The Role of miRNAs in the Development, Proliferation, and Progression of Endometrial Cancer
Abstract
1. Introduction
1.1. Endometrial Cancer
1.2. MicroRNAs
2. MicroRNAs in Endometrial Cancer Patients
2.1. The Process of Carcinogenesis
2.2. Risk Factors and Prognostic Factors
2.2.1. Polycystic Ovary Syndrome (PCOS)
2.2.2. Obesity and Diabetes
2.2.3. Aging of the Body
2.2.4. Involvement of the Lymph Nodes Metastasis
2.2.5. The Impact of miRNA Changes on Survival and Recurrence in Patients with Endometrial Cancer
3. Overview of Selected microRNAs
3.1. MiR-205
3.2. MiR-34
3.3. MiR-21
3.4. MiR-182
3.5. MiR-200
3.6. MiR-103
3.7. MiR-105
3.8. MiR-136
3.9. MiR-155
3.10. MiR-372
3.11. MiR-93
3.12. MiR-125
3.13. MiR-222
3.14. Let-7
3.15. MiR-429
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef]
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial cancer. Nat. Rev. Dis. Primers 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- León-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef] [PubMed]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jürgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef]
- Dowdy, S.C.; Glaser, G.E. Adjuvant therapy for women with high-risk endometrial carcinoma. Lancet Oncol. 2018, 19, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Matei, D.; Filiaci, V.; Randall, M.E.; Mutch, D.; Steinhoff, M.M.; DiSilvestro, P.A.; Moxley, K.M.; Kim, Y.M.; Powell, M.A.; O’Malley, D.M.; et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. N. Engl. J. Med. 2019, 380, 2317–2326. [Google Scholar] [CrossRef]
- Stasenko, M.; Feit, N.; Lee, S.S.K.; Shepherd, C.; Soslow, R.A.; Cadoo, K.A.; Alektiar, K.; Da Silva, E.M.; Martins Sebastião, A.P.; Leitao, M.M.; et al. Clinical patterns and genomic profiling of recurrent ‘ultra-low risk’ endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 717–723. [Google Scholar] [CrossRef]
- Vallone, C.; Rigon, G.; Gulia, C.; Baffa, A.; Votino, R.; Morosetti, G.; Zaami, S.; Briganti, V.; Catania, F.; Gaffi, M.; et al. Non-Coding RNAs and Endometrial Cancer. Genes 2018, 9, 187. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.N. MicroRNA precursors in motion: Exportin-5 mediates their nuclear export. Trends Cell Biol. 2004, 14, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gregory, R.I. MicroRNA biogenesis pathways in cancer. Nat. Rev. Cancer 2015, 15, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Sonenberg, N. The mechanics of miRNA-mediated gene silencing: A look under the hood of miRISC. Nat. Struct. Mol. Biol. 2012, 19, 586–593. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Grzywa, T.M.; Klicka, K.; Włodarski, P.K. Regulators at Every Step-How microRNAs Drive Tumor Cell Invasiveness and Metastasis. Cancers 2020, 12, 3709. [Google Scholar] [CrossRef]
- Chiu, H.C.; Li, C.J.; Yiang, G.T.; Tsai, A.P.; Wu, M.Y. Epithelial to Mesenchymal Transition and Cell Biology of Molecular Regulation in Endometrial Carcinogenesis. J. Clin. Med. 2019, 8, 439. [Google Scholar] [CrossRef]
- Vincent, K.; Pichler, M.; Lee, G.W.; Ling, H. MicroRNAs, genomic instability and cancer. Int. J. Mol. Sci. 2014, 15, 14475–14491. [Google Scholar] [CrossRef] [PubMed]
- Shirjang, S.; Mansoori, B.; Asghari, S.; Duijf, P.H.G.; Mohammadi, A.; Gjerstorff, M.; Baradaran, B. MicroRNAs in cancer cell death pathways: Apoptosis and necroptosis. Free Radic. Biol. Med. 2019, 139, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pedroza-Torres, A.; Romero-Córdoba, S.L.; Justo-Garrido, M.; Salido-Guadarrama, I.; Rodríguez-Bautista, R.; Montaño, S.; Muñiz-Mendoza, R.; Arriaga-Canon, C.; Fragoso-Ontiveros, V.; Álvarez-Gómez, R.M.; et al. MicroRNAs in Tumor Cell Metabolism: Roles and Therapeutic Opportunities. Front. Oncol. 2019, 9, 1404. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Chen, C.; Chu, X. New insights into the regulatory role of microRNA in tumor angiogenesis and clinical implications. Mol. Cancer 2018, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Xu, L.; Jiao, Y.; Luo, S.; Li, A.; Wu, K. The role of cancer-derived microRNAs in cancer immune escape. J. Hematol. Oncol. 2020, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Hirsch, H.A.; Struhl, K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 2009, 139, 693–706. [Google Scholar] [CrossRef]
- Masood, N.; Basharat, Z.; Khan, T.; Yasmin, A. Entangling Relation of Micro RNA-let7, miRNA-200 and miRNA-125 with Various Cancers. Pathol. Oncol. Res. 2017, 23, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Klicka, K.; Grzywa, T.M.; Klinke, A.; Mielniczuk, A.; Włodarski, P.K. The Role of miRNAs in the Regulation of Endometrial Cancer Invasiveness and Metastasis—A Systematic Review. Cancers 2021, 13, 3393. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, X.; Cobb, G.P.; Anderson, T.A. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007, 302, 1–12. [Google Scholar] [CrossRef]
- Ciafrè, S.A.; Galardi, S.; Mangiola, A.; Ferracin, M.; Liu, C.G.; Sabatino, G.; Negrini, M.; Maira, G.; Croce, C.M.; Farace, M.G. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem. Biophys. Res. Commun. 2005, 334, 1351–1358. [Google Scholar] [CrossRef]
- Mendiola-Soto, D.K.; Bárcenas-López, D.A.; Pérez-Amado, C.J.; Cruz-Miranda, G.M.; Mejía-Aranguré, J.M.; Ramírez-Bello, J.; Hidalgo-Miranda, A.; Jiménez-Morales, S. MiRNAs in Hematopoiesis and Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2023, 24, 5436. [Google Scholar] [CrossRef]
- Sajjadi-Dokht, M.; Merza Mohamad, T.A.; Sulaiman Rahman, H.; Suliman Maashi, M.; Danshina, S.; Shomali, N.; Solali, S.; Marofi, F.; Zeinalzadeh, E.; Akbari, M.; et al. MicroRNAs and JAK/STAT3 signaling: A new promising therapeutic axis in blood cancers. Genes Dis. 2022, 9, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, H.; Li, Y.; Su, R. MiR-192-5p-Modified Tumor-Associated Macrophages-Derived Exosome Suppressed Endometrial Cancer Progression Through Targeting IRAK1/NF-κB Signaling. Reprod Sci. 2022, 29, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.X.; Xu, X.X.; Tan, B.Z.; Zhang, Z.; Zhou, X.D. MicroRNA-29b Inhibits Angiogenesis by Targeting VEGFA through the MAPK/ERK and PI3K/Akt Signaling Pathways in Endometrial Carcinoma. Cell Physiol. Biochem. 2017, 41, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Sun, B.; Wu, Q.; Yang, S.; Chen, F. miRNA-miRNA interaction implicates for potential mutual regulatory pattern. Gene 2012, 511, 187–194. [Google Scholar] [CrossRef]
- Hsiao, K.Y.; Sun, H.S.; Tsai, S.J. Circular RNA—New member of noncoding RNA with novel functions. Exp. Biol. Med. 2017, 242, 1136–1141. [Google Scholar] [CrossRef]
- Zheng, Q.; Bao, C.; Guo, W.; Li, S.; Chen, J.; Chen, B.; Luo, Y.; Lyu, D.; Li, Y.; Shi, G.; et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016, 7, 11215. [Google Scholar] [CrossRef]
- Boussios, S.; Ozturk, M.A.; Moschetta, M.; Karathanasi, A.; Zakynthinakis-Kyriakou, N.; Katsanos, K.H.; Christodoulou, D.K.; Pavlidis, N. The Developing Story of Predictive Biomarkers in Colorectal Cancer. J. Pers. Med. 2019, 9, 12. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, L.J.; Zhang, X.Y. Exosomal miRNA-205 promotes breast cancer chemoresistance and tumorigenesis through E2F1. Aging 2021, 13, 18498–18514. [Google Scholar] [CrossRef]
- Konoshenko, M.; Lansukhay, Y.; Krasilnikov, S.; Laktionov, P. MicroRNAs as Predictors of Lung-Cancer Resistance and Sensitivity to Cisplatin. Int. J. Mol. Sci. 2022, 23, 7594. [Google Scholar] [CrossRef]
- Chen, Q.; Xia, H.W.; Ge, X.J.; Zhang, Y.C.; Tang, Q.L.; Bi, F. Serum miR-19a predicts resistance to FOLFOX chemotherapy in advanced colorectal cancer cases. Asian Pac. J. Cancer Prev. 2013, 14, 7421–7426. [Google Scholar] [CrossRef]
- Hansen, T.F.; Carlsen, A.L.; Heegaard, N.H.; Sørensen, F.B.; Jakobsen, A. Changes in circulating microRNA-126 during treatment with chemotherapy and bevacizumab predicts treatment response in patients with metastatic colorectal cancer. Br. J. Cancer 2015, 112, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Che, Q.; Qiu, H.; Bao, W.; Chen, X.; Lu, W.; Li, B.; Wan, X. Elevated MiR-222-3p promotes proliferation and invasion of endometrial carcinoma via targeting ERα. PLoS ONE 2014, 9, e87563. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Jiang, W.; Zhang, R.; Zhang, B.; Silayiding, A.; Duan, X. MicroRNA-135a promotes proliferation, migration, invasion and induces chemoresistance of endometrial cancer cells. Eur. J. Obs. Gynecol. Reprod Biol. X 2020, 5, 100103. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Sharma, I.; Saha, S.C.; Srinivasan, R.; Bhardwaj, P. Role of serum microRNAs as biomarkers for endometriosis, endometrioid carcinoma of ovary & endometrioid endometrial cancer. Indian J. Med. Res. 2022, 156, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Donkers, H.; Hirschfeld, M.; Weiß, D.; Erbes, T.; Jäger, M.; Pijnenborg, J.; Bekkers, R.; Galaal, K. Detection of microRNA in urine to identify patients with endometrial cancer: A feasibility study. Int. J. Gynecol. Cancer 2021, 31, 868–874. [Google Scholar] [CrossRef]
- Donkers, H.; Hirschfeld, M.; Weiß, D.; Erbes, T.; Jaeger, M.; Pijnenborg, J.M.A.; Bekkers, R.; Galaal, K.; ENITEC-consortium. Usefulness of microRNA detection in the diagnostics of endometrial cancer. Acta Obs. Gynecol. Scand 2021, 100, 1148–1154. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Jiang, Y.; Wan, Y.; Zhou, S.; Cheng, W. Tumor-suppressor role of miR-139-5p in endometrial cancer. Cancer Cell Int. 2018, 18, 51. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, K.; Tong, Y.; Dai, X.; Xu, T.; He, D.; Ying, J. Novel miRNA markers for the diagnosis and prognosis of endometrial cancer. J. Cell Mol. Med. 2020, 24, 4533–4546. [Google Scholar] [CrossRef]
- Witek, Ł.; Janikowski, T.; Gabriel, I.; Bodzek, P.; Olejek, A. Analysis of microRNA regulating cell cycle-related tumor suppressor genes in endometrial cancer patients. Hum. Cell 2021, 34, 564–569. [Google Scholar] [CrossRef]
- Johansson, K.; Gagnon, J.D.; Zhou, S.; Fassett, M.S.; Schroeder, A.W.; Kageyama, R.; Bautista, R.A.; Pham, H.; Woodruff, P.G.; Ansel, K.M. An essential role for miR-15/16 in Treg suppression and restriction of proliferation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Zhang, X.; Smith, S.M.; Wang, X.; Zhao, B.; Wu, L.; Hu, X. Three paralogous clusters of the miR-17~92 family of microRNAs restrain IL-12-mediated immune defense. Cell Mol. Immunol. 2021, 18, 1751–1760. [Google Scholar] [CrossRef]
- Navarro, F.; Lieberman, J. miR-34 and p53: New Insights into a Complex Functional Relationship. PLoS ONE 2015, 10, e0132767. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Yu, L.; Chim, C.S. DNA methylation of tumor suppressor miRNA genes: A lesson from the miR-34 family. Epigenomics 2011, 3, 83–92. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zeng, S.; Zhou, Z.W.; He, Z.X.; Zhou, S.F. Hsa-microRNA-181a is a regulator of a number of cancer genes and a biomarker for endometrial carcinoma in patients: A bioinformatic and clinical study and the therapeutic implication. Drug Des. Devel Ther. 2015, 9, 1103–1175. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Mi, P.; Hu, Y. Construction of dysregulated long non-coding RNA-associated competing endogenous RNA network in uterine corpus endometrial carcinoma. Transl. Cancer Res. 2020, 9, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Di Leva, G.; Romano, G.; Nuovo, G.; Suh, S.S.; Ngankeu, A.; Taccioli, C.; Pichiorri, F.; Alder, H.; Secchiero, P.; et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell 2009, 16, 498–509. [Google Scholar] [CrossRef]
- Zhang, R.; He, Y.; Zhang, X.; Xing, B.; Sheng, Y.; Lu, H.; Wei, Z. Estrogen receptor-regulated microRNAs contribute to the BCL2/BAX imbalance in endometrial adenocarcinoma and precancerous lesions. Cancer Lett. 2012, 314, 155–165. [Google Scholar] [CrossRef]
- Ma, L.; Young, J.; Prabhala, H.; Pan, E.; Mestdagh, P.; Muth, D.; Teruya-Feldstein, J.; Reinhardt, F.; Onder, T.T.; Valastyan, S.; et al. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat. Cell Biol. 2010, 12, 247–256. [Google Scholar] [CrossRef]
- Sheedy, P.; Medarova, Z. The fundamental role of miR-10b in metastatic cancer. Am. J. Cancer Res. 2018, 8, 1674–1688. [Google Scholar]
- Chen, H.; Fan, Y.; Xu, W.; Chen, J.; Xu, C.; Wei, X.; Fang, D.; Feng, Y. miR-10b Inhibits Apoptosis and Promotes Proliferation and Invasion of Endometrial Cancer Cells via Targeting HOXB3. Cancer Biother. Radiopharm. 2016, 31, 225–231. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; He, Y. MicroRNA-21-5p promotes epithelial to mesenchymal transition by targeting SRY-box 17 in endometrial cancer. Oncol. Rep. 2020, 43, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Van Sinderen, M.; Griffiths, M.; Menkhorst, E.; Niven, K.; Dimitriadis, E. Restoration of microRNA-29c in type I endometrioid cancer reduced endometrial cancer cell growth. Oncol. Lett. 2019, 18, 2684–2693. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.; Van Sinderen, M.; Rainczuk, K.; Dimitriadis, E. miR-29c overexpression and COL4A1 downregulation in infertile human endometrium reduces endometrial epithelial cell adhesive capacity in vitro implying roles in receptivity. Sci. Rep. 2019, 9, 8644. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Laquintana, V.; Loria, R.; Carosi, M.; de Salvo, L.; Sperduti, I.; Zampa, A.; Cicchillitti, L.; Piaggio, G.; Cutillo, G.; et al. Endometrial cancer prognosis correlates with the expression of L1CAM and miR34a biomarkers. J. Exp. Clin. Cancer Res. 2018, 37, 139. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Huang, K.; Wang, Y.; Li, J.; Yang, X. MicroRNA-34a inhibits cells proliferation and invasion by downregulating Notch1 in endometrial cancer. Oncotarget 2017, 8, 111258–111270. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, F.; Zhang, L.; Jia, Y.; Chen, H. MicroRNA-103 regulates the progression in endometrial carcinoma through ZO-1. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419872621. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Zhang, Y.; Li, S.; Fan, Q.; Qiu, M.; Wang, Y.; Li, Y.; Ji, X.; Yang, Y.; Sang, Z.; et al. miR-107-5p promotes tumor proliferation and invasion by targeting estrogen receptor-α in endometrial carcinoma. Oncol. Rep. 2019, 41, 1575–1585. [Google Scholar] [CrossRef]
- Yue, Z.; Shen, J.J.; Huang, Q.T.; Qin, Y.F.; Li, X.N.; Liu, G.B. [MiR-135b promotes proliferation of endometrial carcinoma cells by targeting FOXO1]. Nan Fang Yi Ke Da Xue Xue Bao 2016, 36, 675–680. [Google Scholar]
- Chang, L.; Yuan, Z.; Shi, H.; Bian, Y.; Guo, R. miR-145 targets the. Am. J. Cancer Res. 2017, 7, 2305–2317. [Google Scholar]
- Li, B.L.; Lu, W.; Qu, J.J.; Ye, L.; Du, G.Q.; Wan, X.P. Loss of exosomal miR-148b from cancer-associated fibroblasts promotes endometrial cancer cell invasion and cancer metastasis. J. Cell Physiol. 2019, 234, 2943–2953. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, L.; Li, L.; Su, Y. miR-148b Functions as a Tumor Suppressor by Targeting Endoplasmic Reticulum Metallo Protease 1 in Human Endometrial Cancer Cells. Oncol. Res. 2018, 27, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Li, X.; Guo, S.; Xie, X. MicroRNA-155 Suppresses the Translation of p38 and Impairs the Functioning of Dendritic Cells in Endometrial Cancer Mice. Cancer Manag. Res. 2020, 12, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.Y.; Tan, M.R.; Zhou, J.; Liang, L.; Liu, X.Y.; Zhao, K.; Bao, E.C. miR-214-3p inhibits epithelial-to-mesenchymal transition and metastasis of endometrial cancer cells by targeting TWIST1. Onco. Targets Ther. 2019, 12, 9449–9458. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; Guo, Z.; Ma, X.; Song, Y.; Guo, Y. Regulation of NEAT1/miR-214-3p on the growth, migration and invasion of endometrial carcinoma cells. Arch. Gynecol. Obs. 2017, 295, 1469–1475. [Google Scholar] [CrossRef]
- Jeffries, J.; Zhou, W.; Hsu, A.Y.; Deng, Q. miRNA-223 at the crossroads of inflammation and cancer. Cancer Lett. 2019, 451, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Li, R.; Li, Y.J.; Yu, X.X.; Sun, Q.N.; Li, A.Y.; Kong, Y. eIF4E-related miR-320a and miR-340-5p inhibit endometrial carcinoma cell metastatic capability by preventing TGF-β1-induced epithelial-mesenchymal transition. Oncol. Rep. 2020, 43, 447–460. [Google Scholar] [CrossRef]
- Yang, C.H.; Pfeffer, S.R.; Sims, M.; Yue, J.; Wang, Y.; Linga, V.G.; Paulus, E.; Davidoff, A.M.; Pfeffer, L.M. The oncogenic microRNA-21 inhibits the tumor suppressive activity of FBXO11 to promote tumorigenesis. J. Biol. Chem. 2015, 290, 6037–6046. [Google Scholar] [CrossRef]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.; Chin, A.R.; et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef]
- Yu, J.; Fan, Q.; Li, L. The MCM3AP-AS1/miR-126/VEGF axis regulates cancer cell invasion and migration in endometrioid carcinoma. World J. Surg. Oncol. 2021, 19, 213. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, M.; Song, Y.; Feng, C. Long Noncoding RNA-ATB Impairs the Function of Tumor Suppressor miR-126-Mediated Signals in Endometrial Cancer for Tumor Growth and Metastasis. Cancer Biother. Radiopharm. 2019, 34, 47–55. [Google Scholar] [CrossRef]
- Wang, J.; Gong, X.; Yang, L.; Li, L.; Gao, X.; Ni, T.; Yang, X.; Fan, Q.; Sun, X.; Wang, Y. Loss of exosomal miR-26a-5p contributes to endometrial cancer lymphangiogenesis and lymphatic metastasis. Clin. Transl. Med. 2022, 12, e846. [Google Scholar] [CrossRef]
- Yang, L.J.; Gao, L.; Guo, Y.N.; Liang, Z.Q.; Li, D.M.; Tang, Y.L.; Liu, Y.H.; Gao, W.J.; Zeng, J.J.; Shi, L.; et al. Upregulation of microRNA miR-141-3p and its prospective targets in endometrial carcinoma: A comprehensive study. Bioengineered 2021, 12, 2941–2956. [Google Scholar] [CrossRef]
- Huang, Y.; Du, K.L.; Guo, P.Y.; Zhao, R.M.; Wang, B.; Zhao, X.L.; Zhang, C.Q. IL-16 regulates macrophage polarization as a target gene of mir-145-3p. Mol. Immunol. 2019, 107, 1–9. [Google Scholar] [CrossRef]
- Kane, N.M.; Howard, L.; Descamps, B.; Meloni, M.; McClure, J.; Lu, R.; McCahill, A.; Breen, C.; Mackenzie, R.M.; Delles, C.; et al. Role of microRNAs 99b, 181a, and 181b in the differentiation of human embryonic stem cells to vascular endothelial cells. Stem Cells 2012, 30, 643–654. [Google Scholar] [CrossRef]
- Zhuang, Y.; Peng, H.; Mastej, V.; Chen, W. MicroRNA Regulation of Endothelial Junction Proteins and Clinical Consequence. Mediat. Inflamm. 2016, 2016, 5078627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Chen, Z.; Wang, W. MicroRNA-424 suppresses estradiol-induced cell proliferation via targeting GPER in endometrial cancer cells. Cell. Mol. Biol. 2015, 61, 96–101. [Google Scholar] [PubMed]
- Korać, P.; Antica, M.; Matulić, M. MiR-7 in Cancer Development. Biomedicines 2021, 9, 325. [Google Scholar] [CrossRef]
- Mitamura, T.; Watari, H.; Wang, L.; Kanno, H.; Kitagawa, M.; Hassan, M.K.; Kimura, T.; Tanino, M.; Nishihara, H.; Tanaka, S.; et al. microRNA 31 functions as an endometrial cancer oncogene by suppressing Hippo tumor suppressor pathway. Mol. Cancer 2014, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Augoff, K.; Das, M.; Bialkowska, K.; McCue, B.; Plow, E.F.; Sossey-Alaoui, K. miR-31 is a broad regulator of β1-integrin expression and function in cancer cells. Mol. Cancer Res. 2011, 9, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Valeri, N.; Gasparini, P.; Fabbri, M.; Braconi, C.; Veronese, A.; Lovat, F.; Adair, B.; Vannini, I.; Fanini, F.; Bottoni, A.; et al. Modulation of mismatch repair and genomic stability by miR-155. Proc. Natl. Acad. Sci. USA 2010, 107, 6982–6987. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Li, J.; Cai, J.; Zhang, H.; Xin, Q.; Wang, N.; Xie, W.; Zhang, Y.; Xu, N. RNA-binding Protein MBNL2 regulates Cancer Cell Metastasis through MiR-182-MBNL2-AKT Pathway. J. Cancer 2021, 12, 6715–6726. [Google Scholar] [CrossRef] [PubMed]
- Penna, E.; Orso, F.; Taverna, D. miR-214 as a key hub that controls cancer networks: Small player, multiple functions. J. Invest. Dermatol. 2015, 135, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Huang, D.; Ma, K.; Deng, X.; Shao, Z. MiR-19a as a prognostic indicator for cancer patients: A meta-analysis. Biosci. Rep. 2019, 39, BSR20182370. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Lai, M.; Chen, M.; Xie, C.; Liao, R.; Kang, Y.J.; Xiao, C.; Hu, W.Y.; Han, J.; Sun, P. The miR-17-92 cluster of microRNAs confers tumorigenicity by inhibiting oncogene-induced senescence. Cancer Res. 2010, 70, 8547–8557. [Google Scholar] [CrossRef] [PubMed]
- Olive, V.; Bennett, M.J.; Walker, J.C.; Ma, C.; Jiang, I.; Cordon-Cardo, C.; Li, Q.J.; Lowe, S.W.; Hannon, G.J.; He, L. miR-19 is a key oncogenic component of mir-17-92. Genes Dev. 2009, 23, 2839–2849. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Nishikawa, R.; Chiyomaru, T.; Goto, Y.; Fukumoto, I.; Usui, H.; Mitsuhashi, A.; Enokida, H.; Nakagawa, M.; Shozu, M.; et al. The tumor-suppressive microRNA-1/133a cluster targets PDE7A and inhibits cancer cell migration and invasion in endometrial cancer. Int. J. Oncol. 2015, 47, 325–334. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Wu, H.J.; Ma, H.D.; Xu, L.P.; Huo, Y.; Yin, L.R. MicroRNA-503 suppresses proliferation and cell-cycle progression of endometrioid endometrial cancer by negatively regulating cyclin D1. FEBS J. 2013, 280, 3768–3779. [Google Scholar] [CrossRef]
- Braun, M.M.; Overbeek-Wager, E.A.; Grumbo, R.J. Diagnosis and Management of Endometrial Cancer. Am. Fam. Physician 2016, 93, 468–474. [Google Scholar]
- Lu, K.H.; Schorge, J.O.; Rodabaugh, K.J.; Daniels, M.S.; Sun, C.C.; Soliman, P.T.; White, K.G.; Luthra, R.; Gershenson, D.M.; Broaddus, R.R. Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J. Clin. Oncol. 2007, 25, 5158–5164. [Google Scholar] [CrossRef]
- Lee, M.; Piao, J.; Jeon, M.J. Risk Factors Associated with Endometrial Pathology in Premenopausal Breast Cancer Patients Treated with Tamoxifen. Yonsei Med. J. 2020, 61, 317–322. [Google Scholar] [CrossRef]
- Piergentili, R.; Zaami, S.; Cavaliere, A.F.; Signore, F.; Scambia, G.; Mattei, A.; Marinelli, E.; Gulia, C.; Perelli, F. Non-Coding RNAs as Prognostic Markers for Endometrial Cancer. Int. J. Mol. Sci. 2021, 22, 3151. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, S.; Tzafettas, J. Anovulation with or without PCO, hyperandrogenaemia and hyperinsulinaemia as promoters of endometrial and breast cancer. Best Prac. Res. Clin. Obs. Gynaecol. 2010, 24, 19–27. [Google Scholar] [CrossRef]
- Chen, B.; Xu, P.; Wang, J.; Zhang, C. The role of MiRNA in polycystic ovary syndrome (PCOS). Gene 2019, 706, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Udesen, P.B.; Sørensen, A.E.; Svendsen, R.; Frisk, N.L.S.; Hess, A.L.; Aziz, M.; Wissing, M.L.M.; Englund, A.L.M.; Dalgaard, L.T. Circulating miRNAs in Women with Polycystic Ovary Syndrome: A Longitudinal Cohort Study. Cells 2023, 12, 983. [Google Scholar] [CrossRef]
- Che, X.; Jian, F.; Chen, C.; Liu, C.; Liu, G.; Feng, W. PCOS serum-derived exosomal miR-27a-5p stimulates endometrial cancer cells migration and invasion. J. Mol. Endocrinol. 2020, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, F.; Catellani, C.; Sartori, C.; Lazzeroni, P.; Amarri, S.; Street, M.E. Obesity, Insulin Resistance, and Colorectal Cancer: Could miRNA Dysregulation Play A Role? Int. J. Mol. Sci. 2019, 20, 2922. [Google Scholar] [CrossRef]
- Sanderson, P.A.; Critchley, H.O.; Williams, A.R.; Arends, M.J.; Saunders, P.T. New concepts for an old problem: The diagnosis of endometrial hyperplasia. Hum. Reprod Update 2017, 23, 232–254. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Hurd, W.W.; Elguero, S.; Barker, N.M.; Zanotti, K.M. Diagnosis and management of endometrial hyperplasia. J. Minim. Invasive Gynecol. 2012, 19, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Dai, X.; Zhan, J.; Zhang, Y.; Zhang, H.; Zeng, S.; Xi, W. Profiling peripheral microRNAs in obesity and type 2 diabetes mellitus. APMIS 2015, 123, 580–585. [Google Scholar] [CrossRef]
- Ouni, M.; Gottmann, P.; Westholm, E.; Schwerbel, K.; Jähnert, M.; Stadion, M.; Rittig, K.; Vogel, H.; Schürmann, A. MiR-205 is up-regulated in islets of diabetes-susceptible mice and targets the diabetes gene Tcf7l2. Acta Physiol. 2021, 232, e13693. [Google Scholar] [CrossRef]
- Pescador, N.; Pérez-Barba, M.; Ibarra, J.M.; Corbatón, A.; Martínez-Larrad, M.T.; Serrano-Ríos, M. Serum circulating microRNA profiling for identification of potential type 2 diabetes and obesity biomarkers. PLoS ONE 2013, 8, e77251. [Google Scholar] [CrossRef]
- Pang, H.; Wang, J.; Wei, Q.; Liu, J.; Chu, X.; Yuan, C.; Yang, B.; Li, M.; Ma, D.; Tang, Y.; et al. miR-548ag functions as an oncogene by suppressing MOB1B in the development of obesity-related endometrial cancer. Cancer Sci. 2023, 114, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Ahn, J.; Choi, Y.M.; Son, H.J.; Choi, W.H.; Cho, H.J.; Yu, J.H.; Seo, J.A.; Jang, Y.J.; Jung, C.H.; et al. Differential circulating and visceral fat microRNA expression of non-obese and obese subjects. Clin. Nutr. 2020, 39, 910–916. [Google Scholar] [CrossRef]
- Williams, A.; Dougal, D.M.; Jenkins, W.; Greene, N.; Williams-DeVane, C.; Kimbro, K.S. Serum miR-17 levels are downregulated in obese, African American women with elevated HbA1c. J. Diabetes Metab. Disord. 2019, 18, 173–179. [Google Scholar] [CrossRef]
- Yu, F.; Chapman, S.; Pham, D.L.; Ko, M.L.; Zhou, B.; Ko, G.Y. Decreased miR-150 in obesity-associated type 2 diabetic mice increases intraocular inflammation and exacerbates retinal dysfunction. BMJ Open Diabetes Res. Care 2020, 8, e001446. [Google Scholar] [CrossRef]
- Kirkwood, T.B. Understanding the odd science of aging. Cell 2005, 120, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.H.; Cameron-Smith, D.; Wessner, B.; Franzke, B. Biomarkers of Aging: From Function to Molecular Biology. Nutrients 2016, 8, 338. [Google Scholar] [CrossRef]
- Kumar, S.; Vijayan, M.; Bhatti, J.S.; Reddy, P.H. MicroRNAs as Peripheral Biomarkers in Aging and Age-Related Diseases. Prog. Mol. Biol. Transl. Sci. 2017, 146, 47–94. [Google Scholar] [CrossRef] [PubMed]
- Gerasymchuk, M.; Cherkasova, V.; Kovalchuk, O.; Kovalchuk, I. The Role of microRNAs in Organismal and Skin Aging. Int. J. Mol. Sci. 2020, 21, 5281. [Google Scholar] [CrossRef] [PubMed]
- Noren Hooten, N.; Fitzpatrick, M.; Wood, W.H.; De, S.; Ejiogu, N.; Zhang, Y.; Mattison, J.A.; Becker, K.G.; Zonderman, A.B.; Evans, M.K. Age-related changes in microRNA levels in serum. Aging 2013, 5, 725–740. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Y.; Yao, L.; Xing, Y.; Yang, H.; Ma, Q. The Role of microRNA-23a-3p in the Progression of Human Aging Process by Targeting FOXO3a. Mol. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, X.; Li, J.; Xiao, R.; Xin, H.; Dai, L.; Zhu, Y.; Bao, W. Serum miRNA-204-5p as a potential non-invasive biomarker for the diagnosis of endometrial cancer with sentinel lymph node mapping. Oncol. Lett. 2022, 24, 248. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Gomez, J.D.; Alektiar, K.M.; Soslow, R.A.; Hensley, M.L.; Leitao, M.M.; Gardner, G.J.; Sonoda, Y.; Chi, D.S.; Barakat, R.R. The incidence of isolated paraaortic nodal metastasis in surgically staged endometrial cancer patients with negative pelvic lymph nodes. Gynecol. Oncol. 2009, 115, 236–238. [Google Scholar] [CrossRef]
- Sato, K.; Miyamoto, M.; Takano, M.; Tsuda, H. MicroRNA-21 expression in cancer cells is an independent biomarker of progression-free survival of endometrioid endometrial carcinoma. Virchows Arch. 2021, 479, 883–891. [Google Scholar] [CrossRef]
- Yoneyama, K.; Ishibashi, O.; Kawase, R.; Kurose, K.; Takeshita, T. miR-200a, miR-200b and miR-429 are onco-miRs that target the PTEN gene in endometrioid endometrial carcinoma. Anticancer Res. 2015, 35, 1401–1410. [Google Scholar]
- Guo, C.M.; Liu, S.Q.; Sun, M.Z. miR-429 as biomarker for diagnosis, treatment and prognosis of cancers and its potential action mechanisms: A systematic literature review. Neoplasma 2020, 67, 215–228. [Google Scholar] [CrossRef]
- Sun, X.; Hou, L.; Qiu, C.; Kong, B. MiR-501 promotes tumor proliferation and metastasis by targeting HOXD10 in endometrial cancer. Cell Mol. Biol. Lett. 2021, 26, 20. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Q.; Kong, B. miRNA-576-5p promotes endometrial cancer cell growth and metastasis by targeting ZBTB4. Clin. Transl. Oncol. 2023, 25, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Delangle, R.; De Foucher, T.; Larsen, A.K.; Sabbah, M.; Azaïs, H.; Bendifallah, S.; Daraï, E.; Ballester, M.; Mehats, C.; Uzan, C.; et al. The Use of microRNAs in the Management of Endometrial Cancer: A Meta-Analysis. Cancers 2019, 11, 832. [Google Scholar] [CrossRef]
- Fu, K.; Li, Y.; Song, J.; Cai, W.; Wu, W.; Ye, X.; Xu, J. Identification of a MicroRNA Signature Associated With Lymph Node Metastasis in Endometrial Endometrioid Cancer. Front. Genet. 2021, 12, 650102. [Google Scholar] [CrossRef]
- Chen, S.; Sun, K.X.; Liu, B.L.; Zong, Z.H.; Zhao, Y. MicroRNA-505 functions as a tumor suppressor in endometrial cancer by targeting TGF-α. Mol. Cancer 2016, 15, 11. [Google Scholar] [CrossRef]
- Constantine, G.D.; Kessler, G.; Graham, S.; Goldstein, S.R. Increased Incidence of Endometrial Cancer Following the Women’s Health Initiative: An Assessment of Risk Factors. J. Women’s Health 2019, 28, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Filiaci, V.L.; Mannel, R.S.; Cohn, D.E.; Matsumoto, T.; Tewari, K.S.; DiSilvestro, P.; Pearl, M.L.; Argenta, P.A.; Powell, M.A.; et al. Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209). J. Clin. Oncol. 2020, 38, 3841–3850. [Google Scholar] [CrossRef]
- Makker, V.; Taylor, M.H.; Aghajanian, C.; Oaknin, A.; Mier, J.; Cohn, A.L.; Romeo, M.; Bratos, R.; Brose, M.S.; DiSimone, C.; et al. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J. Clin. Oncol. 2020, 38, 2981–2992. [Google Scholar] [CrossRef]
- Kong, J.; He, X.; Wang, Y.; Li, J. Effect of microRNA-29b on proliferation, migration, and invasion of endometrial cancer cells. J. Int. Med. Res. 2019, 47, 3803–3817. [Google Scholar] [CrossRef]
- Tsuruta, T.; Kozaki, K.; Uesugi, A.; Furuta, M.; Hirasawa, A.; Imoto, I.; Susumu, N.; Aoki, D.; Inazawa, J. miR-152 is a tumor suppressor microRNA that is silenced by DNA hypermethylation in endometrial cancer. Cancer Res. 2011, 71, 6450–6462. [Google Scholar] [CrossRef]
- Xie, D.; Liang, Y.; Su, Y.; An, Y.; Qu, P. miR-152 inhibits proliferation of human endometrial cancer cells via inducing G2/M phase arrest by suppressing CDC25B expression. Biomed. Pharmacother. 2018, 99, 299–305. [Google Scholar] [CrossRef]
- Karaayvaz, M.; Zhang, C.; Liang, S.; Shroyer, K.R.; Ju, J. Prognostic significance of miR-205 in endometrial cancer. PLoS ONE 2012, 7, e35158. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, W.; Wang, F.; Yang, S.; Hu, J.; Lu, B.; Pan, Z.; Ma, Y.; Zheng, M.; Lei, S.; et al. Plasma-derived exosomal miR-15a-5p as a promising diagnostic biomarker for early detection of endometrial carcinoma. Mol. Cancer 2021, 20, 57. [Google Scholar] [CrossRef]
- Fan, X.; Zou, X.; Liu, C.; Cheng, W.; Zhang, S.; Geng, X.; Zhu, W. MicroRNA expression profile in serum reveals novel diagnostic biomarkers for endometrial cancer. Biosci. Rep. 2021, 41, BSR20210111. [Google Scholar] [CrossRef]
- Ghazala, R.A.; El-Attar, E.A.; Abouzeid, Z.S. Circulating miRNA 27a and miRNA150-5p; a noninvasive approach to endometrial carcinoma. Mol. Biol. Rep. 2021, 48, 4351–4360. [Google Scholar] [CrossRef] [PubMed]
- Rižner, T.L. Discovery of biomarkers for endometrial cancer: Current status and prospects. Expert Rev. Mol. Diagn. 2016, 16, 1315–1336. [Google Scholar] [CrossRef] [PubMed]
- Montagnana, M.; Benati, M.; Danese, E.; Giudici, S.; Perfranceschi, M.; Ruzzenenete, O.; Salvagno, G.L.; Bassi, A.; Gelati, M.; Paviati, E.; et al. Aberrant MicroRNA Expression in Patients With Endometrial Cancer. Int. J. Gynecol. Cancer 2017, 27, 459–466. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, N.; Yin, D.; Li, Y.K.; Guo, L.; Shi, L.P.; Huang, X. Changes in the Expression of Serum MiR-887-5p in Patients With Endometrial Cancer. Int. J. Gynecol. Cancer 2016, 26, 1143–1147. [Google Scholar] [CrossRef]
- Torres, A.; Torres, K.; Pesci, A.; Ceccaroni, M.; Paszkowski, T.; Cassandrini, P.; Zamboni, G.; Maciejewski, R. Diagnostic and prognostic significance of miRNA signatures in tissues and plasma of endometrioid endometrial carcinoma patients. Int. J. Cancer 2013, 132, 1633–1645. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, J.; He, L.; Stiles, B.L. PTEN: Tumor Suppressor and Metabolic Regulator. Front. Endocrinol. 2018, 9, 338. [Google Scholar] [CrossRef]
- Mutter, G.L.; Lin, M.C.; Fitzgerald, J.T.; Kum, J.B.; Baak, J.P.; Lees, J.A.; Weng, L.P.; Eng, C. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J. Natl. Cancer Inst. 2000, 92, 924–930. [Google Scholar] [CrossRef]
- Mutter, G.L. Pten, a protean tumor suppressor. Am. J. Pathol. 2001, 158, 1895–1898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hou, X.; Li, Y.; Zhao, M. MiR-205 inhibits cell apoptosis by targeting phosphatase and tensin homolog deleted on chromosome ten in endometrial cancer Ishikawa cells. BMC Cancer 2014, 14, 440. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Liang, R. miR-205 promotes epithelial-mesenchymal transition by targeting AKT signaling in endometrial cancer cells. J. Obs. Gynaecol. Res. 2015, 41, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Hermeking, H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010, 17, 193–199. [Google Scholar] [CrossRef]
- Li, F.; Chen, H.; Huang, Y.; Zhang, Q.; Xue, J.; Liu, Z.; Zheng, F. miR-34c plays a role of tumor suppressor in HEC-1-B cells by targeting E2F3 protein. Oncol. Rep. 2015, 33, 3069–3074. [Google Scholar] [CrossRef] [PubMed]
- Hiroki, E.; Akahira, J.; Suzuki, F.; Nagase, S.; Ito, K.; Suzuki, T.; Sasano, H.; Yaegashi, N. Changes in microRNA expression levels correlate with clinicopathological features and prognoses in endometrial serous adenocarcinomas. Cancer Sci. 2010, 101, 241–249. [Google Scholar] [CrossRef]
- Maroof, H.; Salajegheh, A.; Smith, R.A.; Lam, A.K. Role of microRNA-34 family in cancer with particular reference to cancer angiogenesis. Exp. Mol. Pathol. 2014, 97, 298–304. [Google Scholar] [CrossRef]
- Tu, J.; Tan, X.; Chen, Y.; Li, Z.; Zhang, Y.; Chen, X.; Yang, H.; Chen, H.; Yu, Z. Growth arrest-specific transcript 5 represses endometrial cancer development by promoting antitumor function of tumor-associated macrophages. Cancer Sci. 2022, 113, 2496–2512. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Tang, Y.; Li, F.; Chen, X. The role of lncRNA-MEG/miR-21-5p/PDCD4 axis in spinal cord injury. Am. J. Transl. Res. 2021, 13, 646–658. [Google Scholar]
- Liu, C.; Zhang, Y.H.; Deng, Q.; Li, Y.; Huang, T.; Zhou, S.; Cai, Y.D. Cancer-Related Triplets of mRNA-lncRNA-miRNA Revealed by Integrative Network in Uterine Corpus Endometrial Carcinoma. Biomed. Res. Int. 2017, 2017, 3859582. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; He, Y.; Peng, F.; Yang, J.; Yuan, C. Endometrial Cancer Cells Promote M2-Like Macrophage Polarization by Delivering Exosomal miRNA-21 under Hypoxia Condition. J. Immunol. Res. 2020, 2020, 9731049. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, J.; Wu, Y.; Tang, X.; Zhu, W. Overexpression of circRNA circFAT1 in Endometrial Cancer Cells Increases Their Stemness by Upregulating miR-21 Through Methylation. Cancer. Biother. Radiopharm. 2022, 37, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liao, Y.; Jia, C.; Ren, J.; Wang, J.; Li, T. MicroRNA-182 promotes tumor cell growth by targeting transcription elongation factor A-like 7 in endometrial carcinoma. Cell. Physiol. Biochem. 2013, 32, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Devor, E.J.; Schickling, B.M.; Reyes, H.D.; Warrier, A.; Lindsay, B.; Goodheart, M.J.; Santillan, D.A.; Leslie, K.K. Cullin-5, a ubiquitin ligase scaffold protein, is significantly underexpressed in endometrial adenocarcinomas and is a target of miR-182. Oncol. Rep. 2016, 35, 2461–2465. [Google Scholar] [CrossRef] [PubMed]
- Myatt, S.S.; Wang, J.; Monteiro, L.J.; Christian, M.; Ho, K.K.; Fusi, L.; Dina, R.E.; Brosens, J.J.; Ghaem-Maghami, S.; Lam, E.W. Definition of microRNAs that repress expression of the tumor suppressor gene FOXO1 in endometrial cancer. Cancer. Res. 2010, 70, 367–377. [Google Scholar] [CrossRef]
- Hurteau, G.J.; Carlson, J.A.; Roos, E.; Brock, G.J. Stable expression of miR-200c alone is sufficient to regulate TCF8 (ZEB1) and restore E-cadherin expression. Cell Cycle 2009, 8, 2064–2069. [Google Scholar] [CrossRef]
- Dai, Y.; Xia, W.; Song, T.; Su, X.; Li, J.; Li, S.; Chen, Y.; Wang, W.; Ding, H.; Liu, X.; et al. MicroRNA-200b is overexpressed in endometrial adenocarcinomas and enhances MMP2 activity by downregulating TIMP2 in human endometrial cancer cell line HEC-1A cells. Nucleic Acid. Ther. 2013, 23, 29–34. [Google Scholar] [CrossRef]
- Yu, D.; Zhou, H.; Xun, Q.; Xu, X.; Ling, J.; Hu, Y. microRNA-103 regulates the growth and invasion of endometrial cancer cells through the downregulation of tissue inhibitor of metalloproteinase 3. Oncol. Lett. 2012, 3, 1221–1226. [Google Scholar] [CrossRef]
- Guo, C.; Song, W.Q.; Sun, P.; Jin, L.; Dai, H.Y. LncRNA-GAS5 induces PTEN expression through inhibiting miR-103 in endometrial cancer cells. J. Biomed. Sci. 2015, 22, 100. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Chen, F.; Hu, T.; Peng, W.; Gu, Q.; Sun, Y. The Diverse Oncogenic and Tumor Suppressor Roles of microRNA-105 in Cancer. Front. Oncol. 2019, 9, 518. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Piao, J.; Ou, J.; Zhu, X. Circ_0109046 promotes the malignancy of endometrial carcinoma cells through the microRNA-105/SOX9/Wnt/β-catenin axis. IUBMB Life 2021, 73, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, X.; Tong, D.; Han, C.; Zhao, R.; He, Y.; Jin, X. miR-136 suppresses tumor invasion and metastasis by targeting RASAL2 in triple-negative breast cancer. Oncol. Rep. 2016, 36, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Qi, Y.; Yin, X.; Gao, J. miR-136 targets MIEN1 and involves the metastasis of colon cancer by suppressing epithelial-to-mesenchymal transition. Onco Targets Ther. 2018, 11, 67–74. [Google Scholar] [CrossRef]
- Shen, S.; Yue, H.; Li, Y.; Qin, J.; Li, K.; Liu, Y.; Wang, J. Upregulation of miR-136 in human non-small cell lung cancer cells promotes Erk1/2 activation by targeting PPP2R2A. Tumour Biol. 2014, 35, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Zong, Z.H.; Liu, Y.; Chen, S.; Zhao, Y. Circ_PUM1 promotes the development of endometrial cancer by targeting the miR-136/NOTCH3 pathway. J. Cell Mol. Med. 2020, 24, 4127–4135. [Google Scholar] [CrossRef]
- Shi, Y.; Jia, L.; Wen, H. Circ_0109046 Promotes the Progression of Endometrial Cancer via Regulating miR-136/HMGA2 Axis. Cancer Manag. Res. 2020, 12, 10993–11003. [Google Scholar] [CrossRef]
- Ma, J.; Li, D.; Kong, F.F.; Yang, D.; Yang, H.; Ma, X.X. miR-302a-5p/367-3p-HMGA2 axis regulates malignant processes during endometrial cancer development. J. Exp. Clin. Cancer Res. 2018, 37, 19. [Google Scholar] [CrossRef]
- Li, Q.; Kong, F.; Cong, R.; Ma, J.; Wang, C.; Ma, X. PVT1/miR-136/Sox2/UPF1 axis regulates the malignant phenotypes of endometrial cancer stem cells. Cell Death Dis. 2023, 14, 177. [Google Scholar] [CrossRef]
- Yamamoto, H.; Imai, K. Microsatellite instability: An update. Arch. Toxicol. 2015, 89, 899–921. [Google Scholar] [CrossRef]
- Tafe, L.J.; Riggs, E.R.; Tsongalis, G.J. Lynch syndrome presenting as endometrial cancer. Clin. Chem. 2014, 60, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Hermyt, E.; Zmarzły, N.; Grabarek, B.; Kruszniewska-Rajs, C.; Gola, J.; Jęda-Golonka, A.; Szczepanek, K.; Mazurek, U.; Witek, A. Interplay between miRNAs and Genes Associated with Cell Proliferation in Endometrial Cancer. Int. J. Mol. Sci. 2019, 20, 6011. [Google Scholar] [CrossRef]
- Choi, C.H.; Park, Y.A.; Choi, J.J.; Song, T.; Song, S.Y.; Lee, Y.Y.; Lee, J.W.; Kim, T.J.; Kim, B.G.; Bae, D.S. Angiotensin II type I receptor and miR-155 in endometrial cancers: Synergistic antiproliferative effects of anti-miR-155 and losartan on endometrial cancer cells. Gynecol. Oncol. 2012, 126, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.L.; Sun, K.X.; Zong, Z.H.; Chen, S.; Zhao, Y. MicroRNA-372 inhibits endometrial carcinoma development by targeting the expression of the Ras homolog gene family member C (RhoC). Oncotarget 2016, 7, 6649–6664. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Li, Q.L.; Pan, W.; Li, J.; Zhang, M.F.; Cao, T.; Su, S.G.; Shen, H. PRMT6 promotes endometrial cancer via AKT/mTOR signaling and indicates poor prognosis. Int. J. Biochem. Cell Biol. 2020, 120, 105681. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Sun, K.X.; Xiu, Y.L.; Liu, B.L.; Feng, M.X.; Sang, X.B.; Zhao, Y. MicroRNA-93 Promotes Epithelial-Mesenchymal Transition of Endometrial Carcinoma Cells. PLoS ONE 2016, 11, e0165776. [Google Scholar] [CrossRef]
- Xu, J.B. MicroRNA-93-5p/IFNAR1 axis accelerates metastasis of endometrial carcinoma by activating the STAT3 pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5657–5666. [Google Scholar] [CrossRef]
- Banzhaf-Strathmann, J.; Edbauer, D. Good guy or bad guy: The opposing roles of microRNA 125b in cancer. Cell Commun. Signal 2014, 12, 30. [Google Scholar] [CrossRef]
- Shang, C.; Lu, Y.M.; Meng, L.R. Erratum: MicroRNA-125b Down-Regulation Mediates Endometrial Cancer Invasion by Targeting ERBB2. Med. Sci. Monit. 2020, 26, e929638. [Google Scholar] [CrossRef]
- Cheung, A.; Shah, S.; Parker, J.; Soor, P.; Limbu, A.; Sheriff, M.; Boussios, S. Non-Epithelial Ovarian Cancers: How Much Do We Really Know? Int. J. Env. Res. Public Health 2022, 19, 1106. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Y.; Chen, L.; Li, R.; Ning, Y.; Zhu, X. Role of metformin in functional endometrial hyperplasia and polycystic ovary syndrome involves the regulation of MEG3/miR-223/GLUT4 and SNHG20/miR-4486/GLUT4 signaling. Mol. Med. Rep. 2022, 26, 218. [Google Scholar] [CrossRef] [PubMed]
- Noren Hooten, N.; Martin-Montalvo, A.; Dluzen, D.F.; Zhang, Y.; Bernier, M.; Zonderman, A.B.; Becker, K.G.; Gorospe, M.; de Cabo, R.; Evans, M.K. Metformin-mediated increase in DICER1 regulates microRNA expression and cellular senescence. Aging Cell 2016, 15, 572–581. [Google Scholar] [CrossRef]
- Udesen, P.B.; Glintborg, D.; Sørensen, A.E.; Svendsen, R.; Nielsen, N.L.S.; Wissing, M.L.M.; Andersen, M.S.; Englund, A.L.M.; Dalgaard, L.T. Metformin decreases miR-122, miR-223 and miR-29a in women with polycystic ovary syndrome. Endocr. Connect. 2020, 9, 1075–1084. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, J.; Liu, S.; Hua, T.; Sun, Q. Corrigendum to “Exercise induced improvements in insulin sensitivity are concurrent with reduced NFE2/miR-432-5p and increased FAM3A” [Life Sci. 207 (2018) 23–29]. Life Sci. 2020, 260, 118515. [Google Scholar] [CrossRef]
- Dos Santos, J.A.C.; Veras, A.S.C.; Batista, V.R.G.; Tavares, M.E.A.; Correia, R.R.; Suggett, C.B.; Teixeira, G.R. Physical exercise and the functions of microRNAs. Life Sci. 2022, 304, 120723. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, X.; Lv, Y.; Fang, Y.; Pan, L.; Gan, Z.; Huang, Z.; Wei, W. A Five-microRNA Signature as Risk Stratification System in Uterine Corpus Endometrial Carcinoma. Comb. Chem. High Throughput. Screen 2021, 24, 187–194. [Google Scholar] [CrossRef]
- Lan, H.; Lu, H.; Wang, X.; Jin, H. MicroRNAs as potential biomarkers in cancer: Opportunities and challenges. Biomed. Res. Int. 2015, 2015, 125094. [Google Scholar] [CrossRef]
- Mall, C.; Rocke, D.M.; Durbin-Johnson, B.; Weiss, R.H. Stability of miRNA in human urine supports its biomarker potential. Biomark. Med. 2013, 7, 623–631. [Google Scholar] [CrossRef]
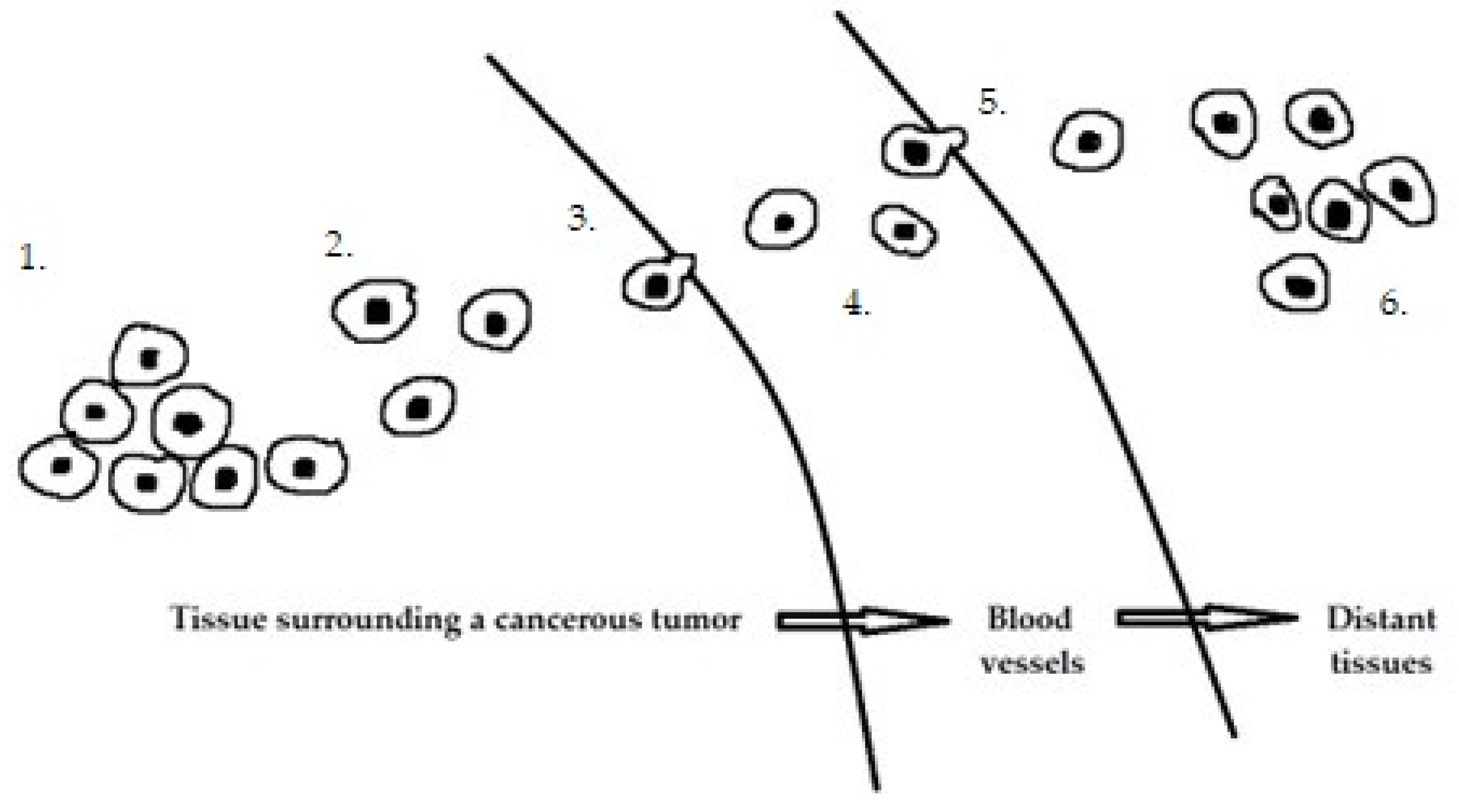
| Primary Tumor Growth | ||
|---|---|---|
| miR-15/16 | ↓ | The miR-15/16 family is a highly expressed tumor suppressor group that targets a large network of genes in T cells to limit their cell cycle, memory formation, and survival. Once activated, miR-15/16 T cells are downregulated, allowing rapid expansion of differentiated effector T cells to mediate a sustained immune response. MiR-15/16 deficiency alters Treg expression of critical functional proteins, including FOXP3, IL2Rα/CD25, CTLA4, PD-1, and IL7Rα/CD127, and results in the accumulation of functionally impaired FOXP3loCD25loCD127hi Tregs [50]. |
| miR-17/91 | ↑ | Involved in immune regulation, three clusters of the miR-17/92 family collectively suppressed IL-12 production in macrophages, and miR-17/92 acts through PTEN to inhibit IL-12 expression by modulating the PI3K-Akt-GSK3 pathway [51]. |
| miR-34 | ↓ | It is involved in the regulation of the cell cycle and apoptosis through p53 signaling [52]. It acts as a tumor suppressor through DNA methylation in both epithelial and hematological malignancies [53]. |
| miR-181a | ↑ | It can interact with H3F3B, ATM, CCDC6, TAM15, RAS, and PLAG1 to promote cell proliferation [54]. |
| miR-200 | ↓ | Targets ZEB1 and blocks the epithelial–mesenchymal transition [55]. |
| miR-211 | ↑ | Targets mRNAs: POU3F2, ZCCHC24, PRLR, ITPR1, and CHRDL1 [56]. |
| miR-222 | ↑ | Targets tumor suppressors PTEN and TIMP3. Targets MMP-2 i MMP-9 [42,57]. |
| Let7 | ↓ | Acting through Lin28, it targets RAS genes. Overexpression of let-7 leads to a decrease in RAS production, accelerating the cell cycle, angiogenesis, and cell adhesion. Therefore, under normal conditions, miR let-7 acts as a tumor suppressor gene and inhibits the activation of oncogenes that can lead to the formation of cancer cells [26,58]. |
| Migration and local invasion | ||
| miR-9 | ↑ | MiR-9, which is upregulated in breast cancer cells, targets CDH1, the mRNA encoding E-cadherin, leading to increased cell motility and invasiveness. The miR-9-mediated downregulation of E-cadherin causes activation of β-catenin signaling, which contributes to the upregulation of growth factor gene expression [59]. |
| miR-10b | ↑ | It increases invasion, migration, and proliferation and inhibits apoptosis in the EC [60]. It targets HOXB3 [61]. |
| miR-21 | ↑ | Overexpression of miR-21-5p promoted epithelial to mesenchymal transition. It works through SOX17 [62]. |
| miR-29c | ↓ | It affects the expression of HBP1, ITGB1, MCL1, MDM2 and SGK1 [63]. Overexpression of miR-29c reduces COL4A1 production in endometrial cells [64]. |
| miR-34a | ↓ | Inverse correlation between miR-34a and L1CAM protein expression. A decrease in miR-34a and an increase in L1CAM are associated with poor [65]. MiR-34a is downregulated in endometrial cancer tissues and is negatively correlated with Notch1 expression [66]. |
| miR-103 | ↑ | Overexpression of miR-103 promotes EC cell proliferation. It works through ZO-1 and triggers its downward adjustments. There is an inverse correlation between ZO-1 and miR-103 [67]. |
| miR-107 | ↑ | MiR-107-5p downregulated Erα mRNA and protein expression [68]. |
| miR-135a | ↑ | MiR-135a can regulate the epithelial-to-mesenchymal transition (EMT) by altering the expression of E-cadherin and N-cadherin. MiR-135a promotes endometrial cancer cell proliferation by regulating PTEN. Expression levels of PTEN and p-AKT in endometrial cancer cells decreased after miR-135a overexpression [43]. |
| miR-135b | ↑ | Upregulation of miR-135b significantly reduced FOXO1 protein and mRNA expression, promoting EC proliferation [69]. |
| miR-145 | ↓ | MiR-145 expression is lower in EC tissues than in neighboring tissues. MiR-145 inhibits SOX11. MiR-145 targets site 3 (3615) of SOX11 3’UTR to affect SOX11 expression [70]. |
| miR-148b | ↓ | Downregulation of miR-148b induced endometrial EMT of the tumor cell as a result of alleviating DNMT1 suppression [71].MiR-148b regulates the expression of endoplasmic reticulum metalloprotease 1 (ERMP1) [72]. |
| miR-155 | ↑ | It impairs the functioning of dendritic cells in endometrial cancer which play an important role in the activation of anticancer immune responses. It acts via the p38MAPK14 pathway [73]. |
| miR-214-3p | ↓ | MiR-214-3p is downregulated and TWIST1 is upregulated in EC tissues and cells. Overexpression of miR-214-3p suppressed migration, invasion, and EMT in EC cells [74].A decrease in miR-214-3p is associated with an increase in NEAT1, HMGA1, and β-catenin [75]. |
| miR-223 | ↑ | MiR-223 modulates the inflammatory response by directly targeting genes mediating signal transduction, including those present in the canonical NF-kB pathway [76]. |
| miR-340 | ↓ | MiR-340-5p is downregulated in the EC compared to adjacent normal tissues. In vitro, miR-340-5p inhibited the migratory capacity of EC cells by downregulating MMP-3 and MMP-9 and prevented TGF-α1-induced EMT by p-eIF4E [77]. |
| Transendothelial migration of cancer cells into vessels | ||
| miR-21 | ↑ | It inhibits the suppressive effect of FBXO11 (a member of the F-box subfamily lacking a clear unifying domain) [78]. |
| miR-105 | ↑ | It targets the ZO-1 protein. In endothelial monolayers, exosome-mediated transfer of tumor-secreted miR-105 effectively disrupts the tight junctions and integrity of these natural barriers to metastasis. Overexpression of miR-105 in non-metastatic cancer cells induces metastasis and vascular permeability in distant organs [79]. |
| miR-126 | ↓ | It is a tumor suppressor and its growth can downregulate VEGF to inhibit EC cell invasion and migration [80].Its decrease correlates with high levels of Lnc-ATB, which induced accelerated tumor growth by regulating the miR-126 PIK3R2 target gene and Sox2-related apoptosis.In the tested RL95 and HEC1A cell lines, the downregulation of Lnc-ATB resulted in the upregulation of miR-126. There was an impairment of cell viability, an increase in caspase-3-related tumor apoptosis, and G1/S arrest [81]. |
| Survival in the circulatory system | ||
| miR-26a | ↓ | Increased peritumoral lymphoid endothelial hyaluronan receptor-1 (LYVE-1) density in LNM patients was negatively associated with the level of miR-26a-5p in primary lesions, indicating that down-expression of miR-26a-5p can induce LNM EC [82]. |
| miR-141 | ↑ | PPP1R12A and PPP1R12B are targeted and degraded. Both are members of the myosin phosphatase (MYPT) targeting protein family [83]. |
| miR-145-3p | ↑ | MiR-145 participates in M2 macrophage polarization by targeting IL-16 and upregulating IL-10 [84]. |
| miR-181-a | ↑ | MiR-181a and miR-181b increased the expression of PECAM-1 mRNA and protein and VE-cadherin accompanying the differentiation of human embryonic stem cells into vascular endothelial cells [85]. By acting on VE-cadherin, it disrupts the barrier in endothelial cells [86]. |
| miR-424 | ↓ | MiR-424 has a protective role in various types of cancer including endometrial cancer, upregulation of miR-424 inactivated PI3K/AKT signaling mediated by G-1 protein-coupled estrogen receptor (GPER) in endometrial cancer. Moreover, the luciferase report confirmed the targeting reaction between miR-424 and GPER [87]. |
| Extravasation | ||
| miR-7 | ↓ | Through the downregulation of the PI3K and MAPK pathways, its dominant role is to inhibit proliferation and survival, stimulate apoptosis, and inhibit migration [88]. |
| miR-21 | ↑ | It inhibits the expression of the SOX17 protein and promotes epithelial-to-mesenchymal transition (EMT) [62]. |
| miR-31 | ↓ | MiR-31 acts as an oncogene in endometrial cancer by suppressing the hippopotamus pathway. MiR-31 significantly suppressed mRNA luciferase activity in conjunction with the LATS2 3′-UTR and consequently promoted the translocation of YAP1, a key molecule in the Hippo pathway, into the nucleus [89]. MiR-31 is a master regulator of integrins as it targets multiple partners of the α subunits (α2, α5, and αV) of β1 integrins as well as β3 integrins, inhibiting cell proliferation in a ligand-dependent manner [90]. |
| miR-155 | ↓ | Targets are MLH1, MSH2, and MSH6 [91]. |
| miR-182 | ↑ | Promotes cancer cell migration and invasion by inhibiting MBNL2 expression [92]. |
| miR-214 | ↑ | Targets are PTEN/AKT, β-catenin, and tyrosine kinase receptor pathways. MiR-214 also regulates the levels of key modulators of gene expression: the epigenetic repressor Ezh2, p53 ”genome guardian”, the transcription factors TFAP2 and another miRNA, miR-148b. Thus, miR-214 seems to play an important role in coordinating tumor proliferation, stem, angiogenesis, invasiveness, extravasation, metastasis, chemoresistance, and microenvironment [93]. |
| Pre-metastatic niche formation | ||
| miR-19a | ↑ | Member of the highly conservative miR-17-92 cluster [94]. The miR-17/miR-20a seed family is responsible for this anti-aging activity [95]. MiR-19a activates the mammalian protein kinase B (AKT) rapamycin (mTOR) pathway, thereby functionally antagonizing PTEN to promote cell survival [96]. |
| miR-126 | ↑ | The target is VEGF. It increases the rate of migration and invasion of EC cells [80]. |
| miR-133a | ↓ | MiR-133a is a suppressor. The miR-1/133a cluster directly regulates PDE7A in EC cells. PDEs are enzymes that regulate the cellular levels of cAMP and cGMP second messengers by controlling their rate of degradation [97]. |
| miR-503 | ↓ | It plays a tumor suppressor role by targeting CCND1 [98]. |
| Upregulation | miR-17 [112] miR-152 [112] miR-205 [113] miR-376a [114] miR-548ag [115] |
| Downregulation | miR-15b [116] miR-17 [117] miR-138 [112] miR-150 [118] miR-593 [112] |
| Upregulation | miR-21 [128] miR-107-5p [68] miR-429 [129,130] miR-501 [131] miR-576-5p [132] |
| Downregulation | miR-24b-5p [133] miR-26a-5p [82] miR-34a [65,66] miR-34b-5p [134] miR-34c-3p [134] miR-34c-5p [134] miR-148b [71] miR-204-5p [126] miR-505 [135] |
| Upregulation | miR-29b [139] miR-126 [81] miR-148b [71,72] miR-152 [140,141] miR-199a-5p [133] miR-214-3p [74] miR-340-5p [77] miR-455-5p [133] miR-505 [135] |
| Downregulation | miR-429 [129] |
| Upregulation | miR-15a-5p [143] miR-20b-5p [144] miR-27a [145] miR-106b-5p [143] miR-107 [143] miR-143 [44,144] miR-143-3p [144] miR-150-5p [145] miR-186 [146,147] miR-195-5p [144] miR-200a [146] miR-203 [146] miR-204 [146] miR-204-5p [144] miR-222 [146,147] miR-223 [146,147] miR-423-3p [144] miR-449 [146] miR-484 [144] miR-887-5p [148] |
| Downregulation | miR-16 [44] miR-99b [44] miR-125 [44] miR-145 [44] miR-204 [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogaczyk, A.; Zawlik, I.; Zuzak, T.; Kluz, M.; Potocka, N.; Kluz, T. The Role of miRNAs in the Development, Proliferation, and Progression of Endometrial Cancer. Int. J. Mol. Sci. 2023, 24, 11489. https://doi.org/10.3390/ijms241411489
Bogaczyk A, Zawlik I, Zuzak T, Kluz M, Potocka N, Kluz T. The Role of miRNAs in the Development, Proliferation, and Progression of Endometrial Cancer. International Journal of Molecular Sciences. 2023; 24(14):11489. https://doi.org/10.3390/ijms241411489
Chicago/Turabian StyleBogaczyk, Anna, Izabela Zawlik, Tomasz Zuzak, Marta Kluz, Natalia Potocka, and Tomasz Kluz. 2023. "The Role of miRNAs in the Development, Proliferation, and Progression of Endometrial Cancer" International Journal of Molecular Sciences 24, no. 14: 11489. https://doi.org/10.3390/ijms241411489
APA StyleBogaczyk, A., Zawlik, I., Zuzak, T., Kluz, M., Potocka, N., & Kluz, T. (2023). The Role of miRNAs in the Development, Proliferation, and Progression of Endometrial Cancer. International Journal of Molecular Sciences, 24(14), 11489. https://doi.org/10.3390/ijms241411489






