D.T.; Wittner, B.S.; Sullivan, J.P.; Aceto, N.; Jordan, N.V.; Yu, M.; Karabacak, N.M.; Comaills, V.; Morris, R.; et al. Expression of β-globin by cancer cells promotes cell survival during blood-borne dissemination. Nat. Commun. 2017, 8, 14344. [Google Scholar] [CrossRef]
- Li, X.; Wu, Z.; Wang, Y.; Mei, Q.; Fu, X.; Han, W. Characterization of adult α- and β-globin elevated by hydrogen peroxide in cervical cancer cells that play a cytoprotective role against oxidative insults. PLoS ONE 2013, 8, e54342. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ye, M.; Liu, H.; Song, C.; Sun, D.; Wu, Y.; Jiang, X.; Chen, R.; Wang, C.; Wang, L.; et al. Phosphoproteome analysis of human liver tissue by long-gradient nanoflow LC coupled with multiple stage MS analysis. Electrophoresis 2010, 31, 1080–1089. [Google Scholar] [PubMed]
- Britton, D.; Zen, Y.; Quaglia, A.; Selzer, S.; Mitra, V.; Löβner, C.; Jung, S.; Böhm, G.; Schmid, P.; Prefot P, et.al. Quantification of pancreatic cancer proteome and phosphorylome: Indicates molecular events likely contributing to cancer and activity of drug targets. PLoS ONE 2014, 9, e90948. [Google Scholar] [CrossRef]
- Mollinedo, F. Neutrophil Degranulation, Plasticity, and Cancer Metastasis. Trends Immunol. 2019, 40, 228–242. [Google Scholar] [CrossRef]
- Ura, B.; Monasta, L.; Arrigoni, G.; Battisti, I.; Licastro, D.; Di Lorenzo, G.; Romano, F.; Aloisio, M.; Peterlunger, I.; Stabile, G.; et al. Phosphoproteins Involved in the Inhibition of Apoptosis and in Cell Survival in the Leiomyoma. J. Clin. Med. 2019, 8, 691. [Google Scholar] [CrossRef]
- Carcoforo, P.; Ura, B.; Mischiati, C.; Squerzanti, M.; Lanzara, V.; Cervellati, C.; Calza, R.; De Laureto, P.P.; Frare, E.; Portinari M, et.al. Comparative proteomic analysis of ductal breast carcinoma demonstrates an altered expression of chaperonins and cytoskeletal proteins. Mol. Med. Rep. 2013, 7, 1700–1704. [Google Scholar] [CrossRef] [PubMed]
- Ura, B.; Scrimin, F.; Franchin, C.; Arrigoni, G.; Licastro, D.; Monasta, L.; Ricci, G. Identification of proteins with different abundance associated with cell migration and proliferation in leiomyoma interstitial fluid by proteomics. Oncol. Lett. 2017, 13, 3912–3920. [Google Scholar] [CrossRef]
- Ura, B.; Scrimin, F.; Arrigoni, G.; Athanasakis, E.; Aloisio, M.; Monasta, L.; Ricci, G. Abnormal expression of leiomyoma cytoskeletal proteins involved in cell migration. Oncol. Rep. 2016, 35, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- Venerando, A.; Franchin, C.; Cant, N.; Cozza, G.; Pagano, M.A.; Tosoni, K.; Zahrani, A.A.; Arrigoni, G.; Ford, R.C.; Mehta, A.; et al. Detection of phospho-sites generated by protein kinase CK2 in CFTR: Mechanistic aspects of Thr1471 phosphorylation. PLoS ONE 2013, 8, e74232. [Google Scholar] [CrossRef] [PubMed]
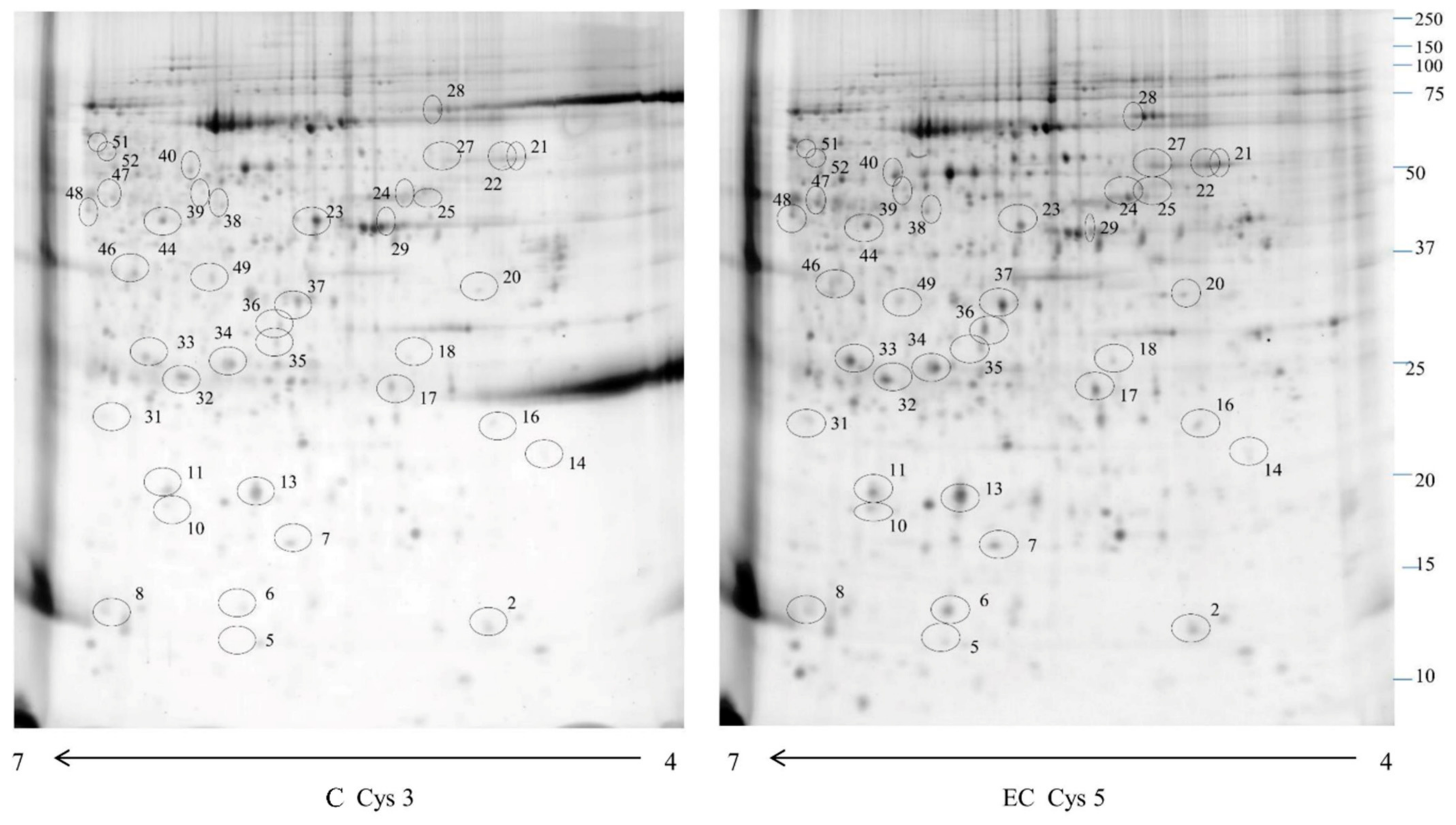


| Spot Number | Accession Number | Protein Description | Gene Symbol | Fold Change | Phosphorylation Sites | p-Value |
|---|---|---|---|---|---|---|
| Spot 6 | P61326 | Protein mago nashi homolog | MAGOH | 16.4 | 0.023 | |
| Spot 16 | P04792 | Heat shock protein beta-1 | HSPB1 1,2 | 10.4 | S3 S15 S82 S83 | 0.045 |
| Spot 37 | P07195 | L-lactate dehydrogenase B chain | LDHB 1 | 9 | S85 S90 | 0.0059 |
| Spot 8 | P68871 | Haemoglobin subunit beta | HBB 1,2 | 5.71 | T5 S10 S45 T124 | 0.045 |
| Spot 14 | P07858 | Cathepsin B | CTSB | 5.34 | 0.013 | |
| Spot 17 | P25788 | Proteasome subunit alpha type-3 | PSMA3 1,2 | 4.4 | S9 | 0.02 |
| Spot 10 | Q9UHV9 | Prefoldin subunit 2 | PFDN2 | 4.18 | 0.04 | |
| Spot 32 | P42126 | Enoyl-CoA delta isomerase 1, mitochondrial | ECI1 | 4.12 | 0.0062 | |
| Spot 31 | P63267 | Actin, gamma-enteric smooth muscle | ACTG2 2 | 3.39 | S23 | 0.012 |
| Spot 7 | O15511 | Actin-related protein 2/3 complex subunit 5 | ARPC5 1 | 3.39 | S64 | 0.01 |
| Spot 34 | P30040 | Endoplasmic reticulum resident protein 29 | ERP29 1 | 3.33 | Y66 | 0.045 |
| Spot 13 | P00738 | Haptoglobin | HP | 3.2 | 0.0019 | |
| Spot 25 | Q15084 | Protein disulfide-isomerase A6 | PDIA6 1,2 | 2.82 | S427 | 0.049 |
| Spot 11 | O14558 | Heat shock protein beta-6 | HSPB6 1 | 2.72 | S16 | 0.0036 |
| Spot 28 | P11021 | Endoplasmic reticulum chaperone BiP | HSPA5 1,2 | 2.64 | S4 S448 S86 T648 | 0.0023 |
| Spot 47 | A0A2R8Y6G6 | 2-phospho-D-glycerate hydro-lyase | ENO1 1,2 | 2.55 | S9 | 0.035 |
| Spot 51 | P31948 | Stress-induced-phosphoprotein 1 | STIP1 1 | 2.5 | S528 S63 | 0.035 |
| Spot 36 | P12429 | Annexin A3 | ANXA3 1 | 2.31 | S19 | 0.031 |
| Spot 21 | P07237 | Protein disulfide-isomerase | P4HB 1,2 | 2.15 | S32 | 0.045 |
| Spot 27 | P06744 | Glucose-6-phosphate isomerase | GPI 1 | 2.12 | S146 S494 S61 T148 T254 | 0.045 |
| Spot 40 | P23381 | Tryptophan--tRNA ligase, cytoplasmic | WARS1 1 | 2.14 | S353 S4 S71 T453 | 0.0063 |
| Spot 20 | P29692 | Elongation factor 1-delta | EEF1D 1,2 | 1.91 | S19 T4 S10 | 0.039 |
| Spot 35 | P47756 | F-actin-capping protein subunit beta | CAPZB 1 | 1.86 | S211 | 0.03 |
| Spot 46 | Q14103 | Heterogeneous nuclear ribonucleoprotein D0 | HNRNPD 1,2 | 1.77 | S19 T4 S10 | 0.0038 |
| Spot 39 | Q9UNH7 | Sorting nexin-6 | SNX6 1 | 1.73 | S67 S206 | 0.045 |
| Spot 38 | P23526 | Adenosylhomocysteinase | AHCY 1 | 1.68 | S68 S70 S80 S84 S85 T82 Y81 | 0.01 |
| Spot 18 | P02760 | Protein AMBP | AMBP | 1.66 | 0.035 | |
| Spot 33 | O75937 | DnaJ homolog subfamily C member 8 | DNJC8 | 1.57 | 0.0045 | |
| Spot 2 | P10599 | Thioredoxin | TXN 1,2 | 1.55 | T9 | 0.045 |
| Spot 49 | Q9GZT8 | NIF3-like protein 1 | NIF3L1 | 1.52 | 0.045 | |
| Spot 44 | P23526 | Adenosylhomocysteinase | AHCY 1 | 1.5 | S68 S70 S80 S84 S85 T82 Y81 | 0.037 |
| Spot 29 | P62736 | Actin, aortic smooth muscle | ACTA2 | 0.47 | 0.045 | |
| Spot 23 | P12277 | Creatine kinase B-type | CKB 1 | 0.28 | S202 S6 T346 T35 | 0.0072 |
| Spot 52 | P62805 | Histone H4 | H4C1 2 | 0.27 | S3 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capaci, V.; Arrigoni, G.; Monasta, L.; Aloisio, M.; Rocca, G.; Di Lorenzo, G.; Licastro, D.; Romano, F.; Ricci, G.; Ura, B. Phospho-DIGE Identified Phosphoproteins Involved in Pathways Related to Tumour Growth in Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 11987. https://doi.org/10.3390/ijms241511987
Capaci V, Arrigoni G, Monasta L, Aloisio M, Rocca G, Di Lorenzo G, Licastro D, Romano F, Ricci G, Ura B. Phospho-DIGE Identified Phosphoproteins Involved in Pathways Related to Tumour Growth in Endometrial Cancer. International Journal of Molecular Sciences. 2023; 24(15):11987. https://doi.org/10.3390/ijms241511987
Chicago/Turabian StyleCapaci, Valeria, Giorgio Arrigoni, Lorenzo Monasta, Michelangelo Aloisio, Giulia Rocca, Giovanni Di Lorenzo, Danilo Licastro, Federico Romano, Giuseppe Ricci, and Blendi Ura. 2023. "Phospho-DIGE Identified Phosphoproteins Involved in Pathways Related to Tumour Growth in Endometrial Cancer" International Journal of Molecular Sciences 24, no. 15: 11987. https://doi.org/10.3390/ijms241511987
APA StyleCapaci, V., Arrigoni, G., Monasta, L., Aloisio, M., Rocca, G., Di Lorenzo, G., Licastro, D., Romano, F., Ricci, G., & Ura, B. (2023). Phospho-DIGE Identified Phosphoproteins Involved in Pathways Related to Tumour Growth in Endometrial Cancer. International Journal of Molecular Sciences, 24(15), 11987. https://doi.org/10.3390/ijms241511987







