Toxic Effects of Two Redox States of Thallium on Immortalised Hypothalamic GT1-7 Neuronal Cells
Abstract
1. Introduction
2. Results
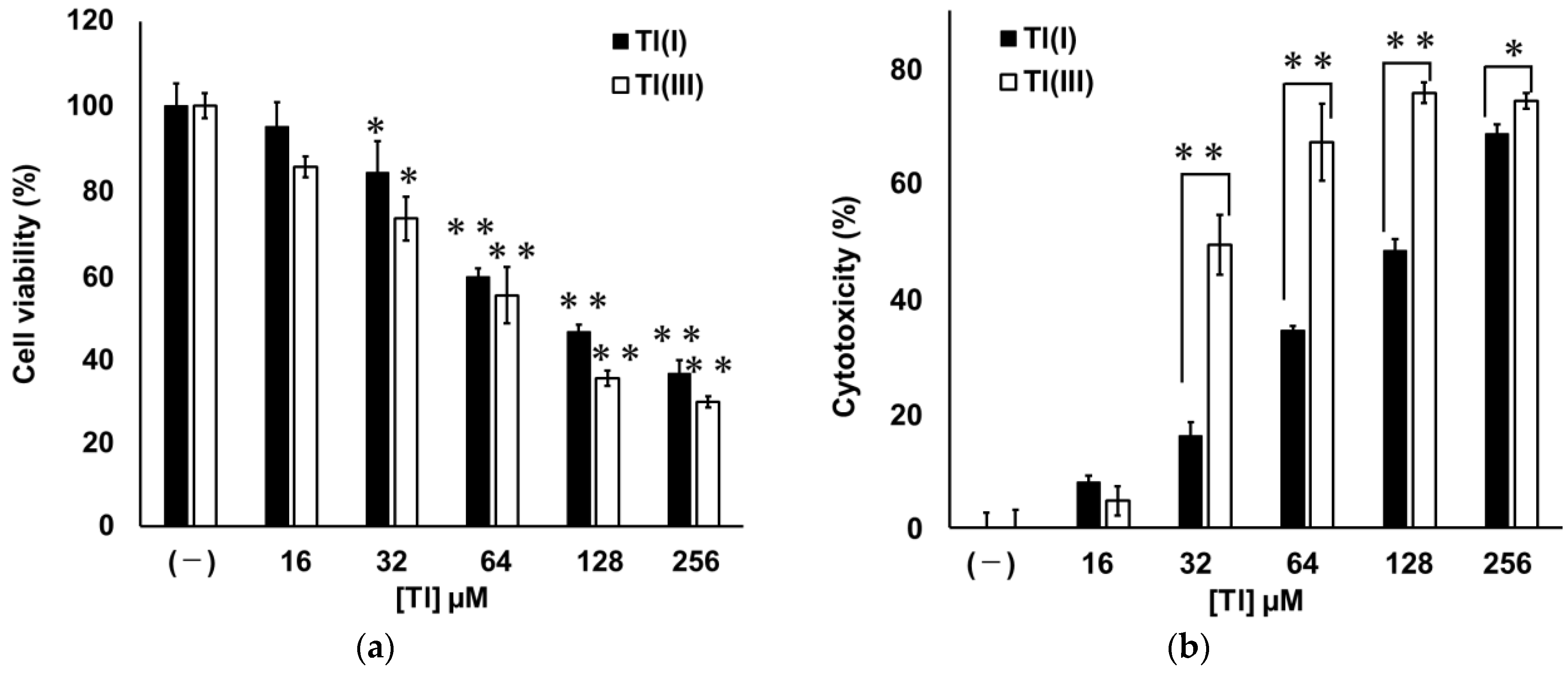
2.1. Cytotoxicities of Tl(I) and Tl(III) in GT1-7 Cells
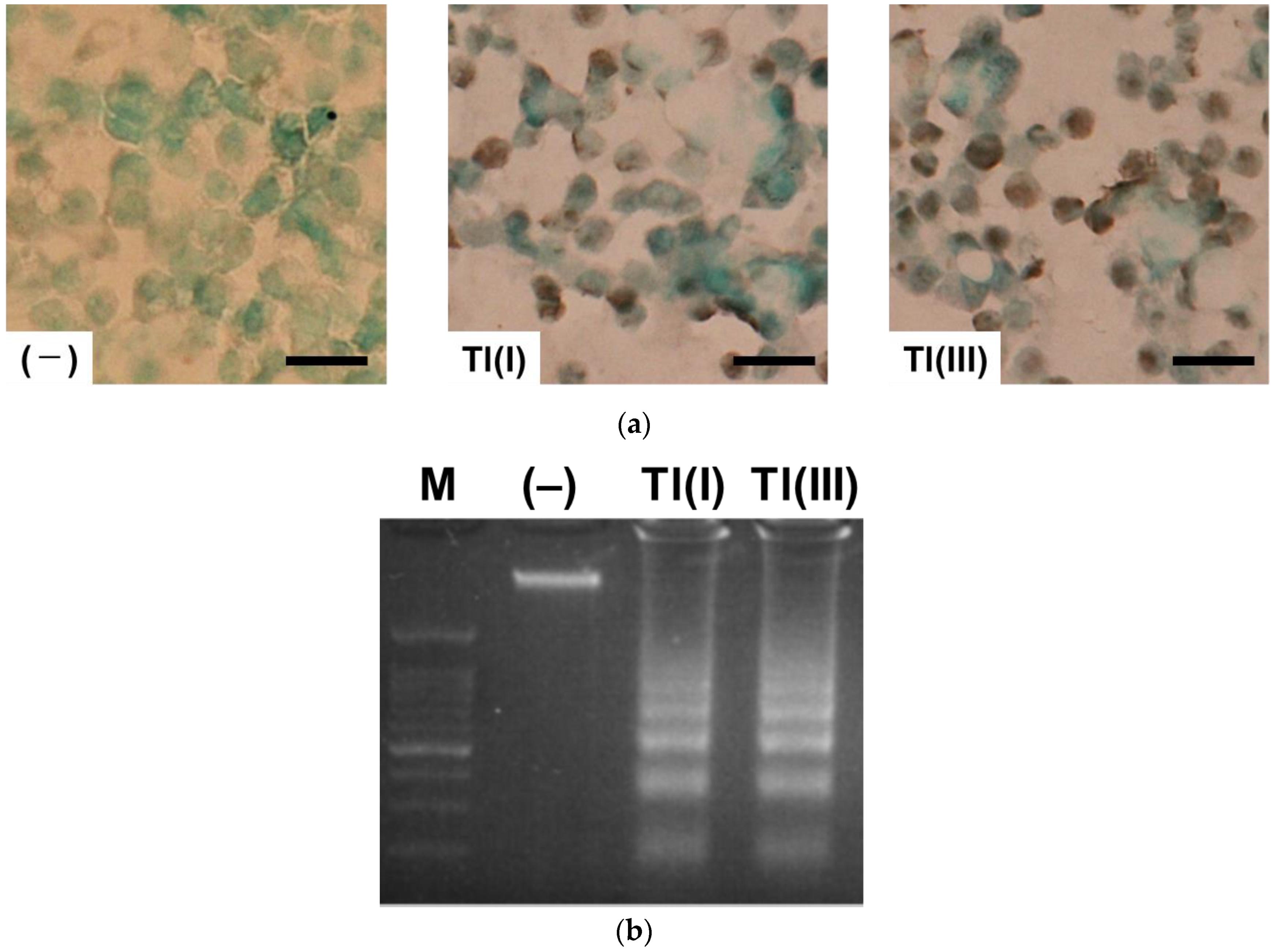
2.2. Mechanisms of GT1-7 Cytotoxicity by Tl(I) and Tl(III)
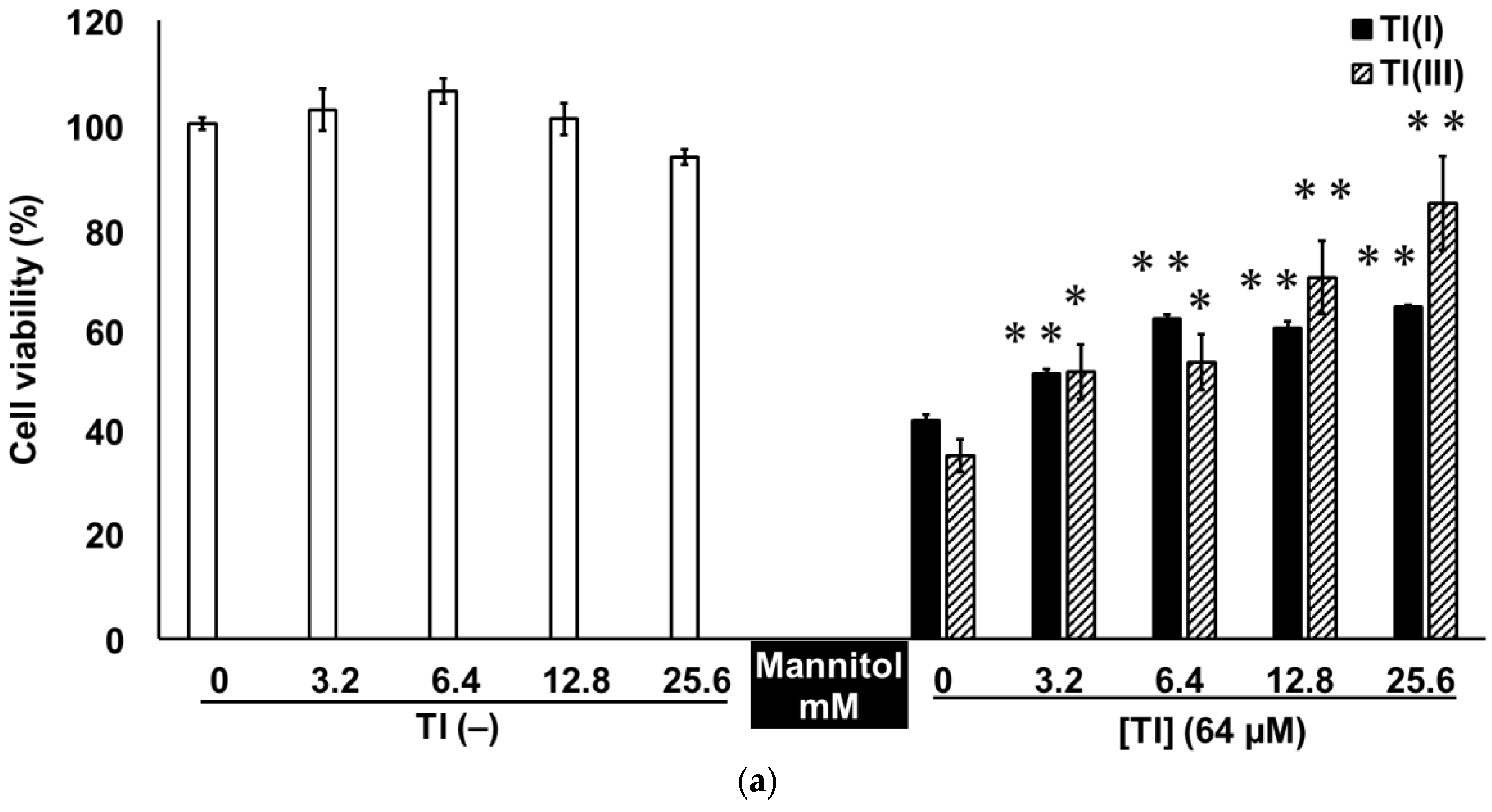
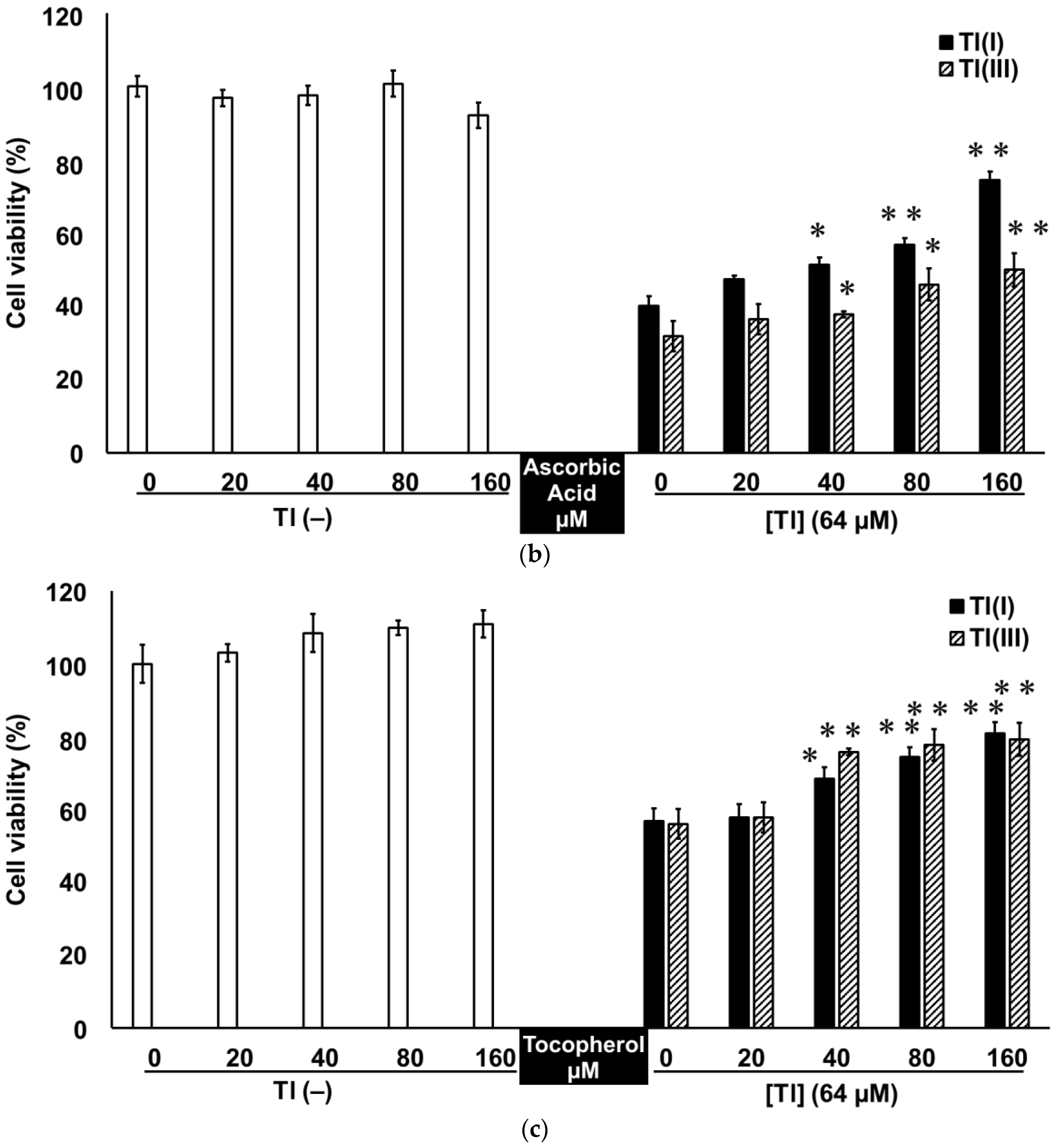
2.3. Tl(I) and Tl(III) Cause Oxidative Stress in GT1-7 Cells
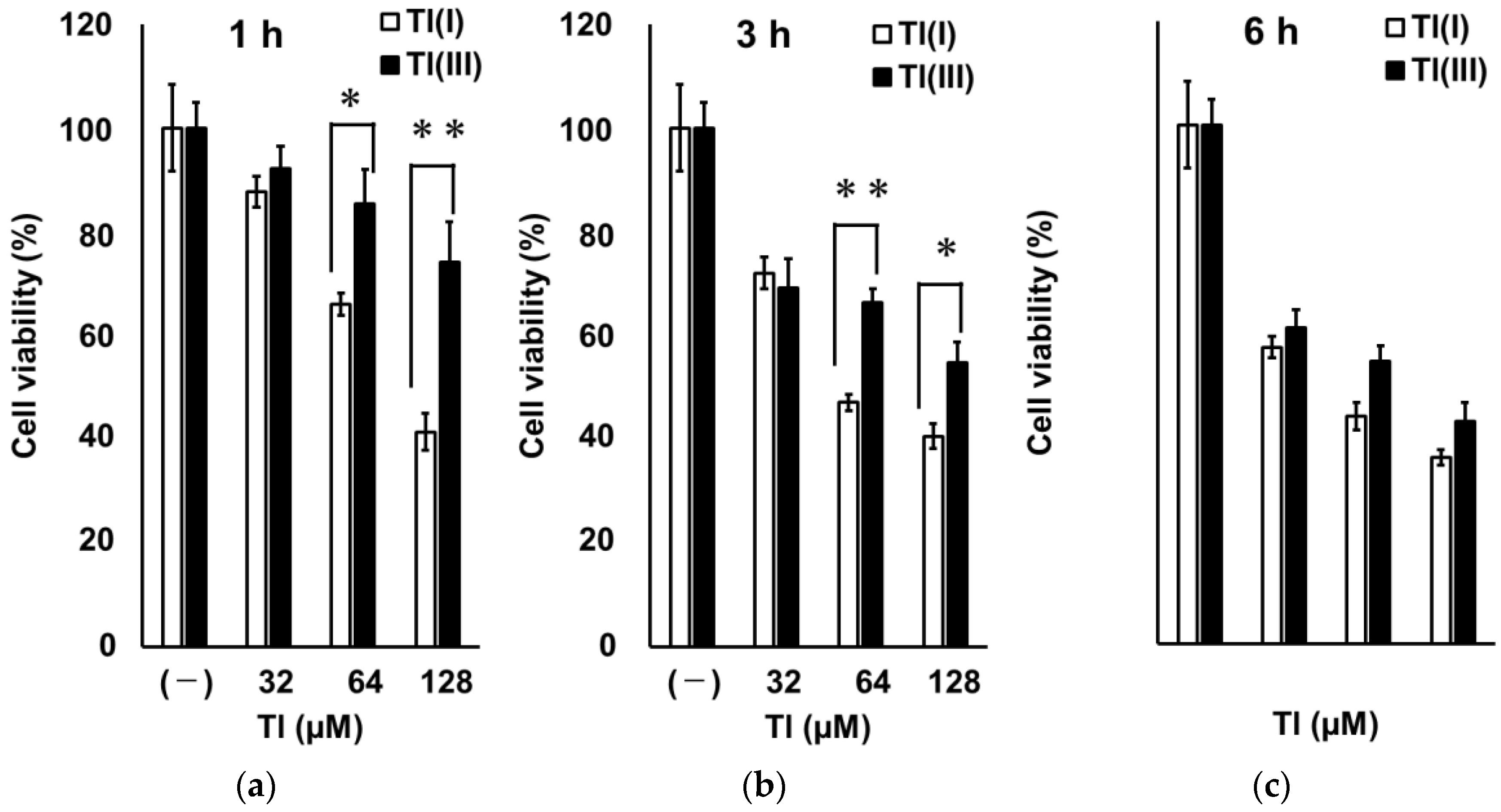
2.4. Time Course Change of GT1-7 Cytotoxicity by Tl(I) and Tl(III)
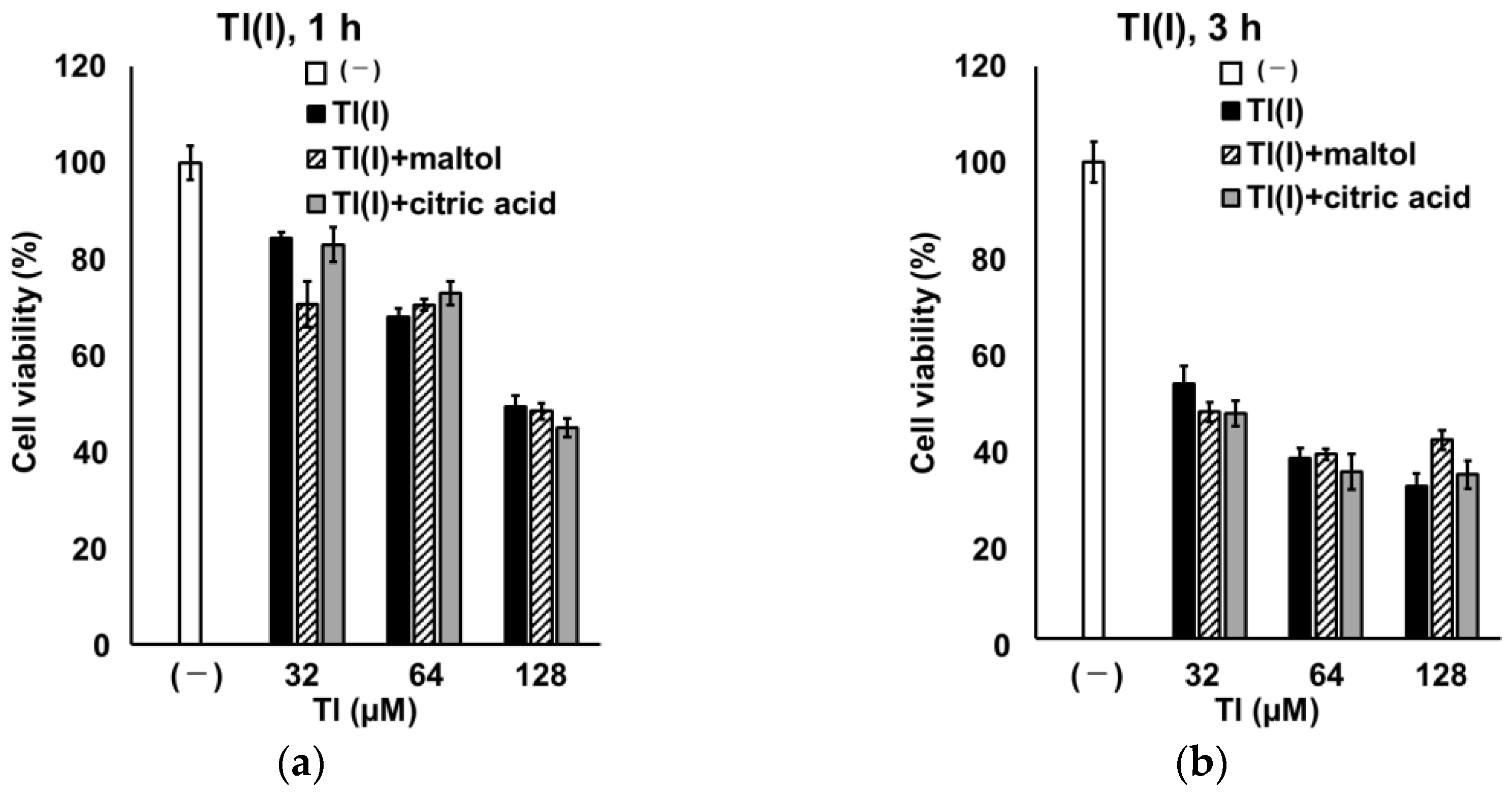
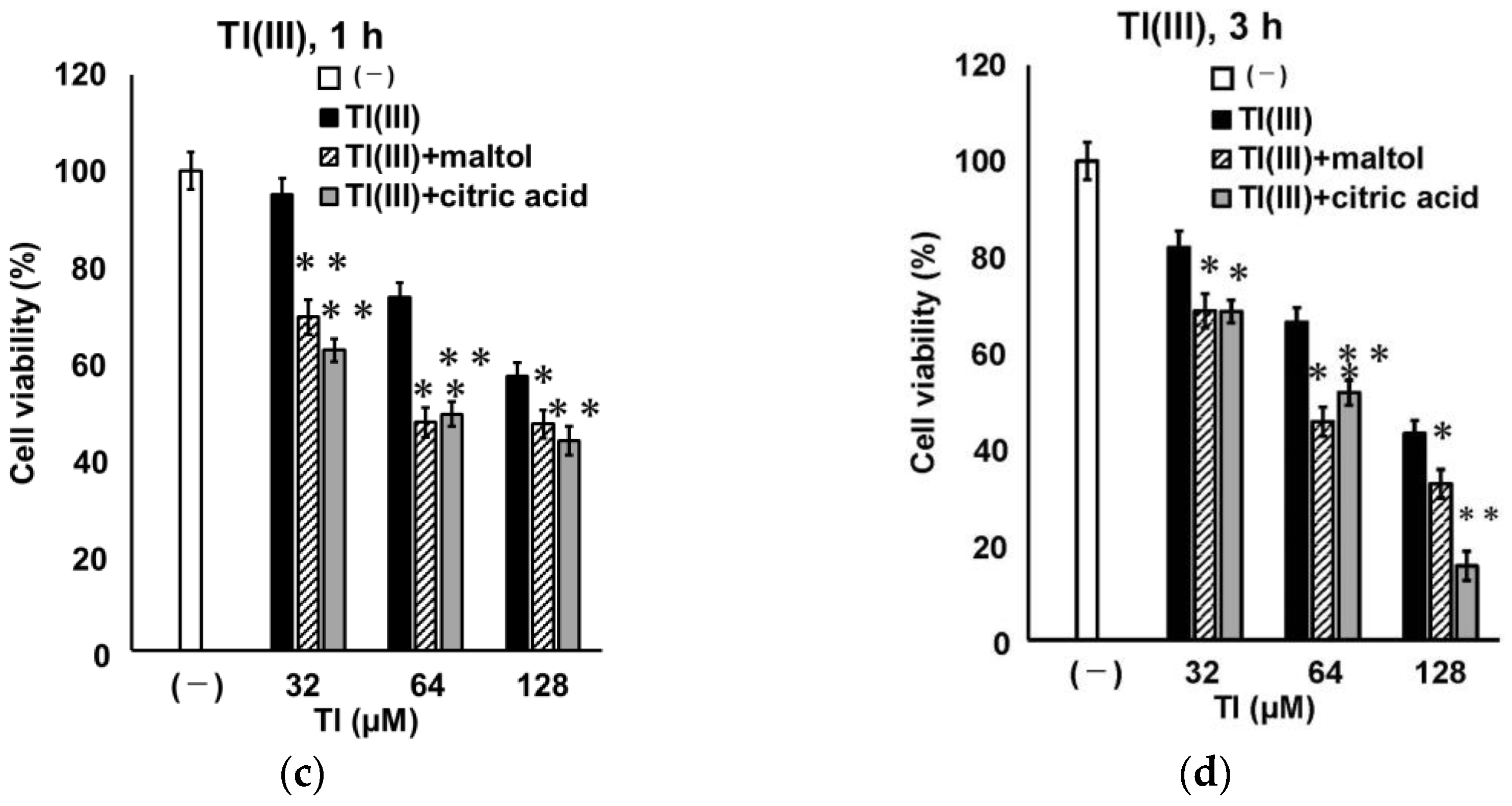
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Evaluation of Cytotoxicity
4.4. Determination of Caspase 3 Activity
4.5. TUNEL Staining
4.6. DNA Fragmentation Assay
4.7. Measurement of SOD Activity
4.8. Measurement of MDA Production
4.9. Measurement of GSH and GSSG Levels
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Małuszyński, M. Thallium in environment. Ochr. Sr. Zasobów Nat. 2009, 40, 31–38. [Google Scholar]
- PCvjetko, P.; Cvjetko, I.; Pavlica, M. Thallium toxicity in humans. Arh. Hig. Rada. Toksikol. 2010, 61, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, F.; Wang, M.; Liu, F.; Liu, W.; Li, X.; Qin, D.; Geng, X.; Huang, X.; Zhang, P. Thallium-induced oxalate secretion from rice (Oryza sativa L.) root contributes to the reduction of Tl(III) to Tl(I). Environ. Exp. Bot. 2018, 155, 387–393. [Google Scholar] [CrossRef]
- Galván-Arzate, S.; Santamaría, A. Thallium toxicity. Toxicol. Lett. 1998, 99, 1–13. [Google Scholar] [CrossRef]
- Luckit, J.; Mir, N.; Hargreaves, M.; Costello, C.; Gazzard, B. Thrombocytopenia associated with thallium poisoning. Hum. Exp. Toxicol. 1990, 9, 47–48. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Thallium; Clement International Corporation: Fairfax, VA, USA, 1999; pp. 1–122. [Google Scholar]
- Karbowska, B. Presence of thallium in the environment: Sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess. 2016, 188, 640. [Google Scholar] [CrossRef]
- Bunzl, K.; Trautmannsheimer, M.; Schramel, P.; Reifenhäuser, W. Availability of arsenic, copper, lead, thallium, and zinc to various vegetables grown in slag-contaminated soils. J. Environ. Qual. 2001, 30, 934–939. [Google Scholar] [CrossRef]
- Heim, M.; Wappelhorst, O.; Markert, B. Thallium in terrestrial environments-occurrence and effects. Ecotoxicology 2002, 11, 369–377. [Google Scholar] [CrossRef]
- Mochizuki, M.; Mori, M.; Akinaga, M.; Yugami, K.; Oya, C.; Hondo, R.; Ueda, F. Thallium contamination in wild ducks in Japan. J. Wildl. Dis. 2005, 41, 664–668. [Google Scholar] [CrossRef]
- Achenbach, C.; Hauswirth, O.; Heindrichs, C.; Ziskoven, R.; Kohler, F.; Barh, U.; Heindrichs, A.; Schulten, H.R. Quantitative measurement of time-dependent thallium distribution in organs of mice by field desorption mass spectrometry. J. Toxicol. Environ. Health 1980, 6, 519–528. [Google Scholar] [CrossRef]
- Osorio-Rico, L.; Santamaria, A.; Galván-Arzate, S. Thallium toxicity: General issues, neurological symptoms, and neurotoxic mechanisms. Adv. Neurobiol. 2017, 18, 345–353. [Google Scholar] [PubMed]
- Pourahmad, J.; Eskandari, M.R.; Daraei, B. A comparison of hepatocyte cytotoxic mechanisms for thallium (I) and thallium (III). Environ. Toxicol. 2010, 25, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, D.; Koyama, H.; Ohkawara, S.; Sadakane, Y.; Kawahara, M. Involvement of trace elements in the pathogenesis of prion diseases. Curr. Pharm. Biotechnol. 2014, 15, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, D.; Kawahara, M. The molecular mechanisms of zinc neurotoxicity and the pathogenesis of vascular type senile dementia. Int. J. Mol. Sci. 2013, 14, 22067–22081. [Google Scholar] [CrossRef]
- Mellon, P.L.; Windle, J.J.; Goldsmith, P.C.; Padula, C.A.; Roberts, J.L.; Weiner, R.I. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 1990, 5, 1–10. [Google Scholar] [CrossRef]
- Mizuno, D.; Kawahara, M. Oligomerization of proteins and neurodegenerative diseases. In Oligomerization of Chemical and Biological Compounds; Lesieur, C., Ed.; InTech: Rijeka, Croatia, 2014; pp. 279–293. [Google Scholar]
- Osorio-Rico, L.; Villeda-Hernández, J.; Santamaría, A.; Königsberg, M.; Galván-Arzate, S. The N-methyl-d-aspartate receptor antagonist MK-801 prevents thallium-induced behavioral and biochemical alterations in the rat brain. Int. J. Toxicol. 2015, 34, 505–513. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Khan, D.A.; Silswal, N.; Saleem, S.; Qureshi, N. Evaluation of Pharmacokinetics, and Bioavailability of Higher Doses of Tocotrienols in Healthy Fed Humans. J. Clin. Exp. Cardiolog. 2016, 7, 434. [Google Scholar] [CrossRef]
- Fiori, G.; Spada, C.; Soru, P.; Eugenio Tontini, G.; Bravi, I.; Mario Cesana, B.; Cesaro, P.; Manes, G.; Orsatti, A.; Prada, A.; et al. Pharmacokinetics of oral mannitol for bowel preparation for colonoscopy. Clin. Transl. Sci. 2022, 15, 2448–2457. [Google Scholar] [CrossRef]
- Garza, A.; Vega, R.; Soto, E. Cellular mechanisms of lead neurotoxicity. Med. Sci. Monit. 2006, 12, RA57–RA65. [Google Scholar]
- Jung, J.Y.; Kim, W.J. Involvement of mitochondrial- and Fas-mediated dual mechanism in CoCl2-induced apoptosis of rat PC12 cells. Neurosci. Lett. 2004, 371, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Catassi, A.; Cesario, A.; Imperatori, A.; Rotolo, N.; Fini, M.; Granone, P.; Dominioni, L. Molecular mechanisms of hexavalent chromium-induced apoptosis in human bronchoalveolar cells. Am. J. Respir. Cell Mol. Biol. 2005, 33, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Savory, J.; Herman, M.M.; Ghribi, O. Intracellular mechanisms underlying aluminum-induced apoptosis in rabbit brain. J. Inorg. Biochem. 2003, 97, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef]
- Hanzel, C.E.; Verstraeten, S.V. Thallium induces hydrogen peroxide generation by impairing mitochondrial function. Toxicol. Appl. Pharmacol. 2006, 216, 485–492. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Puga Molina, L.; Verstraeten, S.V. Thallium(III)-mediated changes in membrane physical properties and lipid oxidation affect cardiolipin-cytochrome c interactions. Biochim. Biophys. Acta 2008, 1778, 2157–2164. [Google Scholar] [CrossRef]
- Del-Valls, T.A.; Sáenz, V.; Arias, A.M.; Biasco, J. Thallium in the marine environment: First ecotoxicological assessments in the Guadalquivir estuary and its potential adverse effect on the Doñana European natural reserve after the Aznalcóllar mining spill (SW Spain). Cienc. Mar. 1999, 25, 161–175. [Google Scholar] [CrossRef]
- Day, J.P.; Barker, J.J.; Evans, L.J.A.; Perks, J.; Seabright, P.J.; Ackrill, P.; Lilley, J.S.; Drumm, P.V.; Newton, G.W.A. Aluminium absorption studied by 26Al tracer. Lancet 1991, 337, 1345. [Google Scholar] [CrossRef]
- Kawahara, M.; Kato-Negishi, M.; Hosoda, R.; Imamura, L.; Tsuda, M.; Kuroda, Y. Brain-derived neurotrophic factor protects cultured rat hippocampal neurons from aluminum maltolate neurotoxicity. J. Inorg. Biochem. 2003, 97, 124–131. [Google Scholar] [CrossRef]
- van Ginkel, M.F.; van der Voet, G.B.; D’Haese, P.C.; De Broe, M.E.; de Wolff, F.A. Effect of citric acid and maltol on the accumulation of aluminum in rat brain and bone. J. Lab. Clin. Med. 1993, 121, 453–460. [Google Scholar]
- Nagasawa, K.; Ito, S.; Kakuda, T.; Nagai, K.; Tamai, I.; Tsuji, A.; Fujimoto, S. Transport mechanism for aluminum citrate at the blood-brain barrier: Kinetic evidence implies involvement of system Xc-in immortalized rat brain endothelial cells. Toxicol. Lett. 2005, 155, 289–296. [Google Scholar] [CrossRef]
- Öhman, L.O.; Martin, R.B. Citrate as the main small molecule binding Al3+ in serum. Clin. Chem. 1994, 40, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Perazzolo, M.; Stella, M.P.; Tapparo, A.; Corain, B.; Favarato, M.; Zatta, P. A long-term toxicological investigation on the effect of tris(maltolate) aluminum(III) in rabbits. Biol. Trace. Elem. Res. 1991, 31, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Kato, M.; Kuroda, Y. Effects of aluminum on the neurotoxicity of primary cultured neurons and on the aggregation of β-amyloid protein. Brain. Res. Bull. 2001, 55, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Bondy, S.C. Low levels of aluminum can lead to behavioral and morphological changes associated with Alzheimer’s disease and age-related neurodegeneration. NeuroToxicology 2016, 52, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.; Mailloux, R.; Puiseux-Dao, S.; Appanna, V.D. Aluminum-induced defective mitochondrial metabolism perturbs cytoskeletal dynamics in human astrocytoma cells. J. Neurosci. Res. 2009, 87, 1474–1483. [Google Scholar] [CrossRef]
- Khare, P.; Mulakaluri, A.; Parsons, S.M. Search for the acetylcholine and vesamicol binding sites in vesicular acetylcholine transporter: The region around the lumenal end of the transport channel. J. Neurochem. 2010, 115, 984–993. [Google Scholar] [CrossRef]
- Rondeau, V. A review of epidemiologic studies on aluminum and silica in relation to Alzheimer’s disease and associated disorders. Rev. Environ. Health 2002, 17, 107–121. [Google Scholar] [CrossRef]
- Kawahara, M.; Kuroda, Y.; Arispe, N.; Rojas, E. Alzheimer’s beta-amyloid, human islet amylin, and prion protein fragment evoke intracellular free calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell line. J. Biol. Chem. 2000, 275, 14077–14083. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ’EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]

| 16 h | Caspase 3 Activity Ratio (vs. (–) Group) | SOD (Unit/mg Protein) | MDA (µM) |
|---|---|---|---|
| (–) | – | 5.5 ± 0.7 | 4.2 ± 0.3 |
| Tl(I) 64 µM | 4.0 ± 0.0 ** | 18.6 ± 2.3 ** | 17.5 ± 1.3 ** |
| Tl(I) 64 µM+ Ascorbic acid 160 µM | 1.5 ± 0.0 ## | 8.1 ± 1.3 ## | 5.8 ± 0.4 ## |
| Tl(I) 64 µM + Tocopherol 160 µM | 1.6 ± 0.3 ## | 8.9 ± 2.3 ## | 5.2 ± 0.1 ## |
| Tl(I) 64 µM + Mannitol 25.6 mM | 1.9 ± 0.1 ## | 8.1 ± 3.5 ## | 5.6 ± 0.1 ## |
| Tl(III) 64 µM | 3.6 ± 0.4 ** | 45.1 ± 6.2 **,## | 26.1 ± 1.1 **,## |
| Tl(III) 64 µM + Ascorbic acid 160 µM | 1.6 ± 0.0 $$ | 10.0 ± 1.2 $$ | 5.5 ± 0.2 ## |
| Tl(III) 64 µM + Tocopherol 160 µM | 1.6 ± 0.2 $$ | 10.4 ± 1.3 $$ | 5.2 ± 0.1 ## |
| Tl(III) 64 µM + Mannitol 25.6 mM | 1.7 ± 0.2 $$ | 9.6 ± 1.6 $$ | 6.0 ± 0.2 ## |
| Intracellular | Extracellular | ||||
|---|---|---|---|---|---|
| GSSG | GSH | GSSG | GSH | ||
| (–) | 2.19 ± 0.24 | 52.57 ± 0.54 | 0.76 ± 0.03 | 2.57 ± 0.11 | |
| 1 h | Tl(I) 64 µM | 3.22 ± 0.15 * | 51.88 ± 0.24 | 0.92 ± 0.06 * | 2.13 ± 0.12 * |
| Tl(III) 64 µM | 3.85 ± 0.20 **,# | 52.71 ± 0.50 | 1.33 ± 0.05 **,## | 1.15 ± 0.12 **,## | |
| (–) | 1.69 ± 0.18 | 54.18 ± 2.03 | 0.70 ± 0.04 | 2.75 ± 0.13 | |
| 3 h | Tl(I) 64 µM | 2.88 ± 0.14 ** | 51.51 ± 0.53 * | 1.11 ± 0.04 ** | 2.36 ± 0.07 * |
| Tl(III) 64 µM | 4.60 ± 0.62 **,# | 47.14 ± 1.81 * | 1.45 ± 0.03 **,# | 1.99 ± 0.14 **,## | |
| (–) | 1.16 ± 0.05 | 67.52 ± 0.70 | 0.72 ± 0.03 | 11.19 ± 0.40 | |
| 16 h | Tl(I) 64 µM | 1.45 ± 0.08 * | 63.77 ± 0.37 * | 1.04 ± 0.07 * | 4.44 ± 0.14 ** |
| Tl(III) 64 µM | 1.82 ± 0.35 ** | 63.26 ± 0.81 ** | 2.04 ± 0.22 **,## | 3.78 ± 0.31 **,## | |
| MDA | 1 h (µM) | 3 h (µM) |
|---|---|---|
| (–) | 3.9 ± 0.4 | 4.4 ± 1.3 |
| Tl(I) 64 µM | 14.0 ± 0.3 ** | 9.4 ± 1.1 ** |
| Tl(I) 64 µM + Maltol 192 µM | 12.6 ± 0.1 ** | 11.4 ± 1.2 ** |
| Tl(I) 64 µM + Citric acid 64 µM | 12.6 ± 0.2 ** | 10.4 ± 0.5 ** |
| Tl(III) 64 µM | 18.2 ± 0.7 **,# | 17.5 ± 0.7 **,# |
| Tl(III) 64 µM + Maltol 192 µM | 8.9 ± 0.2 **,$$ | 11.5 ± 0.6 **,$$ |
| Tl(III) 64 µM + Citric acid 64 µM | 11.9 ± 0.6 **,$$ | 11.5 ± 0.9 **,$$ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuno, D.; Kawahara, M.; Konoha-Mizuno, K.; Ogawara, T.; Hama, R.; Yamazaki, K. Toxic Effects of Two Redox States of Thallium on Immortalised Hypothalamic GT1-7 Neuronal Cells. Int. J. Mol. Sci. 2023, 24, 11583. https://doi.org/10.3390/ijms241411583
Mizuno D, Kawahara M, Konoha-Mizuno K, Ogawara T, Hama R, Yamazaki K. Toxic Effects of Two Redox States of Thallium on Immortalised Hypothalamic GT1-7 Neuronal Cells. International Journal of Molecular Sciences. 2023; 24(14):11583. https://doi.org/10.3390/ijms241411583
Chicago/Turabian StyleMizuno, Dai, Masahiro Kawahara, Keiko Konoha-Mizuno, Terumasa Ogawara, Ryoji Hama, and Kentaro Yamazaki. 2023. "Toxic Effects of Two Redox States of Thallium on Immortalised Hypothalamic GT1-7 Neuronal Cells" International Journal of Molecular Sciences 24, no. 14: 11583. https://doi.org/10.3390/ijms241411583
APA StyleMizuno, D., Kawahara, M., Konoha-Mizuno, K., Ogawara, T., Hama, R., & Yamazaki, K. (2023). Toxic Effects of Two Redox States of Thallium on Immortalised Hypothalamic GT1-7 Neuronal Cells. International Journal of Molecular Sciences, 24(14), 11583. https://doi.org/10.3390/ijms241411583






