A Systematic Review of the Effect of Vericiguat on Patients with Heart Failure
Abstract
1. Introduction
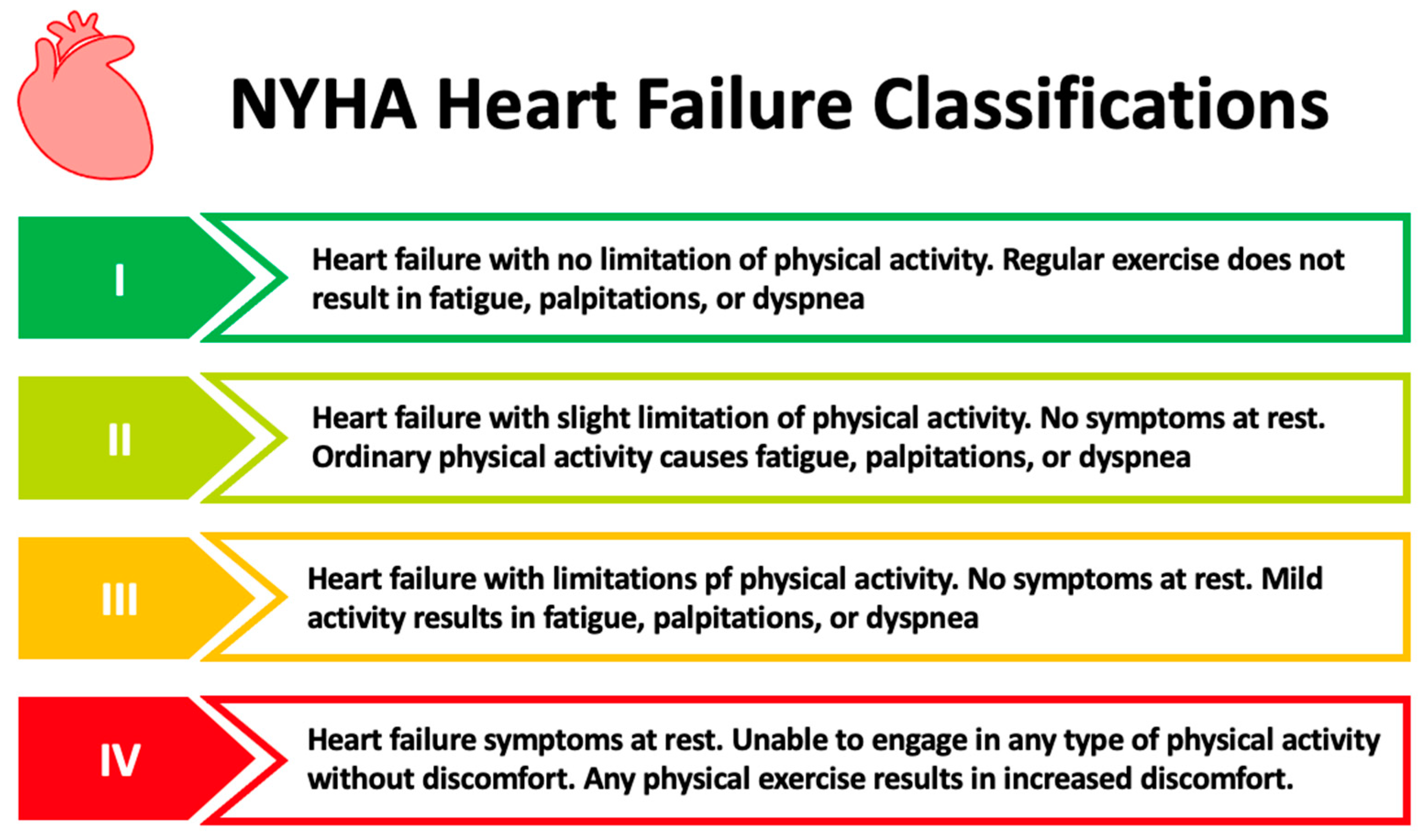
1.1. Chronic Heart Failure
1.2. Standard of Care in CHF
1.3. Mechanism of Action of Vericiguat
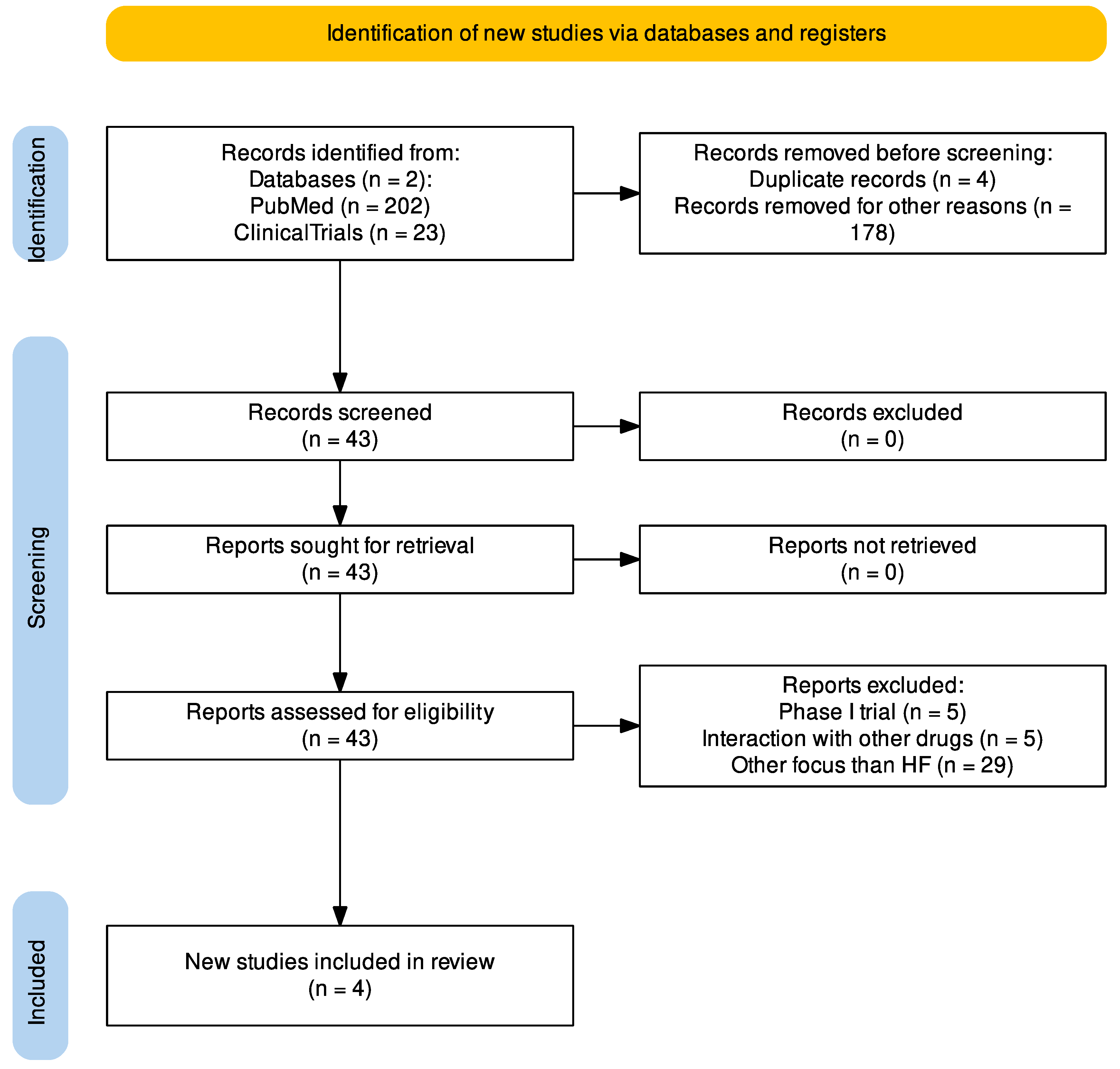
2. Methods
3. Results and Discussion
3.1. The Phase II Trials
3.2. The Phase III Trial
3.3. Ongoing Trials on Vericiguat
3.4. Treatment Strategies and Risk Profiles
3.5. Effect on HFpEF
3.6. Safety of Vericiguat
3.7. NT-proBNP and BNP Levels in HF Patients
3.8. Vericiguat vs. Standard Pharmacological Treatment
3.9. The Effect on Physiological Parameters Compared to Quality of Life
3.10. Limitations and Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roger, V.L. Epidemiology of Heart Failure A Contemporary Perspective. Circ. Res. 2021, 128, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Sorimachi, H.; Harada, T.; Kagami, K.; Saito, Y.; Ishii, H. Epidemiology, Pathophysiology, Diagnosis, and Therapy of Heart Failure With Preserved Ejection Fraction in Japan. J. Card. Fail. 2023, 29, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, A.P.; Dahlstrom, U.; Filippatos, G.; Chioncel, O.; Leiro, M.C.; Drozdz, J.; Fruhwald, F.; Gullestad, L.; Logeart, D.; Fabbri, G.; et al. EURObservational Research Programme: Regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur. J. Heart Fail. 2013, 15, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.R.; Roalfe, A.K.; Adoki, I.; Hobbs, F.D.R.; Taylor, C.J. Survival of patients with chronic heart failure in the community: A systematic review and meta-analysis. Eur. J. Heart Fail. 2019, 21, 1306–1325. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkien, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Greene, S.J.; Butler, J.; Albert, N.M.; DeVore, A.D.; Sharma, P.P.; Duffy, C.I.; Hill, C.L.; McCague, K.; Mi, X.J.; Patterson, J.H.; et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2018, 72, 351–366. [Google Scholar] [CrossRef]
- Merck Announces U.S. FDA Approval of VERQUVO. Available online: https://www.merck.com/news/merck-announces-u-s-fda-approval-of-verquvo-vericiguat/ (accessed on 22 May 2023).
- EMA. Verquvo. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/verquvo (accessed on 22 May 2023).
- Marti, C.N.; Gheorghiade, M.; Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Quyyumi, A.A.; Butler, J. Endothelial dysfunction, arterial stiffness, and heart failure. J. Am. Coll. Cardiol. 2012, 60, 1455–1469. [Google Scholar] [CrossRef]
- Butler, J.; Yang, M.; Manzi, M.A.; Hess, G.P.; Patel, M.J.; Rhodes, T.; Givertz, M.M. Clinical Course of Patients With Worsening Heart Failure With Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2019, 73, 935–944. [Google Scholar] [CrossRef]
- Metra, M.; Teerlink, J.R. Heart failure. Lancet 2017, 390, 1981–1995. [Google Scholar] [CrossRef]
- Classes of Heart Failure. Available online: https://www.heart.org/en/health-topics/heart-failure/what-is-heart-failure/classes-of-heart-failure (accessed on 13 May 2023).
- White, M.; Ducharme, A.; Ibrahim, R.; Whittom, L.; Lavoie, J.; Guertin, M.C.; Racine, N.; He, Y.; Yao, G.Y.; Rouleau, J.L.; et al. Increased systemic inflammation and oxidative stress in patients with worsening congestive heart failure: Improvement after short-term inotropic support. Clin. Sci. 2006, 110, 483–489. [Google Scholar] [CrossRef]
- Munzel, T.; Gori, T.; Keaney, J.F., Jr.; Maack, C.; Daiber, A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur. Heart J. 2015, 36, 2555–2564. [Google Scholar] [CrossRef]
- Sawyer, D.B.; Siwik, D.A.; Xiao, L.; Pimentel, D.R.; Singh, K.; Colucci, W.S. Role of oxidative stress in myocardial hypertrophy and failure. J. Mol. Cell Cardiol. 2002, 34, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, P.S.; Polegato, B.F.; Minicucci, M.F.; Paiva, S.A.R.; Zornoff, L.A.M. Cardiac Remodeling: Concepts, Clinical Impact, Pathophysiological Mechanisms and Pharmacologic Treatment. Arq. Bras. Cardiol. 2016, 106, 62–69. [Google Scholar] [CrossRef]
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.P.; Parissis, J.; Laroche, C.; Piepoli, M.F.; Fonseca, C.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1574–1585. [Google Scholar] [CrossRef]
- Hartupee, J.; Mann, D.L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 2017, 14, 30–38. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byuny, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary. J. Am. Coll. Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef]
- Løgstrup, B.B.; Wolsk, E.; Dridi, N.P. Kronisk Hjertesvigt. Available online: https://nbv.cardio.dk/chf#58-farmakologisk-behandling-af-hfpef-og-hfmref (accessed on 29 May 2023).
- Clinic, C. Aldosterone. Available online: https://my.clevelandclinic.org/health/articles/24158-aldosterone (accessed on 29 May 2023).
- Frishman, W.H. Beta-Adernergic Blockers. Circ. Am. Heart Assoc. J. 2003, 107, e117–e119. [Google Scholar]
- Sica, D.A. Mineralocorticoid Receptor Antagonists for Treatment of Hypertension and Heart Failure. Methodist. Debakey Cardiovasc. J. 2015, 11, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Boron, W.F.; Boulpaep, E.L. Medical Physiology E-Book, 3rd ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Oppermann, M.; Hansen, P.B.; Castrop, H.; Schnermann, J. Vasodilatation of afferent arterioles and paradoxical increase of renal vascular resistance by furosemide in mice. Am. J. Physiol. Renal Physiol. 2007, 293, F279–F287. [Google Scholar] [CrossRef]
- Padda, I.S.; Mahtani, A.U.; Parmar, M. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fala, L. Entresto (Sacubitril/Valsartan): First-in-Class Angiotensin Receptor Neprilysin Inhibitor FDA Approved for Patients with Heart Failure. Am. Health Drug Benefits 2015, 8, 330–334. [Google Scholar]
- EMA. Procoralan (Ivabradine). Available online: tps://www.ema.europa.eu/en/medicines/human/EPAR/procoralan (accessed on 29 May 2023).
- Shah, A.; Gandhi, D.; Srivastava, S.; Shah, K.J.; Mansukhani, R. Heart Failure: A Class Review of Pharmacotherapy. Pharm. Ther. 2017, 42, 464–472. [Google Scholar]
- Breitenstein, S.; Roessig, L.; Sandner, P.; Lewis, K.S. Novel sGC Stimulators and sGC Activators for the Treatment of Heart Failure. Handb. Exp. Pharmacol. 2017, 243, 225–247. [Google Scholar] [CrossRef] [PubMed]
- Evgenov, O.V.; Pacher, P.; Schmidt, P.M.; Hasko, G.; Schmidt, H.H.; Stasch, J.P. NO-independent stimulators and activators of soluble guanylate cyclase: Discovery and therapeutic potential. Nat. Rev. Drug Discov. 2006, 5, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Kassis-George, H.; Verlinden, N.J.; Fu, S.; Kanwar, M. Vericiguat in Heart Failure with a Reduced Ejection Fraction: Patient Selection and Special Considerations. Ther. Clin. Risk Manag. 2022, 18, 315–322. [Google Scholar] [CrossRef]
- Aimo, A.; Castiglione, V.; Borrelli, C.; Saccaro, L.F.; Franzini, M.; Masi, S.; Emdin, M.; Giannoni, A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur. J. Prev. Cardiol. 2020, 27, 494–510. [Google Scholar] [CrossRef]
- Tsigkou, V.; Oikonomou, E.; Anastasiou, A.; Lampsas, S.; Zakynthinos, G.E.; Kalogeras, K.; Katsioupa, M.; Kapsali, M.; Kourampi, I.; Pesiridis, T.; et al. Molecular Mechanisms and Therapeutic Implications of Endothelial Dysfunction in Patients with Heart Failure. Int. J. Mol. Sci. 2023, 24, 4321. [Google Scholar] [CrossRef]
- Mehta, J.K.; Kaur, G.; Buttar, H.S.; Bagabir, H.A.; Bagabir, R.A.; Bagabir, S.A.; Haque, S.; Tuli, H.S.; Telessy, I.G. Role of the renin-angiotensin system in the pathophysiology of coronary heart disease and heart failure: Diagnostic biomarkers and therapy with drugs and natural products. Front. Physiol. 2023, 14, 1034170. [Google Scholar] [CrossRef] [PubMed]
- Tsai, E.J.; Kass, D.A. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol. Ther. 2009, 122, 216–238. [Google Scholar] [CrossRef]
- Follmann, M.; Ackerstaff, J.; Redlich, G.; Wunder, F.; Lang, D.; Kern, A.; Fey, P.; Griebenow, N.; Kroh, W.; Becker-Pelster, E.M.; et al. Discovery of the Soluble Guanylate Cyclase Stimulator Vericiguat (BAY 1021189) for the Treatment of Chronic Heart Failure. J. Med. Chem. 2017, 60, 5146–5161. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Marti, C.N.; Sabbah, H.N.; Roessig, L.; Greene, S.J.; Bohm, M.; Burnett, J.C.; Campia, U.; Cleland, J.G.; Collins, S.P.; et al. Soluble guanylate cyclase: A potential therapeutic target for heart failure. Heart Fail. Rev. 2013, 18, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Gheorghiade, M.; Borlaug, B.A.; Pieske, B.; Vaduganathan, M.; Burnett, J.C.; Roessig, L.; Stasch, J.P.; Solomon, S.D.; Paulus, W.J.; et al. The cGMP Signaling Pathway as a Therapeutic Target in Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2013, 2, e000536. [Google Scholar] [CrossRef] [PubMed]
- Chiles, R.; Al-Horani, R.A. Vericiguat: A New Hope for Heart Failure Patients. Cardiovasc. Ther. 2022, 2022, 1554875. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Butler, J.; Filippatos, G.; Lam, C.; Maggioni, A.P.; Ponikowski, P.; Shah, S.; Solomon, S.; Kraigher-Krainer, E.; Samano, E.T.; et al. Rationale and design of the SOluble guanylate Cyclase stimulatoR in heArT failurE Studies (SOCRATES). Eur. J. Heart Fail. 2014, 16, 1026–1038. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Greene, S.J.; Butler, J.; Filippatos, G.; Lam, C.S.; Maggioni, A.P.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Kraigher-Krainer, E.; et al. Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients With Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial. JAMA 2015, 314, 2251–2262. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Maggioni, A.P.; Lam, C.S.P.; Pieske-Kraigher, E.; Filippatos, G.; Butler, J.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Scalise, A.V.; et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: Results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur. Heart J. 2017, 38, 1119–1127. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Lam, C.S.P.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; O’Connor, C.M.; Pieske, B.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; et al. Effect of Vericiguat vs Placebo on Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction: The VITALITY-HFpEF Randomized Clinical Trial. JAMA 2020, 324, 1512–1521. [Google Scholar] [CrossRef]
- Butler, J.; Lam, C.S.P.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; O’Connor, C.M.; Pieske, B.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; et al. Rationale and Design of the VITALITY-HFpEF Trial. Circ. Heart Fail. 2019, 12, e005998. [Google Scholar] [CrossRef]
- Defilippi, C.R.; Alemayehu, W.G.; Voors, A.A.; Kaye, D.; Blaustein, R.O.; Butler, J.; Ezekowitz, J.A.; Hernandez, A.F.; Lam, C.S.P.; Roessig, L.; et al. Assessment of Biomarkers of Myocardial injury, Inflammation, and Renal Function in Heart Failure With Reduced Ejection Fraction: The VICTORIA Biomarker Substudy. J. Card. Fail. 2023, 29, 448–458. [Google Scholar] [CrossRef]
- Kramer, F.; Voss, S.; Roessig, L.; Igl, B.W.; Butler, J.; Lam, C.S.P.; Maggioni, A.P.; Shah, S.J.; Pieske, B. Evaluation of high-sensitivity C-reactive protein and uric acid in vericiguat-treated patients with heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2020, 22, 1675–1683. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Roessig, L.; Patel, M.J.; Anstrom, K.J.; Butler, J.; Voors, A.A.; Lam, C.S.P.; Ponikowski, P.; Temple, T.; Pieske, B.; et al. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of the Efficacy and Safety of the Oral Soluble Guanylate Cyclase Stimulator: The VICTORIA Trial. JACC Heart Fail. 2018, 6, 96–104. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Giczewska, A.; Sliwa, K.; Edelmann, F.; Refsgaard, J.; Bocchi, E.; Ezekowitz, J.A.; Hernandez, A.F.; O’Connor, C.M.; Roessig, L.; et al. Clinical Outcomes and Response to Vericiguat According to Index Heart Failure Event: Insights From the VICTORIA Trial. JAMA Cardiol. 2021, 6, 706–712. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: Rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur. J. Heart Fail. 2013, 15, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, J.A.; O’Connor, C.M.; Troughton, R.W.; Alemayehu, W.G.; Westerhout, C.M.; Voors, A.A.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Emdin, M.; et al. N-Terminal Pro-B-Type Natriuretic Peptide and Clinical Outcomes: Vericiguat Heart Failure With Reduced Ejection Fraction Study. JACC Heart Fail. 2020, 8, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.W.; Zheng, Y.; Troughton, R.W.; Lund, L.H.; Zhang, J.; Lam, C.S.P.; Westerhout, C.M.; Blaustein, R.O.; Butler, J.; Hernandez, A.F.; et al. Sequential Evaluation of NT-proBNP in Heart Failure: Insights Into Clinical Outcomes and Efficacy of Vericiguat. JACC Heart Fail. 2022, 10, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Stebbins, A.; Melenovsky, V.; Sweitzer, N.K.; Cowie, M.R.; Stehlik, J.; Khan, M.S.; Blaustein, R.O.; Ezekowitz, J.A.; Hernandez, A.F.; et al. Vericiguat and Health-Related Quality of Life in Patients With Heart Failure With Reduced Ejection Fraction: Insights From the VICTORIA Trial. Circ. Heart Fail. 2022, 15, e009337. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Mulder, H.; Reyes, E.; Cowie, M.R.; Lassus, J.; Hernandez, A.F.; Ezekowitz, J.A.; Butler, J.; O’Connor, C.M.; Koglin, J.; et al. Renal function and the effects of vericiguat in patients with worsening heart failure with reduced ejection fraction: Insights from the VICTORIA (Vericiguat Global Study in Subjects with HFrEF) trial. Eur. J. Heart Fail. 2021, 23, 1313–1321. [Google Scholar] [CrossRef]
- Senni, M.; Alemayehu, W.G.; Sim, D.; Edelmann, F.; Butler, J.; Ezekowitz, J.; Hernandez, A.F.; Lam, C.S.P.; O’Connor, C.M.; Pieske, B.; et al. Efficacy and safety of vericiguat in patients with heart failure with reduced ejection fraction treated with sacubitril/valsartan: Insights from the VICTORIA trial. Eur. J. Heart Fail. 2022, 24, 1614–1622. [Google Scholar] [CrossRef]
- LægemiddelStyrelsen. The Clinical Trial Phases. Available online: https://laegemiddelstyrelsen.dk/en/licensing/clinical-trials/clinical-trials-of-medicinal-products/the-clinical-trial-phases/ (accessed on 22 May 2023).
- Milo-Cotter, O.; Cotter-Davison, B.; Lombardi, C.; Sun, H.; Bettari, L.; Bugatti, S.; Rund, M.; Metra, M.; Kaluski, E.; Kobrin, I.; et al. Neurohormonal activation in acute heart failure: Results from VERITAS. Cardiology 2011, 119, 96–105. [Google Scholar] [CrossRef]
- Norre, T.; Grimm, D.; Simonsen, U. Sacubitril/valsartan, sodium-glucose cotransporter 2 inhibitors and vericiguat for congestive heart failure therapy. Basic Clin. Pharmacol. Toxicol. 2022, 130, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Khan, M.S.; Marx, N.; Lam, C.S.P.; Schnaidt, S.; Ofstad, A.P.; Brueckmann, M.; Jamal, W.; et al. Effect of Empagliflozin on Cardiovascular and Renal Outcomes in Patients With Heart Failure by Baseline Diabetes Status: Results From the EMPEROR-Reduced Trial. Circulation 2021, 143, 337–349. [Google Scholar] [CrossRef]
- Butler, J.; Anstrom, K.J.; Armstrong, P.W. Comparing the Benefit of Novel Therapies Across Clinical Trials: Insights from the VICTORIA Trial. Circulation 2020, 142, 717–719. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A Novel Paradigm for Heart Failure With Preserved Ejection Fraction: Comorbidities Drive Myocardial Dysfunction and Remodeling Through Coronary Microvascular Endothelial Inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Heerebeek, L.v.; Borbély, A.; Niessen, H.W.M.; Bronzwaer, J.G.F.; Velden, J.v.d.; Stienen, G.J.M.; Linke, W.A.; Laarman, G.J.; Paulus, W.J. Myocardial Structure and Function Differ in Systolic and Diastolic Heart Failure. Circulation 2006, 113, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Mulder, H.; Lopatin, Y.; Vazquez-Tanus, J.B.; Siu, D.; Ezekowitz, J.; Pieske, B.; O’Connor, C.M.; Roessig, L.; Patel, M.J.; et al. Blood Pressure and Safety Events With Vericiguat in the VICTORIA Trial. J. Am. Heart Assoc. 2021, 10, e021094. [Google Scholar] [CrossRef]
- Boettcher, M.; Gerisch, M.; Lobmeyer, M.; Besche, N.; Thomas, D.; Gerrits, M.; Lemmen, J.; Mueck, W.; Radtke, M.; Becker, C. Metabolism and Pharmacokinetic Drug-Drug Interaction Profile of Vericiguat, A Soluble Guanylate Cyclase Stimulator: Results From Preclinical and Phase I Healthy Volunteer Studies. Clin. Pharmacokinet. 2020, 59, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Frey, R.; Muck, W.; Unger, S.; Artmeier-Brandt, U.; Weimann, G.; Wensing, G. Single-dose pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase stimulator BAY 63-2521: An ascending-dose study in healthy male volunteers. J. Clin. Pharmacol. 2008, 48, 926–934. [Google Scholar] [CrossRef]
- Clinic, C. B-Type Natriuretic Peptide (BNP) Test. Available online: https://my.clevelandclinic.org/health/diagnostics/22629-b-type-natriuretic-peptide (accessed on 28 May 2023).
- Metra, M.; Ravera, A.; Filippatos, G. Understanding worsening heart failure as a therapeutic target: Another step forward? Eur. J. Heart Fail. 2017, 19, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Ryan, J.J. Nitric Oxide Signaling in Heart Failure With Preserved Ejection Fraction. JACC Basic Transl. Sci. 2017, 2, 341–343. [Google Scholar] [CrossRef]


| Class of Drugs | Mechanism of Action | Drug Names |
|---|---|---|
| Angiotensin-converting enzyme inhibitors (ACEi) | The renin–angiotensin–aldosterone system (RAAS) plays a crucial role in maintaining the body’s fluid homeostasis and controlling blood pressure. ACEi block the conversion of angiotensin I to angiotensin II, which in turn prevents the release of aldosterone from the adrenal glands, promoting a decrease in blood pressure [19,20,21]. | Lisinopril, Enalapril, Ramipril Perindopril Trandolapril |
| Angiotensin receptor blockers (ARBs) | ARBs block the AT1 receptors and are often used as an alternative drug in cases where ACEi are not tolerated [5]. | Losartan, Valsartan Candesartan |
| Beta-adrenoceptor antagonists (BAAs) | BAAs bind to beta-adrenoceptors and block the binding of catecholamines (norepinephrine, epinephrine) to these receptors. The inhibition of the binding of catecholamines results in a reduction in HR and also a lowering in SBP [22]. | Carvedilol, Bisoprolol, Metoprolol succinate |
| Mineralocorticoid receptor antagonists (MRAs) | MRAs inhibit aldosterone’s epithelial and nonepithelial actions, thus exerting its function in the heart, kidney, and vascular beds. MRAs have the same outcome as both ACEi and ARBs [23]. | Spironolactone, Eplerenone |
| Diuretics | Diuretics promote diuresis by inhibiting the function of Na–K–Cl cotransporters (NKCC) in the thick ascending tubule of the nephron. They inhibit sodium reabsorption, thus preventing water reabsorption, and the water is excreted in the urine [24]. However, they also increase vasodilatory prostaglandins and the pressure within the proximal tubule [25]. | Furosemide, Bumetanide, Thiazides |
| Sodium–glucose transport protein 2 (SGLT2) inhibitor | SGLT-2 inhibitors increase glucose excretion in the kidneys through selective, reversible inhibition of glucose reabsorption in the proximal renal tubules, thus leading to less water reabsorption. This lowers the blood volume in the body, thus lowering the blood pressure [26]. | Dapagliflozin, Canagliflozin, Empagliflozin |
| Combination drug (ARNi) sacubitril/valsartan | The angiotensin receptor II blocker valsartan and the neprilysin inhibitor sacubitril are used in patients with reduced ejection fraction to reduce the risk of hospitalization and improve survival [27]. | Entresto |
| Ivabradine | Used for the symptomatic management of heart-related long-term stable angina in patients with coronary artery disease. It has shown effectiveness and is only recommended to patients with an HR > 70. Ivabradine is used in addition to other guideline-directed therapy. The drug works by blocking the ion current in the funny channels (also known as current) in the sinoatrial (SA) node. The SA node is the pacemaker of the heart [24], and when treated with ivabradine, the heart rate is lowered. The heart works less and requires less blood that has been oxygenated. Therefore, procoralan lessens or stops the symptoms of angina [28]. | Procoralan |
| Digoxin | Treatment with digoxin inhibits the sodium–potassium ATPase pump, resulting in positive cardiac inotropy in addition to a reduced effect from the sympathetic nervous system and responses from RAAS. The drug is used in cases of atrial fibrillation, atrial flutter, and HF [29]. | Lanoxin |
| Study Title (Clinicaltrials.gov Identifier) | Eligibility | Participants | Dose Regimen | Endpoints | Phase | Status |
|---|---|---|---|---|---|---|
| A study to learn how well the drug vericiguat works and how safe it is under real-world conditions in Indian participants after the worsening of a long-term heart condition in which the left side of the heart does not pump blood as well as it should. (Chronic Heart Failure with Reduced Ejection Fraction) (NCT05658458) | Age ≥ 18 years LVEF < 45%, NYHA class II–IV symptoms | 200 | Starting dose 2.5 mg daily, dose doubled every two weeks, end dose 10 mg daily. | CV death and HF hospitalization | 4 | Recruiting |
| The anti-myocardial fibrosis effect of vericiguat in HFrEF (ANF-HF) (NCT05799638) | Age ≥ 18 years Diagnosed with HFrEF LVEF < 45% NYHA classes II–IV | 60 | Started at 2.5 mg once daily and up-titrated to 5 mg at week 3 and to 10 mg at week 5 | The primary endpoint is the change in extracellular volume (ECV) measured by CMR. | 4 | Recruiting |
| Study of vericiguat (MK-1242) in participants with chronic heart failure with reduced ejection fraction (HFrEF) (MK-1242-035) (VICTOR) (NCT05093933) | (NYHA) Class II–IV LVEF of ≤40% Elevated NT-proBNP levels | 6000 | 2.5, 5.0, or 10.0 mg orally once daily | Composite endpoint of CV death or HF hospitalization | 3 | Recruiting |
| A study to learn more about the safety of the drug vericiguat in Japanese people with chronic heart failure who will be receiving vericiguat under real-world conditions. (NCT05666518) | Child, Adult, Older Adult NT-proBNP ≥ 1000 pg/mL or Brain Natriuretic Peptide (BNP) ≥ 192 pg/mL (sinus rhythm), NT-proBNP ≥ 1600 pg/mL or BNP ≥ 319 pg/mL (atrial fibrillation) NYHA) Class II to IV LVEF of ≤40% | 1400 | Dosage at the discretion of the treating physician | Incidence of CV death for vericiguat arm and control arm | Observational study | Recruiting |
| Efficacy, safety, and pharmacokinetics of vericiguat in pediatric participants with heart failure due to left ventricular systolic dysfunction (MK-1242-036) (NCT05714085) | Children: 29 days to 17 years; history of symptomatic chronic HF resulting from systemic left ventricular (LV) systolic dysfunction LVEF) < 45% assessed within 3 months | 342 | 2.5 mg or 5 mg or 10 mg tablet daily. 0.2 mg/mL or 1 mg/mL in suspension form. | Change from baseline to week 16 in N-terminal pro-brain natriuretic peptide (NT-proBNP) and log-transformed NT-proBNP. | 2/3 | Recruiting |
| The effect of vericiguat on peripheral vascular function, patient health status, and inflammation (NCT05420012) | Age ≥ 18 years, NYHA) Class II to III; LVEF < 45% History of chronic symptomatic HF (ACC/AHA Class C) and New York Heart Association (NYHA) Class II or III symptoms at the time of enrollment. | 24 | Starting dose of vericiguat 2.5 mg, up-titrated to 5 mg and 10 mg in a blinded fashion. | Changes in vascular function using flow-mediated vasodilation (FMD) and the six-minute walk test (6 MWT). | 4 | Recruiting |
| An observational study, called VERI-China, to learn more about how well vericiguat works and how safe it is in a real-world setting in people with chronic heart failure with reduced ejection fraction (HFrEF) in China (VERI-China) (NCT05728502) | Age ≥ 18 years, Patients with HFrEF after a recent decompensation episode (within 6 months of HF hospitalization or within 3 months of intravenous (IV) diuretics for HF not requiring hospitalization) | 2400 | Dosage at the discretion of the treating physician | Time to first occurrence of the composite of cardiovascular (CV) death or first hospitalization due to Heart Failure (HF) | Observational study | Recruiting |
| Impact of vericiguat on the hemodynamics of heart failure (NCT05704478) | Adults 18 years of age or older with NYHA functional class II, III, or IV HFrEF with LVEF < 45% within 12 months of enrollment. Elevated BNP within 30 days of enrollment; History of HF- hospitalization within 6 months of enrollment or increase in diuretic therapy without hospitalization within 3 months of enrollment | 30 | Soluble guanylate cyclase stimulator, dose not mentioned | Cardiac output (L/min) from heart catheterization. Quality-of-life assessment. | 4 | Not Yet Recruiting |
| Trial | AEs | SAE | Treatment-Emergent AEs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hypotension | Syncope | Anemia | Renal Disorder | GIT Disorder | Dizziness | Headache | |||
| SOCRATES-PRESERVED (NCT01951638) | 69.8% | 25% | 4.4% | 0.0% | NA | 5.9% | NA | NA | NA |
| SOCRATES-REDUCED (NCT01951625) | 71.4% | 31.9% | 15.4% | 4.4% | NA | Acute kidney injury 3.3% | NA | NA | NA |
| VITALITY (NCT03547583) | 62.2% | 17.6% | 4.2% | 0.8% | NA | NA | NA | NA | NA |
| VICTORIA (NCT02861534) | 80.5% | 32.8% | 9.1% | 4.0% | 1.6% | 17% | 25.3% | 18.5% | 3.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahana, U.; Wehland, M.; Simonsen, U.; Schulz, H.; Grimm, D. A Systematic Review of the Effect of Vericiguat on Patients with Heart Failure. Int. J. Mol. Sci. 2023, 24, 11826. https://doi.org/10.3390/ijms241411826
Sahana U, Wehland M, Simonsen U, Schulz H, Grimm D. A Systematic Review of the Effect of Vericiguat on Patients with Heart Failure. International Journal of Molecular Sciences. 2023; 24(14):11826. https://doi.org/10.3390/ijms241411826
Chicago/Turabian StyleSahana, Urjosee, Markus Wehland, Ulf Simonsen, Herbert Schulz, and Daniela Grimm. 2023. "A Systematic Review of the Effect of Vericiguat on Patients with Heart Failure" International Journal of Molecular Sciences 24, no. 14: 11826. https://doi.org/10.3390/ijms241411826
APA StyleSahana, U., Wehland, M., Simonsen, U., Schulz, H., & Grimm, D. (2023). A Systematic Review of the Effect of Vericiguat on Patients with Heart Failure. International Journal of Molecular Sciences, 24(14), 11826. https://doi.org/10.3390/ijms241411826








