The Impact of Mild Chronic Stress and Maternal Experience in the Fmr1 Mouse Model of Fragile X Syndrome
Abstract
1. Introduction
2. Results
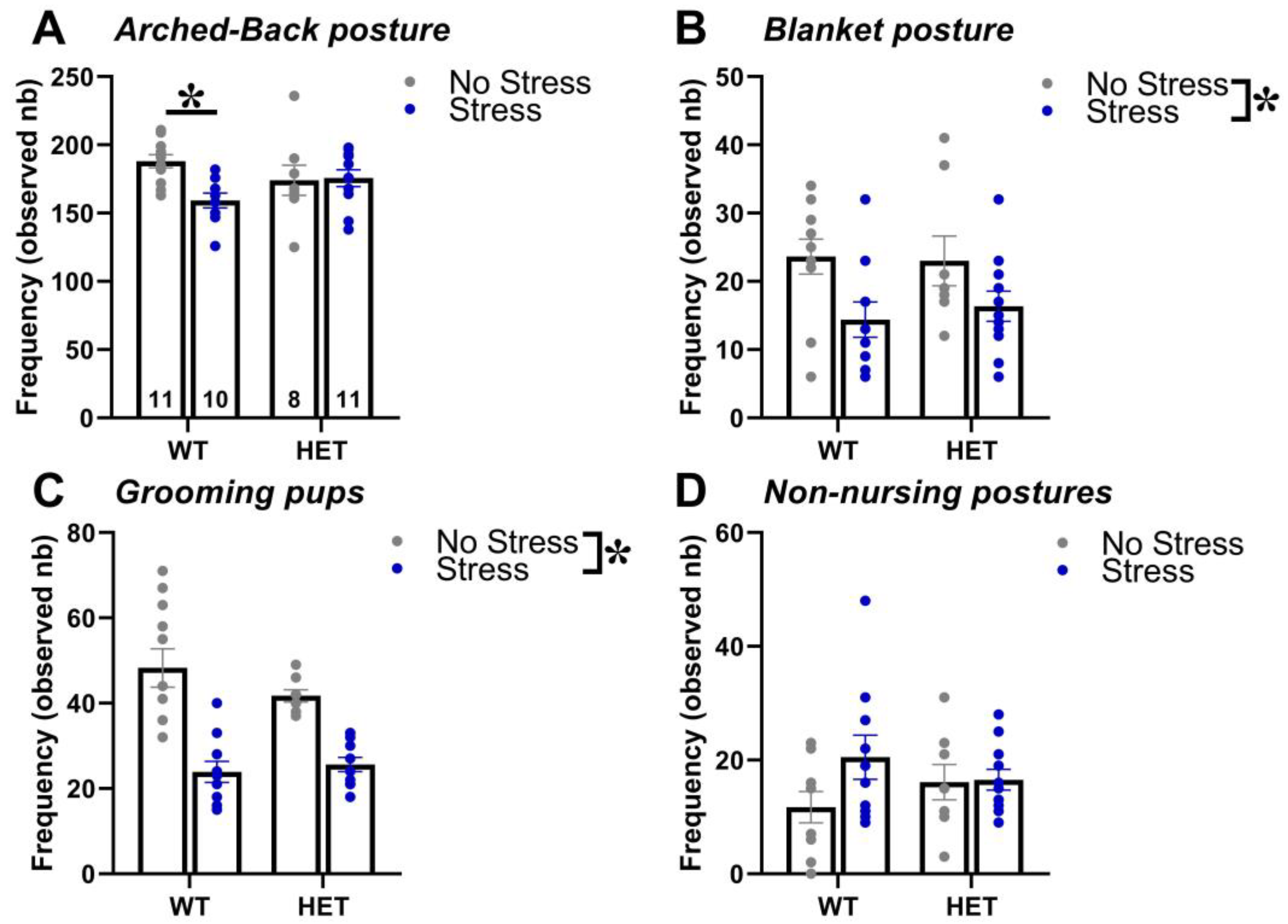
2.1. Maternal Behavior
2.2. Elevated Plus Maze
2.3. Open Field
2.4. Y Maze
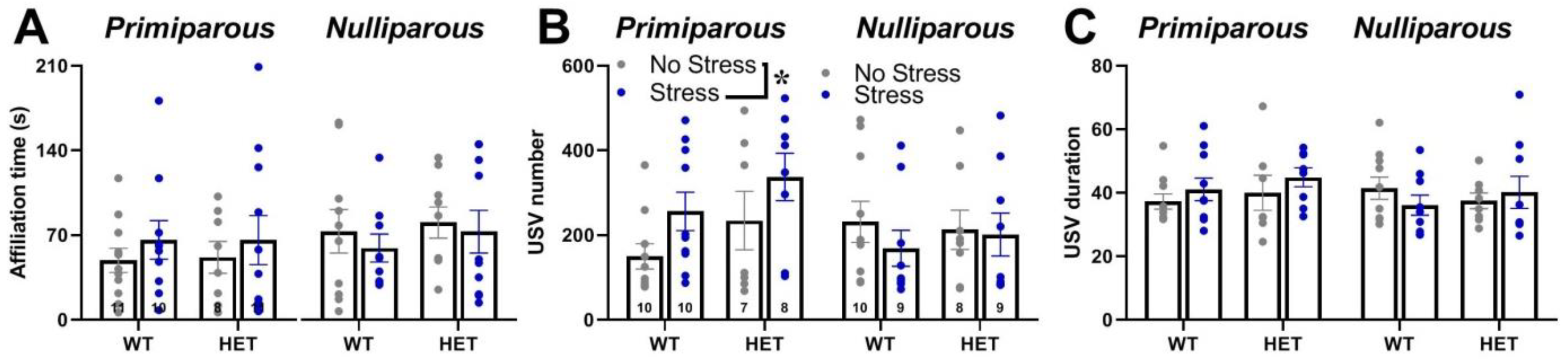
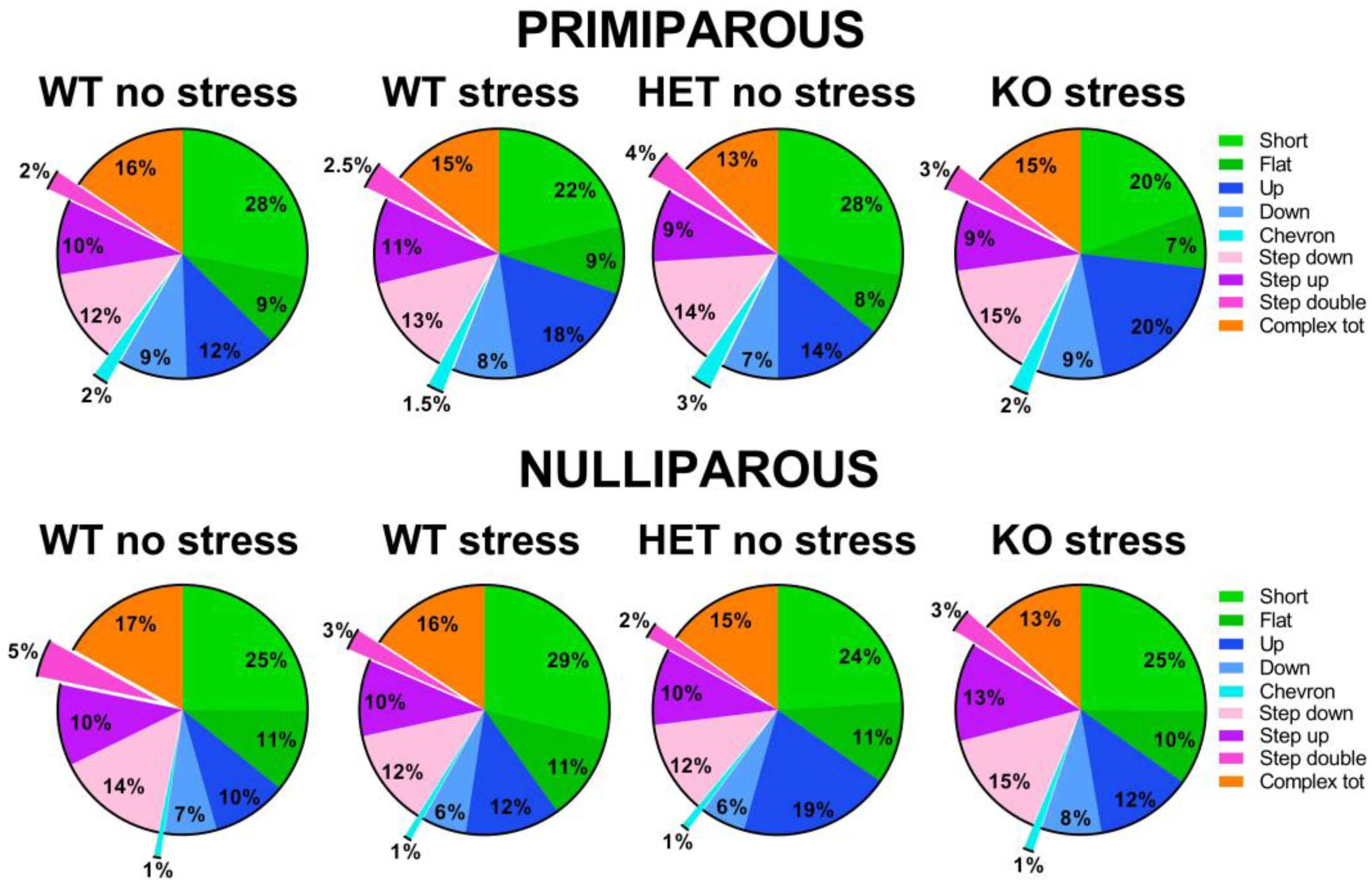
2.5. Social Interaction and Ultrasonic Communication
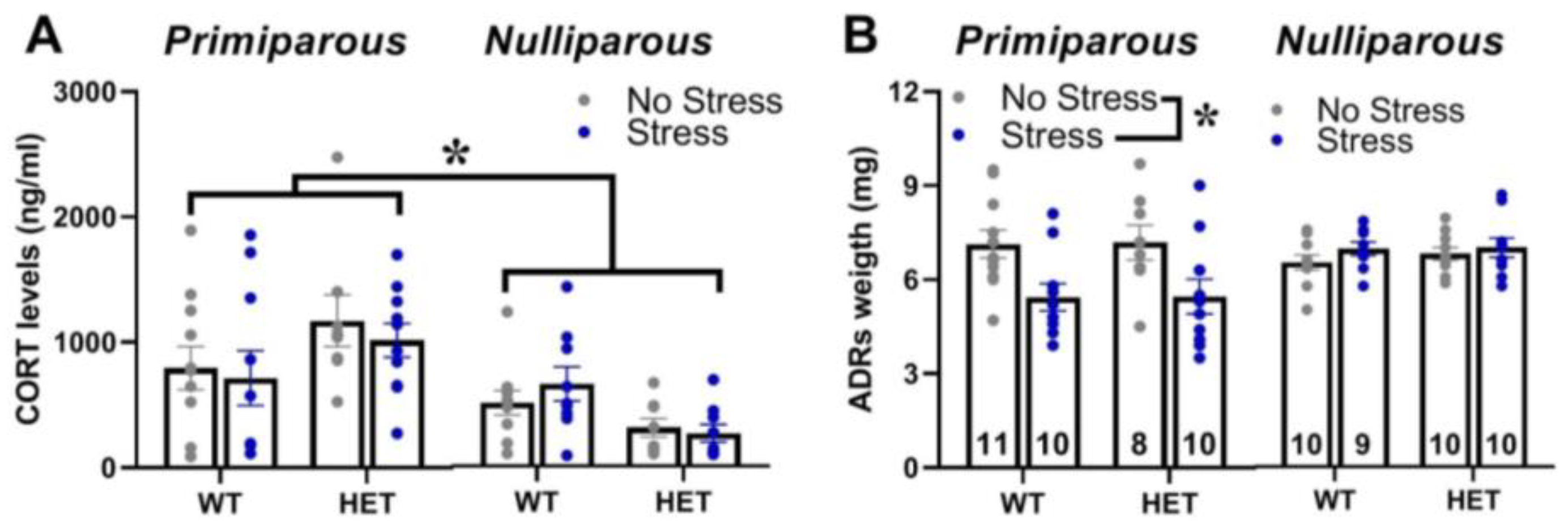
2.6. Corticosterone Blood Levels and Adrenal Gland Weight
3. Discussion
4. Materials and Methods
4.1. Breeding Procedures
4.2. Stress Procedure
- -
- Day 1: 30 min of restrain stress (3 times each day during the light phase, with a 4 h interval) in perforated conical tubes (3 cm in diameter, 11.5 cm long; Becton Dickinson Labware Europe, France), followed by overnight housing with wet bedding (50 mL of water was added to floor sawdust of the home cage at the beginning of the dark phase).
- -
- Day 2: multiple sawdust and cage changes (3 times each day during the light phase, with a 4 h interval), followed by overnight housing with novel objects (12 glass black beads, 1.5 cm in diameter were added in the home cage at the beginning of the dark phase).
4.3. Behavioral Testing Procedures
4.4. Elevated Plus Maze
4.5. Open Field
4.6. Y Maze
4.7. Social Interaction and Ultrasonic Communication
4.8. Assessment of Corticosterone Blood Levels
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verkerk, A.J.; Pieretti, M.; Sutcliffe, J.S.; Fu, Y.-H.; Kuhl, D.P.; Pizzuti, A.; Reiner, O.; Richards, S.; Victoria, M.F.; Zhang, F.; et al. Identification of a gene (FMR1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991, 65, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.J.; Hagerman, P.J. Fragile X Syndrome: Diagnosis, Treatment, and Research; Taylor & Francis: New York, NY, USA, 2002. [Google Scholar]
- Bailey, J.D.B.; Hatton, D.D.; Skinner, M.; Mesibov, G. Autistic Behavior, FMR1 Protein, and Developmental Trajectories in Young Males with Fragile X Syndrome. J. Autism Dev. Disord. 2001, 31, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kerby, D.S.; Dawson, B.L. Autistic features, personality, and adaptive behavior in males with the fragile X syndrome and no autism. Am. J. Ment. Retard. 1994, 98, 455–462. [Google Scholar] [PubMed]
- Mazzocco, M.M.M.; Kates, W.R.; Baumgardner, T.L.; Freund, L.S.; Reiss, A.L. Autistic behaviors among girls with fragile X syndrome. J. Autism Dev. Disord. 1997, 27, 415–435. [Google Scholar] [CrossRef]
- Mazzocco, M.M.M.; Pulsifer, M.; Fiumara, A.; Cocuzza, M.; Nigro, F.; Incorpora, G.; Barone, R. Brief report: Autistic behaviors among children with fragile X or Rett syndrome: Implications for the classification of pervasive developmental disorder. J. Autism Dev. Disord. 1998, 28, 321–328. [Google Scholar] [CrossRef]
- Reiss, A.L.; Freund, L. Behavioral phenotype of fragile X syndrome: DSM-III-R autistic behavior in male children. Am. J. Med. Genet. 1992, 43, 35–46. [Google Scholar] [CrossRef]
- Hatton, D.D.; Sideris, J.; Skinner, M.; Mankowski, J.; Bailey, D.B.; Roberts, J.; Mirrett, P. Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP. Am. J. Med. Genet. Part A 2006, 140A, 1804–1813. [Google Scholar] [CrossRef]
- Turner, G.; Webb, T.; Wake, S.; Robinson, H. Prevalence of fragile X syndrome. Am. J. Med. Genet. 1996, 64, 196–197. [Google Scholar] [CrossRef]
- Wheeler, A.; Raspa, M.; Bann, C.; Bishop, E.; Hessl, D.; Sacco, P.; Bailey, D.B. Anxiety, attention problems, hyperactivity, and the Aberrant Behavior Checklist in fragile X syndrome. Am. J. Med. Genet. Part A 2013, 164, 141–155. [Google Scholar] [CrossRef]
- Loesch, D.Z.; Hay, D.A. Clinical features and reproductive patterns in fragile X female heterozygotes. J. Med. Genet. 1988, 25, 407–414. [Google Scholar] [CrossRef]
- Loesch, D.Z.; Bui, Q.M.; Grigsby, J.; Butler, E.; Epstein, J.; Huggins, R.M.; Taylor, A.K.; Hagerman, R.J. Effect of the Fragile X Status Categories and the Fragile X Mental Retardation Protein Levels on Executive Functioning in Males and Females With Fragile X. Neuropsychology 2003, 17, 646–657. [Google Scholar] [CrossRef]
- Nolin, S.L.; Lewis, F.A., 3rd; Ye, L.L.; Houck, G.E., Jr.; Glicksman, A.E.; Limprasert, P.; Li, S.Y.; Zhong, N.; Ashley, A.E.; Feingold, E.; et al. Familial transmission of the FMR1 CGG repeat. Am. J. Hum. Genet. 1996, 59, 1252–1261. [Google Scholar]
- Reyniers, E.; Vits, L.; De Boulle, K.; Van Roy, B.; Van Velzen, D.; de Graaff, E.; Verkerk, A.J.; Jorens, H.Z.; Darby, J.K.; Oostra, B.; et al. The full mutation in the FMR–1 gene of male fragile X patients is absent in their sperm. Nat. Genet. 1993, 4, 143–146. [Google Scholar] [CrossRef]
- Mazzocco, M.M.; Holden, J.J. Neuropsychological profiles of three sisters homozygous for the fragile X premutation. Am. J. Med. Genet. 1996, 64, 323–328. [Google Scholar] [CrossRef]
- Baker, K.B.; Wray, S.P.; Ritter, R.; Mason, S.; Lanthorn, T.H.; Savelieva, K. Male and female Fmr1 knockout mice on C57 albino background exhibit spatial learning and memory impairments. Genes Brain Behav. 2010, 9, 562–574. [Google Scholar] [CrossRef]
- Ding, Q.; Sethna, F.; Wang, H. Behavioral analysis of male and female Fmr1 knockout mice on C57BL/6 background. Behav. Brain Res. 2014, 271, 72–78. [Google Scholar] [CrossRef]
- Musumeci, S.A.; Bosco, P.; Calabrese, G.; Bakker, C.; Sarro, G.B.; Elia, M.; Ferri, R.; Oostra, B.A. Audiogenic Seizures Susceptibility in Transgenic Mice with Fragile X Syndrome. Epilepsia 2000, 41, 19–23. [Google Scholar] [CrossRef]
- Nguy, S.; Tejada-Simon, M.V. Phenotype analysis and rescue on female FVB. 129-Fmr1 knockout mice. Front. Biol. 2016, 11, 43–52. [Google Scholar] [CrossRef]
- Qin, M.; Kang, J.; Smith, C.B. A null mutation for Fmr1 in female mice: Effects on regional cerebral metabolic rate for glucose and relationship to behavior. Neuroscience 2005, 135, 999–1009. [Google Scholar] [CrossRef]
- Gauducheau, M.; Lemaire-Mayo, V.; D’Amato, F.R.; Oddi, D.; Crusio, W.E.; Pietropaolo, S. Age-specific autistic-like behaviors in heterozygousFmr1-KO female mice. Autism Res. 2017, 10, 1067–1078. [Google Scholar] [CrossRef]
- Petroni, V.; Subashi, E.; Premoli, M.; Wöhr, M.; Crusio, W.E.; Lemaire, V.; Pietropaolo, S. Autistic-like behavioral effects of prenatal stress in juvenile Fmr1 mice: The relevance of sex differences and gene–environment interactions. Sci. Rep. 2022, 12, 7269. [Google Scholar] [CrossRef] [PubMed]
- Pietropaolo, S.; Subashi, E. Mouse models of Fragile X syndrome. In Behavioral Genetics of the Mouse, Pietropaolo; Pietropaolo, S., Sluyter, F., Crusio, W.E., Eds.; Cambridge University Press: Cambridge, UK, 2014; Volume 2, pp. 146–163. [Google Scholar]
- Dawson, G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev. Psychopathol. 2008, 20, 775–803. [Google Scholar] [CrossRef] [PubMed]
- Bagni, C.; Tassone, F.; Neri, G.; Hagerman, R. Fragile X syndrome: Causes, diagnosis, mechanisms, and therapeutics. J. Clin. Investig. 2012, 122, 4314–4322. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.J.; Polussa, J. Treatment of the psychiatric problems associated with fragile X syndrome. Curr. Opin. Psychiatry 2015, 28, 107–112. [Google Scholar] [CrossRef]
- Dyer-Friedman, J.; Glaser, B.; Hessl, D.; Johnston, C.; Huffman, L.C.; Taylor, A.; Wisbeck, J.; Reiss, A.L. Genetic and Environmental Influences on the Cognitive Outcomes of Children With Fragile X Syndrome. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 237–244. [Google Scholar] [CrossRef]
- Hessl, D.; Dyer-Friedman, J.; Glaser, B.; Wisbeck, J.; Barajas, R.G.; Taylor, A.; Reiss, A.L. The Influence of Environmental and Genetic Factors on Behavior Problems and Autistic Symptoms in Boys and Girls With Fragile X Syndrome. Pediatrics 2001, 108, e88. [Google Scholar] [CrossRef]
- Dawson, G.; Webb, S.; Schellenberg, G.D.; Dager, S.; Friedman, S.; Aylward, E.; Richards, T. Defining the broader phenotype of autism: Genetic, brain, and behavioral perspectives. Dev. Psychopathol. 2002, 14, 581–611. [Google Scholar] [CrossRef]
- Restivo, L.; Ferrari, F.; Passino, E.; Sgobio, C.; Bock, J.; Oostra, B.A.; Bagni, C.; Ammassari-Teule, M. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proc. Natl. Acad. Sci. USA 2005, 102, 11557–11562. [Google Scholar] [CrossRef]
- Oddi, D.; Subashi, E.; Middei, S.; Bellocchio, L.; Lemaire-Mayo, V.; Guzmán, M.; Crusio, W.E.; D’Amato, F.R.; Pietropaolo, S. Early Social Enrichment Rescues Adult Behavioral and Brain Abnormalities in a Mouse Model of Fragile X Syndrome. Neuropsychopharmacology 2015, 40, 1113–1122. [Google Scholar] [CrossRef]
- Ward, A.J. A comparison and analysis of the presence of family problems during pregnancy of mothers of “autistic” children and mothers of normal children. Child Psychiatry Hum. Dev. 1990, 20, 279–288. [Google Scholar] [CrossRef]
- Beversdorf, D.Q.; Manning, S.E.; Hillier, A.; Anderson, S.L.; Nordgren, R.E.; Walters, S.E.; Nagaraja, H.N.; Cooley, W.C.; Gaelic, S.E.; Bauman, M.L. Timing of Prenatal Stressors and Autism. J. Autism Dev. Disord. 2005, 35, 471–478. [Google Scholar] [CrossRef]
- Kinney, D.K.; Miller, A.M.; Crowley, D.J.; Huang, E.; Gerber, E. Autism Prevalence Following Prenatal Exposure to Hurricanes and Tropical Storms in Louisiana. J. Autism Dev. Disord. 2007, 38, 481–488. [Google Scholar] [CrossRef]
- Kinney, D.K.; Munir, K.M.; Crowley, D.J.; Miller, A.M. Prenatal stress and risk for autism. Neurosci. Biobehav. Rev. 2008, 32, 1519–1532. [Google Scholar] [CrossRef]
- Beversdorf, D.Q.; Stevens, H.E.; Margolis, K.G.; Van De Water, J. Prenatal Stress and Maternal Immune Dysregulation in Autism Spectrum Disorders: Potential Points for Intervention. Curr. Pharm. Des. 2019, 25, 4331–4343. [Google Scholar] [CrossRef]
- Lemaire-Mayo, V.; Subashi, E.; Henkous, N.; Beracochea, D.; Pietropaolo, S. Behavioral effects of chronic stress in the Fmr1 mouse model for fragile X syndrome. Behav. Brain Res. 2017, 320, 128–135. [Google Scholar] [CrossRef]
- Qin, M.; Xia, Z.; Huang, T.; Smith, C. Effects of chronic immobilization stress on anxiety-like behavior and basolateral amygdala morphology in Fmr1 knockout mice. Neuroscience 2011, 194, 282–290. [Google Scholar] [CrossRef]
- Macbeth, A.H.; Luine, V.N. Changes in anxiety and cognition due to reproductive experience: A review of data from rodent and human mothers. Neurosci. Biobehav. Rev. 2010, 34, 452–467. [Google Scholar] [CrossRef]
- Brunton, P.J.; Russell, J.A. The expectant brain: Adapting for motherhood. Nat. Rev. Neurosci. 2008, 9, 11–25. [Google Scholar] [CrossRef]
- Kinsley, C.H. The neuroplastic maternal brain. Horm. Behav. 2008, 54, 1–4. [Google Scholar] [CrossRef]
- Darnaudéry, M.; Perez-Martin, M.; Del Favero, F.; Gomez-Roldan, C.; Garcia-Segura, L.M.; Maccari, S. Early motherhood in rats is associated with a modification of hippocampal function. Psychoneuroendocrinology 2007, 32, 803–812. [Google Scholar] [CrossRef]
- Lemaire, V.; Billard, J.-M.; Dutar, P.; George, O.; Piazza, P.V.; Epelbaum, J.; Le Moal, M.; Mayo, W. Motherhood-induced memory improvement persists across lifespan in rats but is abolished by a gestational stress. Eur. J. Neurosci. 2006, 23, 3368–3374. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, V.; Lamarque, S.; Le Moal, M.; Piazza, P.-V.; Abrous, D.N. Postnatal Stimulation of the Pups Counteracts Prenatal Stress-Induced Deficits in Hippocampal Neurogenesis. Biol. Psychiatry 2006, 59, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Muir, J.L.; Pfister, H.P.; Ivinskis, A. Effects of prepartum stress and postpartum enrichment on mother–infant interaction and offspring problem-solving ability in Rattus norvegicus. J. Comp. Psychol. 1985, 99, 468–478. [Google Scholar] [CrossRef]
- Maccari, S.; Piazza, P.; Kabbaj, M.; Barbazanges, A.; Simon, H.; Le Moal, M. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J. Neurosci. 1995, 15, 110–116. [Google Scholar] [CrossRef]
- Smith, J.W.; Seckl, J.R.; Evans, A.T.; Costall, B.; Smythe, J.W. Gestational stress induces post-partum depression-like behaviour and alters maternal care in rats. Psychoneuroendocrinology 2004, 29, 227–244. [Google Scholar] [CrossRef]
- Misdrahi, D.; Pardon, M.C.; Pérez-Diaz, F.; Hanoun, N.; Cohen-Salmon, C. Prepartum chronic ultramild stress increases corticosterone and estradiol levels in gestating mice: Implications for postpartum depressive disorders. Psychiatry Res. 2005, 137, 123–130. [Google Scholar] [CrossRef]
- Negroni, J.; Venault, P.; Pardon, M.C.; Pérez-Diaz, F.; Chapouthier, G.; Cohen-Salmon, C. Chronic ultra-mild stress improves locomotor performance of B6D2F1 mice in a motor risk situation. Behav. Brain Res. 2004, 155, 265–273. [Google Scholar] [CrossRef]
- Pardon, M.-C.; Pérez-Diaz, F.; Joubert, C.; Cohen-Salmon, C. Age-dependent effects of a chronic ultramild stress procedure on open-field behaviour in B6D2F1 female mice. Physiol. Behav. 2000, 70, 7–13. [Google Scholar] [CrossRef]
- Pardon, M.C.; Pérez-Diaz, F.; Joubert, C.; Cohen-Salmon, C. Influence of a chronic ultramild stress procedure on decision-making in mice. J. Psychiatry Neurosci. 2000, 25, 167–177. [Google Scholar]
- Champagne, F.A.; Curley, J.P.; Keverne, E.B.; Bateson, P.P. Natural variations in postpartum maternal care in inbred and outbred mice. Physiol. Behav. 2007, 91, 325–334. [Google Scholar] [CrossRef]
- Curley, J.; Jensen, C.; Franks, B.; Champagne, F. Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Horm. Behav. 2012, 61, 454–461. [Google Scholar] [CrossRef]
- Maggio, J.C.; Whitney, G. Ultrasonic vocalizing by adult female mice (Mus musculus). J. Comp. Psychol. 1985, 99, 420–436. [Google Scholar] [CrossRef]
- Whitney, G.; Coble, J.R.; Stockton, M.D.; Tilson, E.F. Ultrasonic emissions: Do they facilitate courtship of mice? J. Comp. Physiol. Psychol. 1973, 84, 445–452. [Google Scholar] [CrossRef]
- Giannoccaro, S.; Ferraguto, C.; Petroni, V.; Marcelly, C.; Nogues, X.; Campuzano, V.; Pietropaolo, S. Early Neurobehavioral Characterization of the CD Mouse Model of Williams–Beuren Syndrome. Cells 2023, 12, 391. [Google Scholar] [CrossRef]
- Petroni, V.; Subashi, E.; Premoli, M.; Memo, M.; Lemaire, V.; Pietropaolo, S. Long-term behavioral effects of prenatal stress in the Fmr1-knock-out mouse model for fragile X syndrome. Front. Cell. Neurosci. 2022, 16, 917183. [Google Scholar] [CrossRef]
- Premoli, M.; Petroni, V.; Bulthuis, R.; Bonini, S.A.; Pietropaolo, S. Ultrasonic Vocalizations in Adult C57BL/6J Mice: The Role of Sex Differences and Repeated Testing. Front. Behav. Neurosci. 2022, 16, 883353. [Google Scholar] [CrossRef]
- Kat, R.; Arroyo-Araujo, M.; de Vries, R.B.; Koopmans, M.A.; de Boer, S.F.; Kas, M.J. Translational validity and methodological underreporting in animal research: A systematic review and meta-analysis of the Fragile X syndrome (Fmr1 KO) rodent model. Neurosci. Biobehav. Rev. 2022, 139, 104722. [Google Scholar] [CrossRef]
- Zupan, B.; Sharma, A.; Frazier, A.; Klein, S.; Toth, M. Programming social behavior by the maternal fragile X protein. Genes, Brain Behav. 2016, 15, 578–587. [Google Scholar] [CrossRef]
- Zupan, B.; Toth, M. Wild-Type Male Offspring of fmr-1+/− Mothers Exhibit Characteristics of the Fragile X Phenotype. Neuropsychopharmacology 2008, 33, 2667–2675. [Google Scholar] [CrossRef]
- Zupan, B.; Toth, M. Fmr-1 as an Offspring Genetic and a Maternal Environmental Factor in Neurodevelopmental Disease. In Modeling Fragile X Syndrome; Springer: Berlin/Heidelberg, Germany, 2012; Volume 54, pp. 243–253. [Google Scholar] [CrossRef]
- Bowman, R.; Frankfurt, M.; Luine, V. Sex differences in cognition following variations in endocrine status. Learn. Mem. 2022, 29, 234–245. [Google Scholar] [CrossRef]
- Bowman, R.E. Stress-Induced Changes in Spatial Memory are Sexually Differentiated and Vary Across the Lifespan. J. Neuroendocr. 2005, 17, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Bowman, R.E.; Beck, K.D.; Luine, V.N. Chronic stress effects on memory: Sex differences in performance and monoaminergic activity. Horm. Behav. 2003, 43, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.M.; Evans, J.J.; Derber, W.J.; Johnston, K.A.; Laudenslager, M.L.; Crnic, L.S.; Maclean, K.N. Mouse model of fragile X syndrome: Behavioral and hormonal response to stressors. Behav. Neurosci. 2009, 123, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Smith, C.B. Unaltered hormonal response to stress in a mouse model of fragile X syndrome. Psychoneuroendocrinology 2008, 33, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Sandi, C.; Haller, J. Stress and the social brain: Behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 2015, 16, 290–304. [Google Scholar] [CrossRef]
- Weinstock, M. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 2008, 32, 1073–1086. [Google Scholar] [CrossRef]
- Laviola, G.; Adriani, W.; Morley-Fletcher, S.; Terranova, M. Peculiar response of adolescent mice to acute and chronic stress and to amphetamine: Evidence of sex differences. Behav. Brain Res. 2002, 130, 117–125. [Google Scholar] [CrossRef]
- Dallman, M.F.; Akana, S.F.; Strack, A.M.; Scribner, K.S.; Pecoraro, N.; LA Fleur, S.; Houshyar, H.; Gomez, F. Chronic Stress-Induced Effects of Corticosterone on Brain: Direct and Indirect. Ann. N. Y. Acad. Sci. 2004, 1018, 141–150. [Google Scholar] [CrossRef]
- Nair, B.B.; Aung, Z.K.; Porteous, R.; Prescott, M.; Glendining, K.A.; Jenkins, D.E.; Augustine, R.A.; Silva, M.S.; Yip, S.H.; Bouwer, G.T.; et al. Impact of chronic variable stress on neuroendocrine hypothalamus and pituitary in male and female C57BL/6J mice. J. Neuroendocr. 2021, 33, e12972. [Google Scholar] [CrossRef]
- Marchette, R.C.N.; Bicca, M.A.; Santos, E.C.D.S.; de Lima, T.C.M. Distinctive stress sensitivity and anxiety-like behavior in female mice: Strain differences matter. Neurobiol. Stress 2018, 9, 55–63. [Google Scholar] [CrossRef]
- Safari, M.A.; Jahromi, M.K.; Rezaei, R.; Aligholi, H.; Brand, S. The Effect of Swimming on Anxiety-Like Behaviors and Corticosterone in Stressed and Unstressed Rats. Int. J. Environ. Res. Public Health 2020, 17, 6675. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Juruena, M.F.; Eror, F.; Cleare, A.J.; Young, A.H. The role of early life stress in HPA axis and anxiety. In Advances in Experimental Medicine and Biology; Springer: Amsterdam, The Netherlands, 2020; pp. 141–153. [Google Scholar] [CrossRef]
- Pawluski, J.L.; Lambert, K.G.; Kinsley, C.H. Neuroplasticity in the maternal hippocampus: Relation to cognition and effects of repeated stress. Horm. Behav. 2016, 77, 86–97. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Guilleminot, A.; Martin, B.; D’Amato, F.R.; Crusio, W.E. Genetic-Background Modulation of Core and Variable Autistic-Like Symptoms in Fmr1 Knock-Out Mice. PLoS ONE 2011, 6, e17073. [Google Scholar] [CrossRef]
- Burkholder, T.; Foltz, C.; Karlsson, E.; Linton, C.G.; Smith, J.M. Health Evaluation of Experimental Laboratory Mice. Curr. Protoc. Mouse Biol. 2012, 2, 145–165. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Crusio, W.E. Strain-Dependent Changes in Acoustic Startle Response and its Plasticity Across Adolescence in Mice. Behav. Genet. 2009, 39, 623–631. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Goubran, M.; Joffre, C.; Aubert, A.; Lemaire-Mayo, V.; Crusio, W.E.; Layé, S. Dietary supplementation of omega-3 fatty acids rescues fragile X phenotypes in Fmr1-Ko mice. Psychoneuroendocrinology 2014, 49, 119–129. [Google Scholar] [CrossRef]
- Sanderson, D.J.; Gray, A.; Simon, A.; Taylor, A.M.; Deacon, R.M.J.; Seeburg, P.H.; Sprengel, R.; Good, M.A.; Rawlins, J.N.P.; Bannerman, D.M. Deletion of glutamate receptor-A (GluR-A) AMPA receptor subunits impairs one-trial spatial memory. Behav. Neurosci. 2007, 121, 559–569. [Google Scholar] [CrossRef]
- Moles, A.; D’Amato, F.R. Ultrasonic vocalization by female mice in the presence of a conspecific carrying food cues. Anim. Behav. 2000, 60, 689–694. [Google Scholar] [CrossRef]
- Moles, A.; Costantini, F.; Garbugino, L.; Zanettini, C.; D’Amato, F.R. Ultrasonic vocalizations emitted during dyadic interactions in female mice: A possible index of sociability? Behav. Brain Res. 2007, 182, 223–230. [Google Scholar] [CrossRef]
- Gaudissard, J.; Ginger, M.; Premoli, M.; Memo, M.; Frick, A.; Pietropaolo, S. Behavioral abnormalities in the Fmr1-KO2 mouse model of fragile X syndrome: The relevance of early life phases. Autism Res. 2017, 10, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, F.R.; Moles, A. Ultrasonic vocalizations as an index of social memory in female mice. Behav. Neurosci. 2001, 115, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Premoli, M.; Bonini, S.A.; Mastinu, A.; Maccarinelli, G.; Aria, F.; Paiardi, G.; Memo, M. Specific profile of ultrasonic communication in a mouse model of neurodevelopmental disorders. Sci. Rep. 2019, 9, 15912. [Google Scholar] [CrossRef] [PubMed]
- Scattoni, M.L.; Ricceri, L.; Crawley, J.N. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011, 10, 44–56. [Google Scholar] [CrossRef]
- Caruso, A.; Ricceri, L.; Scattoni, M.L. Ultrasonic vocalizations as a fundamental tool for early and adult behavioral phenotyping of Autism Spectrum Disorder rodent models. Neurosci. Biobehav. Rev. 2020, 116, 31–43. [Google Scholar] [CrossRef]
- Caligioni, C.S. Assessing Reproductive Status/Stages in Mice. Curr. Protoc. Neurosci. 2009, 48, A.4I.1–A.4I.8. [Google Scholar] [CrossRef]
- Vandesquille, M.; Baudonnat, M.; Decorte, L.; Louis, C.; Lestage, P.; Beracochea, D. Working memory deficits and related disinhibition of the cAMP/PKA/CREB are alleviated by prefrontal alpha4beta2*-nAChRs stimulation in aged mice. Neurobiol. Aging 2013, 34, 1599–1609. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subashi, E.; Lemaire, V.; Petroni, V.; Pietropaolo, S. The Impact of Mild Chronic Stress and Maternal Experience in the Fmr1 Mouse Model of Fragile X Syndrome. Int. J. Mol. Sci. 2023, 24, 11398. https://doi.org/10.3390/ijms241411398
Subashi E, Lemaire V, Petroni V, Pietropaolo S. The Impact of Mild Chronic Stress and Maternal Experience in the Fmr1 Mouse Model of Fragile X Syndrome. International Journal of Molecular Sciences. 2023; 24(14):11398. https://doi.org/10.3390/ijms241411398
Chicago/Turabian StyleSubashi, Enejda, Valerie Lemaire, Valeria Petroni, and Susanna Pietropaolo. 2023. "The Impact of Mild Chronic Stress and Maternal Experience in the Fmr1 Mouse Model of Fragile X Syndrome" International Journal of Molecular Sciences 24, no. 14: 11398. https://doi.org/10.3390/ijms241411398
APA StyleSubashi, E., Lemaire, V., Petroni, V., & Pietropaolo, S. (2023). The Impact of Mild Chronic Stress and Maternal Experience in the Fmr1 Mouse Model of Fragile X Syndrome. International Journal of Molecular Sciences, 24(14), 11398. https://doi.org/10.3390/ijms241411398






