The Sigma Receptors in Alzheimer’s Disease: New Potential Targets for Diagnosis and Therapy
Abstract
1. Introduction
2. Sigma Receptors in AD
2.1. The Sigma (σ) Receptors
2.2. Sigma-1 Receptor in AD
2.3. Sigma-2 Receptor in AD
3. Ligands Targeting σ1 or σ2 Receptors
3.1. Agonists/Antagonists of σ Receptors and Their Therapeutic Potential in Clinical Trials
| Agents | Property | Disease | Clinical Trials ID | Phase/Status |
|---|---|---|---|---|
| Pridopidine | σ1 receptor agonist | HD | NCT03019289 NCT00724048 NCT04556656 NCT00665223 NCT02006472 NCT01306929 NCT02494778 | I/Completed II/III/Completed III/Recruiting III/Completed II/Completed II/Completed II/Terminated |
| Levodopa-induced dyskinesia (PD) | NCT03922711 | II/Terminated | ||
| ALS | NCT04297683 NCT04615923 | II/III/Recruiting II/III/Active, not recruiting | ||
| Dextromethorphan (AVP-923) b | σ1 receptor agonist mu (μ) opioid agonist and NMDA receptor antagonist [195] | AD | NCT00788047 NCT01584440 NCT01832350 NCT02446132 NCT02442778 NCT02442765 NCT00726726 NCT04947553 NCT05557409 NCT04797715 NCT00056524 | I/Completed II/Completed IV/Terminated III/Recruiting III/Completed III/Completed I/Completed III/Recruiting III/Recruiting III/Completed III/Completed |
| SA4503 | σ1 receptor agonist | Ischemic stroke | NCT00639249 | II/Completed |
| MDD | NCT00551109 | II/Completed | ||
| MR309 (E-52862) | σ1 receptor antagonist | Oxaliplatin-induced neuropathy | Ref. [196] | IIa/Completed |
| ANAVEX2-73 (blarcamesine) | σ1 receptor agonist muscarinic receptor modulator | Moderate AD | NCT04314934 NCT03790709 NCT02756858 NCT02244541 | IIb/III/Recruiting IIb/III/Completed II/Completed IIa/Completed |
| Rett syndrome | NCT04304482 NCT03941444 NCT03758924 | II/Recruiting III/Completed II/Completed | ||
| PD | NCT03774459 | II/Completed | ||
| Edonerpic maleate (T-817MA) | σ1 receptor activation | Mild-to-moderate AD | NCT00663936 NCT04191486 | II/Completed II/Recruiting |
| Aβ inhibitor | NCT02079909 | II/Completed | ||
| Hepatic impairment | NCT02693197 | I/Completed | ||
| Roluperidone (MIN-101) | σ2 receptor antagonist and 5-HT2A receptor antagonist | Negative symptoms of schizophrenia | NCT03397134 | III/Completed |
| NCT02232529 | I/Completed | |||
| Schizophrenia | NCT03038646 | I/Completed | ||
| Healthy subjects | NCT03072056 | I/Completed | ||
| CT1812 | σ2 receptor antagonist | Healthy volunteers | NCT03716427 | I/Completed |
| AD | NCT05531656 | II/Not recruiting | ||
| NCT04735536 | II/Completed | |||
| NCT02907567 | I/II/Completed | |||
| NCT05248672 | I/Completed | |||
| NCT05225389 | I/Completed | |||
| NCT03507790 | II/Recruiting | |||
| NCT03493282 | I/II/Completed | |||
| NCT03522129 | I/Completed | |||
| Age-related macular degeneration | NCT05893537 | II/Recruiting | ||
| Dementia with Lewy bodies | NCT05225415 | II/Recruiting | ||
| Cognitive impairment | NCT02570997 | I/Completed |
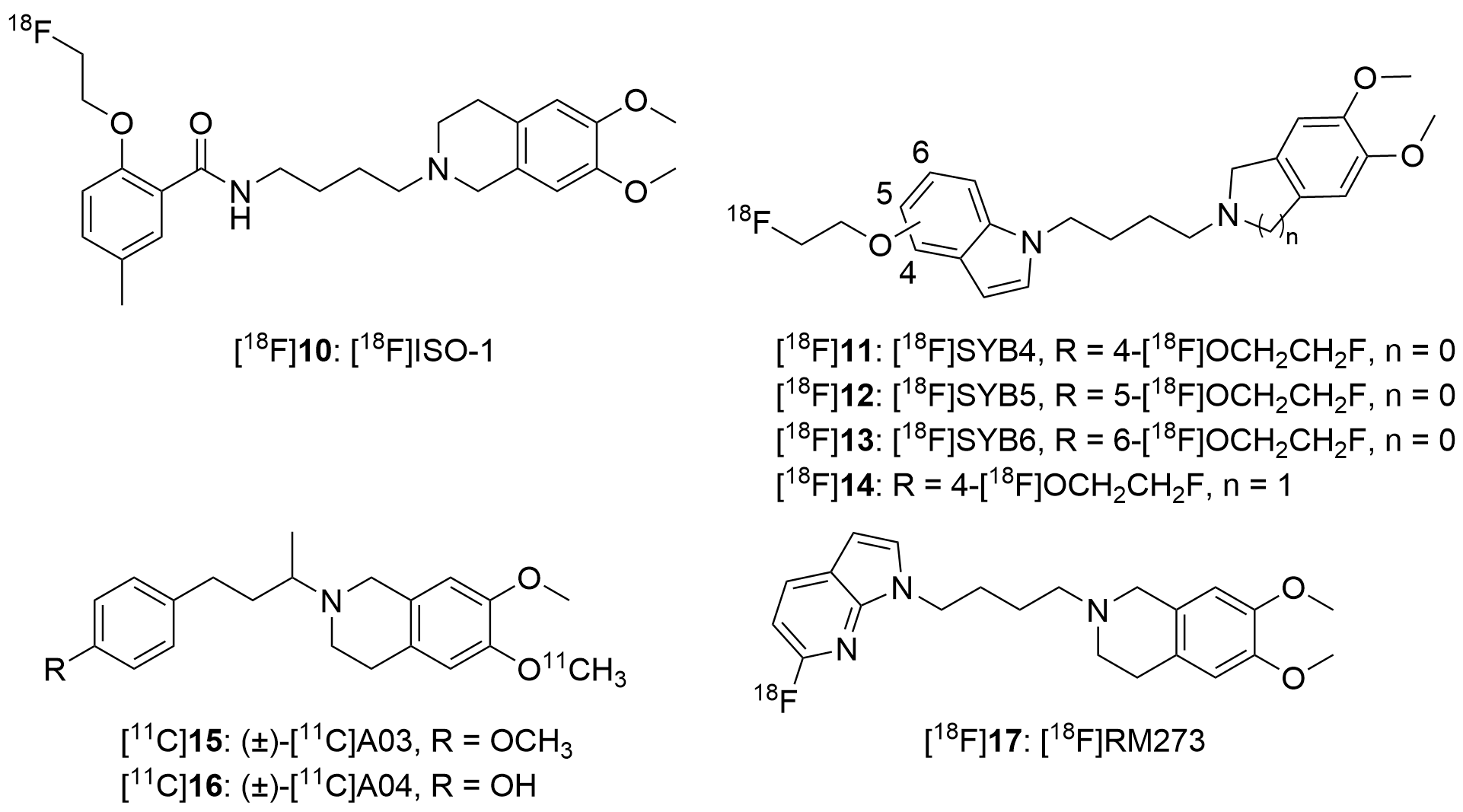
3.2. Development of Radioligands for Neuroimaging of σ Receptors
3.2.1. Characteristics of Optimal σ1 or σ2 Receptor Radioligands for PET Imaging in AD
3.2.2. Radioligands Targeting the σ1 Receptor
| Ligand | Ki(σ1) | Ki(σ2) | Selectivity | Log D | fP | BPND |
|---|---|---|---|---|---|---|
| [18F]4 a | 0.79 | 277 | 351 | 2.55 | 8% | - |
| [18F]5 a | 2.30 | 327 | 142 | 2.50 | 18% | 2.78–5.21 |
| [18F]6 a | 2.30 | 897 | 390 | 2.80 | 2% | 0.77–1.85 |
| [18F]7 a | 0.57 | 1650 | 2895 | 2.80 | 2% | |
| [18F]8 b | 2.26 | 299 | 127 | 0.76 c | 73% | 6.3–14.8 |
| [18F]9 b | 1.61 | 246 | 152 | 0.76 c | 67% | 9.6–27.7 |
3.2.3. Radioligands Targeting the σ2 Receptor
| Ligand | Ki(σ1) | Ki(σ2) | Selectivity | Log D |
|---|---|---|---|---|
| [18F]SYB4 a | 371 | 1.79 | 207 | 2.43 b |
| [18F]SYB5 a | 187 | 3.27 | 57 | 2.29 b |
| [18F]SYB6 a | 376 | 2.63 | 143 | 2.17 |
| [18F]ISO-1 c | 330 | 6.95 | 48 | 3.06 |
| [18F]ISO-1 d [18F]ISO-1 e | 102 95.1 | 28.2 13.3 | 4 7 | 3.06 3.06 |
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AChEI | Acetylcholinesterase inhibitors |
| APP | Amyloid precursor protein |
| ApoE | Apolipoprotein E gene |
| ARIA | Amyloid-related imaging abnormalities |
| Aβ | β-amyloid |
| AβOs | Aβ oligomers |
| ALS | Amyotrophic lateral sclerosis |
| BBB | Blood–brain barrier |
| BuChE | Butyrylcholinesterase |
| BiP | Binding immunoglobulin protein |
| CNS | Central nervous system |
| CDK5 | Cyclin-dependent kinase 5 |
| DA | Dopamine |
| ER | Endoplasmic reticulum |
| EPR | Emopamil binding protein |
| GI | Gastrointestinal |
| GPCRs | G protein-coupled transporters |
| GRP78 | Glucose-regulated protein 78 |
| HD | Huntington’s Disease |
| IP3R | inositol 1,4,5-trisphosphate receptor |
| IRE1 | Inositol-requiring enzyme 1 |
| LDLR | Low-density lipoprotein receptor |
| LRP-1 | Low-density lipoprotein receptor-related protein 1 |
| LXRs | Liver X receptors |
| mAb | Monoclonal antibody |
| MAM | Mitochondria-associated ER membrane |
| MAC30 | Meningioma-associated protein 30 |
| MDD | Major depressive disorder |
| MS | Multiple sclerosis |
| MCI | Mild cognitive impairment |
| NFTs | Neurofibrillary tangles |
| NMDA | N-methyl-D-aspartate receptor |
| NGF | Nerve growth factor |
| PD | Parkinson’s disease |
| PET | Positron Emission Tomography |
| PGRMC1 | Progesterone receptor membrane component 1 |
| ROS | Reactive oxygen species |
| SAM | Senescence-accelerated mouse |
| TMEM97 | Transmembrane protein 97 |
| VEGF | Vascular endothelial growth factor |
| VAChT | Vesicular acetylcholine transporter |
| VLDLR | Very-low-density lipoprotein receptor |
References
- Gauthier, S.; Webster, C.; Servaes, S.; Morais, J.A.; Rosa-Neto, P. World Alzheimer Report 2022: Life after Diagnosis: Navigating Treatment, Care and Support; Alzheimer’s Disease International: London, UK, 2022. [Google Scholar]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Haeberlein, S.B.; Carrillo, M.C.; Hendrix, J.A.; Kerchner, G.; Margolin, R.; Maruff, P.; Miller, D.S.; Tong, G.; Tome, M.B.; et al. The national institute on aging and the Alzheimer’s association research framework for Alzheimer’s disease: Perspectives from the research roundtable. Alzheimers Dement. 2018, 14, 563–575. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Grundke-Iqbal, I.; Iqbal, K.; Tung, Y.C.; Quinlan, M.; Wisniewski, H.M.; Binder, L.I. Abnormal phosphorylation of the microtubule-associated protein tau in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 1986, 83, 4913–4917. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Maloney, A.J. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976, 2, 1403. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Price, D.L.; DeLong, M.R. Alzheimer’s disease: A disorder of cortical cholinergic innervation. Science 1983, 219, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.G. Pharmacology of memory: Cholinergic-glutamatergic interactions. Curr. Opin. Neurobiol. 1995, 5, 155–160. [Google Scholar] [CrossRef]
- Bartus, R.T.; Dean, R.L., 3rd; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Khachaturian, Z.S. Calcium, membranes, aging, and Alzheimer’s disease. Introduction and overview. Ann. N. Y. Acad. Sci. 1989, 568, 1–4. [Google Scholar] [CrossRef]
- Cascella, R.; Cecchi, C. Calcium dyshomeostasis in Alzheimer’s disease pathogenesis. Int. J. Mol. Sci. 2021, 22, 4914. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: Implications for therapy. Acta Neuropathol. 2013, 126, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Wong-Guerra, M.; Calfio, C.; Maccioni, R.B.; Rojo, L.E. Revisiting the neuroinflammation hypothesis in Alzheimer’s disease: A focus on the druggability of current targets. Front. Pharmacol. 2023, 14, 1161850. [Google Scholar] [CrossRef] [PubMed]
- Area-Gomez, E.; Schon, E.A. On the pathogenesis of Alzheimer’s disease: The MAM hypothesis. FASEB J. 2017, 31, 864–867. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and mitochondrial cascades in Alzheimer’s disease. J. Alzheimers Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef]
- Sugimoto, H.; Iimura, Y.; Yamanishi, Y.; Yamatsu, K. Synthesis and structure-activity relationships of acetylcholinesterase inhibitors: 1-benzyl-4-[(5,6-dimethoxy-1-oxoindan-2-yl)methyl]piperidine hydrochloride and related compounds. J. Med. Chem. 1995, 38, 4821–4829. [Google Scholar] [CrossRef]
- Ishikawa, M.; Sakata, M.; Ishii, K.; Kimura, Y.; Oda, K.; Toyohara, J.; Wu, J.; Ishiwata, K.; Iyo, M.; Hashimoto, K. High occupancy of sigma-1 receptors in the human brain after single oral administration of donepezil: A positron emission tomography study using [11C]SA4503. Int. J. Neuropsychopharmacol. 2009, 12, 1127–1131. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E.; Brunetti, L.; Piemontese, L.; Pereira-Santos, A.R.; Cardoso, S.M.; Madrid, Y.; Chaves, S.; Santos, M.A. Novel rivastigmine derivatives as promising multi-target compounds for potential treatment of Alzheimer’s disease. Biomedicines 2022, 10, 1510. [Google Scholar] [CrossRef]
- Enz, A.; Amstutz, R.; Boddeke, H.; Gmelin, G.; Malanowski, J. Brain selective inhibition of acetylcholinesterase: A novel approach to therapy for Alzheimer’s disease. Prog. Brain Res. 1993, 98, 431–438. [Google Scholar]
- Thomsen, T.; Kewitz, H. Selective inhibition of human acetylcholinesterase by galanthamine in vitro and in vivo. Life Sci. 1990, 46, 1553–1558. [Google Scholar] [CrossRef]
- Maelicke, A.; Albuquerque, E.X. Allosteric modulation of nicotinic acetylcholine receptors as a treatment strategy for Alzheimer’s disease. Eur. J. Pharmacol. 2000, 393, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Brewster, J.T., 2nd; Dell’Acqua, S.; Thach, D.Q.; Sessler, J.L. Classics in chemical neuroscience: Donepezil. ACS Chem. Neurosci. 2019, 10, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Haake, A.; Nguyen, K.; Friedman, L.; Chakkamparambil, B.; Grossberg, G.T. An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert Opin. Drug Saf. 2020, 19, 147–157. [Google Scholar] [CrossRef]
- Vecchio, I.; Sorrentino, L.; Paoletti, A.; Marra, R.; Arbitrio, M. The state of the art on acetylcholinesterase inhibitors in the treatment of Alzheimer’s disease. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211029113. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E.; Cuello, A.C.; Fisher, A. Reimagining cholinergic therapy for Alzheimer’s disease. Brain 2022, 145, 2250–2275. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A. The molecular basis of memantine action in Alzheimer’s disease and other neurologic disorders: Low-affinity, uncompetitive antagonism. Curr. Alzheimer Res. 2005, 2, 155–165. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Liu, R.; Huang, Y.; Zhang, N.; Zhang, R. Memantine, donepezil, or combination therapy-what is the best therapy for Alzheimer’s disease? A network meta-analysis. Brain Behav. 2020, 10, e01831. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and management of dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef]
- Pardo-Moreno, T.; González-Acedo, A.; Rivas-Domínguez, A.; García-Morales, V.; García-Cozar, F.J.; Ramos-Rodríguez, J.J.; Melguizo-Rodríguez, L. Therapeutic approach to Alzheimer’s disease: Current treatments and new perspectives. Pharmaceutics 2022, 14, 1117. [Google Scholar] [CrossRef]
- Rossom, R.; Adityanjee; Dysken, M. Efficacy and tolerability of memantine in the treatment of dementia. Am. J. Geriatr. Pharmacother. 2004, 2, 303–312. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Aisen, P.; Apostolova, L.G.; Atri, A.; Salloway, S.; Weiner, M. Aducanumab: Appropriate use recommendations. J. Prev. Alzheimers Dis. 2021, 8, 398–410. [Google Scholar] [CrossRef]
- Cummings, J.; Apostolova, L.; Rabinovici, G.D.; Atri, A.; Aisen, P.; Greenberg, S.; Hendrix, S.; Selkoe, D.; Weiner, M.; Petersen, R.C.; et al. Lecanemab: Appropriate use recommendations. J. Prev. Alzheimers Dis. 2023, 10, 362–377. [Google Scholar] [CrossRef]
- Blasco, D.; Roberts, J.S. Editorial: Implications of emerging uses of genetic testing for Alzheimer’s disease. J. Prev. Alzheimers Dis. 2023, 10, 359–361. [Google Scholar]
- Vogt, A.S.; Jennings, G.T.; Mohsen, M.O.; Vogel, M.; Bachmann, M.F. Alzheimer’s disease: A brief history of immunotherapies targeting amyloid β. Int. J. Mol. Sci. 2023, 24, 3895. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Sodium oligomannate: First approval. Drugs 2020, 80, 441–444. [Google Scholar] [CrossRef]
- Cheng, H.-R.; Wen, C.-Y.; Zhang, C.; Kwapong, W. The use of GV-971 induces liver injury in an Alzheimer’s disease patient. Authorea 2020. preprints. [Google Scholar] [CrossRef]
- Schreiner, B.; Hedskog, L.; Wiehager, B.; Ankarcrona, M. Amyloid-β peptides are generated in mitochondria-associated endoplasmic reticulum membranes. J. Alzheimers Dis. 2015, 43, 369–374. [Google Scholar] [CrossRef]
- Alon, A.; Schmidt, H.R.; Wood, M.D.; Sahn, J.J.; Martin, S.F.; Kruse, A.C. Identification of the gene that codes for the σ2 receptor. Proc. Natl. Acad. Sci. USA 2017, 114, 7160–7165. [Google Scholar] [CrossRef]
- Riad, A.; Zeng, C.; Weng, C.C.; Winters, H.; Xu, K.; Makvandi, M.; Metz, T.; Carlin, S.; Mach, R.H. Sigma-2 receptor/TMEM97 and PGRMC-1 increase the rate of internalization of LDL by LDL receptor through the formation of a ternary complex. Sci. Rep. 2018, 8, 16845. [Google Scholar] [CrossRef]
- Riad, A.; Lengyel-Zhand, Z.; Zeng, C.; Weng, C.C.; Lee, V.M.; Trojanowski, J.Q.; Mach, R.H. The sigma-2 receptor/TMEM97, PGRMC1, and LDL receptor complex are responsible for the cellular uptake of Aβ42 and its protein aggregates. Mol. Neurobiol. 2020, 57, 3803–3813. [Google Scholar] [CrossRef] [PubMed]
- Fehér, Á.; Juhász, A.; László, A.; Kálmán, J., Jr.; Pákáski, M.; Kálmán, J.; Janka, Z. Association between a variant of the sigma-1 receptor gene and Alzheimer’s disease. Neurosci. Lett. 2012, 517, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.L.; Fang, M.; Zhao, Y.X.; Liu, X.Y. Roles of sigma-1 receptors in Alzheimer’s disease. Int. J. Clin. Exp. Med. 2015, 8, 4808–4820. [Google Scholar]
- Marrazzo, A.; Caraci, F.; Salinaro, E.T.; Su, T.P.; Copani, A.; Ronsisvalle, G. Neuroprotective effects of sigma-1 receptor agonists against beta-amyloid-induced toxicity. Neuroreport 2005, 16, 1223–1226. [Google Scholar] [CrossRef]
- An, Y.; Qi, Y.; Li, Y.; Li, Z.; Yang, C.; Jia, D. Activation of the sigma-1 receptor attenuates blood-brain barrier disruption by inhibiting amyloid deposition in Alzheimer’s disease mice. Neurosci. Lett. 2022, 774, 136528. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Y.; Huang, Y. Imaging sigma receptors in the brain: New opportunities for diagnosis of Alzheimer’s disease and therapeutic development. Neurosci. Lett. 2019, 691, 3–10. [Google Scholar] [CrossRef]
- Ye, N.; Qin, W.; Tian, S.; Xu, Q.; Wold, E.A.; Zhou, J.; Zhen, X.C. Small molecules selectively targeting sigma-1 receptor for the treatment of neurological diseases. J. Med. Chem. 2020, 63, 15187–15217. [Google Scholar] [CrossRef]
- Bartz, F.; Kern, L.; Erz, D.; Zhu, M.; Gilbert, D.; Meinhof, T.; Wirkner, U.; Erfle, H.; Muckenthaler, M.; Pepperkok, R.; et al. Identification of cholesterol-regulating genes by targeted RNAi screening. Cell Metab. 2009, 10, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Li, J.; Xie, X.; Yang, H.; Zhang, M.; Wang, B.; Kent, K.C.; Plutzky, J.; Guo, L.W. BRD2 regulation of sigma-2 receptor upon cholesterol deprivation. Life Sci. Alliance 2021, 4, e201900540. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.H.; Chen, A.F.; Xie, X.Y.; Huang, Y.S. Sigma ligands as potent inhibitors of Aβ and AβOs in neurons and promising therapeutic agents of Alzheimer’s disease. Neuropharmacology 2021, 190, 108342. [Google Scholar] [CrossRef]
- Martin, W.R.; Eades, C.G.; Thompson, J.A.; Huppler, R.E.; Gilbert, P.E. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976, 197, 517–532. [Google Scholar] [PubMed]
- Vaupel, D.B. Naltrexone fails to antagonize the sigma effects of PCP and SKF 10,047 in the dog. Eur. J. Pharmacol. 1983, 92, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Quirion, R.; Bowen, W.D.; Itzhak, Y.; Junien, J.L.; Musacchio, J.M.; Rothman, R.B.; Su, T.P.; Tam, S.W.; Taylor, D.P. A proposal for the classification of sigma binding sites. Trends Pharmacol. Sci. 1992, 13, 85–86. [Google Scholar] [CrossRef]
- Bouchard, P.; Quirion, R. [3H]1,3-di(2-tolyl)guanidine and [3H](+)pentazocine binding sites in the rat brain: Autoradiographic visualization of the putative sigma-1 and sigma-2 receptor subtypes. Neuroscience 1997, 76, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Ishiwata, K.; Tajima, H.; Ishii, S.; Matsuno, K.; Homma, Y.; Senda, M. In vivo evaluation of [11C]SA4503 as a PET ligand for mapping CNS sigma-1 receptors. Nucl. Med. Biol. 2000, 27, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Novakova, M.; Ela, C.; Barg, J.; Vogel, Z.; Hasin, Y.; Eilam, Y. Inotropic action of sigma receptor ligands in isolated cardiac myocytes from adult rats. Eur. J. Pharmacol. 1995, 286, 19–30. [Google Scholar] [CrossRef]
- Hellewell, S.B.; Bruce, A.; Feinstein, G.; Orringer, J.; Williams, W.; Bowen, W.D. Rat liver and kidney contain high densities of sigma-1 and sigma-2 receptors: Characterization by ligand binding and photoaffinity labeling. Eur. J. Pharmacol. 1994, 268, 9–18. [Google Scholar] [CrossRef]
- Liu, Y.; Whitlock, B.B.; Pultz, J.A.; Wolfe, S.A., Jr. Sigma-1 receptors modulate functional activity of rat splenocytes. J. Neuroimmunol. 1995, 59, 143–154. [Google Scholar] [CrossRef]
- Wolfe, S.A., Jr.; Ha, B.K.; Whitlock, B.B.; Saini, P. Differential localization of three distinct binding sites for sigma receptor ligands in rat spleen. J. Neuroimmunol. 1997, 72, 45–58. [Google Scholar] [CrossRef]
- Schmidt, H.R.; Kruse, A.C. The molecular function of σ receptors: Past, present, and future. Trends Pharmacol. Sci. 2019, 40, 636–654. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Bowen, W.D.; Goldstein, S.R.; Roberts, A.H.; Patrick, S.L.; Hohmann, A.G.; DeCosta, B. Autoradiographic distribution of [3H](+)-pentazocine and [3H]1,3-di-o-tolylguanidine (DTG) binding sites in guinea pig brain: A comparative study. Brain Res. 1992, 581, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Søby, K.K.; Mikkelsen, J.D.; Meier, E.; Thomsen, C. Lu 28-179 labels a σ2-site in rat and human brain. Neuropharmacology 2002, 43, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Leitner, M.L.; Hohmann, A.G.; Patrick, S.L.; Walker, J.M. Regional variation in the ratio of sigma-1 to sigma-2 binding in rat brain. Eur. J. Pharmacol. 1994, 259, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Izzo, N.J.; Colom-Cadena, M.; Riad, A.A.; Xu, J.; Singh, M.; Abate, C.; Cahill, M.A.; Spires-Jones, T.L.; Bowen, W.D.; Mach, R.H.; et al. Proceedings from the fourth international symposium on σ2 receptors: Role in health and disease. eNeuro 2020, 7, ENEURO.0317-20.2020. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Xu, J.; Mach, R.H. Sigma-2 receptors: An emerging target for CNS PET imaging studies. In PET and SPECT of Neurobiological Systems; Dierckx, R.A.J.O., Otte, A., de Vries, E.F.J., van Waarde, A., Lammertsma, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 973–991. [Google Scholar]
- Weber, E.; Sonders, M.; Quarum, M.; McLean, S.; Pou, S.; Keana, J.F. 1,3-Di(2-[5-3H]tolyl)guanidine: A selective ligand that labels sigma-type receptors for psychotomimetic opiates and antipsychotic drugs. Proc. Natl. Acad. Sci. USA 1986, 83, 8784–8788. [Google Scholar] [CrossRef]
- Seth, P.; Fei, Y.J.; Li, H.W.; Huang, W.; Leibach, F.H.; Ganapathy, V. Cloning and functional characterization of a sigma receptor from rat brain. J. Neurochem. 1998, 70, 922–931. [Google Scholar] [CrossRef]
- Hanner, M.; Moebius, F.F.; Flandorfer, A.; Knaus, H.G.; Striessnig, J.; Kempner, E.; Glossmann, H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. USA 1996, 93, 8072–8077. [Google Scholar] [CrossRef]
- Seth, P.; Leibach, F.H.; Ganapathy, V. Cloning and structural analysis of the cDNA and the gene encoding the murine type 1 sigma receptor. Biochem. Biophys. Res. Commun. 1997, 241, 535–540. [Google Scholar] [CrossRef]
- Kekuda, R.; Prasad, P.D.; Fei, Y.J.; Leibach, F.H.; Ganapathy, V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1). Biochem. Biophys. Res. Commun. 1996, 229, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef]
- Su, T.P.; Su, T.C.; Nakamura, Y.; Tsai, S.Y. The sigma-1 receptor as a pluripotent modulator in living systems. Trends Pharmacol. Sci. 2016, 37, 262–278. [Google Scholar] [CrossRef]
- Schmidt, H.R.; Zheng, S.; Gurpinar, E.; Koehl, A.; Manglik, A.; Kruse, A.C. Crystal structure of the human sigma-1 receptor. Nature 2016, 532, 527–530. [Google Scholar] [CrossRef]
- Fontanilla, D.; Johannessen, M.; Hajipour, A.R.; Cozzi, N.V.; Jackson, M.B.; Ruoho, A.E. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 2009, 323, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Colabufo, N.A.; Berardi, F.; Abate, C.; Contino, M.; Niso, M.; Perrone, R. Is the sigma-2 receptor a histone binding protein? J. Med. Chem. 2006, 49, 4153–4158. [Google Scholar] [CrossRef] [PubMed]
- Abate, C.; Elenewski, J.; Niso, M.; Berardi, F.; Colabufo, N.A.; Azzariti, A.; Perrone, R.; Glennon, R.A. Interaction of the sigma-2 receptor ligand PB28 with the human nucleosome: Computational and experimental probes of interaction with the H2A/H2B dimer. ChemMedChem 2010, 5, 268–273. [Google Scholar] [CrossRef]
- Xu, J.; Zeng, C.; Chu, W.; Pan, F.; Rothfuss, J.M.; Zhang, F.; Tu, Z.; Zhou, D.; Zeng, D.; Vangveravong, S.; et al. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat. Commun. 2011, 2, 380. [Google Scholar] [CrossRef]
- Zeng, C.; Garg, N.; Mach, R.H. The PGRMC1 protein level correlates with the binding activity of a sigma-2 fluorescent probe (SW120) in rat brain cells. Mol. Imaging Biol. 2016, 18, 172–179. [Google Scholar] [CrossRef]
- Alon, A.; Lyu, J.; Braz, J.M.; Tummino, T.A.; Craik, V.; O’Meara, M.J.; Webb, C.M.; Radchenko, D.S.; Moroz, Y.S.; Huang, X.P.; et al. Structures of the σ2 receptor enable docking for bioactive ligand discovery. Nature 2021, 600, 759–764. [Google Scholar] [CrossRef]
- Son, K.N.; Lee, H.; Shah, D.; Kalmodia, S.; Miller, R.C.; Ali, M.; Balasubramaniam, A.; Cologna, S.M.; Kong, H.; Shukla, D.; et al. Histatin-1 is an endogenous ligand of the sigma-2 receptor. FEBS J. 2021, 288, 6815–6827. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Zhang, T.; Ma, X.; Pratuangtham, S.; Zhang, G.C.; Ondrus, A.A.; Mafi, A.; Lomenick, B.; Jones, J.J.; Ondrus, A.E. A proteome-wide map of 20(S)-hydroxycholesterol interactors in cell membranes. Nat. Chem. Biol. 2021, 17, 1271–1280. [Google Scholar] [CrossRef]
- Maurice, T.; Goguadze, N. Sigma-1 (σ1) receptor in memory and neurodegenerative diseases. Handb. Exp. Pharmacol. 2017, 244, 81–108. [Google Scholar]
- Piechal, A.; Jakimiuk, A.; Mirowska-Guzel, D. Sigma receptors and neurological disorders. Pharmacol. Rep. 2021, 73, 1582–1594. [Google Scholar] [CrossRef]
- Maurice, T.; Goguadze, N. Role of σ1 receptors in learning and memory and Alzheimer’s disease-type dementia. Adv. Exp. Med. Biol. 2017, 964, 213–233. [Google Scholar]
- Nguyen, L.; Lucke-Wold, B.P.; Mookerjee, S.; Kaushal, N.; Matsumoto, R.R. Sigma-1 receptors and neurodegenerative diseases: Towards a hypothesis of sigma-1 receptors as amplifiers of neurodegeneration and neuroprotection. Adv. Exp. Med. Biol. 2017, 964, 133–152. [Google Scholar]
- Yang, K.; Wang, C.; Sun, T. The roles of intracellular chaperone proteins, sigma receptors, in Parkinson’s disease (PD) and major depressive disorder (MDD). Front. Pharmacol. 2019, 10, 528. [Google Scholar] [CrossRef]
- Wilson, H.; Pagano, G.; de Natale, E.R.; Mansur, A.; Caminiti, S.P.; Polychronis, S.; Middleton, L.T.; Price, G.; Schmidt, K.F.; Gunn, R.N.; et al. Mitochondrial complex 1, sigma-1, and synaptic vesicle 2A in early drug-naive Parkinson’s disease. Mov. Disord. 2020, 35, 1416–1427. [Google Scholar] [CrossRef]
- Francardo, V.; Geva, M.; Bez, F.; Denis, Q.; Steiner, L.; Hayden, M.R.; Cenci, M.A. Pridopidine induces functional neurorestoration via the sigma-1 receptor in a mouse model of Parkinson’s disease. Neurotherapeutics 2019, 16, 465–479. [Google Scholar] [CrossRef]
- Guo, C.H.; Cao, T.; Zheng, L.T.; Waddington, J.L.; Zhen, X.C. Development and characterization of an inducible Dicer conditional knockout mouse model of Parkinson’s disease: Validation of the antiparkinsonian effects of a sigma-1 receptor agonist and dihydromyricetin. Acta Pharmacol. Sin. 2020, 41, 499–507. [Google Scholar] [CrossRef]
- Siddiqui, T.; Bhatt, L.K. Targeting Sigma-1 receptor: A promising strategy in the treatment of Parkinson’s disease. Neurochem. Res. 2023, 1–11. [Google Scholar] [CrossRef]
- Eddings, C.R.; Arbez, N.; Akimov, S.; Geva, M.; Hayden, M.R.; Ross, C.A. Pridopidine protects neurons from mutant-huntingtin toxicity via the sigma-1 receptor. Neurobiol. Dis. 2019, 129, 118–129. [Google Scholar] [CrossRef]
- Kraskovskaya, N.A.; Bezprozvanny, I.B. Normalization of calcium balance in striatal neurons in Huntington’s disease: Sigma-1 receptor as a potential target for therapy. Biochemistry 2021, 86, 471–479. [Google Scholar] [CrossRef]
- Herrando-Grabulosa, M.; Gaja-Capdevila, N.; Vela, J.M.; Navarro, X. Sigma 1 receptor as a therapeutic target for amyotrophic lateral sclerosis. Br. J. Pharmacol. 2021, 178, 1336–1352. [Google Scholar] [CrossRef]
- Mavlyutov, T.A.; Guo, L.W.; Epstein, M.L.; Ruoho, A.E. Role of the sigma-1 receptor in amyotrophic lateral sclerosis (ALS). J. Pharmacol. Sci. 2015, 127, 10–16. [Google Scholar] [CrossRef]
- Ellis, D.Z.; Li, L.; Park, Y.; He, S.; Mueller, B.; Yorio, T. Sigma-1 receptor regulates mitochondrial function in glucose- and oxygen-deprived retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2017, 58, 2755–2764. [Google Scholar] [CrossRef]
- Smith, S.B.; Wang, J.; Cui, X.; Mysona, B.A.; Zhao, J.; Bollinger, K.E. Sigma-1 receptor: A novel therapeutic target in retinal disease. Prog. Retin. Eye Res. 2018, 67, 130–149. [Google Scholar] [CrossRef]
- Jiang, G.; Mysona, B.; Dun, Y.; Gnana-Prakasam, J.P.; Pabla, N.; Li, W.; Dong, Z.; Ganapathy, V.; Smith, S.B. Expression, subcellular localization, and regulation of sigma receptor in retinal muller cells. Invest. Ophthalmol. Vis. Sci. 2006, 47, 5576–5582. [Google Scholar] [CrossRef]
- Mavlyutov, T.A.; Epstein, M.; Guo, L.W. Subcellular localization of the sigma-1 receptor in retinal neurons—An electron microscopy study. Sci. Rep. 2015, 5, 10689. [Google Scholar] [CrossRef]
- Brimson, J.M.; Brimson, S.; Chomchoei, C.; Tencomnao, T. Using sigma-ligands as part of a multi-receptor approach to target diseases of the brain. Expert Opin. Ther. Targets 2020, 24, 1009–1028. [Google Scholar] [CrossRef]
- Wang, Y.M.; Xia, C.Y.; Jia, H.M.; He, J.; Lian, W.W.; Yan, Y.; Wang, W.P.; Zhang, W.K.; Xu, J.K. Sigma-1 receptor: A potential target for the development of antidepressants. Neurochem. Int. 2022, 159, 105390. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Muñoz, M.; Onetti, Y.; Cortés-Montero, E.; Garzón, J.; Sánchez-Blázquez, P. Cannabidiol enhances morphine antinociception, diminishes NMDA-mediated seizures and reduces stroke damage via the sigma-1 receptor. Mol. Brain 2018, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Wei, Q.; Leng, S.; Li, C.; Han, B.; Bai, Y.; Zhang, H.; Yao, H. Activation of sigma-1 receptor enhanced pericyte survival via the interplay between apoptosis and autophagy: Implications for blood-brain barrier integrity in stroke. Transl. Stroke Res. 2020, 11, 267–287. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Jiao, Y.; Tang, T.; Yang, L.; Lu, C.; Zhang, Y.; Zhang, Y.; Bai, Y.; Chao, J.; et al. An increase of sigma-1 receptor in the penumbra neuron after acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 1981–1987. [Google Scholar] [CrossRef]
- Romero, L.; Merlos, M.; Vela, J.M. Antinociception by sigma-1 receptor antagonists: Central and peripheral effects. Adv. Pharmacol. 2016, 75, 179–215. [Google Scholar]
- Merlos, M.; Burgueño, J.; Portillo-Salido, E.; Plata-Salamán, C.R.; Vela, J.M. Pharmacological modulation of the sigma-1 receptor and the treatment of pain. Adv. Exp. Med. Biol. 2017, 964, 85–107. [Google Scholar]
- Skuza, G. Ethanol withdrawal-induced depressive symptoms in animals and therapeutic potential of sigma1 receptor ligands. Pharmacol. Rep. 2013, 65, 1681–1687. [Google Scholar] [CrossRef]
- Bai, T.; Lei, P.; Zhou, H.; Liang, R.; Zhu, R.; Wang, W.; Zhou, L.; Sun, Y. Sigma-1 receptor protects against ferroptosis in hepatocellular carcinoma cells. J. Cell. Mol. Med. 2019, 23, 7349–7359. [Google Scholar] [CrossRef]
- Soriani, O.; Kourrich, S. The sigma-1 receptor: When adaptive regulation of cell electrical activity contributes to stimulant addiction and cancer. Front. Neurosci. 2019, 13, 1186. [Google Scholar] [CrossRef]
- Brimson, J.M.; Akula, K.K.; Abbas, H.; Ferry, D.R.; Kulkarni, S.K.; Russell, S.T.; Tisdale, M.J.; Tencomnao, T.; Safrany, S.T. Simple ammonium salts acting on sigma-1 receptors yield potential treatments for cancer and depression. Sci. Rep. 2020, 10, 9251. [Google Scholar] [CrossRef]
- Pontisso, I.; Combettes, L. Role of sigma-1 receptor in calcium modulation: Possible involvement in cancer. Genes 2021, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Gong, K.; Yan, Y.; Song, B.; Zhang, X.; Gong, Y. Mitochondrial modulation of store-operated Ca2+ entry in model cells of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2012, 426, 196–202. [Google Scholar] [CrossRef]
- Hung, C.H.; Ho, Y.S.; Chang, R.C. Modulation of mitochondrial calcium as a pharmacological target for Alzheimer’s disease. Ageing Res. Rev. 2010, 9, 447–456. [Google Scholar] [CrossRef]
- Guan, P.P.; Cao, L.L.; Wang, P. Elevating the levels of calcium ions exacerbate Alzheimer’s disease via inducing the production and aggregation of β-Amyloid protein and phosphorylated tau. Int. J. Mol. Sci. 2021, 22, 5900. [Google Scholar] [CrossRef]
- Bezprozvanny, I. Calcium signaling and neurodegenerative diseases. Trends Mol. Med. 2009, 15, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association Calcium Hypothesis Workgroup; Khachaturian, Z.S. Calcium hypothesis of Alzheimer’s disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimers Dement. 2017, 13, 178–182.e17. [Google Scholar] [CrossRef]
- van Waarde, A.; Ramakrishnan, N.K.; Rybczynska, A.A.; Elsinga, P.H.; Ishiwata, K.; Nijholt, I.M.; Luiten, P.G.; Dierckx, R.A. The cholinergic system, sigma-1 receptors and cognition. Behav. Brain Res. 2011, 221, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Callens, M.; Loncke, J.; Bultynck, G. Dysregulated Ca2+ homeostasis as a central theme in neurodegeneration: Lessons from Alzheimer’s disease and wolfram syndrome. Cells 2022, 11, 1963. [Google Scholar] [CrossRef]
- Resende, R.; Fernandes, T.; Pereira, A.C.; Marques, A.P.; Pereira, C.F. Endoplasmic reticulum-mitochondria contacts modulate reactive oxygen species-mediated signaling and oxidative stress in brain disorders: The key role of sigma-1 receptor. Antioxid. Redox Signal. 2022, 37, 758–780. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Hayashi, T.; Harvey, B.K.; Wang, Y.; Wu, W.W.; Shen, R.F.; Zhang, Y.; Becker, K.G.; Hoffer, B.J.; Su, T.P. Sigma-1 receptors regulate hippocampal dendritic spine formation via a free radical-sensitive mechanism involving Rac1xGTP pathway. Proc. Natl. Acad. Sci. USA 2009, 106, 22468–22473. [Google Scholar] [CrossRef]
- Weng, T.Y.; Tsai, S.A.; Su, T.P. Roles of sigma-1 receptors on mitochondrial functions relevant to neurodegenerative diseases. J. Biomed. Sci. 2017, 24, 74. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Hayashi, T.; Hayashi, E.; Su, T.P. Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS One. 2013, 8, e76941. [Google Scholar] [CrossRef] [PubMed]
- Voronin, M.V.; Abramova, E.V.; Verbovaya, E.R.; Vakhitova, Y.V.; Seredenin, S.B. Chaperone-dependent mechanisms as a pharmacological target for neuroprotection. Int. J. Mol. Sci. 2023, 24, 823. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, B.; Zhu, Y.; Chen, L.; Sokabe, M.; Chen, L. DHEA prevents Aβ25-35-impaired survival of newborn neurons in the dentate gyrus through a modulation of PI3K-Akt-mTOR signaling. Neuropharmacology 2010, 59, 323–333. [Google Scholar] [CrossRef]
- Nishimura, T.; Ishima, T.; Iyo, M.; Hashimoto, K. Potentiation of nerve growth factor-induced neurite outgrowth by fluvoxamine: Role of sigma-1 receptors, IP3 receptors and cellular signaling pathways. PLoS One. 2008, 3, e2558. [Google Scholar] [CrossRef]
- Lahmy, V.; Meunier, J.; Malmström, S.; Naert, G.; Givalois, L.; Kim, S.H.; Villard, V.; Vamvakides, A.; Maurice, T. Blockade of Tau hyperphosphorylation and Aβ₁₋₄₂ generation by the aminotetrahydrofuran derivative ANAVEX2-73, a mixed muscarinic and σ₁ receptor agonist, in a nontransgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology 2013, 38, 1706–1723. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Pokrass, M.J.; Klauer, N.R.; Nohara, H.; Su, T.P. Sigma-1 receptor regulates tau phosphorylation and axon extension by shaping p35 turnover via myristic acid. Proc. Natl. Acad. Sci. USA 2015, 112, 6742–6747. [Google Scholar] [CrossRef]
- Ciesielski, J.; Su, T.P.; Tsai, S.Y. Myristic acid hitchhiking on sigma-1 receptor to fend off neurodegeneration. Recept. Clin. Investig. 2016, 3, e1114. [Google Scholar]
- Sha, S.; Qu, W.J.; Li, L.; Lu, Z.H.; Chen, L.; Yu, W.F.; Chen, L. Sigma-1 receptor knockout impairs neurogenesis in dentate gyrus of adult hippocampus via down-regulation of NMDA receptors. CNS Neurosci. Ther. 2013, 19, 705–713. [Google Scholar] [CrossRef]
- Couly, S.; Goguadze, N.; Yasui, Y.; Kimura, Y.; Wang, S.M.; Sharikadze, N.; Wu, H.E.; Su, T.P. Knocking out sigma-1 receptors reveals diverse health problems. Cell Mol. Neurobiol. 2022, 42, 597–620. [Google Scholar] [CrossRef]
- Raulin, A.C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.C. ApoE in Alzheimer’s disease: Pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.L.; Faull, R.L.; Storey, P.; Leslie, R.A. Loss of sigma binding sites in the CA1 area of the anterior hippocampus in Alzheimer’s disease correlates with CA1 pyramidal cell loss. Brain Res. 1993, 623, 299–302. [Google Scholar] [CrossRef]
- Antonini, V.; Marrazzo, A.; Kleiner, G.; Coradazzi, M.; Ronsisvalle, S.; Prezzavento, O.; Ronsisvalle, G.; Leanza, G. Anti-amnesic and neuroprotective actions of the sigma-1 receptor agonist (-)-MR22 in rats with selective cholinergic lesion and amyloid infusion. J. Alzheimers Dis. 2011, 24, 569–586. [Google Scholar] [CrossRef]
- Antonini, V.; Prezzavento, O.; Coradazzi, M.; Marrazzo, A.; Ronsisvalle, S.; Arena, E.; Leanza, G. Anti-amnesic properties of (+/−)-PPCC, a novel sigma receptor ligand, on cognitive dysfunction induced by selective cholinergic lesion in rats. J. Neurochem. 2009, 109, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, L.; Wang, H.; Xu, B.; Tomimoto, H.; Chen, L. Anti-amnesic effect of neurosteroid PREGS in Aβ25–35-injected mice through σ1 receptor- and α7nAChR-mediated neuroprotection. Neuropharmacology 2012, 63, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Maurice, T.; Su, T.P.; Privat, A. Sigma1 (σ1) receptor agonists and neurosteroids attenuate β25–35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience 1998, 83, 413–428. [Google Scholar] [CrossRef]
- Meunier, J.; Ieni, J.; Maurice, T. The anti-amnesic and neuroprotective effects of donepezil against amyloid β25-35 peptide-induced toxicity in mice involve an interaction with the σ1 receptor. Br. J. Pharmacol. 2006, 149, 998–1012. [Google Scholar] [CrossRef]
- Villard, V.; Espallergues, J.; Keller, E.; Vamvakides, A.; Maurice, T. Anti-amnesic and neuroprotective potentials of the mixed muscarinic receptor/sigma 1 (σ1) ligand ANAVEX2-73, a novel aminotetrahydrofuran derivative. J. Psychopharmacol. 2011, 25, 1101–1117. [Google Scholar] [CrossRef]
- Villard, V.; Espallergues, J.; Keller, E.; Alkam, T.; Nitta, A.; Yamada, K.; Nabeshima, T.; Vamvakides, A.; Maurice, T. Antiamnesic and neuroprotective effects of the aminotetrahydrofuran derivative ANAVEX1-41 against amyloid beta25-35-induced toxicity in mice. Neuropsychopharmacology 2009, 34, 1552–1566. [Google Scholar] [CrossRef]
- Ukai, M.; Maeda, H.; Nanya, Y.; Kameyama, T.; Matsuno, K. Beneficial effects of acute and repeated administrations of sigma receptor agonists on behavioral despair in mice exposed to tail suspension. Pharmacol. Biochem. Behav. 1998, 61, 247–252. [Google Scholar] [CrossRef]
- Hayashi, T.; Maurice, T.; Su, T.P. Ca2+ signaling via sigma1-receptors: Novel regulatory mechanism affecting intracellular Ca2+ concentration. J. Pharmacol. Exp. Ther. 2000, 293, 788–798. [Google Scholar] [PubMed]
- Maurice, T. Beneficial effect of the sigma1 receptor agonist PRE-084 against the spatial learning deficits in aged rats. Eur. J. Pharmacol. 2001, 431, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Maurice, T.; Roman, F.J.; Su, T.P.; Privat, A. Beneficial effects of sigma agonists on the age-related learning impairment in the senescence-accelerated mouse (SAM). Brain Res. 1996, 733, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.F.; Ma, W.H.; Xie, X.Y.; Huang, Y.S. Sigma-2 Receptor as a potential drug target. Curr. Med. Chem. 2021, 28, 4172–4189. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, C.; Wang, C.; Sun, M.; Yin, D.; Sun, T. Sigma-2 receptor-A potential target for cancer/Alzheimer’s disease treatment via its regulation of cholesterol homeostasis. Molecules 2020, 25, 5439. [Google Scholar] [CrossRef]
- Lizama, B.N.; Kahle, J.; Catalano, S.M.; Caggiano, A.O.; Grundman, M.; Hamby, M.E. Sigma-2 receptors-from basic biology to therapeutic target: A focus on age-related degenerative diseases. Int. J. Mol. Sci. 2023, 24, 6251. [Google Scholar] [CrossRef]
- Mach, R.H.; Zeng, C.; Hawkins, W.G. The σ2 receptor: A novel protein for the imaging and treatment of cancer. J. Med. Chem. 2013, 56, 7137–7160. [Google Scholar] [CrossRef]
- Mach, R.H.; Smith, C.R.; al-Nabulsi, I.; Whirrett, B.R.; Childers, S.R.; Wheeler, K.T. Sigma-2 receptors as potential biomarkers of proliferation in breast cancer. Cancer Res. 1997, 57, 156–161. [Google Scholar]
- Al-Nabulsi, I.; Mach, R.H.; Wang, L.M.; Wallen, C.A.; Keng, P.C.; Sten, K.; Childers, S.R.; Wheeler, K.T. Effect of ploidy, recruitment, environmental factors, and tamoxifen treatment on the expression of sigma-2 receptors in proliferating and quiescent tumour cells. Br. J. Cancer 1999, 81, 925–933. [Google Scholar] [CrossRef]
- Wheeler, K.T.; Wang, L.M.; Wallen, C.A.; Childers, S.R.; Cline, J.M.; Keng, P.C.; Mach, R.H. Sigma-2 receptors as a biomarker of proliferation in solid tumours. Br. J. Cancer 2000, 82, 1223–1232. [Google Scholar] [CrossRef]
- Shaghaghi, Z.; Alvandi, M.; Ghanbarimasir, Z.; Farzipour, S.; Emami, S. Current development of sigma-2 receptor radioligands as potential tumor imaging agents. Bioorg. Chem. 2021, 115, 105163. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Arbez, N.; Sahn, J.J.; Lu, Y.; Linkens, K.T.; Hodges, T.R.; Tang, A.; Wiseman, R.; Martin, S.F.; Ross, C.A. Neuroprotective effects of σ2R/TMEM97 receptor modulators in the neuronal model of Huntington’s disease. ACS Chem. Neurosci. 2022, 13, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Limegrover, C.S.; Yurko, R.; Izzo, N.J.; LaBarbera, K.M.; Rehak, C.; Look, G.; Rishton, G.; Safferstein, H.; Catalano, S.M. Sigma-2 receptor antagonists rescue neuronal dysfunction induced by Parkinson’s patient brain-derived α-synuclein. J. Neurosci. Res. 2021, 99, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Papp, M. The selective sigma2 ligand Lu 28-179 has an antidepressant-like profile in the rat chronic mild stress model of depression. Behav. Pharmacol. 2000, 11, 117–124. [Google Scholar] [CrossRef]
- Davidson, M.; Saoud, J.; Staner, C.; Noel, N.; Luthringer, E.; Werner, S.; Reilly, J.; Schaffhauser, J.Y.; Rabinowitz, J.; Weiser, M.; et al. Efficacy and safety of MIN-101: A 12-week randomized, double-blind, placebo-controlled trial of a new drug in development for the treatment of negative symptoms in schizophrenia. Am. J. Psychiatry 2017, 174, 1195–1202. [Google Scholar] [CrossRef]
- Sahn, J.J.; Mejia, G.L.; Ray, P.R.; Martin, S.F.; Price, T.J. Sigma-2 receptor/Tmem97 agonists produce long lasting antineuropathic pain effects in mice. ACS Chem. Neurosci. 2017, 8, 1801–1811. [Google Scholar] [CrossRef]
- Wilson, L.L.; Alleyne, A.R.; Eans, S.O.; Cirino, T.J.; Stacy, H.M.; Mottinelli, M.; Intagliata, S.; McCurdy, C.R.; McLaughlin, J.P. Characterization of CM-398, a novel selective sigma-2 receptor ligand, as a potential therapeutic for neuropathic pain. Molecules 2022, 27, 3617. [Google Scholar] [CrossRef]
- Izzo, N.J.; Xu, J.; Zeng, C.; Kirk, M.J.; Mozzoni, K.; Silky, C.; Rehak, C.; Yurko, R.; Look, G.; Rishton, G.; et al. Alzheimer’s therapeutics targeting amyloid beta 1-42 oligomers II: Sigma-2/PGRMC1 receptors mediate abeta 42 oligomer binding and synaptotoxicity. PLoS ONE 2014, 9, e111899. [Google Scholar] [CrossRef]
- Esparza, T.J.; Zhao, H.; Cirrito, J.R.; Cairns, N.J.; Bateman, R.J.; Holtzman, D.M.; Brody, D.L. Amyloid-β oligomerization in Alzheimer dementia versus high-pathology controls. Ann. Neurol. 2013, 73, 104–119. [Google Scholar] [CrossRef]
- Lacor, P.N.; Buniel, M.C.; Furlow, P.W.; Clemente, A.S.; Velasco, P.T.; Wood, M.; Viola, K.L.; Klein, W.L. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J. Neurosci. 2007, 27, 796–807. [Google Scholar] [CrossRef]
- DaRocha-Souto, B.; Scotton, T.C.; Coma, M.; Serrano-Pozo, A.; Hashimoto, T.; Serenó, L.; Rodríguez, M.; Sánchez, B.; Hyman, B.T.; Gómez-Isla, T. Brain oligomeric β-amyloid but not total amyloid plaque burden correlates with neuronal loss and astrocyte inflammatory response in amyloid precursor protein/tau transgenic mice. J. Neuropathol. Exp. Neurol. 2011, 70, 360–376. [Google Scholar] [CrossRef]
- Izzo, N.J.; Staniszewski, A.; To, L.; Fa, M.; Teich, A.F.; Saeed, F.; Wostein, H.; Walko, T., 3rd; Vaswani, A.; Wardius, M.; et al. Alzheimer’s therapeutics targeting amyloid beta 1-42 oligomers I: Abeta 42 oligomer binding to specific neuronal receptors is displaced by drug candidates that improve cognitive deficits. PLoS One. 2014, 9, e111898. [Google Scholar] [CrossRef] [PubMed]
- Colom-Cadena, M.; Tulloch, J.; Jackson, R.J.; Catterson, J.H.; Rose, J.; Davies, C.; Hooley, M.; Anton-Fernandez, A.; Dunnett, S.; Tempelaar, R.; et al. TMEM97 increases in synapses and is a potential synaptic Aβ binding partner in human Alzheimer’s disease. bioRxiv 2021, 2021.02.01.428238. [Google Scholar] [CrossRef]
- Xiong, H.; Callaghan, D.; Jones, A.; Walker, D.G.; Lue, L.F.; Beach, T.G.; Sue, L.I.; Woulfe, J.; Xu, H.; Stanimirovic, D.B.; et al. Cholesterol retention in Alzheimer’s brain is responsible for high beta- and gamma-secretase activities and Abeta production. Neurobiol. Dis. 2008, 29, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Lane-Donovan, C.; Philips, G.T.; Herz, J. More than cholesterol transporters: Lipoprotein receptors in CNS function and neurodegeneration. Neuron 2014, 83, 771–787. [Google Scholar] [CrossRef]
- Pappolla, M.A.; Bryant-Thomas, T.K.; Herbert, D.; Pacheco, J.; Fabra Garcia, M.; Manjon, M.; Girones, X.; Henry, T.L.; Matsubara, E.; Zambon, D.; et al. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology 2003, 61, 199–205. [Google Scholar] [CrossRef]
- Nicholson, A.M.; Ferreira, A. Increased membrane cholesterol might render mature hippocampal neurons more susceptible to beta-amyloid-induced calpain activation and tau toxicity. J. Neurosci. 2009, 29, 4640–4651. [Google Scholar] [CrossRef]
- Sawamura, N.; Gong, J.S.; Chang, T.Y.; Yanagisawa, K.; Michikawa, M. Promotion of tau phosphorylation by MAP kinase Erk1/2 is accompanied by reduced cholesterol level in detergent-insoluble membrane fraction in Niemann-Pick C1-deficient cells. J. Neurochem. 2003, 84, 1086–1096. [Google Scholar] [CrossRef]
- Thejer, B.M.; Infantino, V.; Santarsiero, A.; Pappalardo, I.; Abatematteo, F.S.; Teakel, S.; Van Oosterum, A.; Mach, R.H.; Denora, N.; Lee, B.C.; et al. Sigma-2 receptor ligand binding modulates association between TSPO and TMEM97. Int. J. Mol. Sci. 2023, 24, 6381. [Google Scholar] [CrossRef]
- Steffensen, K.R.; Jakobsson, T.; Gustafsson, J. Targeting liver X receptors in inflammation. Expert Opin. Ther. Targets 2013, 17, 977–990. [Google Scholar] [CrossRef]
- Vilner, B.J.; Bowen, W.D. Modulation of cellular calcium by sigma-2 receptors: Release from intracellular stores in human SK-N-SH neuroblastoma cells. J. Pharmacol. Exp. Ther. 2000, 292, 900–911. [Google Scholar] [PubMed]
- Cassano, G.; Gasparre, G.; Contino, M.; Niso, M.; Berardi, F.; Perrone, R.; Colabufo, N.A. The sigma-2 receptor agonist PB28 inhibits calcium release from the endoplasmic reticulum of SK-N-SH neuroblastoma cells. Cell Calcium 2006, 40, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Cassano, G.; Gasparre, G.; Niso, M.; Contino, M.; Scalera, V.; Colabufo, N.A. F281, synthetic agonist of the sigma-2 receptor, induces Ca2+ efflux from the endoplasmic reticulum and mitochondria in SK-N-SH cells. Cell Calcium 2009, 45, 340–345. [Google Scholar] [CrossRef]
- Yi, B.; Sahn, J.J.; Ardestani, P.M.; Evans, A.K.; Scott, L.L.; Chan, J.Z.; Iyer, S.; Crisp, A.; Zuniga, G.; Pierce, J.T.; et al. Small molecule modulator of sigma-2 receptor is neuroprotective and reduces cognitive deficits and neuroinflammation in experimental models of Alzheimer’s disease. J. Neurochem. 2017, 140, 561–575. [Google Scholar] [CrossRef]
- Guo, L.; Zhen, X. Sigma-2 receptor ligands: Neurobiological effects. Curr. Med. Chem. 2015, 22, 989–1003. [Google Scholar] [CrossRef]
- Terada, K.; Migita, K.; Matsushima, Y.; Sugimoto, Y.; Kamei, C.; Matsumoto, T.; Mori, M.; Matsunaga, K.; Takata, J.; Karube, Y. Cholinesterase inhibitor rivastigmine enhances nerve growth factor-induced neurite outgrowth in PC12 cells via sigma-1 and sigma-2 receptors. PLoS One 2018, 13, e0209250. [Google Scholar] [CrossRef]
- Ju, Y.; Tam, K.Y. Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 543–549. [Google Scholar]
- Terada, K.; Migita, K.; Matsushima, Y.; Kamei, C. Sigma-2 receptor as a potential therapeutic target for treating central nervous system disorders. Neural Regen. Res. 2019, 14, 1893–1894. [Google Scholar] [CrossRef]
- Kargbo, R.B. Sigma-1 and Sigma-2 receptor modulators as potential therapeutics for Alzheimer’s disease. ACS Med. Chem. Lett. 2021, 12, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, M.I.; Malar, D.S.; Tencomnao, T.; Brimson, J.M. The emerging role of the sigma-1 receptor in autophagy: Hand-in-hand targets for the treatment of Alzheimer’s. Expert Opin. Ther. Targets 2021, 25, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2020. Alzheimers Dement. 2020, 6, e12050. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement. 2022, 8, e12295. [Google Scholar] [CrossRef]
- Malar, D.S.; Thitilertdecha, P.; Ruckvongacheep, K.S.; Brimson, S.; Tencomnao, T.; Brimson, J.M. Targeting sigma receptors for the treatment of neurodegenerative and neurodevelopmental disorders. CNS Drugs 2023, 37, 399–440. [Google Scholar] [CrossRef]
- McClure, E.W.; Daniels, R.N. Classics in chemical neuroscience: Dextromethorphan (DXM). ACS Chem. Neurosci. 2023, 14, 2256–2270. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Dinis-Oliveira, R.J. Pharmacokinetics and pharmacodynamics of dextromethorphan: Clinical and forensic aspects. Drug Metab. Rev. 2020, 52, 258–282. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R. Deuterated dextromethorphan/quinidine for agitation in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1013–1014. [Google Scholar] [CrossRef]
- Hampel, H.; Williams, C.; Etcheto, A.; Goodsaid, F.; Parmentier, F.; Sallantin, J.; Kaufmann, W.E.; Missling, C.U.; Afshar, M. A precision medicine framework using artificial intelligence for the identification and confirmation of genomic biomarkers of response to an Alzheimer’s disease therapy: Analysis of the blarcamesine (ANAVEX2-73) Phase 2a clinical study. Alzheimers Dement. 2020, 6, e12013. [Google Scholar] [CrossRef]
- Fukushima, T. Pharmacological properties of T-817MA, a novel neurotrophic agent, for treatment of Alzheimer’s disease. Nihon Yakurigaku Zasshi 2010, 136, 11–14. [Google Scholar] [CrossRef]
- Yano, T.; Tanabe, H.; Kobayashi, K.; Kobayashi, H.; Nabetani, A.; Sakai, Y.; Okuda, T.; Nakagawa, M.; Nakamura, T. P4-210: Sigma-1 receptor is a molecular target for novel neuroprotectant T-817MA. Alzheimers Dement. 2015, 11, P861. [Google Scholar] [CrossRef]
- Fukushima, T.; Nakamura, A.; Iwakami, N.; Nakada, Y.; Hattori, H.; Hoki, S.; Yamaguchi, H.; Nakagawa, M.; Terashima, N.; Narita, H. T-817MA, a neuroprotective agent, attenuates the motor and cognitive impairments associated with neuronal degeneration in P301L tau transgenic mice. Biochem. Biophys. Res. Commun. 2011, 407, 730–734. [Google Scholar] [CrossRef]
- Rishton, G.M.; Look, G.C.; Ni, Z.J.; Zhang, J.; Wang, Y.; Huang, Y.; Wu, X.; Izzo, N.J.; LaBarbera, K.M.; Limegrover, C.S.; et al. Discovery of investigational drug CT1812, an antagonist of the sigma-2 receptor complex for Alzheimer’s disease. ACS Med. Chem. Lett. 2021, 12, 1389–1395. [Google Scholar] [CrossRef]
- Izzo, N.J.; Yuede, C.M.; LaBarbera, K.M.; Limegrover, C.S.; Rehak, C.; Yurko, R.; Waybright, L.; Look, G.; Rishton, G.; Safferstein, H.; et al. Preclinical and clinical biomarker studies of CT1812: A novel approach to Alzheimer’s disease modification. Alzheimers Dement. 2021, 17, 1365–1382. [Google Scholar] [CrossRef]
- Lauterbach, E.C. Dextromethorphan as a potential rapid-acting antidepressant. Med. Hypotheses. 2011, 76, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Bruna, J.; Videla, S.; Argyriou, A.A.; Velasco, R.; Villoria, J.; Santos, C.; Nadal, C.; Cavaletti, G.; Alberti, P.; Briani, C.; et al. Efficacy of a novel sigma-1 receptor antagonist for oxaliplatin-induced neuropathy: A randomized, double-blind, placebo-controlled phase IIa clinical trial. Neurotherapeutics. 2018, 15, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT imaging: A literature review over the last decade. Int. J. Mol. Sci. 2022, 23, 5023. [Google Scholar] [CrossRef]
- Shojaie, M.; Tabarestani, S.; Cabrerizo, M.; DeKosky, S.T.; Vaillancourt, D.E.; Loewenstein, D.; Duara, R.; Adjouadi, M. PET imaging of tau pathology and amyloid-β, and MRI for Alzheimer’s disease feature fusion and multimodal classification. J. Alzheimers Dis. 2021, 84, 1497–1514. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Ishiwata, K. Sigma receptor ligands: Possible application as therapeutic drugs and as radiopharmaceuticals. Curr. Pharm. Des. 2006, 12, 3857–3876. [Google Scholar] [PubMed]
- Agha, H.; McCurdy, C.R. In vitro and in vivo sigma 1 receptor imaging studies in different disease states. RSC Med. Chem. 2021, 12, 154–177. [Google Scholar] [CrossRef]
- Mach, R.H.; Wheeler, K.T. Development of molecular probes for imaging sigma-2 receptors in vitro and in vivo. Cent. Nerv. Syst. Agents Med. Chem. 2009, 9, 230–245. [Google Scholar] [CrossRef]
- Sakata, M.; Kimura, Y.; Naganawa, M.; Oda, K.; Ishii, K.; Chihara, K.; Ishiwata, K. Mapping of human cerebral sigma-1 receptors using positron emission tomography and [11C]SA4503. Neuroimage 2007, 35, 1–8. [Google Scholar] [CrossRef]
- Shiba, K.; Ogawa, K.; Ishiwata, K.; Yajima, K.; Mori, H. Synthesis and binding affinities of methylvesamicol analogs for the acetylcholine transporter and sigma receptor. Bioorg. Med. Chem. 2006, 14, 2620–2626. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, K.; Nakazawa, M.; Okamoto, K.; Kawashima, Y.; Mita, S. Binding properties of SA4503, a novel and selective sigma-1 receptor agonist. Eur. J. Pharmacol. 1996, 306, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, K.; Mita, S. SA4503: A novel sigma₁ receptor agonist. CNS Drug Rev. 1998, 4, 1–24. [Google Scholar] [CrossRef]
- Hirata, M.; Mori, T.; Soga, S.; Umeda, T.; Ohmomo, Y. Synthesis and in vitro evaluation of iodinated derivatives of piperazine as a new ligand for sigma receptor imaging by single photon emission computed tomography. Chem. Pharm. Bull. 2006, 54, 470–475. [Google Scholar] [CrossRef][Green Version]
- Lever, J.R.; Gustafson, J.L.; Xu, R.; Allmon, R.L.; Lever, S.Z. Sigma-1 and sigma-2 receptor binding affinity and selectivity of SA4503 and fluoroethyl SA4503. Synapse 2006, 59, 350–358. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Deuther-Conrad, W.; Lu, J.; Xie, Y.; Jia, B.; Cui, M.; Steinbach, J.; Brust, P.; Liu, B.; et al. Novel cyclopentadienyl tricarbonyl 99mTc complexes containing 1-piperonylpiperazine moiety: Potential imaging probes for sigma-1 receptors. J. Med. Chem. 2014, 57, 7113–7125. [Google Scholar] [CrossRef] [PubMed]
- Weissman, A.D.; Su, T.P.; Hedreen, J.C.; London, E.D. Sigma receptors in post-mortem human brains. J. Pharmacol. Exp. Ther. 1988, 247, 29–33. [Google Scholar]
- Kornhuber, J.; Schoppmeyer, K.; Bendig, C.; Riederer, P. Characterization of [3H]pentazocine binding sites in post-mortem human frontal cortex. J. Neural. Transm. 1996, 103, 45–53. [Google Scholar] [CrossRef]
- Kawamura, K.; Ishiwata, K.; Shimada, Y.; Kimura, Y.; Kobayashi, T.; Matsuno, K.; Homma, Y.; Senda, M. Preclinical evaluation of [11C]SA4503: Radiation dosimetry, in vivo selectivity and PET imaging of sigma1 receptors in the cat brain. Ann. Nucl. Med. 2000, 14, 285–292. [Google Scholar] [CrossRef]
- Ishiwata, K.; Tsukada, H.; Kawamura, K.; Kimura, Y.; Nishiyama, S.; Kobayashi, T.; Matsuno, K.; Senda, M. Mapping of CNS sigma1 receptors in the conscious monkey: Preliminary PET study with [11C]SA4503. Synapse 2001, 40, 235–237. [Google Scholar] [CrossRef]
- Kawamura, K.; Kimura, Y.; Tsukada, H.; Kobayashi, T.; Nishiyama, S.; Kakiuchi, T.; Ohba, H.; Harada, N.; Matsuno, K.; Ishii, K.; et al. An increase of sigma receptors in the aged monkey brain. Neurobiol. Aging 2003, 24, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Toyohara, J.; Kobayashi, T.; Mita, S.; Ishiwata, K. Application of [¹¹C]SA4503 to selection of novel σ₁ selective agonists. Nucl. Med. Biol. 2012, 39, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Toyohara, J.; Sakata, M.; Ishiwata, K. Imaging of sigma1 receptors in the human brain using PET and [11C]SA4503. Cent. Nerv. Syst. Agents Med. Chem. 2009, 9, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Mishina, M.; Ohyama, M.; Ishii, K.; Kitamura, S.; Kimura, Y.; Oda, K.; Kawamura, K.; Sasaki, T.; Kobayashi, S.; Katayama, Y.; et al. Low density of sigma-1 receptors in early Alzheimer’s disease. Ann. Nucl. Med. 2008, 22, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, A.V.; Mansur, A.; Rizzo, G.; Bishop, C.; Lewis, Y.; Kocagoncu, E.; Lingford-Hughes, A.; Huiban, M.; Passchier, J.; Rowe, J.B.; et al. Widespread cell stress and mitochondrial dysfunction occur in patients with early Alzheimer’s disease. Sci. Transl. Med. 2022, 14, eabk1051. [Google Scholar] [CrossRef]
- Mishina, M.; Ishiwata, K.; Ishii, K.; Kitamura, S.; Kimura, Y.; Kawamura, K.; Oda, K.; Sasaki, T.; Sakayori, O.; Hamamoto, M.; et al. Function of sigma1 receptors in Parkinson’s disease. Acta Neurol. Scand 2005, 112, 103–107. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ishiwata, K.; Ishii, K.; Kimura, Y.; Sakata, M.; Naganawa, M.; Oda, K.; Miyatake, R.; Fujisaki, M.; Shimizu, E.; et al. High occupancy of sigma-1 receptors in the human brain after single oral administration of fluvoxamine: A positron emission tomography study using [11C]SA4503. Biol. Psychiatry 2007, 62, 878–883. [Google Scholar] [CrossRef]
- Kunitachi, S.; Fujita, Y.; Ishima, T.; Kohno, M.; Horio, M.; Tanibuchi, Y.; Shirayama, Y.; Iyo, M.; Hashimoto, K. Phencyclidine-induced cognitive deficits in mice are ameliorated by subsequent subchronic administration of donepezil: Role of sigma-1 receptors. Brain Res. 2009, 1279, 189–196. [Google Scholar] [CrossRef]
- Shen, B.; Park, J.H.; Hjørnevik, T.; Cipriano, P.W.; Yoon, D.; Gulaka, P.K.; Holly, D.; Behera, D.; Avery, B.A.; Gambhir, S.S.; et al. Radiosynthesis and first-in-human PET/MRI evaluation with clinical-grade [18F]FTC-146. Mol. Imaging Biol. 2017, 19, 779–786. [Google Scholar] [CrossRef]
- Hjørnevik, T.; Cipriano, P.W.; Shen, B.; Park, J.H.; Gulaka, P.; Holley, D.; Gandhi, H.; Yoon, D.; Mittra, E.S.; Zaharchuk, G.; et al. Biodistribution and radiation dosimetry of 18F-FTC-146 in humans. J. Nucl. Med. 2017, 58, 2004–2009. [Google Scholar] [CrossRef]
- Waterhouse, R.N.; Nobler, M.S.; Zhou, Y.; Chang, R.C.; Morales, O.; Kuwabawa, H.; Kumar, A.; VanHeertum, R.L.; Wong, D.F.; Sackeim, H.A. First evaluation of the sigma-1 receptor radioligand [18F]1-3-fluoropropyl-4-((4-cyanophenoxy)-methyl) piperidine ([18F]FPS) in healthy humans. Neuroimage 2004, 22, T29–T30. [Google Scholar]
- Li, Y.; Wang, X.; Zhang, J.; Deuther-Conrad, W.; Xie, F.; Zhang, X.; Liu, J.; Qiao, J.; Cui, M.; Steinbach, J.; et al. Synthesis and evaluation of novel 18F-labeled spirocyclic piperidine derivatives as σ1 receptor ligands for positron emission tomography imaging. J. Med. Chem. 2013, 56, 3478–3491. [Google Scholar] [CrossRef] [PubMed]
- Holl, K.; Falck, E.; Köhler, J.; Schepmann, D.; Humpf, H.U.; Brust, P.; Wünsch, B. Synthesis, characterization, and metabolism studies of fluspidine enantiomers. ChemMedChem 2013, 8, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Maier, C.A.; Wünsch, B. Novel spiropiperidines as highly potent and subtype selective sigma-receptor ligands. Part 1. J. Med. Chem. 2002, 45, 438–448. [Google Scholar] [CrossRef]
- Maier, C.A.; Wünsch, B. Novel sigma receptor ligands. Part 2. SAR of spiro[[2]benzopyran-1,4′-piperidines] and spiro[[2]benzofuran-1,4′-piperidines] with carbon substituents in position 3. J. Med. Chem. 2002, 45, 4923–4930. [Google Scholar] [CrossRef]
- Wiese, C.; Grosse Maestrup, E.; Schepmann, D.; Vela, J.M.; Holenz, J.; Buschmann, H.; Wünsch, B. Pharmacological and metabolic characterisation of the potent sigma1 receptor ligand 1′-benzyl-3-methoxy-3H-spiro[[2]benzofuran-1,4′-piperidine]. J. Pharm. Pharmacol. 2009, 61, 631–640. [Google Scholar] [CrossRef]
- Fischer, S.; Wiese, C.; Maestrup, E.G.; Hiller, A.; Deuther-Conrad, W.; Scheunemann, M.; Schepmann, D.; Steinbach, J.; Wünsch, B.; Brust, P. Molecular imaging of σ receptors: Synthesis and evaluation of the potent σ1 selective radioligand [18F]fluspidine. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 540–551. [Google Scholar] [CrossRef]
- Brust, P.; Deuther-Conrad, W.; Becker, G.; Patt, M.; Donat, C.K.; Stittsworth, S.; Fischer, S.; Hiller, A.; Wenzel, B.; Dukic-Stefanovic, S.; et al. Distinctive in vivo kinetics of the new σ1 receptor ligands (R)-(+)- and (S)-(-)-18F-fluspidine in porcine brain. J. Nucl. Med. 2014, 55, 1730–1736. [Google Scholar] [CrossRef]
- Kranz, M.; Sattler, B.; Wüst, N.; Deuther-Conrad, W.; Patt, M.; Meyer, P.M.; Fischer, S.; Donat, C.K.; Wünsch, B.; Hesse, S.; et al. Evaluation of the enantiomer specific biokinetics and radiation doses of [18F]fluspidine-A new tracer in clinical translation for imaging of σ₁ receptors. Molecules 2016, 21, 1164. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Wang, X.; Zhang, J.M.; Deuther-Conrad, W.; Zhang, X.J.; Huang, Y.; Li, Y.; Ye, J.J.; Cui, M.C.; Steinbach, J.; et al. Synthesis and evaluation of a 18F-labeled spirocyclic piperidine derivative as promising σ1 receptor imaging agent. Bioorg. Med. Chem. 2014, 22, 5270–5278. [Google Scholar] [CrossRef]
- Baum, E.; Cai, Z.; Bois, F.; Holden, D.; Lin, S.F.; Lara-Jaime, T.; Kapinos, M.; Chen, Y.; Deuther-Conrad, W.; Fischer, S.; et al. PET imaging evaluation of four σ1 radiotracers in nonhuman primates. J. Nucl. Med. 2017, 58, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Strauss, M.; Becker, G.; Hesse, S.; Bednasch, K.; Ettrich, B.; Zientek, F.; Rullmann, M.; Wilke, S.; Luthardt, J.; et al. IIncreased sigma-1 receptor (Sig-1R) binding in the brain of unmedicated patients with acute major depressive disorder (MDD) using the novel Sig-1R-specific radioligand (-)-[18F]Fluspidine and PET. J. Nucl. Med. 2018, 59, 551. [Google Scholar]
- Grachev, I.D.; Meyer, P.M.; Becker, G.A.; Bronzel, M.; Marsteller, D.; Pastino, G.; Voges, O.; Rabinovich, L.; Knebel, H.; Zientek, F.; et al. Sigma-1 and dopamine D2/D3 receptor occupancy of pridopidine in healthy volunteers and patients with Huntington disease: A [18F] fluspidine and [18F] fallypride PET study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Glennon, R.A. Pharmacophore identification for sigma-1 (σ1) receptor binding: Application of the “deconstruction-reconstruction-elaboration” approach. Mini Rev. Med. Chem. 2005, 5, 927–940. [Google Scholar] [CrossRef]
- Wunsch, B. Pharmacophore models and development of spirocyclic ligands for σ1 receptors. Curr. Pharm. Des. 2012, 18, 930–937. [Google Scholar] [CrossRef]
- Xie, F.; Bergmann, R.; Kniess, T.; Deuther-Conrad, W.; Mamat, C.; Neuber, C.; Liu, B.; Steinbach, J.; Brust, P.; Pietzsch, J.; et al. 18F-Labeled 1,4-dioxa-8-azaspiro [4.5]decane derivative: Synthesis and biological evaluation of a σ1 receptor radioligand with low lipophilicity as potent tumor imaging agent. J. Med. Chem. 2015, 58, 5395–5407. [Google Scholar] [CrossRef]
- Tian, J.; He, Y.; Deuther-Conrad, W.; Fu, H.; Xie, F.; Zhang, Y.; Wang, T.; Zhang, X.; Zhang, J.; Brust, P.; et al. Synthesis and evaluation of new 1-oxa-8-azaspiro [4.5]decane derivatives as candidate radioligands for sigma-1 receptors. Bioorg. Med. Chem. 2020, 28, 115560. [Google Scholar] [CrossRef]
- He, Y.; Xie, F.; Ye, J.; Deuther-Conrad, W.; Cui, B.; Wang, L.; Lu, J.; Steinbach, J.; Brust, P.; Huang, Y.; et al. 1-(4-[18F]Fluorobenzyl)-4-[(tetrahydrofuran-2-yl)methyl]piperazine: A novel suitable radioligand with low lipophilicity for imaging σ1 receptors in the brain. J. Med. Chem. 2017, 60, 4161–4172. [Google Scholar] [CrossRef]
- Jia, H.; Cai, Z.; Holden, D.; He, Y.; Lin, S.F.; Li, S.; Baum, E.; Shirali, A.; Kapinos, M.; Gao, H.; et al. Positron emission tomography imaging evaluation of a novel 18F-labeled sigma-1 receptor radioligand in cynomolgus monkeys. ACS Chem. Neurosci. 2020, 11, 1673–1681. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Zhang, X.; Chen, L.; Zheng, M.; Zhang, J.; Brust, P.; Deuther-Conrad, W.; Huang, Y.; Jia, H. Synthesis and characterization of the two enantiomers of a chiral sigma-1 receptor radioligand:(S)-(+)-and (R)-(-)-[18F]FBFP. Chin. Chem. Lett. 2022, 33, 3543–3548. [Google Scholar] [CrossRef]
- Zheng, M.; Holden, D.; Alluri, S.R.; Wang, T.; Felchner, Z.; Gao, H.; Zhang, L.; Labaree, D.; Ropchan, J.; Carson, R.; et al. Assessing the chiral selectivity of a sigma-1 receptor radiotracer: A PET imaging study of (R)- and (S)-18F-FBFP in non-human primates. J. Nucl. Med. 2022, 63 (Suppl. 2), 2324. [Google Scholar]
- Tu, Z.; Xu, J.; Jones, L.A.; Li, S.; Dumstorff, C.; Vangveravong, S.; Chen, D.L.; Wheeler, K.T.; Welch, M.J.; Mach, R.H. Fluorine-18-labeled benzamide analogues for imaging the σ2 receptor status of solid tumors with positron emission tomography. J. Med. Chem. 2007, 50, 3194–3204. [Google Scholar] [CrossRef]
- Dehdashti, F.; Laforest, R.; Gao, F.; Shoghi, K.I.; Aft, R.L.; Nussenbaum, B.; Kreisel, F.H.; Bartlett, N.L.; Cashen, A.; Wagner-Johnston, N.; et al. Assessment of cellular proliferation in tumors by PET using 18F-ISO-1. J. Nucl. Med. 2013, 54, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Grundman, M.; Morgan, R.; Lickliter, J.D.; Schneider, L.S.; DeKosky, S.; Izzo, N.J.; Guttendorf, R.; Higgin, M.; Pribyl, J.; Mozzoni, K.; et al. A phase 1 clinical trial of the sigma-2 receptor complex allosteric antagonist CT1812, a novel therapeutic candidate for Alzheimer’s disease. Alzheimers Dement. 2019, 5, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, R.P.; Gündel, D.; Teodoro, R.; Ludwig, F.A.; Fischer, S.; Toussaint, M.; Schepmann, D.; Wünsch, B.; Brust, P.; Deuther-Conrad, W. Design, radiosynthesis and preliminary biological evaluation in mice of a brain-penetrant 18F-labelled σ2 receptor ligand. Int. J. Mol. Sci. 2021, 22, 5447. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Zhang, X.; Deuther-Conrad, W.; Fu, H.; Cui, M.; Zhang, J.; Brust, P.; Huang, Y.; Jia, H. Discovery and development of brain-penetrant 18F-labeled radioligands for neuroimaging of the sigma-2 receptors. Acta Pharm. Sin. B 2022, 12, 1406–1415. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, J.Y.; Hsieh, C.-J.; Riad, A.; Izzo, N.J.; Catalano, S.M.; Graham, T.J.A.; Mach, R.H. Screening of σ2 receptor ligands and in vivo evaluation of 11C-labeled 6,7-dimethoxy-2-[4-(4-methoxyphenyl)butan-2-yl]-1,2,3,4-tetrahydroisoquinoline for potential use as a σ2 receptor brain PET tracer. J. Med. Chem. 2022, 65, 6261–6272. [Google Scholar] [CrossRef]
- Wang, L.; Ye, J.; He, Y.; Deuther-Conrad, W.; Zhang, J.; Zhang, X.; Cui, M.; Steinbach, J.; Huang, Y.; Brust, P.; et al. 18F-Labeled indole-based analogs as highly selective radioligands for imaging sigma-2 receptors in the brain. Bioorg. Med. Chem. 2017, 25, 3792–3802. [Google Scholar] [CrossRef]
- Xie, F.; Kniess, T.; Neuber, C.; Deuther-Conrad, W.; Mamat, C.; Lieberman, B.P.; Liu, B.; Mach, R.H.; Brust, P.; Steinbach, J.; et al. Novel indole-based sigma-2 receptor ligands: Synthesis, structure–affinity relationship and antiproliferative activity. MedChemComm 2015, 6, 1093–1103. [Google Scholar] [CrossRef]
- Lee, I.; Lieberman, B.P.; Li, S.; Hou, C.; Makvandi, M.; Mach, R.H. Comparative evaluation of 4 and 6-carbon spacer conformationally flexible tetrahydroisoquinolinyl benzamide analogues for imaging the sigma-2 receptor status of solid tumors. Nucl. Med. Biol. 2016, 43, 721–731. [Google Scholar] [CrossRef]
- Alluri, S.R.; Zheng, M.; Holden, D.; Zhang, Y.; Li, S.; Felchner, Z.; Zhang, L.; Ropchan, J.; Carson, R.; Jia, H.; et al. Quantitative evaluation of a novel brain-penetrant sigma-2 receptor radioligand in non-human primates. J. Nucl. Med. 2022, 63 (Suppl. 2), 2845. [Google Scholar]
- Alluri, S.R.; Zheng, M.-Q.; Holden, D.; Zhang, Y.; Li, S.; Felchner, Z.; Kapinos, M.; Ropchan, J.; Carson, R.E.; Jia, H.; et al. Imaging brain sigma-2 receptor: Evaluation of 18F-radiotracers in nonhuman primates. In Proceedings of the Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging, SNMMI, Chicago, IL, USA, 24–27 June 2023; Available online: https://s3.amazonaws.com/amz.xcdsystem.com/706224A1-90F0-EFDE-9D12FA836B3BDEDF_abstract_File1523/PresentationPoster_P655_0613015515.pdf (accessed on 21 July 2023).
- Klyucherev, T.O.; Olszewski, P.; Shalimova, A.A.; Chubarev, V.N.; Tarasov, V.V.; Attwood, M.M.; Syvänen, S.; Schiöth, H.B. Advances in the development of new biomarkers for Alzheimer’s disease. Transl. Neurodegener. 2022, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Eysert, F.; Kinoshita, P.F.; Mary, A.; Vaillant-Beuchot, L.; Checler, F.; Chami, M. Molecular dysfunctions of mitochondria-associated membranes (MAMs) in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 9521. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cao, Y.; Pei, H.; Ma, L.; Yang, Y.; Li, H. The contribution of mitochondria-associated endoplasmic reticulum membranes (MAMs) dysfunction in Alzheimer’s disease and the potential countermeasure. Front. Neurosci. 2023, 17, 1158204. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jin, H.; Huang, Y. Mitochondria-associated membranes (MAMs): A potential therapeutic target for treating Alzheimer’s disease. Clin. Sci. 2021, 135, 109–126. [Google Scholar] [CrossRef]
- Delprat, B.; Crouzier, L.; Su, T.P.; Maurice, T. At the crossing of ER stress and MAMs: A key role of sigma-1 receptor? Adv. Exp. Med. Biol. 2020, 1131, 699–718. [Google Scholar]
- Maurice, T.; Meunier, J.; Feng, B.; Ieni, J.; Monaghan, D.T. Interaction with sigma1 protein, but not N-methyl-D-aspartate receptor, is involved in the pharmacological activity of donepezil. J. Pharmacol. Exp. Ther. 2006, 317, 606–614. [Google Scholar] [CrossRef]


| Agent | Target a | Mechanism b | Chemical Structure |
|---|---|---|---|
| Donepezil | AChE | AChE reversible inhibition |  |
| Galantamine | AChE | AChE reversible inhibition | 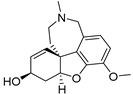 |
| Rivastigmine | AChE | AChE reversible inhibition |  |
| Memantine | NMDA receptors | NMDA non-competitive antagonist | 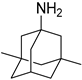 |
| Aducanumab (Aduhelm) c | Aβ plaque | mAb immunotherapy against Aβ | - |
| Lecanemab (Leqembi) d | Protofibrillar and oligomeric forms of Aβ plaque | mAb immunotherapy against Aβ | - |
| GV-971 e | Gut microbiota | yet to be fully elucidated | marine-derived oligosaccharide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Jia, H. The Sigma Receptors in Alzheimer’s Disease: New Potential Targets for Diagnosis and Therapy. Int. J. Mol. Sci. 2023, 24, 12025. https://doi.org/10.3390/ijms241512025
Wang T, Jia H. The Sigma Receptors in Alzheimer’s Disease: New Potential Targets for Diagnosis and Therapy. International Journal of Molecular Sciences. 2023; 24(15):12025. https://doi.org/10.3390/ijms241512025
Chicago/Turabian StyleWang, Tao, and Hongmei Jia. 2023. "The Sigma Receptors in Alzheimer’s Disease: New Potential Targets for Diagnosis and Therapy" International Journal of Molecular Sciences 24, no. 15: 12025. https://doi.org/10.3390/ijms241512025
APA StyleWang, T., & Jia, H. (2023). The Sigma Receptors in Alzheimer’s Disease: New Potential Targets for Diagnosis and Therapy. International Journal of Molecular Sciences, 24(15), 12025. https://doi.org/10.3390/ijms241512025





