Abstract
Glycosylation is a widespread glycosyl modification that regulates gene expression and metabolite bioactivity in all life processes of plants. Phosphoribosylation is a special glycosyl modification catalyzed by phosphoribosyltransferase (PRTase), which functions as a key step in the biosynthesis pathway of purine and pyrimidine nucleotides, histidine, tryptophan, and coenzyme NAD(P)+ to control the production of these essential metabolites. Studies in the past decades have reported that PRTases are indispensable for plant survival and thriving, whereas the complicated physiological role of PRTases in plant life and their crosstalk is not well understood. Here, we comprehensively overview and critically discuss the recent findings on PRTases, including their classification, as well as the function and crosstalk in regulating plant development, abiotic stress response, and the balance of growth and stress responses. This review aims to increase the understanding of the role of plant PRTase and also contribute to future research on the trade-off between plant growth and stress response.
1. Introduction
Glycosylation, the transfer of a sugar moiety to an acceptor molecule, is a widespread modification in plants that regulates gene expression and metabolite bioactivity. This glycosyl modification occurs on carbohydrates, proteins, lipids, hormones, and other secondary metabolites to alter their important properties, such as solubility, stability, biological activity, or intermolecular interactions [1,2,3,4]. Moreover, glycosylation plays a crucial physiological role in all life processes of plants, including growth, development, and stress response [2,3].
Phosphoribosylation, a special glycosyl modification catalyzed by phosphoribosyltransferases (PRTase), is a key step in the biosynthetic pathways of purine and pyrimidine nucleotides, tryptophan (Try) and histidine (His), and cofactor NAD(P)+ to control the production of these metabolites [5]. These metabolites are essential for plants because purine and pyrimidine nucleotides, as well as Try and His are the basic constituent units of nucleic acids and proteins, respectively. In addition, Try is also a key precursor of auxin biosynthesis [6], while NAD(P)+ is the core substance of energy metabolism [7].
The physiological functions of many plant PRTases have been clarified [8,9,10,11,12]. In addition, PRTases can collectively contribute to plant life processes since different PRTases crosstalk with each other by sharing 5-phosphoribosyl-1-pyrophosphate (PRPP) as sugar donors. However, research on plant PRTases in the past few decades mainly focuses on their role in plant survival and thriving, with little discussion about their role and crosstalk in plant development and abiotic stress responses [13,14]. In fact, recent studies clearly demonstrate that PRTase-related metabolic pathways are critical for chloroplast development, gametophyte development, salt, and osmotic stress response [15,16,17,18]. Herein, we summarize the recent advances in the function and crosstalk of plant PRTases in regulating plant development and abiotic stress response, aiming to provide new insights into the complicated role of PRTase in plant life processes.
2. Classification and Characteristics of PRTase
To date, studies have shown that PRTase is responsible for catalyzing the transfer of ribose-5-phosphate from the glycosyl donor PRPP to acceptor molecules (e.g., adenine, guanine, uracil) to form glycosidic bonds, which rely on divalent cations [5,19]. PRTase belongs to the PRT family and is identified at the amino acid sequence level [5]. Almost all PRTases contain a 13-residue sequence motif that is predicted to be a PRPP-binding site, which is composed of four hydrophobic amino acids, two acidic amino acids and seven variable characteristic amino acids [5,20].
PRTases can be divided into four categories based on the similarity of the three-dimensional structures, as shown in Figure 1A [5,19]. Class I PRTase is a homodimer formed by the N-terminal domain of one monomer adjoining the C-terminal domain of the other monomer, which shares a common α/β-barrel domain [5]. Class II PRTase monomers have two α/β-barrel domains that form homodimers in a similar manner to class I PRTase [21]. Class III PRTase monomers contain a small N-terminal α-helical domain and a large C-terminal α/β-barrel domain, with the N-terminal domain of one monomer close to the N-terminal domain of the other monomer, forming homodimers [19,22,23]. Class IV PRTase are homohexamers, assembled from six monomers, each containing three α/β barrel domains [24,25].
As shown in Figure 1B and Table 1, different classes of PRTase are involved in different metabolic pathways. Class I PRTase is responsible for purine and pyrimidine nucleotide biosynthesis pathways, including amidophosphoribosyltransferase (ATase), adenine phosphoribosyltransferase (APRT), hypoxanthine-guanine phosphoribosyltransferase (HGPRT), orotate phosphoribosyltransferase (OPRT), and uracil phosphoribosyltransferase (UPRT) [5,26,27]. Class II PRTase is involved in the NAD(P)+ biosynthesis pathway, including quinolinic acid phosphoribosyltransferase (QPRT) and nicotinamide phosphoribosyltransferase (NaPRT) [21,28]. Class III PRTase participates in the Try biosynthesis pathway, with only anthranilate phosphoribosyltransferase (AnPRT) [19,22,23,29]. Class IV PRTase is responsible for the His biosynthesis pathway, with only ATP phosphoribosyltransferase (ATP–PRT) [24,25].

Table 1.
Classification of PRTases, their related metabolic pathways and experimental evidence of gene function.
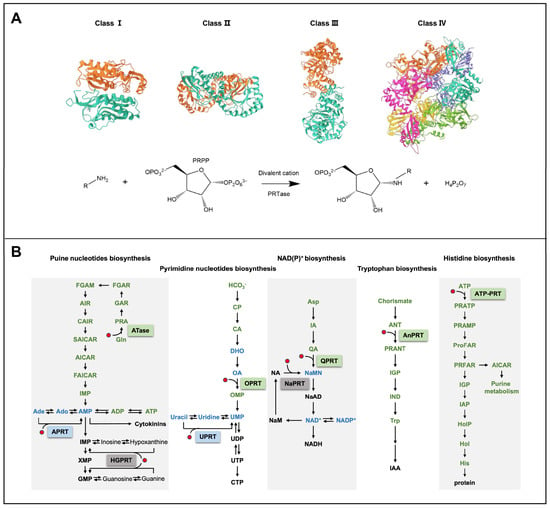
Figure 1.
The protein structure and the catalytic reaction of PRTase, and its related metabolic pathways in plants [30,31,32,33]. (A) Protein structure and the catalytic reaction of PRTase. Different colors represent different monomers in the homodimer or homomultimer. (B) The metabolic pathways that PRTases are involved in. PRTases are shown within boxes. The green box indicates that it is chloroplast-localized, the blue box indicates that it can be chloroplast- and cytoplasm-localized, and the gray box indicates that it is cytoplasm-localized. The red dot represents 5-phosphoribosyl-1-pyrophosphate (PRPP), the common substrate of PRTases. Abbreviations for metabolites are shown in the Abbreviations list.
3. Functions of PRTase and Related Metabolic Pathways in Plants
3.1. Class I PRTase
Class I PRTase, which comprises more family members compared with the other class of PRTase, is responsible for the biosynthesis of all nucleotides, including adenine, guanine, cytosine, and uracil nucleotides. As is known, nucleotides are essential for all organisms because they are the fundamental building blocks of nucleic acids as well as ribosomes. Furthermore, adenosine triphosphate (ATP) is required for the biosynthesis of all other nucleotides, and adenine nucleotides have a greater effect on plant physiology than other nucleotides [30]. Thus, this section will focus on the role of adenine nucleotide biosynthesis in plant growth and development.
3.1.1. The Role of Class I PRTase in the Adenine Nucleotide Biosynthesis Pathway
In class I PRTase, only ATase and APRT are involved in adenine nucleotide biosynthesis. ATase and APRT are key factors that promote adenine nucleotide accumulation in plants [41]. ATase catalyzes the first and rate-limiting step in the adenine nucleotide de novo biosynthesis pathway [42]. In Arabidopsis (Arabidopsis thaliana), three ATase genes have been identified. AtATase1 is specifically expressed in flowers and roots, and AtATase2 is rather constitutively expressed in leaves, flowers, and roots, while AtATase3 is weakly expressed in silique, cauline leaves, and roots [8,42]. APRT is a key enzyme in the adenine salvage pathway that converts adenine to adenosine monophosphate (AMP) [13]. Three APRT genes have been identified in Arabidopsis, with AtAPRT1 contributing most of the APRT activity, as the APRT activity of the AtAPRT1 knockout mutant was reduced by >95% [13,43]. In addition to Arabidopsis, APRT genes have been found in barley, rice, wheat, oilseed rape, maize, and tomato [44].
3.1.2. Adenine Nucleotide Regulates Plant Growth and Development
Altered adenine nucleotide biosynthesis causes changes in the intracellular level of adenine nucleotides, thus regulating plant growth and development. Reduced biosynthesis of adenine nucleotides, especially ATP, strongly inhibits RNA synthesis in plants because ATP is the basis for the biosynthesis of all other nucleotides. Notably, phosphate is critical for the biosynthesis of ATP [30]. In Arabidopsis, phosphate starvation leads to a significant reduction in intracellular ATP levels and a nearly 90% reduction in the RNA content of shoots, resulting in a growth arrest in seedlings [45]. In addition, transcriptional expression of the APRT gene in the adenine nucleotide biosynthesis pathway was significantly induced. A similar growth arrest phenotype also appears in the suspension-cultured Catharanthus roseus cells under phosphate starvation conditions [46]. The addition of phosphate could rapidly increase the RNA content in the Catharanthus roseus cells within 24 h, and the cells would be able to grow again [46]. Thus, adenine nucleotide is a key factor that regulates RNA synthesis and plant growth.
In addition to reducing RNA synthesis, defects in adenine nucleotide biosynthesis also inhibit ribosome biogenesis in plants. Adenine nucleotide starvation caused by ATase deficiency in the de novo biosynthesis pathway can activate autophagy and inhibit the evolutionarily conserved TOR (target of rapamycin) kinase activity, a core regulator of ribosome biogenesis and plant growth [47]. In addition, silencing the expression of the PRPP synthetase (PRS4) in N. benthamiana reduces the adenine nucleotide biosynthesis and thus inhibits TOR activity and ribosome biogenesis, resulting in pleiotropic phenotypes including dwarfism, abnormal leaf shape, and delayed flowering [48]. Hence, adenine nucleotide contributes to plant growth and development by regulating TOR activity and ribosome biogenesis (Figure 2).
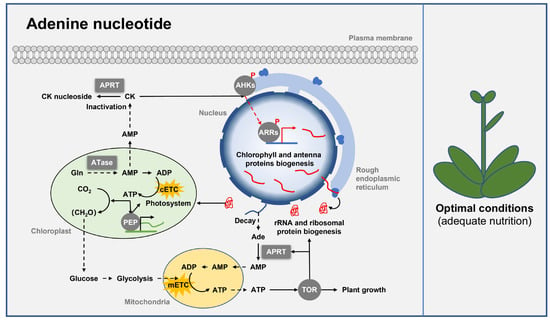
Figure 2.
Class Ⅰ PRTase in adenine nucleotide biosynthesis pathways regulates plant growth and development. Class Ⅰ PRTase ATase and APRT regulate chloroplast development, ribosome biogenesis, and plant growth under nutrient-adequate conditions by influencing the intracellular levels of adenine nucleotides and CK. ATase and APRT are represented by square boxes, and other proteins are represented by round boxes. Solid arrows indicate direct interactions or one-step reactions, and dashed arrows indicate indirect interactions, multistep reactions, or transmembrane transport. Abbreviations for metabolites are shown in the Abbreviations list. Other abbreviations are as follows: adenine phosphoribosyltransferase (APRT), amidophosphoribosyl transferase (ATase), plastid-encoded RNA polymerase (PEP), Arabidopsis histidine kinase (AHK), Arabidopsis response regulator (ARR), target of rapamycin (TOR), chloroplastic electron transport chain (cETC), mitochondrial electron transport chain (mETC).
As derivatives of adenine, the synthesis of cytokinins also requires class I PRTase [49,50]. Notably, ATase is critical for cytokinin synthesis. Arabidopsis ATase2 mutants have shown decreased cytokinin content, cell number, and the level of plastid-encoded RNA polymerase (PEP)-dependent transcript in leave cells, resulting in impaired chloroplast development, growth retardation, and bleached/etiolated seedling phenotype, and similar phenotypes have also been found in transgenic tobacco plants [8,11,15,34,35]. In addition, APRT participates in cytokinin inactivation. Arabidopsis APRT1 mutants have elevated levels of activated cytokinin, which triggers cytokinin responses, resulting in increased chlorophyll and anthocyanin contents, and delayed leaf senescence [13,14]. Furthermore, APRT also plays an important role in gametophyte development in Arabidopsis, wheat, rice and Vitis amurensis [16,36,37,51]. Overall, ATase and APRT play a key role in plant chloroplast and gametophyte development by regulating the accumulation of adenine nucleotides and cytokinins (Figure 2).
3.2. Class II PRTase
3.2.1. The Role of Class II PRTase in the NAD(P)+ Biosynthesis Pathway
QPRT and NaPRT, the only two members of class II PRTase, are rate-limiting enzymes in the biosynthesis pathways of NAD(P)+ [7,9]. QPRT is responsible for the de novo biosynthesis pathway of NAD(P)+, while NaPRT is involved in the salvage pathway of NAD(P)+. Class II PRTase promotes the intracellular accumulation of NAD(P)+ and is essential for maintaining NAD(P)+ homeostasis in plant cells since the half-life of NAD(P)+ can be as short as 15 min [52,53].
3.2.2. NAD(P)+ Regulates Plant Growth and Development
The change of intracellular NAD(P)+ level strongly affects plant growth and development. NAD+, an indispensable coenzyme and redox carrier in plants, is involved in many redox reactions in cellular glycolysis, the tricarboxylic acid cycle, and other energy metabolic processes. In these processes, NAD+ is reduced to NADH, which is the basis for mitochondrial respiration to generate ATP through oxidative phosphorylation. Decreased free NAD+ levels severely affect mitochondrial-related metabolic processes, thereby greatly inhibiting ATP biosynthesis and cellular energy metabolism [7]. Furthermore, enzymes in the NAD+ biosynthesis pathway are essential for the plant, such that loss-of-function mutants are embryo-lethal [9].
In addition to its role in energy metabolism, NAD(P)+ also contributes to chloroplast development and photosynthesis. Recent studies have reported that NAD+ cap modification at the 5′ end of mRNA can regulate mRNA stability and translation in a DOX1-dependent manner [54,55,56,57]. Arabidopsis Atdxo1 mutant has pleiotropic phenotypes, such as dwarfism, leaf yellowing/albinism, and various developmental defects [17]. This is due to the increased NAD+-RNA stability in the mutant Atdxo1, and thus, enhances post-transcriptional silencing of specific transcripts mainly involved in porphyrin and chlorophyll metabolism, as well as photosynthesis [17,58]. Additionally, NADP+ is an indispensable coenzyme in plant photosynthesis [7,59], because NADP+ is reduced in the photoreaction to NADPH that is required for the CO2 fixation in the dark reaction. Silencing of the QPRT gene reduces the content of NAD(P)+, leading to a decrease in chlorophyll content, inhibition of photosynthesis, and delayed growth and development in Nicotiana tabacum [39]. Additionally, mutation of rice OsNaPRT1 results in dwarfism and early leaf senescence [12]. These results indicate that QPRT and NaPRT are important contributors to plant growth and development by maintaining moderate intracellular NAD(P)+ levels (Figure 3).
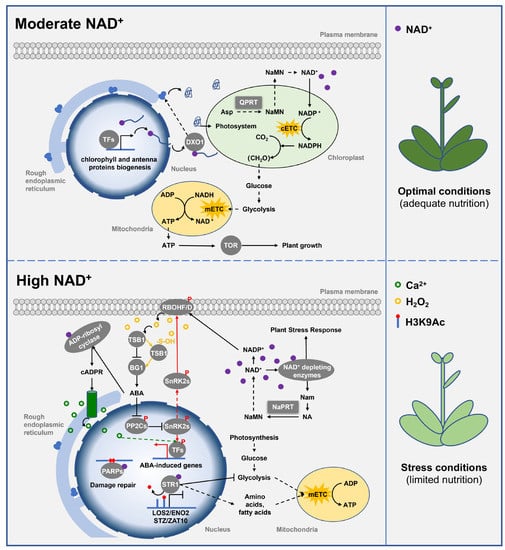
Figure 3.
Class II PRTase in NAD(P)+ biosynthesis pathways regulate plant growth, development, and abiotic stress response. Class II PRTase QPRT and NaPRT control the biosynthesis of NAD(P)+ to regulate its intracellular levels. Under nutrient-adequate conditions, moderate levels of NAD+ participate in mRNA modification and ATP biosynthesis to promote chloroplast development and plant growth. However, under nutrient-limiting conditions, elevated levels of NAD+ enable NAD+-dependent enzymes to trigger calcium signaling and histone deacetylation, thereby activating ABA signaling, and ROS signaling to promote plant systemic stress responses. QPRT and NaPRT are represented by square boxes, and other proteins are represented by round boxes. Solid arrows indicate direct interactions or one-step reactions, and dashed arrows indicate indirect interactions, multistep reactions, or transmembrane transport. Abbreviations for proteins are as follows: quinolinic acid phosphoribosyltransferase (QPRT), decapping exonuclease 1 (DXO1), transcription factors (TFs), target of rapamycin (TOR), NADPH oxidase (RBOHF/D), tryptophan synthase β subunit 1 (TSB1), β-glucosidase 1 (BG1), sucrose non-fermenting 1-related protein kinase 2 (SnRK2), nicotinamide phosphoribosyltransferase (NaPRT), protein phosphatase 2C (PP2C). Abbreviations for metabolites are shown in the Abbreviations list.
3.2.3. NAD(P)+ Regulates Plant Abiotic Stress Response
Recent studies have found that the crosstalk between NAD(P)+ and abscisic acid (ABA) is involved in regulating plant abiotic stress responses. In plants, ABA is the most important hormone in response to abiotic stress, with functions such as stress resistance, growth inhibition, seed germination inhibition, and senescence promotion [60]. Interestingly, high levels of NAD(P)+ can promote ABA synthesis and signal transduction, as well as enhance plant response to ABA and stress. In turn, ABA signaling can prevent the further increase in NAD(P)+ levels through the downstream transcription factor ABI4 specifically inhibiting the expression of quinolinate synthase gene in the NAD(P)+ de novo biosynthesis pathway [60].
High levels of NAD(P)+ contribute to plant abiotic stress response by promoting ABA synthesis and signal transduction (Figure 3). ABA levels increase in an NAD+-dependent manner in seedlings grown on mediums with different concentrations of NAD+ [60,61,62]. Under abiotic stress conditions, ABA activates reactive oxygen species (ROS) signaling; in turn, ROS can inactivate tryptophan synthase TSB1 through sulfinylation, thereby relieving its inhibition on the activity of β-glucosidase BG1 and promoting the release of ABA from ABA-GE to form a positive feedback loop [63]. Importantly, ABA-activated ROS signaling, which is critical for plants to respond to salt, drought, high light and other stresses, is mainly produced by NADPH oxidase (RBOHF and RBOHD) [60,64,65,66]. In fact, RBOHF and RBOHD require NADPH electrons to reduce oxygen to superoxide anions, so the continuous supply of NADPH is a necessary fuel to maintain ROS production [67]. Defects in NAD(P)+ biosynthesis lead to RBOHF and RBOHD inactivation, resulting in the inhibition of ROS production. NADP+ and ROS levels have been observed to be reduced in the Atfin4 and Atfin4-4 mutants with impaired NAD(P)+ de novo biosynthesis [67]. Thus, high levels of NAD(P)+ promote ABA synthesis and ABA-activated ROS signaling under abiotic stress conditions.
In addition to promoting ABA synthesis and signaling, high levels of NAD+ enhance plant responses to ABA and abiotic stress by activating NAD+-dependent enzymes (Figure 3). NAD+ acts as a coenzyme involved in ADP-ribosyl cyclase-mediated calcium-responsive second messenger cyclic ADP-ribose (cADPR) synthesis [68], sirtuins-mediated protein deacetylation [69], and poly (ADP-ribose) polymerase (PARP)-mediated protein single- and poly-ADP ribosylation [70]. These NAD+-dependent enzymes consume NAD+ and are sensitive to free NAD+ levels. Elevating NAD+ levels by silencing the expression of AtPARP2 could promote ADP-ribosyl cyclase-mediated cADPR synthesis to activate Ca2+ signaling, thereby enhancing plant response to ABA [71]. cADPR is a key regulator of the ABA signaling pathway in plants and induces the expression of more than 100 ABA-responsive genes which subsequently enhance the activity of ADP-ribosyl cyclase, further amplifying cADPR signaling [72]. In addition, the NAD+-dependent histone, H3K9 deacetylase SRT1, also positively regulates the expression of ABA-responsive genes and plant stress responses. For example, the Arabidopsis SRT1 mutant, Atsrt1, and its RNAi-silenced lines have exhibited decreased sensitivity to ABA and stress, while the overexpressing lines have exhibited hypersensitivity to salt and osmotic stress due to their excessive response to ABA [18]. Similarly, Oryza sativa (rice) SRT1 is also involved in the epigenetic regulation of stress-responsive genes [73,74,75]. Altogether, high levels of NAD+-induced calcium signaling and histone deacetylation greatly enhance plant responses to ABA and abiotic stress.
Interestingly, NAD+ can also improve plant stress resistance. Under abiotic stress conditions, NAD+ is mainly consumed by PARP-mediated modification of nuclear proteins, thereby regulating DNA synthesis and repair, chromatin structure, gene transcription, and cell cycle [7]. This PARP-mediated modification is one of the major causes of energy depletion and death in mammalian cells [71,76]. In Arabidopsis and Brassica napus, RNAi silencing of PARP expression has been found to reduce NAD+ depletion resulted in broad-spectrum stress-resistant plants without negative effects on plant growth, development, and seed production [71]. In addition, overexpression of the ADP-ribose pyrophosphatase gene NUDX2, which recycles AMP and ribose-5-phosphate from free ADP-ribose molecules has been found to maintain ATP and NAD+ levels and enhance tolerance to oxidative stress in Arabidopsis [77]. Thus, maintaining NAD+ levels under abiotic stress can greatly improve plant stress resistance and thus regulate plant growth-defense balance.
3.3. Class III PRTase
3.3.1. The Role of Class III PRTase in the Tryptophan Biosynthesis Pathway
AnPRT is the only member of class III PRTase and catalyzes the second step of Try biosynthesis, which transfers the phosphoribosyl group of PRPP to anthranilate to form phosphoribosyl anthranilate [23]. Overexpression of PRPP synthetase gene to increase PRPP content in Arabidopsis can promote Try biosynthesis, indicating that this step is the key to determining the flow of this pathway [78]. Thus, AnPRT is a critical factor regulating Try accumulation.
3.3.2. Tryptophan Regulates Plant Growth and Development
As the primary precursor of IAA synthesis, the level of Try is an important determinant of IAA synthesis [6,32,79]. Changes in Try biosynthesis affect its intracellular levels and thus regulate the synthesis of IAA, a major plant hormone that regulates plant growth [80]. In the Attsb1 mutant whose mutation occurred in the tryptophan synthase β-subunit (TSB) loci, both IAA biosynthesis and plant growth are severely inhibited [63]. Furthermore, the Try biosynthesis-deficient mutant, tdd1, is embryonic-lethal in rice [81]. On the contrary, transgenic Brassica oleracea overexpressing BoTSB1 or BoTSB2 can accumulate more Try and IAA, so it exhibits phenotypes such as long hypocotyls, large plants, and a high number of lateral roots [82]. Thus, Try biosynthesis is essential for maintaining moderate IAA levels and plant growth.
Moderate levels of IAA in the cytoplasm and apoplasts can jointly promote plant growth and development through signal transduction. On the one hand, cytoplasmic IAA is recognized by the receptor TIR1 and promotes the degradation of canonical AUX/IAA inhibitors, thereby releasing auxin response factors (ARFs) to promote plant growth [83]. In addition, Solanum lycopersicum (tomato) ARFs have been found to be involved in chloroplast development, since SlARF4, SlARF10, and SlARF6A promote chlorophyll synthesis, photosynthesis, and increase starch accumulation [84,85,86]. On the other hand, unlike TIR1-dependent cytoplasmic signaling, moderate levels of IAA in the apoplast activate the small G protein ROP2 (Rho-related protein in plant 2) via transmembrane kinase 1 (TMK1), thereby activating TOR to promote plant growth [87,88]. Furthermore, TOR phosphorylates and stabilizes the IAA efflux carrier, pin-formed2 (PIN2), and its gradient distribution in Arabidopsis taproots, stimulating root tip elongation [89]. Overall, moderate levels of IAA promote plant growth and development by activating ARFs and TOR (Figure 4).
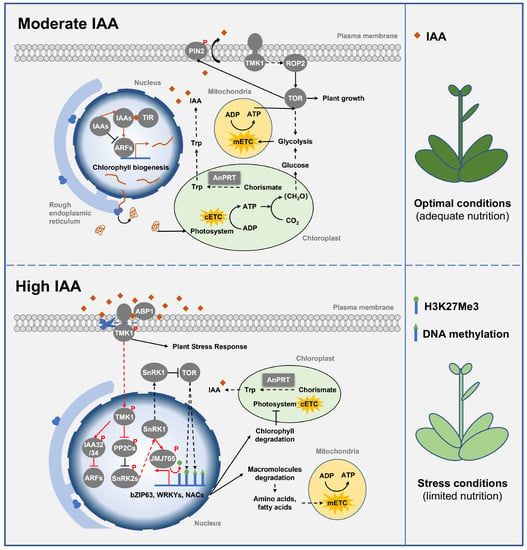
Figure 4.
Class Ⅲ PRTase in the tryptophan biosynthesis pathway regulates plant growth, development, and abiotic stress response. Class Ⅲ PRTase AnPRT controls the biosynthesis of Try to regulate the level of IAA. Under nutrient-adequate conditions, moderate levels of IAA promote ARFs expression and TMK1-mediated TOR activation to promote chloroplast development and plant growth. However, under nutrient-limiting conditions, elevated levels of IAA mediate C-terminal cleavage of TMK1 to repress the expression of ARFs and activate ABA signaling, thus activating SnRK1 and inactivating TOR to promote plant abiotic stress responses. AnPRT is represented by a square box, and other proteins are represented by round boxes. Solid arrows indicate direct interactions or one-step reactions, and dashed arrows indicate indirect interactions, multistep reactions, or transmembrane transport. Abbreviations for proteins are as follows: pin-formed 2 (PIN2), transmembrane kinase 1 (TMK1), rho-related protein in plant 2 (ROP2), target of rapamycin (TOR), indole-3-acetic acid (IAA), Toll–interleukin 1 receptor (TIR), auxin response factor (ARF), anthranilate phosphoribosyltransferase (AnPRT), auxin-binding protein 1 (ABP1), sucrose non-fermenting 1-related protein kinase 1 (SnRK1), protein phosphatase 2C (PP2C), sucrose non-fermenting 1-related protein kinase 2 (SnRK2). Abbreviations for metabolites are shown in the Abbreviations list.
3.3.3. Tryptophan Regulates Plant Abiotic Stress Response
Try contributes to plant abiotic stress responses by promoting the accumulation of high levels of IAA. In Arabidopsis, loss of function of anthranilate synthase α and β subunits (WEI2/ASA1 and WEI7/ASB1) can block excessive IAA accumulation and corresponding phenotypes, such as growth inhibition of seedling roots, epinasty of the cotyledons, and adventitious root formation [90]. Moreover, these phenotypes can be complemented by supplementing Try in the medium [90]. Hence, excess biosynthesis of Try is essential for the accumulation of high levels of IAA.
High levels of IAA inhibit IAA signaling and activate ABA signaling to prompt plant abiotic stress responses and inhibit plant growth (Figure 4). In Arabidopsis, high levels of IAA in the apoplast binding to the receptor ABP1 trigger the C-terminal cleavage of TMK1 (TMK1-C) [91]. TMK1-C subsequently enters the nucleus to activate non-canonical IAA32 and IAA34, thus repressing the expression of ARFs [83]. Moreover, TMK1-C also facilitates the phosphorylation and inactivation of the ABA signaling negative regulator PP2Cs (protein phosphatase 2C, ABI1/2), which releases its interacting proteins SnRK2s, thereby activating SnRK1 to inhibit TOR [92,93]. Notably, the inhibition of plant growth by ABA signaling is largely dependent on the evolutionarily conserved energy sensor, SnRK1, and its inhibition of TOR. Under normal conditions (nutrition adequacy), ABA signaling and SnRK1 signaling are blocked, thereby maintaining TOR activity and promoting plant growth, whereas under nutrient limitation conditions, ABA signaling and SnRK1 are activated, and activated SnRK1 inhibits TOR activity and plant growth [92,94]. Specifically, SnRK1 activates specific transcription factors (such as bZIP63, WRKYs, and NACs) and epigenetic factors (such as H3K27me3 demethylase JMJ705) [95,96,97,98,99], and inactivates TOR to reduce global H3K27me3 and DNA methylation, as well as allows EIN2 to negatively regulate the expression of genes related to plant cell division and elongation [100,101,102,103,104], thereby promoting catabolism and inhibiting anabolism to maintain cellular energy homeostasis under stress. Taken together, the level of IAA is a decisive factor in the trade-off between plant growth and stress response.
3.4. Class IV PRTase
3.4.1. The Role of Class IV PRTase in the Histidine Biosynthesis Pathway
ATP–PRT, the only member of class IV PRTase, catalyzes the first step of His biosynthesis, which is the condensation of ATP and PRPP to form phosphoribosyl ATP (PRATP) [40]. ATP–PRT is the rate-limiting enzyme in the His biosynthesis pathway and is feedback inhibited by His [105]. ATP–PRT is a key regulator of His accumulation since the level of His in plants largely depends on ATP–PRT activity [33]. There are two ATP–PRT homologous proteins in Arabidopsis with 74.6% similarity, and both contain chloroplast transit peptides at the N-terminus [106]. Overexpression of either isoform ATP–PRT is sufficient to increase free His level by 41-fold in Arabidopsis bud tissue without altering the content of other amino acids [33].
3.4.2. Histidine Regulates Plant Growth and Development
His is an essential amino acid for plant growth and development. In Arabidopsis, mutations at the His biosynthesis pathway gene are lethal [33]. His residue functions as a conserved catalytic site for many enzymes due to its imidazole functional group, which gives it a unique advantage in participating in acid-base catalyzed reactions [33]. Among these enzymes, histidine kinases such as cytokinin receptors, ethylene receptors, and phytochromes are widely involved in signal transduction during plant growth and development [107]. The biosynthesis of His appears to be a limiting factor for the biosynthesis of these important histidine kinases since His is one of the least abundant amino acid residues in proteins [33]. Thus, changes in His biosynthesis may influence cytokinin receptor synthesis to regulate cytokinin signaling and chloroplast development (Figure 5).
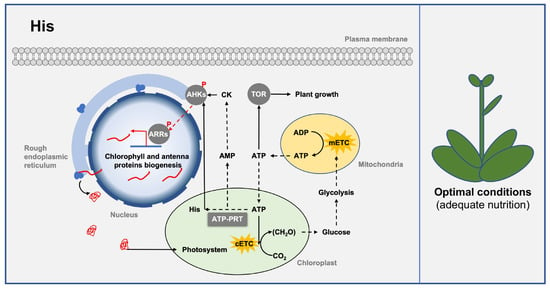
Figure 5.
Class Ⅳ PRTase in the histidine biosynthesis pathway regulates plant growth and development. Class Ⅳ PRTase ATP–PRT regulates chloroplast development and plant growth under nutrient-adequate conditions by influencing intracellular His level and AMP/ATP metabolism. ATP–PRT is represented by a square box, and other proteins are represented by round boxes. Solid arrows indicate direct interactions or one-step reactions, and dashed arrows indicate indirect interactions, multistep reactions, or transmembrane transport. Abbreviations for proteins are as follows: Arabidopsis histidine kinase (AHK), Arabidopsis response regulator (ARR), target of rapamycin (TOR), ATP phosphoribosyltransferase (ATP–PRT). Abbreviations for metabolites are shown in the Abbreviations list.
His biosynthesis may also affect cellular energy homeostasis (Figure 5). The biosynthesis of His is an energy-consuming process, since it is estimated that 31–41 ATPs are required for one His molecule, and cells with uncontrolled His biosynthesis consume 2.5% of its total metabolic energy [33,108,109]. Furthermore, the His biosynthesis pathway also releases the adenine nucleotide intermediate AlCAR into the de novo adenine nucleotide biosynthesis pathway to generate AMP [33], thereby downregulating the intracellular energy charge. Importantly, a significant negative correlation between biomass and His content has been observed in Arabidopsis overexpressing AtATP–PRT [33]. These results suggest that moderate His biosynthesis is essential to maintain energy homeostasis and plant growth.
4. Crosstalk between the PRTase-Related Metabolic Pathways
Due to sharing the substrate PRPP, PRTase leads to crosstalk between related metabolic pathways. Furthermore, PRTase-related metabolic pathways play a synergistic role in regulating plant growth, development, and abiotic stress response.
4.1. Common Substrate of PRTases
PRPP, the intersection of PRTase-related metabolic pathways, is essential for all organisms. The competitive utilization of the common substrate PRPP by different PRTase makes it flow to the corresponding metabolic pathway. Hove-Jensen et al. [110] reported that PRPP mainly flows to the purine and pyrimidine nucleotide biosynthesis pathways. In Escherichia coli, purine and pyrimidine nucleotide biosynthesis each consume 30% to 40% of PRPP, His and Try biosynthesis each consume 10% to 15% of PRPP, and NAD(P)+ biosynthesis consumes approximately 1% of PRPP [110]. However, the competition and distribution of PRPP in PRTase-related metabolic pathways in plants remain to be elucidated. Notably, the content of PRPP is an important factor limiting plant growth since overexpression of the PRPP synthetase gene significantly increases biomass accumulation in Arabidopsis and Nicotiana tabacum [78]. Hence, the competitive utilization of limited PRPP by PRTase regulates the biosynthesis of PRTase-related metabolites during plant growth.
4.2. Common Function of PRTase-Related Metabolic Pathways
The PRTase-related metabolic pathways coordinately promote plant growth and development (Figure 6A). Adenine nucleotide and His, Try, NAD(P)+ promote cytokinin signaling, IAA signaling, and mRNA 5′ NAD+ cap modification, respectively, and thus participate in chloroplast development and photosynthesis. Moreover, photosynthesis-derived glucose generates ATP through oxidative phosphorylation, which together with moderate levels of IAA activates TOR [111]. Importantly, TOR promotes central carbon and energy metabolism, as well as key anabolic processes, including nucleotide, amino acid, lipid, and cell wall synthesis, which are essential for rapid growth [88,100,101,111,112,113,114,115]. Overall, the PRTase-related metabolic pathways are jointly involved in plant growth and development by promoting chloroplast development and activating TOR.
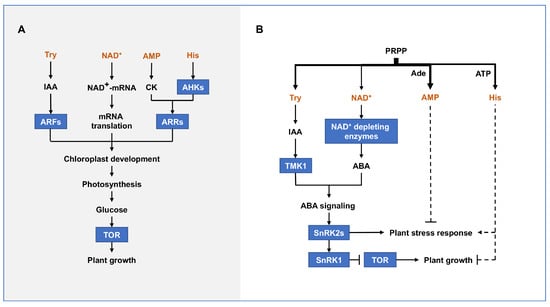
Figure 6.
The PRTase-related metabolic pathways co-regulate plant growth (A) and abiotic stress response (B). Proteins are represented by square boxes. Dotted lines indicate unclear roles and mechanisms. Abbreviations for proteins are as follows: auxin response factor (ARF), Arabidopsis histidine kinase (AHK), Arabidopsis response regulator (ARR), target of rapamycin (TOR), transmembrane kinase 1 (TMK1), sucrose non-fermenting 1-related protein kinase 2 (SnRK2), sucrose non-fermenting 1-related protein kinase 1 (SnRK1). Abbreviations for metabolites are shown in the Abbreviations list.
PRTase-related metabolic pathways also synergistically affect plant abiotic stress responses and the switching between growth and stress responses (Figure 6B). Under abiotic stress conditions, high levels of NAD+ and IAA directly activates ABA signaling in response to nutrient deficiencies and energy limitations. Importantly, changes in the flow of any PRTase-related metabolic pathway may alter the flux of PRPP to NAD+ or IAA biosynthesis pathways, leading to plant abiotic stress responses. For example, although the NAD+ content of multiple NAD+ biosynthesis-deficient mutants in Arabidopsis and rice is significantly reduced, they instead have an ABA hypersensitivity phenotype and excessive accumulation of ROS, resulting in growth inhibition and premature leaf senescence [12,60,116,117]. In addition, mutations in the adenine nucleotide salvage enzyme AtAPRT1 lead to enhanced oxidation and high-temperature tolerance in Arabidopsis [13]. His biosynthesis is also a potentially important factor in inhibiting plant growth under energy-limited conditions [33].
In a mechanism similar to that of promoting plant abiotic stress response, PRTases may participate in fruit ripening (Figure 7). To date, significant increases in free NAD+ and IAA levels have been observed before the initiation of tomato fruit ripening (i.e., system II ethylene production) [31,118]. High levels of NAD+ and IAA may be critical for system II ethylene production and fruit ripening since they can trigger ABA signaling that mediates SlSnRK1 activation. On the one hand, activated SlSnRK1 may promote the expression of the key regulator SlRIN in the autocatalytic loop of system II ethylene and the ethylene biosynthesis genes by inactivating SlTOR and activating demethylase to remove H3K27me3 and DNA methylation [99,119]. In fact, heterologous overexpression of Malus hupehensis SnRK1 advanced tomato fruit ripening by about 10 days [120,121], whereas the silencing of SlSnRK1 expression in tomato fruit by VIGS delayed or completely inhibited ripening [122]. On the other hand, in addition to increased ethylene biosynthesis, activated SlSnRK1 may also enhance fruit sensitivity to ethylene, which is necessary for fruit ripening [123,124]. Specifically, SlSnRK1-mediated SlTOR inactivation stabilizes SlEIN3/EIL1 in a SlEIN2-dependent manner to induce downstream ethylene-responsive factors expression, resulting in increased fruit sensitivity to ethylene [101,125]. Huang et al. [126] constructed the tomato SlEIN2 mutant slein2-1 and found it was insensitive to ethylene and completely arrested fruit ripening. Taken together, PRTase-related metabolic pathways are potentially critical in fruit ripening, mediating SlSnRK1 activation and SlTOR inactivation through the activation of ABA signaling.
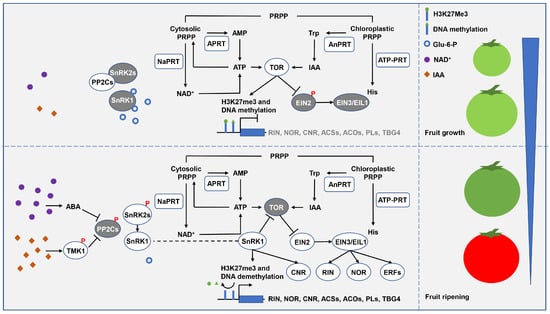
Figure 7.
A possible model for the regulation of fruit growth and ripening by the PRTase-related metabolic pathways. Under nutrient-enriched conditions, SnRK1 activity is inhibited, and ATP and moderate levels of IAA synergistically activate TOR to promote fruit growth. However, under nutrient-limited conditions, high levels of NAD+ and IAA activate ABA signaling, so activated SnRK1 inhibits TOR activity and promotes fruit ripening. PRTases are represented by square boxes, other proteins are represented by round boxes. Active proteins are white, and inactivated proteins are grey. Abbreviations for proteins and genes are as follows: transmembrane kinase 1 (TMK1), protein phosphatase 2C (PP2C), sucrose non-fermenting 1-related protein kinase 2 (SnRK2), sucrose non-fermenting 1-related protein kinase 1 (SnRK1), nicotinamide phosphoribosyl transferase (NaPRT), adenine phosphoribosyltransferase (APRT), target of rapamycin (TOR), anthranilate phosphoribosyltransferase (AnPRT), ATP phosphoribosyltransferase (ATP–PRT), ethylene insensitive 2 (EIN2), ethylene-insensitive proteins (EIN3/EIL1), colorless non-ripening (CNR), ripening inhibitor (RIN), non-ripening (NOR), ethylene-responsive factor (ERF), ACC synthase (ACS), ACC oxidase (ACO), pectate lyases (PL), tomato beta-galactosidase 4 (TBG4). Abbreviations for metabolites are shown in the Abbreviations list.
5. Conclusions and Perspectives
PRTases contribute to nucleic acid and protein synthesis, energy metabolism, hormonal signaling, and epigenetics by promoting the biosynthesis of adenine nucleotides, NAD(P)+, Try, and His. Importantly, these metabolites play an essential role in chloroplast development and plant growth through cytokinin signaling, IAA signaling, and NAD+ cap modification of mRNA. In addition, PRTases co-regulate plant abiotic stress response via crosstalk with each other. Under abiotic stress conditions, increased biosynthesis of NAD+ and Try leads to elevated levels of free NAD+ and IAA, which govern the transition of plants from growth to stress response through ABA signaling and epigenetic remodeling. In this process, NAD-dependent enzymes and the conserved SnRK1-TOR axis regulatory modules are key factors, down-regulating anabolism and up-regulating catabolism through histone acetylation/methylation remodeling and transcriptional reprogramming to coordinate nutrient supply and plant growth.
It is a brilliant strategy for plants to use the levels of PRTase-related metabolites that are necessary for growth as a signal to guide the transition between plant growth and stress response. Specifically, plants can sense a stressful environment by monitoring elevated levels of these intracellular metabolites, rapidly turning on the switch of stress response, which then triggers a reduction in these metabolite levels, further turning off the switch of growth, and vice versa. Until now, studies have found that levels of NAD+ and Try play a decisive role in the trade-off between plant growth and stress response, but the importance of adenine nucleotides and His has been underestimated. Elucidating the role and mechanism of adenine nucleotide and His biosynthesis in plant growth defense tradeoffs is an urgent but challenging goal. This knowledge will guide us in precisely regulating the levels of PRTase-related metabolites in plants to help maximize plant yield under fluctuating environmental conditions.
Author Contributions
Writing—original draft preparation, Y.L.; writing—conceived and revised, W.W. and B.Z.; writing—review and editing, P.W. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China National Key R&D Program during the 14th Five-year Plan Period (Grant No. 2022YFD2100103) and the National Natural Science Foundation of China (Grant NO. 32272373 and 31871847) to B.Z.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Component | Abbreviation |
| Glutamine | Gln |
| Formylglycinamide ribonucleotide | FGAR |
| 4-carboxy aminoimidazole ribonucleotide | CAIR |
| 5-formaminoimidazole-4-carboxamide ribonucleotide | FAICAR |
| Adenine | Ade |
| Adenosine triphosphate | ATP |
| Carbamoyl phosphate | CP |
| Orotic acid | OA |
| Uridine diphosphate | UDP |
| Aspartate | Asp |
| Nicotinic acid mononucleotide nicotinamide mononucleotide | NaMN |
| Nicotinamide | Nam |
| Nicotinamide adenine dinucleotide | NADH |
| 1-(2-carboxyphenylamino)-1-deoxyribulose-5-phosphate | IGP |
| Auxin | IAA |
| Pro-phosphoribosyl formimino-5-aminoimidazole-4-carboxamide ribonucleotide | ProFAR |
| Imidazoleacetol phosphate | IAP |
| Histidine | His |
| Abscisic acid | ABA |
| Glycinamide ribonucleotide | GAR |
| 5-aminoimidazole ribonucleotide | AIR |
| 5-aminoimidazole-4-carboxamide ribonucleotide | AICAR |
| Adenosine monophosphate | AMP |
| Adenosine diphosphate | ADP |
| Guanosine monophosphate | GMP |
| Dihydro-orotate | DHO |
| Uridine monophosphate | UMP |
| Cytosine triphosphate | CTP |
| 5-phosphoribosylamine | PRA |
| Formylglycinamidine ribonucleotide | FGAM |
| N-succinyl-5-aminoimidazole-4-carboxamide ribonucleotide | SAICAR |
| Inosine monophosphate | IMP |
| Adenosine | Ado |
| Xanthosine monophosphate | XMP |
| Carbamoyl aspartate | CA |
| Orotidine 5′-monophosphate | OMP |
| Uridine triphosphate | UTP |
| Iminoaspartate | IA |
| Nicotinic acid adenine dinucleotide | NaAD |
| Nicotinate | NA |
| Anthranilate | ANT |
| Indole-3-glycerol-phosphate | IND |
| 5-phosphoribosyl ATP | PRATP |
| Phosphoribulosyl formimino-5-aminoimidazole-4-carboxamide ribonucleotide | PRFAR |
| Histidinol phosphate | HolP |
| Cytokinin | CK |
| Glucose 6-phosphate | Glu-6-P |
| Quinolinate | QA |
| Nicotinamide adenine dinucleotide | NAD+ |
| Nicotinamide adenine dinucleotide phosphate | NADP+ |
| 5-phosphoribosyl anthranilate | PRANT |
| tryptophan | Try |
| 5-phosphoribosyl AMP | PRAMP |
| Imidazoleglycerol phosphate | IGP |
| Histidinol | Hol |
| 5-phosphoribosyl-1-pyrophosphate | PRPP |
References
- Scheible, W.R.; Pauly, M. Glycosyltransferases and cell wall biosynthesis: Novel players and insights. Curr. Opin. Plant Biol. 2004, 7, 285–295. [Google Scholar] [CrossRef]
- Lairson, L.L.; Henrissat, B.; Davies, G.J.; Withers, S.G. Glycosyltransferases: Structures, functions, and mechanisms. Annu. Rev. Biochem. 2008, 77, 521–555. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Sangwan, R.S.; Sangwan, N.S. Plant secondary metabolism linked glycosyltransferases: An update on expanding knowledge and scopes. Biotechnol. Adv. 2016, 34, 714–739. [Google Scholar] [CrossRef] [PubMed]
- Moremen, K.W.; Haltiwanger, R.S. Emerging structural insights into glycosyltransferase-mediated synthesis of glycans. Nat. Chem. Biol. 2019, 15, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.C.; Smith, J.L. The PRT protein family. Curr. Opin. Struct. Biol. 2001, 11, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, H. Current aspects of auxin biosynthesis in plants. Biosci. Biotechnol. Biochem. 2016, 80, 34–42. [Google Scholar] [CrossRef]
- Gakière, B.; Fernie, A.R.; Pétriacq, P. More to NAD+ than meets the eye: A regulator of metabolic pools and gene expression in Arabidopsis. Free Radic. Biol. Med. 2018, 122, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.F.; Chen, L.J.; Boldt, R.; Sun, C.W.; Li, H.M. Characterization of Arabidopsis glutamine phosphoribosyl pyrophosphate amidotransferase-deficient mutants. Plant Physiol. 2004, 135, 1314–1323. [Google Scholar] [CrossRef]
- Katoh, A.; Uenohara, K.; Akita, M.; Hashimoto, T. Early steps in the biosynthesis of NAD in Arabidopsis start with aspartate and occur in the plastid. Plant Physiol. 2006, 141, 851–857. [Google Scholar] [CrossRef]
- Mainguet, S.E.; Gakière, B.; Majira, A.; Pelletier, S.; Bringel, F.; Guérard, F.; Caboche, M.; Berthomé, R.; Renou, J.P. Uracil salvage is necessary for early Arabidopsis development. Plant J. 2009, 60, 280–291. [Google Scholar] [CrossRef]
- Woo, N.S.; Gordon, M.J.; Graham, S.R.; Rossel, J.B.; Badger, M.R.; Pogson, B.J. A mutation in the purine biosynthetic enzyme ATASE2 impacts high light signalling and acclimation responses in green and chlorotic sectors of Arabidopsis leaves. Funct. Plant Biol. 2011, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ren, D.; Hu, S.; Li, G.; Dong, G.; Jiang, L.; Hu, X.; Ye, W.; Cui, Y.; Zhu, L.; et al. Down-Regulation of a Nicotinate Phosphoribosyltransferase Gene, OsNaPRT1, Leads to Withered Leaf Tips. Plant Physiol. 2016, 171, 1085–1098. [Google Scholar] [CrossRef]
- Sukrong, S.; Yun, K.Y.; Stadler, P.; Kumar, C.; Facciuolo, T.; Moffatt, B.A.; Falcone, D.L. Improved growth and stress tolerance in the Arabidopsis oxt1 mutant triggered by altered adenine metabolism. Mol. Plant 2012, 5, 1310–1332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Lin, X.; Hong, X.; Zhu, Y.; Li, W.; He, W.; An, F.; Guo, H. Adenine phosphoribosyl transferase 1 is a key enzyme catalyzing cytokinin conversion from nucleobases to nucleotides in Arabidopsis. Mol. Plant 2013, 6, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shang, Z.; Wang, L.; Lu, Q.; Wen, X.; Chi, W.; Zhang, L.; Lu, C. Purine biosynthetic enzyme ATase2 is involved in the regulation of early chloroplast development and chloroplast gene expression in Arabidopsis. Photosynth. Res. 2015, 126, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Li, J.R.; Shen, H.L.; Yang, Y.M.; Fan, S.T.; Li, K.; Guo, Y.S.; Lin, H.; Liu, Z.D.; Guo, X.W. VaAPRT3 Gene is Associated With Sex Determination in Vitis amurensis. Front. Genet. 2021, 12, 727260. [Google Scholar] [CrossRef]
- Pan, S.; Li, K.E.; Huang, W.; Zhong, H.; Wu, H.; Wang, Y.; Zhang, H.; Cai, Z.; Guo, H.; Chen, X.; et al. Arabidopsis DXO1 possesses deNADding and exonuclease activities and its mutation affects defense-related and photosynthetic gene expression. J. Integr. Plant Biol. 2020, 62, 967–983. [Google Scholar] [CrossRef]
- Liu, X.; Wei, W.; Zhu, W.; Su, L.; Xiong, Z.; Zhou, M.; Zheng, Y.; Zhou, D.X. Histone Deacetylase AtSRT1 Links Metabolic Flux and Stress Response in Arabidopsis. Mol. Plant 2017, 10, 1510–1522. [Google Scholar] [CrossRef]
- Perveen, S.; Rashid, N.; Tang, X.F.; Imanaka, T.; Papageorgiou, A.C. Anthranilate phosphoribosyltransferase from the hyperthermophilic archaeon Thermococcus kodakarensis shows maximum activity with zinc and forms a unique dimeric structure. FEBS Open Bio 2017, 7, 1217–1230. [Google Scholar] [CrossRef]
- Lohkamp, B.; McDermott, G.; Campbell, S.A.; Coggins, J.R.; Lapthorn, A.J. The structure of Escherichia coli ATP-phosphoribosyltransferase: Identification of substrate binding sites and mode of AMP inhibition. J. Mol. Biol. 2004, 336, 131–144. [Google Scholar] [CrossRef]
- Shin, D.H.; Oganesyan, N.; Jancarik, J.; Yokota, H.; Kim, R.; Kim, S.H. Crystal structure of a nicotinate phosphoribosyltransferase from Thermoplasma acidophilum. J. Biol. Chem. 2005, 280, 18326–18335. [Google Scholar] [CrossRef]
- Marino, M.; Deuss, M.; Svergun, D.I.; Konarev, P.V.; Sterner, R.; Mayans, O. Structural and mutational analysis of substrate complexation by anthranilate phosphoribosyltransferase from Sulfolobus solfataricus. J. Biol. Chem. 2006, 281, 21410–21421. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, M.; Kuang, Z.; Yue, J.; Xue, L.; Zhu, M.; Zhu, Z.; Khan, M.H.; Niu, L. Crystal structures of anthranilate phosphoribosyltransferase from Saccharomyces cerevisiae. Acta Crystallogr. F Struct. Biol. Commun. 2021, 77, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Mittelstädt, G.; Jiao, W.; Livingstone, E.K.; Moggré, G.J.; Nazmi, A.R.; Parker, E.J. A dimeric catalytic core relates the short and long forms of ATP-phosphoribosyltransferase. Biochem. J. 2018, 475, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Ruszkowski, M. Guarding the gateway to Histidine biosynthesis in plants: Medicago truncatula ATP-phosphoribosyltransferase in relaxed and tense states. Biochem. J. 2018, 475, 2681–2697. [Google Scholar] [CrossRef] [PubMed]
- Vos, S.; Parry, R.J.; Burns, M.R.; de Jersey, J.; Martin, J.L. Structures of free and complexed forms of Escherichia coli xanthine-guanine phosphoribosyltransferase. J. Mol. Biol. 1998, 282, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Silva, C.H.; Iulek, J.; Oliva, G.; Thiemann, O.H. Crystal structure of adenine phosphoribosyltransferase from Leishmania tarentolae: Potential implications for APRT catalytic mechanism. Biochim. Biophys. Acta 2004, 1696, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.S.; Patterson, D.N.; Ncube, Z.; Toth, E.A. The crystal structure of human quinolinic acid phosphoribosyltransferase in complex with its inhibitor phthalic acid. Proteins 2014, 82, 405–414. [Google Scholar] [CrossRef]
- Scully, T.W.; Jiao, W.; Mittelstädt, G.; Parker, E.J. Structure, mechanism and inhibition of anthranilate phosphoribosyltransferase. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220039. [Google Scholar] [CrossRef]
- Zrenner, R.; Stitt, M.; Sonnewald, U.; Boldt, R. Pyrimidine and purine biosynthesis and degradation in plants. Annu. Rev. Plant Biol. 2006, 57, 805–836. [Google Scholar] [CrossRef]
- Decros, G.; Beauvoit, B.; Colombié, S.; Cabasson, C.; Bernillon, S.; Arrivault, S.; Guenther, M.; Belouah, I.; Prigent, S.; Baldet, P.; et al. Regulation of Pyridine Nucleotide Metabolism During Tomato Fruit Development Through Transcript and Protein Profiling. Front. Plant Sci. 2019, 10, 1201. [Google Scholar] [CrossRef]
- Mano, Y.; Nemoto, K. The pathway of auxin biosynthesis in plants. J. Exp. Bot. 2012, 63, 2853–2872. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.D.; Ingle, R.A.; Smith, J.A. Relative contributions of nine genes in the pathway of Histidine biosynthesis to the control of free Histidine concentrations in Arabidopsis thaliana. Plant Biotechnol. J. 2009, 7, 499–511. [Google Scholar] [CrossRef] [PubMed]
- van der Graaff, E.; Hooykaas, P.; Lein, W.; Lerchl, J.; Kunze, G.; Sonnewald, U.; Boldt, R. Molecular analysis of “de novo” purine biosynthesis in solanaceous species and in Arabidopsis thaliana. Front. Biosci. 2004, 9, 1803–1816. [Google Scholar] [CrossRef]
- Rosar, C.; Kanonenberg, K.; Nanda, A.M.; Mielewczik, M.; Bräutigam, A.; Novák, O.; Strnad, M.; Walter, A.; Weber, A.P. The leaf reticulate mutant dov1 is impaired in the first step of purine metabolism. Mol. Plant 2012, 5, 1227–1241. [Google Scholar] [CrossRef]
- Xing, Q.; Ru, Z.; Li, J.; Zhou, C.; Jin, D.; Sun, Y.; Wang, B. Cloning of a second form of adenine phosphoribosyltransferase gene (TaAPT2) from wheat and analysis of its association with thermo-sensitive genic male sterility (TGMS). Plant Sci. 2005, 169, 37–45. [Google Scholar] [CrossRef]
- Zhou, C.J.; Li, J.; Zou, J.C.; Liang, F.S.; Ye, C.J.; Jin, D.M.; Weng, M.L.; Wang, B. Cloning and characterization of a second form of the rice adenine phosphoribosyl transferase gene (OsAPT2) and its association with TGMS. Plant Mol. Biol. 2006, 60, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qian, W.; Liu, X.; Qin, H.; Wang, D. Molecular and functional analysis of hypoxanthine-guanine phosphoribosyltransferase from Arabidopsis thaliana. New Phytol. 2007, 175, 448–461. [Google Scholar] [CrossRef]
- Khan, S.; Pandey, S.S.; Jyotshna Shanker, K.; Khan, F.; Rahman, L.U. Cloning and functional characterization of quinolinic acid phosphoribosyl transferase (QPT) gene of Nicotiana tabacum. Physiol. Plant. 2017, 160, 253–265. [Google Scholar] [CrossRef]
- Muralla, R.; Sweeney, C.; Stepansky, A.; Leustek, T.; Meinke, D. Genetic dissection of Histidine biosynthesis in Arabidopsis. Plant Physiol. 2007, 144, 890–903. [Google Scholar] [CrossRef]
- Moffatt, B.A.; Ashihara, H. Purine and pyrimidine nucleotide synthesis and metabolism. Arab. Book 2002, 1, e0018. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Shiraishi, H.; Okada, K.; Shimura, Y. Two amidophosphoribosyltransferase genes of Arabidopsis thaliana expressed in different organs. Plant Mol. Biol. 1994, 26, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, B.; Somerville, C. Positive selection for male-sterile mutants of Arabidopsis lacking adenine phosphoribosyl transferase activity. Plant Physiol. 1988, 86, 1150–1154. [Google Scholar] [CrossRef]
- Wu, S.; Yu, Z.; Wang, F.; Li, W.; Yang, Q.; Ye, C.; Sun, Y.; Jin, D.; Zhao, J.; Wang, B. Identification and characterization of a novel adenine phosphoribosyltransferase gene (ZmAPT2) from maize (Zea mays L.). DNA Seq. 2008, 19, 357–365. [Google Scholar] [CrossRef]
- Hewitt, M.M.; Carr, J.M.; Williamson, C.L.; Slocum, R.D. Effects of phosphate limitation on expression of genes involved in pyrimidine synthesis and salvaging in Arabidopsis. Plant Physiol. Biochem. 2005, 43, 91–99. [Google Scholar] [CrossRef]
- Yin, Y.; Shimano, F.; Ashihara, H. Involvement of rapid nucleotide synthesis in recovery from phosphate starvation of Catharanthus roseus cells. J. Exp. Bot. 2007, 58, 1025–1033. [Google Scholar] [CrossRef]
- Mimura, K.; Sakamaki, J.I.; Morishita, H.; Kawazu, M.; Mano, H.; Mizushima, N. Genome-wide CRISPR screening reveals nucleotide synthesis negatively regulates autophagy. J. Biol. Chem. 2021, 296, 100780. [Google Scholar] [CrossRef]
- Busche, M.; Scarpin, M.R.; Hnasko, R.; Brunkard, J.O. TOR coordinates nucleotide availability with ribosome biogenesis in plants. Plant Cell 2021, 33, 1615–1632. [Google Scholar] [CrossRef]
- Astot, C.; Dolezal, K.; Nordström, A.; Wang, Q.; Kunkel, T.; Moritz, T.; Chua, N.H.; Sandberg, G. An alternative cytokinin biosynthesis pathway. Proc. Natl. Acad. Sci. USA 2000, 97, 14778–14783. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; Qin, W.; Moreau, F.; Moffatt, B. Adenine phosphoribosyltransferase isoforms of Arabidopsis and their potential contributions to adenine and cytokinin metabolism. Physiol. Plant. 2002, 115, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, C.; Moffatt, B.A.; Blacker, M.; Laloue, M. Male sterility associated with APRT deficiency in Arabidopsis thaliana results from a mutation in the gene APT1. Mol. Genet. Genom. 1998, 257, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Bockwoldt, M.; Houry, D.; Niere, M.; Gossmann, T.I.; Reinartz, I.; Schug, A.; Ziegler, M.; Heiland, I. Identification of evolutionary and kinetic drivers of NAD-dependent signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 15957–15966. [Google Scholar] [CrossRef]
- Yang, L.; Shen, J.; Liu, C.; Kuang, Z.; Tang, Y.; Qian, Z.; Guan, M.; Yang, Y.; Zhan, Y.; Li, N.; et al. Nicotine rebalances NAD+ homeostasis and improves aging-related symptoms in male mice by enhancing NAMPT activity. Nat. Commun. 2023, 14, 900. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Doamekpor, S.K.; Bird, J.G.; Nickels, B.E.; Tong, L.; Hart, R.P.; Kiledjian, M. 5′ End Nicotinamide Adenine Dinucleotide Cap in Human Cells Promotes RNA Decay through DXO-Mediated deNADding. Cell 2017, 168, 1015–1027.e10. [Google Scholar] [CrossRef]
- Kwasnik, A.; Wang, V.Y.; Krzyszton, M.; Gozdek, A.; Zakrzewska-Placzek, M.; Stepniak, K.; Poznanski, J.; Tong, L.; Kufel, J. Arabidopsis DXO1 links RNA turnover and chloroplast function independently of its enzymatic activity. Nucleic Acids Res. 2019, 47, 4751–4764. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Zhao, Y.; You, C.; Le, B.; Gong, Z.; Mo, B.; Xia, Y.; Chen, X. NAD+-capped RNAs are widespread in the Arabidopsis transcriptome and can probably be translated. Proc. Natl. Acad. Sci. USA 2019, 116, 12094–12102. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, H.; Zhang, S.; Shao, X.; Ni, M.; Cai, Z.; Chen, X.; Xia, Y. NAD tagSeq reveals that NAD+-capped RNAs are mostly produced from a large number of protein-coding genes in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 12072–12077. [Google Scholar] [CrossRef]
- Yu, X.; Willmann, M.R.; Vandivier, L.E.; Trefely, S.; Kramer, M.C.; Shapiro, J.; Guo, R.; Lyons, E.; Snyder, N.W.; Gregory, B.D. Messenger RNA 5′ NAD+ Capping Is a Dynamic Regulatory Epitranscriptome Mark That Is Required for Proper Response to Abscisic Acid in Arabidopsis. Dev. Cell 2021, 56, 125–140.e6. [Google Scholar] [CrossRef]
- Fernie, A.; Hashida, S.-N.; Yoshimura, K.; Gakière, B.; Mou, Z.; Pétriacq, P. Editorial: NAD Metabolism and Signaling in Plants. Front. Plant Sci. 2020, 11, 146. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, Z.; Shi, H.; Yao, J.; Liu, X.; Wang, F.; Zeng, L.; Xie, Z.; Zhu, J.K. Reciprocal regulation between nicotinamide adenine dinucleotide metabolism and abscisic acid and stress response pathways in Arabidopsis. PLoS Genet. 2020, 16, e1008892. [Google Scholar] [CrossRef]
- Waadt, R.; Hitomi, K.; Nishimura, N.; Hitomi, C.; Adams, S.R.; Getzoff, E.D.; Schroeder, J.I. FRET-based reporters for the direct visualization of abscisic acid concentration changes and distribution in Arabidopsis. eLife 2014, 3, e01739. [Google Scholar] [CrossRef]
- Feitosa-Araujo, E.; da Fonseca-Pereira, P.; Pena, M.M.; Medeiros, D.B.; Perez de Souza, L.; Yoshida, T.; Weber, A.P.M.; Araújo, W.L.; Fernie, A.R.; Schwarzländer, M.; et al. Changes in intracellular NAD status affect stomatal development in an abscisic acid-dependent manner. Plant J. 2020, 104, 1149–1168. [Google Scholar] [CrossRef]
- Liu, W.C.; Song, R.F.; Zheng, S.Q.; Li, T.T.; Zhang, B.L.; Gao, X.; Lu, Y.T. Coordination of plant growth and abiotic stress responses by Tryptophan Synthase β Subunit1 through modulating tryptophan and ABA homeostasis in Arabidopsis. Mol. Plant 2022, 15, 973–990. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fichman, Y.; Mittler, R. Vascular Bundles Mediate Systemic Reactive Oxygen Signaling during Light Stress. Plant Cell 2020, 32, 3425–3435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Li, P.; Zhao, L.; Qi, F.; Htwe, N.M.P.S.; Li, Q.; Zhang, D.; Lin, F.; Shang-Guan, K.; Liang, Y. The receptor-like cytoplasmic kinase RIPK regulates broad-spectrum ROS signaling in multiple layers of plant immune system. Mol. Plant. 2021, 14, 1652–1667. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Li, P.; Hong, X.; Xu, C.; Wang, R.; Liang, Y. The receptor-like cytosolic kinase RIPK activates NADP-malic enzyme 2 to generate NADPH for fueling ROS production. Mol. Plant 2022, 15, 887–903. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, Z.; Yu, X.; Ye, P.; Liu, H.; Xue, X.; Yang, L.; Li, Z.; Wu, Y.; Fang, C.; et al. Direct Gating of the TRPM2 Channel by cADPR via Specific Interactions with the ADPR Binding Pocket. Cell Rep. 2019, 27, 3684–3695.e4. [Google Scholar] [CrossRef]
- Kupis, W.; Pałyga, J.; Tomal, E.; Niewiadomska, E. The role of sirtuins in cellular homeostasis. J. Physiol. Biochem. 2016, 72, 371–380. [Google Scholar] [CrossRef]
- Briggs, A.G.; Bent, A.F. Poly(ADP-ribosyl)ation in plants. Trends Plant Sci. 2011, 16, 372–380. [Google Scholar] [CrossRef]
- Vanderauwera, S.; De Block, M.; Van de Steene, N.; van de Cotte, B.; Metzlaff, M.; Van Breusegem, F. Silencing of poly(ADP-ribose) polymerase in plants alters abiotic stress signal transduction. Proc. Natl. Acad. Sci. USA 2007, 104, 15150–15155. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.P.; Duque, P.; Chua, N.H. ABA activates ADPR cyclase and cADPR induces a subset of ABA-responsive genes in Arabidopsis. Plant J. 2004, 38, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Sun, Q.; Qin, F.; Li, C.; Zhao, Y.; Zhou, D.X. Down-regulation of a SILENT INFORMATION REGULATOR2-related Histone deacetylase gene, OsSRT1, induces DNA fragmentation and cell death in rice. Plant Physiol. 2007, 144, 1508–1519. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, H.; Zhao, Y.; Sun, Q.; Hu, Y.; Peng, H.; Zhou, D.X. The rice NAD(+)-dependent Histone deacetylase OsSRT1 targets preferentially to stress- and metabolism-related genes and transposable elements. PLoS ONE 2013, 8, e66807. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhou, D.X. Rice NAD+-dependent Histone deacetylase OsSRT1 represses glycolysis and regulates the moonlighting function of GAPDH as a transcriptional activator of glycolytic genes. Nucleic Acids Res. 2017, 45, 12241–12255. [Google Scholar] [CrossRef]
- Ha, H.C.; Snyder, S.H. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc. Natl. Acad. Sci. USA 1999, 96, 13978–13982. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ishikawa, K.; Harada, K.; Fukusaki, E.; Yoshimura, K.; Shigeoka, S. Overexpression of an ADP-ribose pyrophosphatase, AtNUDX2, confers enhanced tolerance to oxidative stress in Arabidopsis plants. Plant J. 2009, 57, 289–301. [Google Scholar] [CrossRef]
- Koslowsky, S.; Riegler, H.; Bergmüller, E.; Zrenner, R. Higher biomass accumulation by increasing phosphoribosylpyrophosphate synthetase activity in Arabidopsis thaliana and Nicotiana tabacum. Plant Biotechnol. J. 2008, 6, 281–294. [Google Scholar] [CrossRef]
- Morino, K.; Matsuda, F.; Miyazawa, H.; Sukegawa, A.; Miyagawa, H.; Wakasa, K. Metabolic profiling of tryptophan-overproducing rice calli that express a feedback-insensitive alpha subunit of anthranilate synthase. Plant Cell Physiol. 2005, 46, 514–521. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Sazuka, T.; Kamiya, N.; Nishimura, T.; Ohmae, K.; Sato, Y.; Imamura, K.; Nagato, Y.; Koshiba, T.; Nagamura, Y.; Ashikari, M.; et al. A rice tryptophan deficient dwarf mutant, tdd1, contains a reduced level of indole acetic acid and develops abnormal flowers and organless embryos. Plant J. 2009, 60, 227–241. [Google Scholar] [CrossRef]
- Li, R.; Jiang, J.; Jia, S.; Zhu, X.; Su, H.; Li, J. Overexpressing broccoli tryptophan biosynthetic genes BoTSB1 and BoTSB2 promotes biosynthesis of IAA and indole glucosinolates. Physiol. Plant. 2020, 168, 174–187. [Google Scholar] [CrossRef]
- Cao, M.; Chen, R.; Li, P.; Yu, Y.; Zheng, R.; Ge, D.; Zheng, W.; Wang, X.; Gu, Y.; Gelová, Z.; et al. TMK1-mediated auxin signalling regulates differential growth of the apical hook. Nature 2019, 568, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Sagar, M.; Chervin, C.; Mila, I.; Hao, Y.; Roustan, J.P.; Benichou, M.; Gibon, Y.; Biais, B.; Maury, P.; Latché, A.; et al. SlARF4, an auxin response factor involved in the control of sugar metabolism during tomato fruit development. Plant Physiol. 2013, 161, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Mei, L.; Wu, M.; Wei, W.; Shan, W.; Gong, Z.; Zhang, Q.; Yang, F.; Yan, F.; Zhang, Q.; et al. SlARF10, an auxin response factor, is involved in chlorophyll and sugar accumulation during tomato fruit development. J. Exp. Bot. 2018, 69, 5507–5518. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Gong, Z.; Tang, Y.; Wu, M.; Yan, F.; Zhang, X.; Zhang, Q.; Yang, F.; Hu, X.; et al. Auxin response factor 6A regulates photosynthesis, sugar accumulation, and fruit development in tomato. Hortic. Res. 2019, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Dai, N.; Chen, J.; Nagawa, S.; Cao, M.; Li, H.; Zhou, Z.; Chen, X.; De Rycke, R.; Rakusová, H.; et al. Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 2014, 343, 1025–1028. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Liu, Y.; Li, H.; Fu, L.; Liu, Z.; Xu, L.; Liu, H.; Xu, T.; Xiong, Y. Differential TOR activation and cell proliferation in Arabidopsis root and shoot apexes. Proc. Natl. Acad. Sci. USA 2017, 114, 2765–2770. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, P.; Yu, Y.; Xiong, Y. Glucose-TOR signaling regulates PIN2 stability to orchestrate auxin gradient and cell expansion in Arabidopsis root. Proc. Natl. Acad. Sci. USA 2020, 117, 32223–32225. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Hoyt, J.M.; Hamilton, A.A.; Alonso, J.M. A Link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 2005, 17, 2230–2242. [Google Scholar] [CrossRef]
- Friml, J.; Gallei, M.; Gelová, Z.; Johnson, A.; Mazur, E.; Monzer, A.; Rodriguez, L.; Roosjen, M.; Verstraeten, I.; Živanović, B.D.; et al. ABP1-TMK auxin perception for global phosphorylation and auxin canalization. Nature 2022, 609, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Belda-Palazón, B.; Adamo, M.; Valerio, C.; Ferreira, L.J.; Confraria, A.; Reis-Barata, D.; Rodrigues, A.; Meyer, C.; Rodriguez, P.L.; Baena-González, E. A dual function of SnRK2 kinases in the regulation of SnRK1 and plant growth. Nat. Plants. 2020, 6, 1345–1353. [Google Scholar] [CrossRef]
- Yang, J.; He, H.; He, Y.; Zheng, Q.; Li, Q.; Feng, X.; Wang, P.; Qin, G.; Gu, Y.; Wu, P.; et al. TMK1-based auxin signaling regulates abscisic acid responses via phosphorylating ABI1/2 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2102544118. [Google Scholar] [CrossRef] [PubMed]
- Nukarinen, E.; Nägele, T.; Pedrotti, L.; Wurzinger, B.; Mair, A.; Landgraf, R.; Börnke, F.; Hanson, J.; Teige, M.; Baena-Gonzalez, E.; et al. Quantitative phosphoproteomics reveals the role of the AMPK plant ortholog SnRK1 as a metabolic master regulator under energy deprivation. Sci. Rep. 2016, 6, 31697. [Google Scholar] [CrossRef]
- Hey, S.J.; Byrne, E.; Halford, N.G. The interface between metabolic and stress signalling. Ann. Bot. 2010, 105, 197–203. [Google Scholar] [CrossRef]
- Cho, Y.H.; Hong, J.W.; Kim, E.C.; Yoo, S.D. Regulatory functions of SnRK1 in stress-responsive gene expression and in plant growth and development. Plant Physiol. 2012, 158, 1955–1964. [Google Scholar] [CrossRef]
- Broeckx, T.; Hulsmans, S.; Rolland, F. The plant energy sensor: Evolutionary conservation and divergence of SnRK1 structure, regulation, and function. J. Exp. Bot. 2016, 67, 6215–6252. [Google Scholar] [CrossRef]
- Ramon, M.; Dang, T.V.T.; Broeckx, T.; Hulsmans, S.; Crepin, N.; Sheen, J.; Rolland, F. Default Activation and Nuclear Translocation of the Plant Cellular Energy Sensor SnRK1 Regulate Metabolic Stress Responses and Development. Plant Cell 2019, 31, 1614–1632. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.; Li, J.; Zhang, X.; Hu, F.; Zhao, Y.; Zhou, D.X. SnRK1 stimulates the Histone H3K27me3 demethylase JMJ705 to regulate a transcriptional switch to control energy homeostasis. Plant Cell 2021, 33, 3721–3742. [Google Scholar] [CrossRef]
- Zhu, T.; Li, L.; Feng, L.; Mo, H.; Ren, M. Target of Rapamycin Regulates Genome Methylation Reprogramming to Control Plant Growth in Arabidopsis. Front. Genet. 2020, 11, 186. [Google Scholar] [CrossRef]
- Fu, L.; Liu, Y.; Qin, G.; Wu, P.; Zi, H.; Xu, Z.; Zhao, X.; Wang, Y.; Li, Y.; Yang, S.; et al. The TOR-EIN2 axis mediates nuclear signalling to modulate plant growth. Nature 2021, 591, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, J.; Li, G. Dynamic epigenetic modifications in plant sugar signal transduction. Trends Plant Sci. 2022, 27, 379–390. [Google Scholar] [CrossRef]
- Belda-Palazón, B.; Costa, M.; Beeckman, T.; Rolland, F.; Baena-González, E. ABA represses TOR and root meristem activity through nuclear exit of the SnRK1 kinase. Proc. Natl. Acad. Sci. USA 2022, 119, e2204862119. [Google Scholar] [CrossRef]
- Ye, R.; Wang, M.; Du, H.; Chhajed, S.; Koh, J.; Liu, K.H.; Shin, J.; Wu, Y.; Shi, L.; Xu, L.; et al. Glucose-driven TOR-FIE-PRC2 signalling controls plant development. Nature 2022, 609, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Pisco, J.P.; de Chiara, C.; Pacholarz, K.J.; Garza-Garcia, A.; Ogrodowicz, R.W.; Walker, P.A.; Barran, P.E.; Smerdon, S.J.; de Carvalho, L.P.S. Uncoupling conformational states from activity in an allosteric enzyme. Nat. Commun. 2017, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Ohta, D.; Fujimori, K.; Mizutani, M.; Nakayama, Y.; Kunpaisal-Hashimoto, R.; Münzer, S.; Kozaki, A. Molecular cloning and characterization of ATP-phosphoribosyl transferase from Arabidopsis, a key enzyme in the Histidine biosynthetic pathway. Plant Physiol. 2000, 122, 907–914. [Google Scholar] [CrossRef]
- Hoang, X.L.T.; Prerostova, S.; Thu, N.B.A.; Thao, N.P.; Vankova, R.; Tran, L.P. Histidine Kinases: Diverse Functions in Plant Development and Responses to Environmental Conditions. Annu. Rev. Plant Biol. 2021, 72, 297–323. [Google Scholar] [CrossRef]
- Akashi, H.; Gojobori, T. Metabolic efficiency and amino acid composition in the proteomes of Escherichia coli and Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2002, 99, 3695–3700. [Google Scholar] [CrossRef]
- Swire, J. Selection on synthesis cost affects interprotein amino acid usage in all three domains of life. J. Mol. Evol. 2007, 64, 558–571. [Google Scholar] [CrossRef]
- Hove-Jensen, B.; Andersen, K.R.; Kilstrup, M.; Martinussen, J.; Switzer, R.L.; Willemoës, M. Phosphoribosyl Diphosphate (PRPP): Biosynthesis, Enzymology, Utilization, and Metabolic Significance. Microbiol. Mol. Biol. Rev. 2016, 81, e00040-16. [Google Scholar] [CrossRef]
- Xiong, Y.; McCormack, M.; Li, L.; Hall, Q.; Xiang, C.; Sheen, J. Glucose-TOR signalling reprograms the transcriptome and activates meristems. Nature 2013, 496, 181–186. [Google Scholar] [CrossRef]
- Ben-Sahra, I.; Howell, J.J.; Asara, J.M.; Manning, B.D. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 2013, 339, 1323–1328. [Google Scholar] [CrossRef]
- Ben-Sahra, I.; Hoxhaj, G.; Ricoult, S.J.H.; Asara, J.M.; Manning, B.D. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 2016, 351, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, A.M.; Christen, S.; Shimobayashi, M.; Cornu, M.; Fava, L.L.; Moes, S.; Prescianotto-Baschong, C.; Sauer, U.; Jenoe, P.; Hall, M.N. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science 2013, 339, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.M.; Oh, G.G.K.; Lee, C.P.; Millar, A.H. Metabolite Regulatory Interactions Control Plant Respiratory Metabolism via Target of Rapamycin (TOR) Kinase Activation. Plant Cell 2020, 32, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Pichersky, E. Nicotinamidase participates in the salvage pathway of NAD biosynthesis in Arabidopsis. Plant J. 2007, 49, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Zhuang, Y.; Li, H.; Li, P.; Huo, H.; Shu, D.; Huang, W.; Wang, S. The cloning and characterization of hypersensitive to salt stress mutant, affected in quinolinate synthase, highlights the involvement of NAD in stress-induced accumulation of ABA and proline. Plant J. 2020, 102, 85–98. [Google Scholar] [CrossRef]
- Pattison, R.J.; Catalá, C. Evaluating auxin distribution in tomato (Solanum lycopersicum) through an analysis of the PIN and AUX/LAX gene families. Plant J. 2012, 70, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; How-Kit, A.; Stammitti, L.; Teyssier, E.; Rolin, D.; Mortain-Bertrand, A.; Halle, S.; Liu, M.; Kong, J.; Wu, C.; et al. A DEMETER-like DNA demethylase governs tomato fruit ripening. Proc. Natl. Acad. Sci. USA 2015, 112, 10804–10809. [Google Scholar] [CrossRef]
- Li, G.; Peng, F.; Zhang, L.; Shi, X.; Wang, Z. Cloning and characterization of a SnRK1-encoding gene from Malus hupehensis Rehd. and heterologous expression in tomato. Mol. Biol. Rep. 2010, 37, 947–954. [Google Scholar] [CrossRef]
- Wang, X.; Peng, F.; Li, M.; Yang, L.; Li, G. Expression of a heterologous SnRK1 in tomato increases carbon assimilation, nitrogen uptake and modifies fruit development. J. Plant Physiol. 2012, 169, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Wang, X.; Ye, B.; Jin, M.; Chen, W.; Wang, Y.; Zhou, Y.; Blanks, A.M.; Gu, M.; Zhang, P.; et al. Molecular and functional characterization of the SBP-box transcription factor SPL-CNR in tomato fruit ripening and cell death. J. Exp. Bot. 2020, 71, 2995–3011. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J.J. Fruit ripening mutants yield insights into ripening control. Curr. Opin. Plant Biol. 2007, 10, 283–289. [Google Scholar] [CrossRef]
- Shin, J.H.; Mila, I.; Liu, M.; Rodrigues, M.A.; Vernoux, T.; Pirrello, J.; Bouzayen, M. The RIN-regulated Small Auxin-Up RNA SAUR69 is involved in the unripe-to-ripe phase transition of tomato fruit via enhancement of the sensitivity to ethylene. New Phytol. 2019, 222, 820–836. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, F.; Xiong, F.; Deng, K.; Li, Z.; Ren, M. Target of Rapamycin (TOR) Negatively Regulates Ethylene Signals in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 2680. [Google Scholar] [CrossRef]
- Huang, W.; Hu, N.; Xiao, Z.; Qiu, Y.; Yang, Y.; Yang, J.; Mao, X.; Wang, Y.; Li, Z.; Guo, H. A molecular framework of ethylene-mediated fruit growth and ripening processes in tomato. Plant Cell 2022, 34, 3280–3300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).