The Contribution of Vascular Proteoglycans to Atherothrombosis: Clinical Implications
Abstract
1. Introduction
2. Atherosclerosis
2.1. Plaque Rupture
2.2. Plaque Erosion
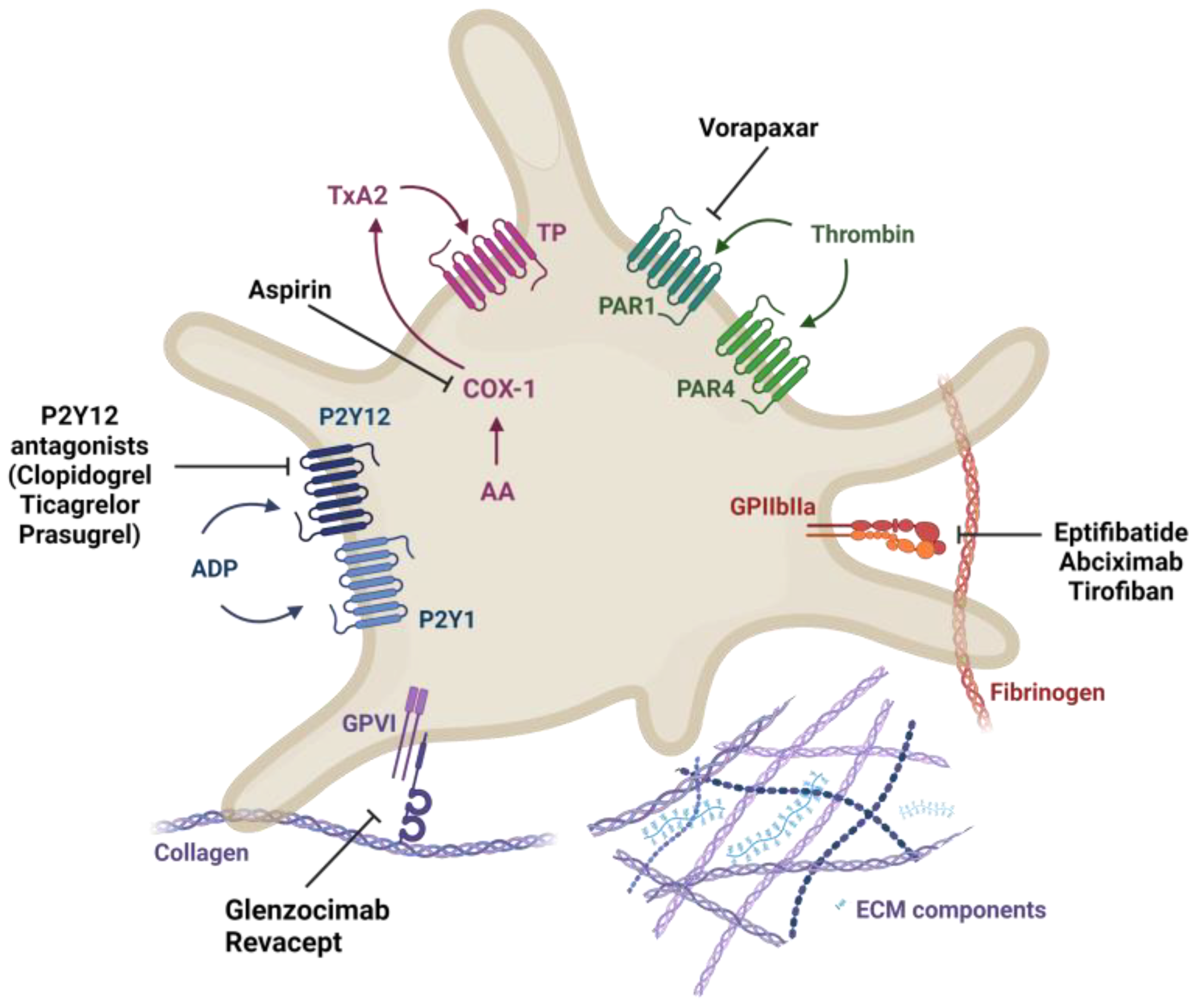
2.3. Current Treatment for Atherothrombosis
3. Proteoglycans and Atherothrombosis
3.1. Perlecan
3.2. Biglycan
3.3. Decorin
3.4. Versican
3.5. Hyaluronan
| Proteoglycan | Platelet | Endothelial Cell | Smooth Muscle |
|---|---|---|---|
| Perlecan | ↓ activation via G6b-B [52] | ↑ proliferation and angiogenesis [51] | ↓ proliferation [44,47] |
| Biglycan | ↓ activation (indirectly by reducing thrombin levels) [39,59,60] | ↑ proliferation and angiogenesis [55,56] | ↓ proliferation ↑ migration [38] ↑ autophagy [42] |
| Decorin | ↑ adhesion via platelet α2β1 [76] | ↑ proliferation and angiogenesis [55] | ↓ proliferation [37] |
| ↑ activation via PLCγ [36,77] | ↑ autophagy [75] | ↑ migration [38] | |
| ↓ platelet adhesion to collagen by masking collagen binding sites [64] | ↑ calcification [132] | ||
| Versican | ↑ adhesion (V1 and V2 isoforms) [9,61] | ↑ angiogenesis [96] | ↑ proliferation and migration (V1) [97] ↑ Differentiation and anti-inflammatory (V3) [101] |
| Hyaluronan | ↓ platelet activation via CD44 (HMW) [61,126] | ↑ adhesion, proliferation, and migration [108,113] ↓ proliferation and angiogenesis [93,110,116] ↓ inflammation (HMW) ↑ inflammation (LMW) [93,119] | ↑ migration (HMW) [93,121,122] |
4. Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kischel, P.; Waltregny, D.; Dumont, B.; Turtoi, A.; Greffe, Y.; Kirsch, S.; De Pauw, E.; Castronovo, V. Versican overexpression in human breast cancer lesions: Known and new isoforms for stromal tumor targeting. Int. J. Cancer 2010, 126, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Farb, A.; Burke, A.P.; Tang, A.L.; Liang, Y.; Mannan, P.; Smialek, J.; Virmani, R. Coronary Plaque Erosion Without Rupture into a Lipid Core. Circulation 1996, 93, 1354–1363. [Google Scholar] [CrossRef]
- Farb, A.; Kolodgie, F.D.; Hwang, J.Y.; Burke, A.P.; Tefera, K.; Weber, D.K.; Wight, T.N.; Virmani, R. Extracellular Matrix Changes in Stented Human Coronary Arteries. Circulation 2004, 110, 940–947. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47 (Suppl. S8), C13–C18. [Google Scholar] [CrossRef] [PubMed]
- Pedicino, D.; Vinci, R.; Giglio, A.F.; Pisano, E.; Porto, I.; Vergallo, R.; Russo, G.; Ruggio, A.; D’Aiello, A.; Flego, D.; et al. Alterations of Hyaluronan Metabolism in Acute Coronary Syndrome: Implications for Plaque Erosion. J. Am. Coll. Cardiol. 2018, 72, 1490–1503. [Google Scholar] [CrossRef]
- Newby, A.C.; Johnson, T.W.; White, S.J. Endothelial erosion of plaques as a substrate for coronary thrombosis. Thromb. Haemost. 2016, 115, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Fahed, A.C.; Jang, I.-K. Plaque erosion and acute coronary syndromes: Phenotype, molecular characteristics and future directions. Nat. Rev. Cardiol. 2021, 18, 724–734. [Google Scholar] [CrossRef]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In Vivo Diagnosis of Plaque Erosion and Calcified Nodule in Patients with Acute Coronary Syndrome by Intravascular Optical Coherence Tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Burke, A.P.; Farb, A.; Weber, D.K.; Kutys, R.; Wight, T.N.; Virmani, R. Differential accumulation of proteoglycans and hyaluronan in culprit lesions: Insights into plaque erosion. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1642–1648. [Google Scholar] [CrossRef]
- Otsuka, F.; Yasuda, S.; Noguchi, T.; Ishibashi-Ueda, H. Pathology of coronary atherosclerosis and thrombosis. Cardiovasc. Diagn. Ther. 2016, 6, 396–408. [Google Scholar] [CrossRef]
- Quillard, T.; Franck, G.; Mawson, T.; Folco, E.; Libby, P. Mechanisms of erosion of atherosclerotic plaques. Curr. Opin. Infect. Dis. 2017, 28, 434–441. [Google Scholar]
- Subbotin, V.M. Excessive intimal hyperplasia in human coronary arteries before intimal lipid depositions is the initiation of coronary atherosclerosis and constitutes a therapeutic target. Drug Discov. Today 2016, 21, 1578–1595. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Kastelein, J.J.; Ginsberg, H.N.; Chapman, M.J.; Nicholls, S.J.; Ray, K.K.; Packard, C.J.; Laufs, U.; Brook, R.D.; Oliver-Williams, C.; et al. Association of Genetic Variants Related to CETP Inhibitors and Statins with Lipoprotein Levels and Cardiovascular Risk. JAMA 2017, 318, 947–956. [Google Scholar]
- Weber, C.; Badimon, L.; Mach, F.; van der Vorst, E.P.C. Therapeutic strategies for atherosclerosis and atherothrombosis: Past, present and future. Thromb. Haemost. 2017, 117, 1258–1264. [Google Scholar] [CrossRef]
- Cahill, P.A.; Redmond, E.M. Vascular endothelium—Gatekeeper of vessel health. Atherosclerosis 2016, 248, 97–109. [Google Scholar] [CrossRef]
- Olie, R.H.; van der Meijden, P.E.; Cate, H.T. The coagulation system in atherothrombosis: Implications for new therapeutic strategies. Res. Prac. Thromb. Haemost. 2018, 2, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Libby, P.; Tabas, I. Inflammation and plaque vulnerability. J. Intern. Med. 2015, 278, 483–493. [Google Scholar] [CrossRef]
- Camaré, C.; Pucelle, M.; Nègre-Salvayre, A.; Salvayre, R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017, 12, 18–34. [Google Scholar] [CrossRef]
- Ten Cate, H.; Hemker, H.C. Thrombin Generation and Atherothrombosis: What Does the Evidence Indicate? J. Am. Heart Assoc. 2016, 5, e003553. [Google Scholar]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar]
- Mastenbroek, T.G.; van Geffen, J.; Heemskerk, J.W.M.; Cosemans, J.M.E.M. Acute and persistent platelet and coagulant activities in atherothrombosis. J. Thromb. Haemost. 2015, 13 (Suppl. S1), S272–S280. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, G.; Nakano, M.; Prati, F.; Niccoli, G.; Mallus, M.T.; Ramazzotti, V.; Montone, R.A.; Kolodgie, F.D.; Virmani, R.; Crea, F.; et al. Response to Letter Regarding Article, “High Levels of Systemic Myeloperoxidase Are Associated with Coronary Plaque Erosion in Patients with Acute Coronary Syndromes: A Clinicopathological Study”. Circulation 2010, 124, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.; Yonetsu, T.; Kakuta, T.; Soeda, T.; Saito, Y.; Yan, B.P.; Kurihara, O.; Takano, M.; Niccoli, G.; Higuma, T.; et al. Clinical and Laboratory Predictors for Plaque Erosion in Patients with Acute Coronary Syndromes. J. Am. Hear. Assoc. 2019, 8, e012322. [Google Scholar] [CrossRef]
- Satta, S.; Beal, R.; Smith, R.; Luo, X.; Ferris, G.R.; Langford-Smith, A.; Teasdale, J.; Ajime, T.T.; Serré, J.; Hazell, G.; et al. A Nrf2-OSGIN1&2-HSP70 axis mediates cigarette smoke-induced endothelial detachment: Implications for plaque erosion. Cardiovasc. Res. 2023, cvad022. [Google Scholar] [CrossRef]
- Kramer, M.C.; Rittersma, S.Z.; de Winter, R.J.; Ladich, E.R.; Fowler, D.R.; Liang, Y.-H.; Kutys, R.; Carter-Monroe, N.; Kolodgie, F.D.; van der Wal, A.C.; et al. Relationship of Thrombus Healing to Underlying Plaque Morphology in Sudden Coronary Death. J. Am. Coll. Cardiol. 2010, 55, 122–132. [Google Scholar] [CrossRef]
- Rittersma, S.Z.; van der Wal, A.C.; Koch, K.T.; Piek, J.J.; Henriques, J.P.; Mulder, K.J.; Ploegmakers, J.P.; Meesterman, M.; de Winter, R.J. Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis: A pathological thrombectomy study in primary percutaneous coronary intervention. Circulation 2005, 111, 1160–1165. [Google Scholar] [CrossRef]
- Patrono, C.; Morais, J.; Baigent, C.; Collet, J.-P.; Fitzgerald, D.; Halvorsen, S.; Rocca, B.; Siegbahn, A.; Storey, R.F.; Vilahur, G. Antiplatelet Agents for the Treatment and Prevention of Coronary Atherothrombosis. J. Am. Coll. Cardiol. 2017, 70, 1760–1776. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Badimon, J. Overview of Aspirin and Platelet Biology. Am. J. Cardiol. 2021, 144 (Suppl. S1), S2–S9. [Google Scholar] [CrossRef]
- Mayer, K.; Hein-Rothweiler, R.; Schüpke, S.; Janisch, M.; Bernlochner, I.; Ndrepepa, G.; Sibbing, D.; Gori, T.; Borst, O.; Holdenrieder, S.; et al. Efficacy and Safety of Revacept, a Novel Lesion-Directed Competitive Antagonist to Platelet Glycoprotein VI, in Patients Undergoing Elective Percutaneous Coronary Intervention for Stable Ischemic Heart Disease: The Randomized, Double-blind, Placebo-Controlled ISAR-PLASTER Phase 2 Trial. JAMA Cardiol. 2021, 6, 753–761. [Google Scholar]
- Billiald, P.; Slater, A.; Welin, M.; Clark, J.C.; Loyau, S.; Pugnière, M.; Jiacomini, I.G.; Rose, N.; Lebozec, K.; Toledano, E.; et al. Targeting platelet GPVI with glenzocimab: A novel mechanism for inhibition. Blood Adv. 2023, 7, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Wight, T.N. A Role for Extracellular Matrix in Atherosclerotic Plaque Erosion. J. Am. Coll. Cardiol. 2018, 72, 1504–1505. [Google Scholar] [CrossRef]
- Nijst, P.; Verbrugge, F.H.; Grieten, L.; Dupont, M.; Steels, P.; Tang, W.W.; Mullens, W. The Pathophysiological Role of Interstitial Sodium in Heart Failure. J. Am. Coll. Cardiol. 2015, 65, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.S.; Shrestha, P.; Sarpong, K.A.; Yazdani, S.; El Masri, R.; De Jong, W.H.A.; Navis, G.; Vivès, R.R.; Born, J.V.D. High sodium diet converts renal proteoglycans into pro-inflammatory mediators in rats. PLoS ONE 2017, 12, e0178940. [Google Scholar] [CrossRef] [PubMed]
- Et-Taouil, K.; Schiavi, P.; Lévy, B.I.; Plante, G.E. Sodium Intake, Large Artery Stiffness, and Proteoglycans in the Spontaneously Hypertensive Rat. Hypertension 2001, 38, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.W.; Kinsella, M.G.; Levkau, B.; Clowes, A.W.; Wight, T.N. Retroviral Overexpression of Decorin Differentially Affects the Response of Arterial Smooth Muscle Cells to Growth Factors. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 777–784. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guidetti, G.; Bertoni, A.; Viola, M.; Tira, E.; Balduini, C.; Torti, M. The small proteoglycan decorin supports adhesion and activation of human platelets. Blood 2002, 100, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Beck, K.-F.; Raslik, I.; Walpen, S.; Mihalik, D.; Micegova, M.; Macakova, K.; Schönherr, E.; Seidler, D.G.; Varga, G.; et al. Biglycan, a Nitric Oxide-regulated Gene, Affects Adhesion, Growth, and Survival of Mesangial Cells. J. Biol. Chem. 2003, 278, 26227–26237. [Google Scholar] [CrossRef]
- D’Antoni, M.L.; Risse, P.-A.; Ferraro, P.; Martin, J.G.; Ludwig, M.S. Effects of decorin and biglycan on human airway smooth muscle cell adhesion. Matrix Biol. 2012, 31, 101–112. [Google Scholar] [CrossRef]
- Grandoch, M.; Kohlmorgen, C.; Melchior-Becker, A.; Feldmann, K.; Homann, S.; Müller, J.; Kiene, L.S.; Zeng-Brouwers, J.; Schmitz, F.; Nagy, N.; et al. Loss of Biglycan Enhances Thrombin Generation in Apolipoprotein E-Deficient Mice: Implications for Inflammation and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, e41–e50. [Google Scholar] [CrossRef]
- O’brien, K.D.; Lewis, K.; Fischer, J.W.; Johnson, P.; Hwang, J.-Y.; Knopp, E.A.; Kinsella, M.G.; Barrett, P.H.R.; Chait, A.; Wight, T.N. Smooth muscle cell biglycan overexpression results in increased lipoprotein retention on extracellular matrix: Implications for the retention of lipoproteins in atherosclerosis. Atherosclerosis 2004, 177, 29–35. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Johnson, P.Y.; Braun, K.R.; Hinek, A.; Fischer, J.W.; O’Brien, K.D.; Starcher, B.; Clowes, A.W.; Merrilees, M.J.; Wight, T.N. Retrovirally Mediated Overexpression of Glycosaminoglycan-Deficient Biglycan in Arterial Smooth Muscle Cells Induces Tropoelastin Synthesis and Elastic Fiber Formation in Vitro and in Neointimae after Vascular Injury. Am. J. Pathol. 2008, 173, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Poluzzi, C.; Nastase, M.V.; Zeng-Brouwers, J.; Roedig, H.; Hsieh, L.T.H.; Michaelis, J.B.; Buhl, E.M.; Rezende, F.; Manavski, Y.; Bleich, A.; et al. Biglycan evokes autophagy in macrophages via a novel CD44/Toll-like receptor 4 signaling axis in ischemia/reperfusion injury. Kidney Int. 2019, 95, 540–562. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Pillarisetti, S. Lipoprotein modulation of subendothelial heparan sulfate proteoglycans (perlecan) and atherogenicity. Trends Cardiovasc. Med. 2000, 10, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Nakada, S.; Yoshihara, T.; Nara, T.; Furuya, N.; Miida, T.; Hattori, N.; Arikawa-Hirasawa, E. Perlecan, a heparan sulfate proteoglycan, regulates systemic metabolism with dynamic changes in adipose tissue and skeletal muscle. Sci. Rep. 2018, 8, 7766. [Google Scholar] [CrossRef]
- Tran, P.-K.; Agardh, H.E.; Tran-Lundmark, K.; Ekstrand, J.; Roy, J.; Henderson, B.; Gabrielsen, A.; Hansson, G.K.; Swedenborg, J.; Paulsson-Berne, G.; et al. Reduced perlecan expression and accumulation in human carotid atherosclerotic lesions. Atherosclerosis 2007, 190, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Naso, M.F.; Zimmermann, D.R.; Iozzo, R.V. Characterization of the complete genomic structure of the human versican gene and functional analysis of its promoter. J. Biol. Chem. 1994, 269, 32999–33008. [Google Scholar] [CrossRef]
- Nugent, M.A.; Nugent, H.M.; Iozzo, R.V.; Sanchack, K.; Edelman, E.R. Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc. Natl. Acad. Sci. USA 2000, 97, 6722–6727. [Google Scholar] [CrossRef]
- Talusan, P.; Bedri, S.; Yang, S.; Kattapuram, T.; Silva, N.; Roughley, P.J.; Stone, J.R. Analysis of Intimal Proteoglycans in Atherosclerosis-prone and Atherosclerosis-resistant Human Arteries by Mass Spectrometry. Mol. Cell. Proteom. 2005, 4, 1350–1357. [Google Scholar] [CrossRef]
- Bix, G.; Iozzo, R.A.; Woodall, B.; Burrows, M.; McQuillan, A.; Campbell, S.; Fields, G.B.; Iozzo, R.V. Endorepellin, the C-terminal angiostatic module of perlecan, enhances collagen-platelet responses via the alpha2beta1-integrin receptor. Blood 2007, 109, 3745–3748. [Google Scholar] [CrossRef]
- Iozzo, R.V.; San Antonio, J.D. Heparan sulfate proteoglycans: Heavy hitters in the angiogenesis arena. J. Clin. Investig. 2001, 108, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Vögtle, T.; Sharma, S.; Mori, J.; Nagy, Z.; Semeniak, D.; Scandola, C.; Geer, M.J.; Smith, C.W.; Lane, J.; Pollack, S.; et al. Heparan sulfates are critical regulators of the inhibitory megakaryocyte-platelet receptor G6b-B. Elife 2019, 8, e46840. [Google Scholar] [CrossRef]
- Segev, A.; Nili, N.; Strauss, B.H. The role of perlecan in arterial injury and angiogenesis. Cardiovasc. Res. 2004, 63, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Wight, T.N.; Sueishi, K. Early atherosclerosis in humans: Role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc. Res. 2008, 79, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Chui, A.; Gunatillake, T.; Brennecke, S.P.; Ignjatovic, V.; Monagle, P.T.; Whitelock, J.M.; Van Zanten, D.E.; Eijsink, J.; Wang, Y.; Deane, J.; et al. Expression of Biglycan in First Trimester Chorionic Villous Sampling Placental Samples and Altered Function in Telomerase-Immortalized Microvascular Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1168–1179. [Google Scholar] [CrossRef][Green Version]
- Berendsen, A.D.; Pinnow, E.L.; Maeda, A.; Brown, A.C.; McCartney-Francis, N.; Kram, V.; Owens, R.T.; Robey, P.G.; Holmbeck, K.; de Castro, L.F.; et al. Biglycan modulates angiogenesis and bone formation during fracture healing. Matrix Biol. 2014, 35, 223–231. [Google Scholar] [CrossRef]
- Schaefer, L.; Babelova, A.; Kiss, E.; Hausser, H.-J.; Baliova, M.; Krzyzankova, M.; Marsche, G.; Young, M.F.; Mihalik, D.; Götte, M.; et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J. Clin. Investig. 2005, 115, 2223–2233. [Google Scholar] [CrossRef]
- Murthi, P.; Faisal, F.; Rajaraman, G.; Stevenson, J.; Ignjatovic, V.; Monagle, P.; Brennecke, S.; Said, J. Placental Biglycan Expression is Decreased in Human Idiopathic Fetal Growth Restriction. Placenta 2010, 31, 712–717. [Google Scholar] [CrossRef]
- McGuire, E.A.; Tollefsen, D.M. Activation of heparin cofactor II by fibroblasts and vascular smooth muscle cells. J. Biol. Chem. 1987, 262, 169–175. [Google Scholar] [CrossRef]
- He, L.; Giri, T.K.; Vicente, C.P.; Tollefsen, D.M. Vascular dermatan sulfate regulates the antithrombotic activity of heparin cofactor II. Blood 2008, 111, 4118–4125. [Google Scholar] [CrossRef]
- Mazzucato, M.; Cozzi, M.R.; Pradella, P.; Perissinotto, D.; Malmström, A.; Mörgelin, M.; Spessotto, P.; Colombatti, A.; Marco, L.; Perris, R. Vascular PG-M/versican variants promote platelet adhesion at low shear rates and cooperate with collagens to induce aggregation. FASEB J. 2002, 16, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.A.; Sun, M.; Barnum, C.E.; Weiss, S.N.; Huegel, J.; Shetye, S.S.; Lin, L.; Saez, D.; Adams, S.M.; Iozzo, R.V.; et al. Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biol. 2017, 64, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Keene, D.R.; San Antonio, J.D.; Mayne, R.; McQuillan, D.J.; Sarris, G.; Santoro, S.A.; Iozzo, R.V. Decorin Binds Near the C Terminus of Type I Collagen. J. Biol. Chem. 2000, 275, 21801–21804. [Google Scholar] [CrossRef]
- Paderi, J.E.; Stuart, K.; Sturek, M.; Park, K.; Panitch, A. The inhibition of platelet adhesion and activation on collagen during balloon angioplasty by collagen-binding peptidoglycans. Biomaterials 2011, 32, 2516–2523. [Google Scholar] [CrossRef]
- Kalamajski, S.; Aspberg, A.; Oldberg, Å. The Decorin Sequence SYIRIADTNIT Binds Collagen Type I. J. Biol. Chem. 2007, 282, 16062–16067. [Google Scholar] [CrossRef]
- Raspanti, M.; Viola, M.; Forlino, A.; Tenni, R.; Gruppi, C.; Tira, M.E. Glycosaminoglycans show a specific periodic interaction with type I collagen fibrils. J. Struct. Biol. 2008, 164, 134–139. [Google Scholar] [CrossRef]
- Neill, T.; Schaefer, L.; Iozzo, R.V. Decorin: A guardian from the matrix. Am. J. Pathol. 2012, 181, 380–387. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Mann, D.M.; Ruoslahti, E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature 1990, 346, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Baghy, K.; Horváth, Z.; Regős, E.; Kiss, K.; Schaff, Z.; Iozzo, R.V.; Kovalszky, I. Decorin interferes with platelet-derived growth factor receptor signaling in experimental hepatocarcinogenesis. FEBS J. 2013, 280, 2150–2164. [Google Scholar] [CrossRef]
- Scott, R.A.; Panitch, A. Decorin Mimic Regulates Platelet-Derived Growth Factor and Interferon-γ Stimulation of Vascular Smooth Muscle Cells. Biomacromolecules 2014, 15, 2090–2103. [Google Scholar] [CrossRef]
- Nili, N.; Cheema, A.N.; Giordano, F.J.; Barolet, A.W.; Babaei, S.; Hickey, R.; Eskandarian, M.R.; Smeets, M.; Butany, J.; Pasterkamp, G.; et al. Decorin inhibition of PDGF-stimulated vascular smooth muscle cell function: Potential mechanism for inhibition of intimal hyperplasia after balloon angioplasty. Am. J. Pathol. 2003, 163, 869–878. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Moscatello, D.K.; McQuillan, D.J.; Eichstetter, I. Decorin Is a Biological Ligand for the Epidermal Growth Factor Receptor. J. Biol. Chem. 1999, 274, 4489–4492. [Google Scholar] [CrossRef]
- Chui, A.; Murthi, P.; Gunatillake, T.; Brennecke, S.P.; Ignjatovic, V.; Monagle, P.T.; Whitelock, J.M.; Said, J.M. Altered decorin leads to disrupted endothelial cell function: A possible mechanism in the pathogenesis of fetal growth restriction? Placenta 2014, 35, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.A.; Ramaswamy, A.K.; Park, K.; Panitch, A. Decorin mimic promotes endothelial cell health in endothelial monolayers and endothelial–smooth muscle co-cultures. J. Tissue Eng. Regen. Med. 2015, 11, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Buraschi, S.; Neill, T.; Goyal, A.; Poluzzi, C.; Smythies, J.; Owens, R.T.; Schaefer, L.; Torres, A.; Iozzo, R.V. Decorin causes autophagy in endothelial cells via Peg3. Proc. Natl. Acad. Sci. USA 2013, 110, E2582–E2591. [Google Scholar] [CrossRef] [PubMed]
- De Witt, S.M.; Swieringa, F.; Cavill, R.; Lamers, M.M.; Van Kruchten, R.; Mastenbroek, T.; Baaten, C.; Coort, S.; Pugh, N.; Schulz, A.; et al. Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat. Commun. 2014, 5, 4257. [Google Scholar] [CrossRef]
- Speich, H.E.; Grgurevich, S.; Kueter, T.J.; Earhart, A.D.; Slack, S.M.; Jennings, L.K. Platelets undergo phosphorylation of Syk at Y525/526 and Y352 in response to pathophysiological shear stress. Am. J. Physiol. Cell Physiol. 2008, 295, C1045–C1054. [Google Scholar] [CrossRef]
- Kuznetsova, S.A.; Issa, P.; Perruccio, E.M.; Zeng, B.; Sipes, J.M.; Ward, Y.; Seyfried, N.T.; Fielder, H.L.; Day, A.J.; Wight, T.N.; et al. Versican-thrombospondin-1 binding in vitro and colocalization in microfibrils induced by inflammation on vascular smooth muscle cells. J. Cell Sci. 2006, 119 Pt 21, 4499–4509. [Google Scholar] [CrossRef]
- Wight, T.N. Versican: A versatile extracellular matrix proteoglycan in cell biology. Curr. Opin. Cell Biol. 2002, 14, 617–623. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Zheng, P.S.; Yang, B.B. beta 1-Integrin-mediated glioma cell adhesion and free radical-induced apoptosis are regulated by binding to a C-terminal domain of PG-M/versican. J. Biol. Chem. 2002, 277, 12294–12301. [Google Scholar] [CrossRef]
- Zheng, P.-S.; Reis, M.; Sparling, C.; Lee, D.Y.; La Pierre, D.P.; Wong, C.-K.A.; Deng, Z.; Kahai, S.; Wen, J.; Yang, B.B. Versican G3 Domain Promotes Blood Coagulation through Suppressing the Activity of Tissue Factor Pathway Inhibitor-1. J. Biol. Chem. 2006, 281, 8175–8182. [Google Scholar] [CrossRef]
- Dours-Zimmermann, M.T.; Zimmermann, D.R. A novel glycosaminoglycan attachment domain identified in two alternative splice variants of human versican. J. Biol. Chem. 1994, 269, 32992–32998. [Google Scholar] [CrossRef] [PubMed]
- Bogen, O.; Bender, O.; Alvarez, P.; Kern, M.; Tomiuk, S.; Hucho, F.; Levine, J.D. Expression of a novel versican variant in dorsal root ganglia from spared nerve injury rats. Mol. Pain 2019, 15, 1744806919874557. [Google Scholar] [CrossRef] [PubMed]
- Kenagy, R.D.; Plaas, A.H.; Wight, T.N. Versican Degradation and Vascular Disease. Trends Cardiovasc. Med. 2006, 16, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Nandadasa, S.; Foulcer, S.; Apte, S.S. The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis. Matrix Biol. 2014, 35, 34–41. [Google Scholar] [CrossRef]
- Islam, S.; Chuensirikulchai, K.; Khummuang, S.; Keratibumrungpong, T.; Kongtawelert, P.; Kasinrerk, W.; Hatano, S.; Nagamachi, A.; Honda, H.; Watanabe, H. Accumulation of versican facilitates wound healing: Implication of its initial ADAMTS-cleavage site. Matrix Biol. 2019, 87, 77–93. [Google Scholar] [CrossRef]
- Jonsson-Rylander, A.C.; Nilsson, T.; Fritsche-Danielson, R.; Hammarström, A.; Behrendt, M.; Andersson, J.O.; Lindgren, K.; Andersson, A.K.; Wallbrandt, P.; Rosengren, B.; et al. Role of ADAMTS-1 in atherosclerosis: Remodeling of carotid artery, immunohistochemistry, and proteolysis of versican. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 180–185. [Google Scholar] [CrossRef]
- McCulloch, D.R.; Nelson, C.M.; Dixon, L.J.; Silver, D.L.; Wylie, J.D.; Lindner, V.; Sasaki, T.; Cooley, M.A.; Argraves, W.S.; Apte, S.S. ADAMTS Metalloproteases Generate Active Versican Fragments that Regulate Interdigital Web Regression. Dev. Cell 2009, 17, 687–698. [Google Scholar] [CrossRef]
- Schmitt, M. Versican vs versikine: Tolerance vs attack. Blood 2016, 128, 612–613. [Google Scholar] [CrossRef]
- Hope, C.; Foulcer, S.; Jagodinsky, J.; Chen, S.X.; Jensen, J.L.; Patel, S.; Leith, C.; Maroulakou, I.; Callander, N.; Miyamoto, S.; et al. Immunoregulatory roles of versican proteolysis in the myeloma microenvironment. Blood 2016, 128, 680–685. [Google Scholar] [CrossRef]
- Coulson-Thomas, V.J.; Gesteira, T.F.; Hascall, V.; Kao, W. Umbilical cord mesenchymal stem cells suppress host rejection: The role of the glycocalyx. J. Biol. Chem. 2014, 289, 23465–23481. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a crucial regulator of inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Wight, T.N. Provisional matrix: A role for versican and hyaluronan. Matrix Biol. 2017, 60–61, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Schmalfeldt, M.; Bandtlow, C.; Dours-Zimmermann, M.; Winterhalter, K.; Zimmermann, D. Brain derived versican V2 is a potent inhibitor of axonal growth. J. Cell Sci. 2000, 113, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Dours-Zimmermann, M.T.; Maurer, K.; Rauch, U.; Stoffel, W.; Fässler, R.; Zimmermann, D.R. Versican V2 Assembles the Extracellular Matrix Surrounding the Nodes of Ranvier in the CNS. J. Neurosci. 2009, 29, 7731–7742. [Google Scholar] [CrossRef]
- Arciniegas, E.; Neves, C.Y.; Candelle, D.; Parada, D. Differential versican isoforms and aggrecan expression in the chicken embryo aorta. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 279, 592–600. [Google Scholar] [CrossRef]
- Wight, T.N.; Kinsella, M.G.; Evanko, S.P.; Potter-Perigo, S.; Merrilees, M.J. Versican and the regulation of cell phenotype in disease. Biochim. et Biophys. Acta BBA Bioenerg. 2014, 1840, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Yoon, D.W.; Braun, K.R.; Wight, T.N. Expression of versican V3 by arterial smooth muscle cells alters tumor growth factor β (TGFβ)-, epidermal growth factor (EGF)-, and nuclear factor κB (NFκB)-dependent signaling pathways, creating a microenvironment that resists monocyte adhesion. J. Biol. Chem. 2014, 289, 15393–15404. [Google Scholar] [CrossRef] [PubMed]
- Merrilees, M.J.; Beaumont, B.W.; Braun, K.R.; Thomas, A.C.; Kang, I.; Hinek, A.; Passi, A.; Wight, T.N. Neointima formed by arterial smooth muscle cells expressing versican variant V3 is resistant to lipid and macrophage accumulation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1309–1316. [Google Scholar] [CrossRef]
- Merrilees, M.J.; Lemire, J.M.; Fischer, J.W.; Kinsella, M.G.; Braun, K.R.; Clowes, A.W.; Wight, T.N. Retrovirally Mediated Overexpression of Versican V3 by Arterial Smooth Muscle Cells Induces Tropoelastin Synthesis and Elastic Fiber Formation In Vitro and In Neointima After Vascular Injury. Circ. Res. 2002, 90, 481–487. [Google Scholar] [CrossRef]
- Kang, I.; Barth, J.L.; Sproul, E.P.; Yoon, D.W.; Workman, G.A.; Braun, K.R.; Argraves, W.S.; Wight, T.N. Expression of V3 Versican by Rat Arterial Smooth Muscle Cells Promotes Differentiated and Anti-inflammatory Phenotypes. J. Biol. Chem. 2015, 290, 21629–21641. [Google Scholar] [CrossRef] [PubMed]
- Pirvu, T.N.; Schroeder, J.E.; Peroglio, M.; Verrier, S.; Kaplan, L.; Richards, R.G.; Alini, M.; Grad, S. Platelet-rich plasma induces annulus fibrosus cell proliferation and matrix production. Eur. Spine J. 2014, 23, 745–753. [Google Scholar] [CrossRef]
- Cardoso, L.E.; Little, P.J.; Ballinger, M.L.; Chan, C.K.; Braun, K.R.; Potter-Perigo, S.; Bornfeldt, K.E.; Kinsella, M.G.; Wight, T.N. Platelet-derived Growth Factor Differentially Regulates the Expression and Post-translational Modification of Versican by Arterial Smooth Muscle Cells through Distinct Protein Kinase C and Extracellular Signal-regulated Kinase Pathways. J. Biol. Chem. 2010, 285, 6987–6995. [Google Scholar] [CrossRef] [PubMed]
- Osman, N.; Getachew, R.; Thach, L.; Wang, H.; Su, X.; Zheng, W.; Little, P.J. Platelet-derived growth factor-stimulated versican synthesis but not glycosaminoglycan elongation in vascular smooth muscle is mediated via Akt phosphorylation. Cell. Signal. 2014, 26, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Ivey, M.E.; Little, P.J. Thrombin regulates vascular smooth muscle cell proteoglycan synthesis via PAR-1 and multiple downstream signalling pathways. Thromb. Res. 2008, 123, 288–297. [Google Scholar] [CrossRef]
- Kawashima, H.; Hirose, M.; Hirose, J.; Nagakubo, D.; Plaas, A.H.; Miyasaka, M. Binding of a Large Chondroitin Sulfate/Dermatan Sulfate Proteoglycan, Versican, to L-selectin, P-selectin, and CD44. J. Biol. Chem. 2000, 275, 35448–35456. [Google Scholar] [CrossRef]
- Frenette, P.S.; Denis, C.V.; Weiss, L.; Jurk, K.; Subbarao, S.; Kehrel, B.; Hartwig, J.H.; Vestweber, D.; Wagner, D.D. P-Selectin Glycoprotein Ligand 1 (Psgl-1) Is Expressed on Platelets and Can Mediate Platelet–Endothelial Interactions in Vivo. J. Exp. Med. 2000, 191, 1413–1422. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef]
- Liang, J.; Jiang, D.; Noble, P.W. Hyaluronan as a therapeutic target in human diseases. Adv. Drug Deliv. Rev. 2016, 97, 186–203. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar]
- De La Motte, C.; Nigro, J.; Vasanji, A.; Rho, H.; Kessler, S.; Bandyopadhyay, S.; Danese, S.; Fiocchi, C.; Stern, R. Platelet-derived hyaluronidase 2 cleaves hyaluronan into fragments that trigger monocyte-mediated production of proinflammatory cytokines. Am. J. Pathol. 2009, 174, 2254–2264. [Google Scholar] [CrossRef] [PubMed]
- Evanko, S.P.; Johnson, P.Y.; Braun, K.R.; Underhill, C.B.; Dudhia, J.; Wight, T.N. Platelet-Derived Growth Factor Stimulates the Formation of Versican–Hyaluronan Aggregates and Pericellular Matrix Expansion in Arterial Smooth Muscle Cells. Arch. Biochem. Biophys. 2001, 394, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Turley, E.A. Extracellular matrix remodeling: Multiple paradigms in vascular disease. Circ. Res. 2001, 88, 2–4. [Google Scholar] [CrossRef]
- D’Agostino, A.; Stellavato, A.; Corsuto, L.; Diana, P.; Filosa, R.; La Gatta, A.; De Rosa, M.; Schiraldi, C. Is molecular size a discriminating factor in hyaluronan interaction with human cells? Carbohydr. Polym. 2017, 157, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Slevin, M.; Krupinski, J.; Gaffney, J.; Matou, S.; West, D.; Delisser, H.; Savani, R.C.; Kumar, S. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007, 26, 58–68. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’ascola, A.; Nastasi, G.; Calatroni, A. Small hyaluronan oligosaccharides induce inflammation by engaging both toll-like-4 and CD44 receptors in human chondrocytes. Biochem. Pharmacol. 2010, 80, 480–490. [Google Scholar] [CrossRef]
- Petrey, A.C.; Obery, D.R.; Kessler, S.P.; Flamion, B.; de la Motte, C.A. Hyaluronan Depolymerization by Megakaryocyte Hyaluronidase-2 Is Required for Thrombopoiesis. Am. J. Pathol. 2016, 186, 2390–2403. [Google Scholar] [CrossRef]
- Li, J.; Wu, F.; Zhang, K.; He, Z.; Zou, D.; Luo, X.; Fan, Y.; Yang, P.; Zhao, A.; Huang, N. Controlling Molecular Weight of Hyaluronic Acid Conjugated on Amine-rich Surface: Toward Better Multifunctional Biomaterials for Cardiovascular Implants. ACS Appl. Mater. Interfaces 2017, 9, 30343–30358. [Google Scholar] [CrossRef]
- Weigel, P.H.; Fuller, G.M.; LeBoeuf, R.D. A model for the role of hyaluronic acid and fibrin in the early events during the inflammatory response and wound healing. J. Theor. Biol. 1986, 119, 219–234. [Google Scholar] [CrossRef]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef]
- Evanko, S.P.; Angello, J.C.; Wight, T.N. Formation of Hyaluronan- and Versican-Rich Pericellular Matrix Is Required for Proliferation and Migration of Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Koshiishi, I.; Shizari, M.; Underhill, C.B. CD44 can mediate the adhesion of platelets to hyaluronan. Blood 1994, 84, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Holm, A.-C.B.S.; Bengtsson, T.; Grenegård, M.; Lindström, E.G. Hyaluronic acid influence on platelet-induced airway smooth muscle cell proliferation. Exp. Cell Res. 2012, 318, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Verheye, S.; Markou, C.P.; Salame, M.Y.; Wan, B.; KingIII, S.B.; Robinson, K.A.; Chronos, N.A.F.; Hanson, S.R. Reduced Thrombus Formation by Hyaluronic Acid Coating of Endovascular Devices. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1168–1172. [Google Scholar] [CrossRef]
- Liu, G.; Liu, G.; Alzoubi, K.; Chatterjee, M.; Walker, B.; Muenzer, P.; Luo, D.; Umbach, A.T.; Elvira, B.; Chen, H.; et al. CD44 sensitivity of platelet activation, membrane scrambling and adhesion under high arterial shear rates. Thromb. Haemost. 2016, 115, 99–108. [Google Scholar]
- Lauer, M.E.; Glant, T.T.; Mikecz, K.; DeAngelis, P.L.; Haller, F.M.; Husni, M.E.; Hascall, V.C.; Calabro, A. Irreversible Heavy Chain Transfer to Hyaluronan Oligosaccharides by Tumor Necrosis Factor-stimulated Gene-6. J. Biol. Chem. 2013, 288, 205–214. [Google Scholar] [CrossRef]
- Petrey, A.C.; Obery, D.R.; Kessler, S.P.; Zawerton, A.; Flamion, B.; De La Motte, C.A. Platelet hyaluronidase-2 regulates the early stages of inflammatory disease in colitis. Blood 2019, 134, 765–775. [Google Scholar] [CrossRef]
- Chowdhury, B.; Hemming, R.; Hombach-Klonisch, S.; Flamion, B.; Triggs-Raine, B. Murine Hyaluronidase 2 Deficiency Results in Extracellular Hyaluronan Accumulation and Severe Cardiopulmonary Dysfunction. J. Biol. Chem. 2013, 288, 520–528. [Google Scholar] [CrossRef]
- Meteva, D.; Vinci, R.; Seppelt, C.; Abdelwahed, Y.S.; Pedicino, D.; Nelles, G.; Skurk, C.; Haghikia, A.; Rauch-Kröhnert, U.; Gerhardt, T.; et al. Toll-like receptor 2, hyaluronan, and neutrophils play a key role in plaque erosion: The OPTICO-ACS study. Eur. Heart J. 2023, ehad379. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.W.; Steitz, S.A.; Johnson, P.Y.; Burke, A.; Kolodgie, F.; Virmani, R.; Giachelli, C.; Wight, T.N. Decorin Promotes Aortic Smooth Muscle Cell Calcification and Colocalizes to Calcified Regions in Human Atherosclerotic Lesions. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2391–2396. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Yamamoto, E.; Sugiyama, T.; Jia, H.; Ma, L.; Hu, S.; Wang, C.; Zhu, Y.; Li, L.; Xu, M.; et al. EROSION Study (Effective Anti-Thrombotic Therapy Without Stenting: Intravascular Optical Coherence Tomography-Based Management in Plaque Erosion): A 1-Year Follow-Up Report. Circ. Cardiovasc. Interv. 2017, 10, e005860. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drysdale, A.; Unsworth, A.J.; White, S.J.; Jones, S. The Contribution of Vascular Proteoglycans to Atherothrombosis: Clinical Implications. Int. J. Mol. Sci. 2023, 24, 11854. https://doi.org/10.3390/ijms241411854
Drysdale A, Unsworth AJ, White SJ, Jones S. The Contribution of Vascular Proteoglycans to Atherothrombosis: Clinical Implications. International Journal of Molecular Sciences. 2023; 24(14):11854. https://doi.org/10.3390/ijms241411854
Chicago/Turabian StyleDrysdale, Amelia, Amanda J. Unsworth, Stephen J. White, and Sarah Jones. 2023. "The Contribution of Vascular Proteoglycans to Atherothrombosis: Clinical Implications" International Journal of Molecular Sciences 24, no. 14: 11854. https://doi.org/10.3390/ijms241411854
APA StyleDrysdale, A., Unsworth, A. J., White, S. J., & Jones, S. (2023). The Contribution of Vascular Proteoglycans to Atherothrombosis: Clinical Implications. International Journal of Molecular Sciences, 24(14), 11854. https://doi.org/10.3390/ijms241411854






