Use of Enzymatically Activated Carbon Monoxide Donors for Sensitizing Drug-Resistant Tumor Cells
Abstract
1. Introduction
2. Results
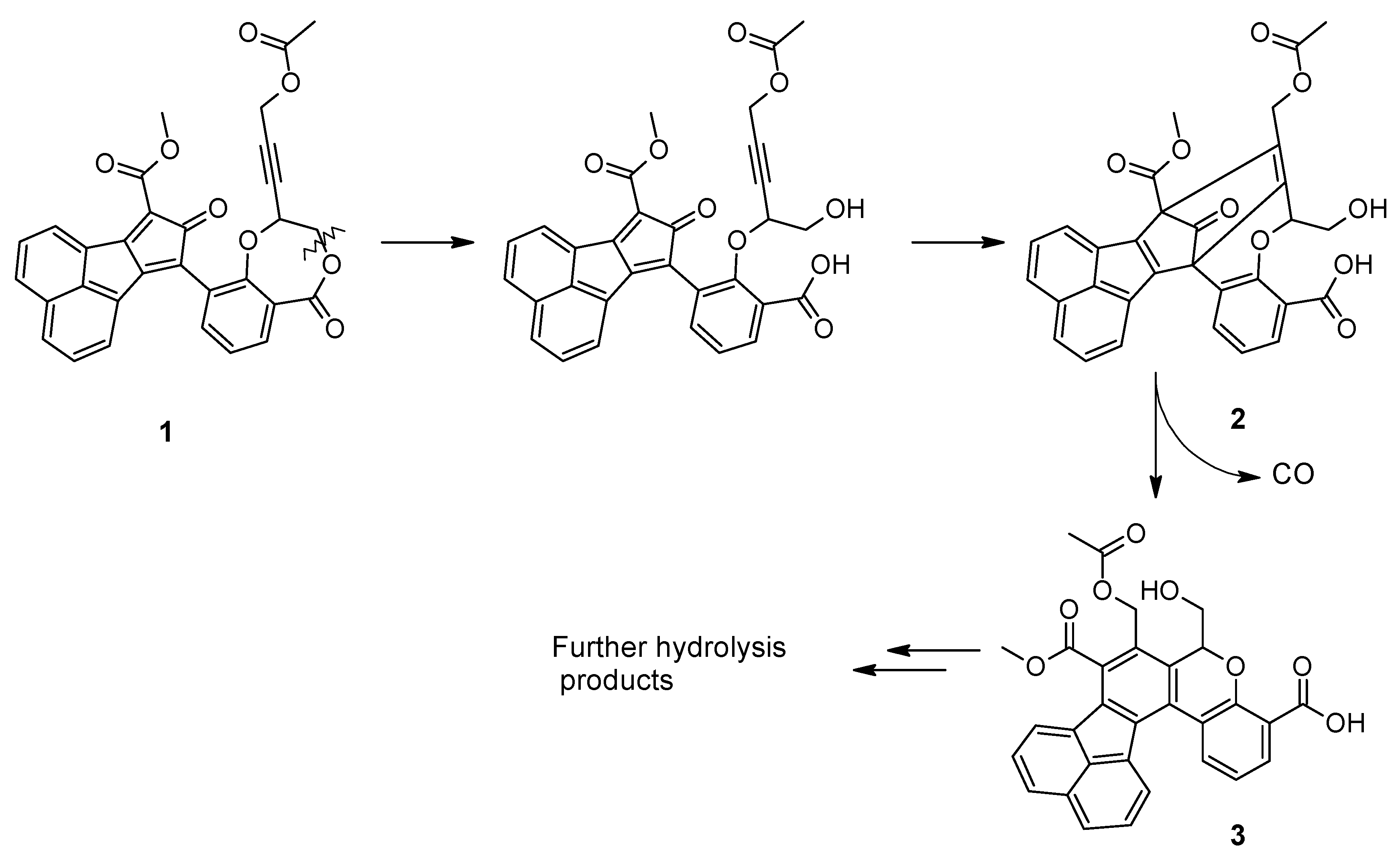
2.1. Chemistry
2.2. Stability Studies
2.3. Biological Studies
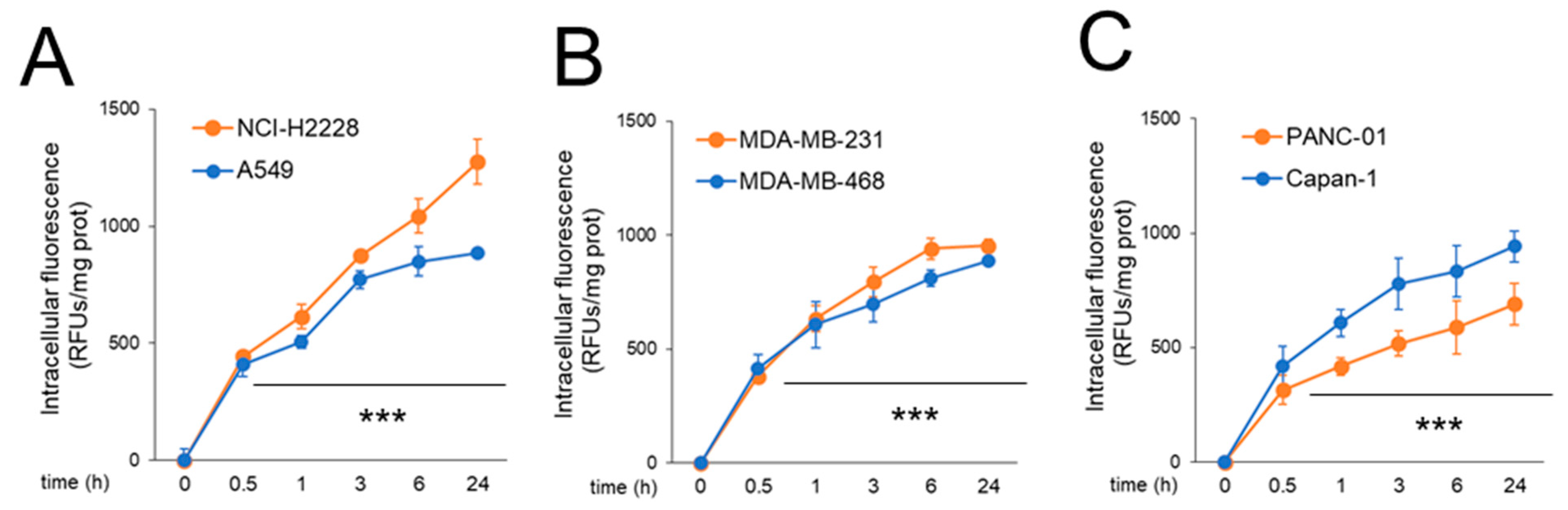
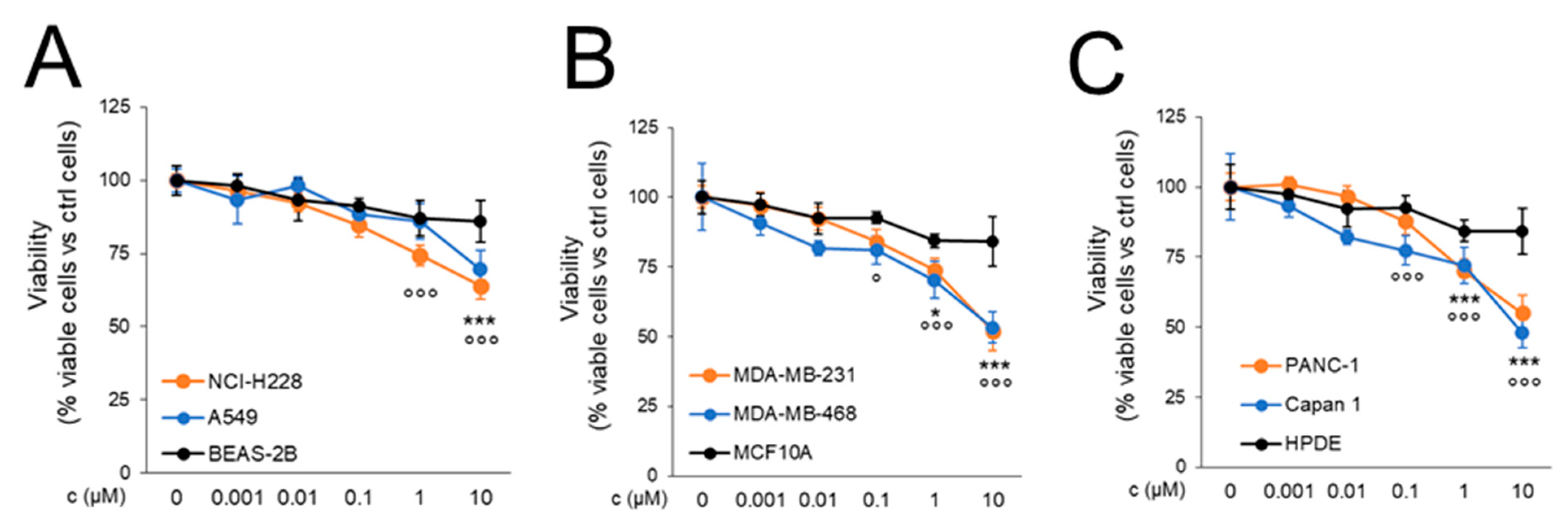
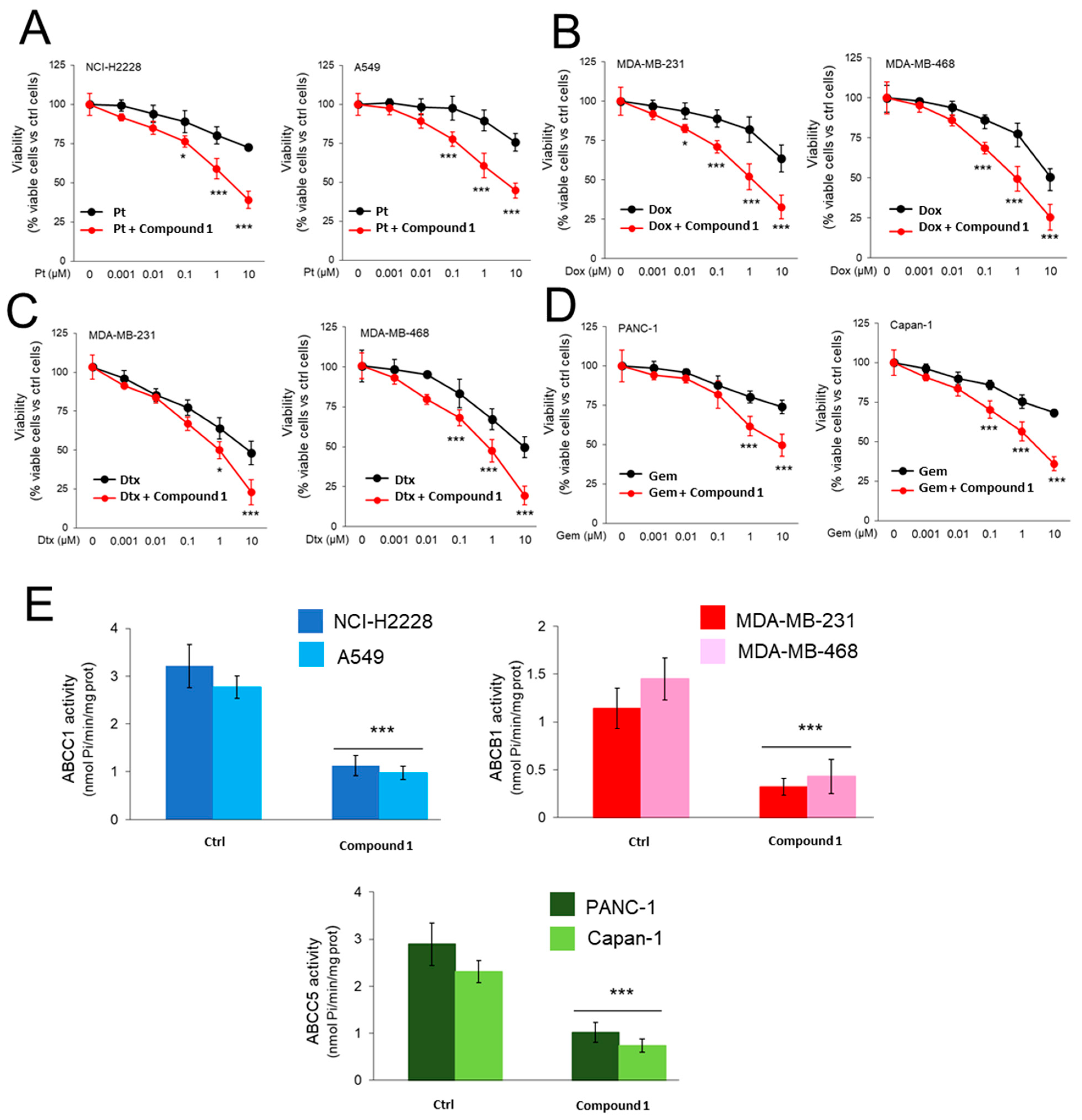
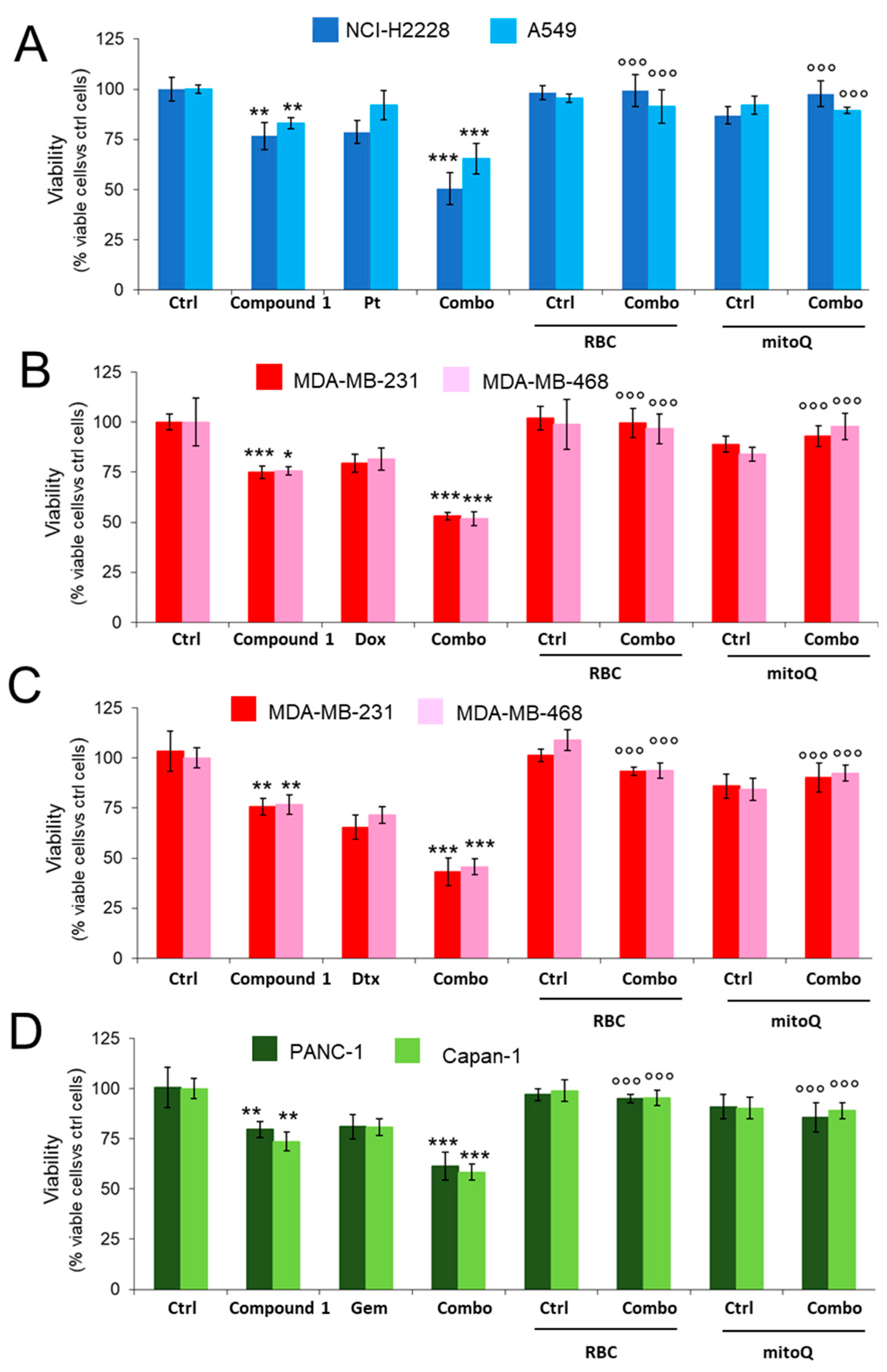
2.3.1. CORM Is Effectively Taken Up by Drug-Resistant Cancer Cells Lines and Overcomes Resistance to Multiple Chemotherapeutic Drugs
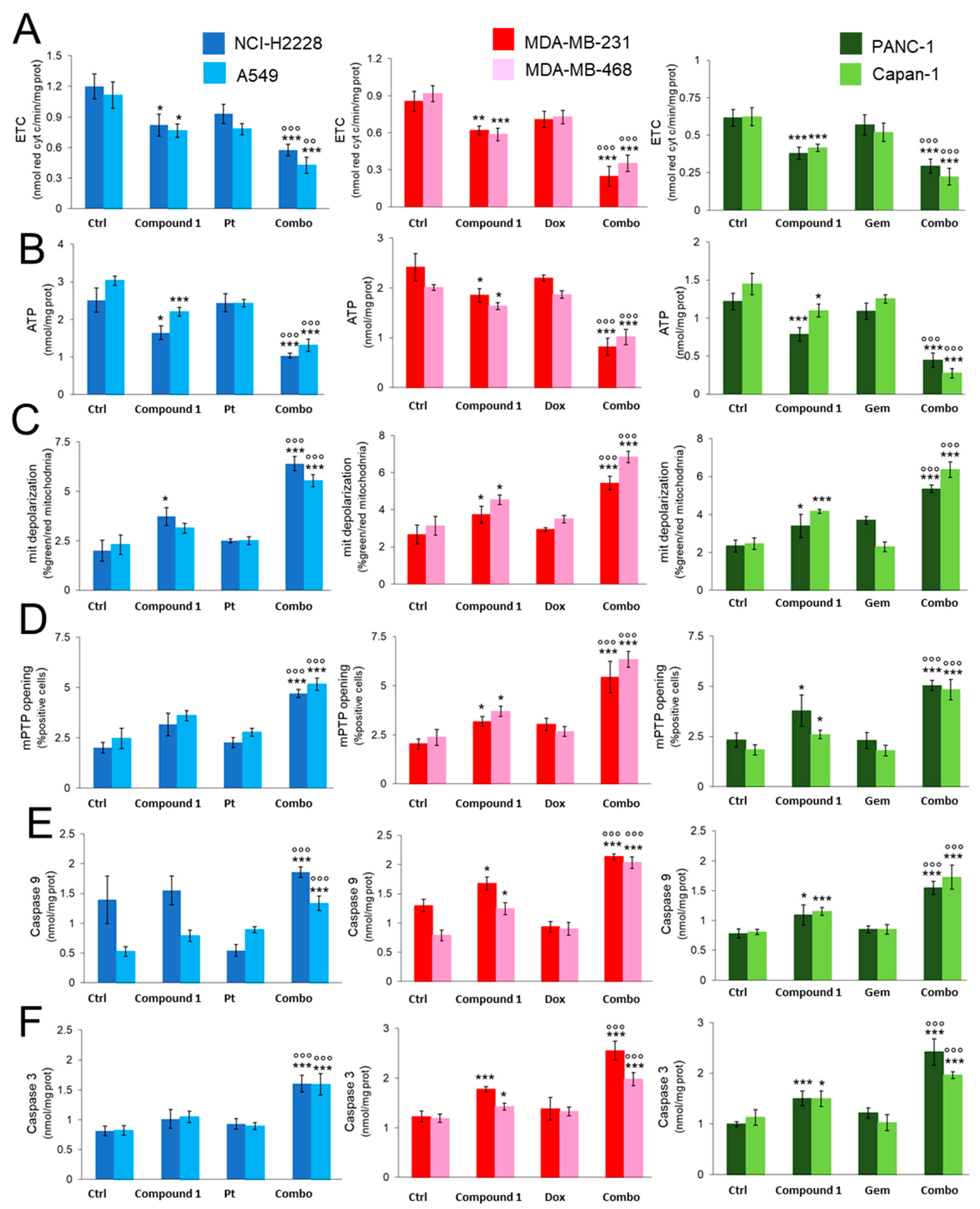
2.3.2. CORM Alters Mitochondrial Energy Metabolism and Enhances Chemotherapy-Dependent Mitochondrial Apoptosis in Resistance Cells
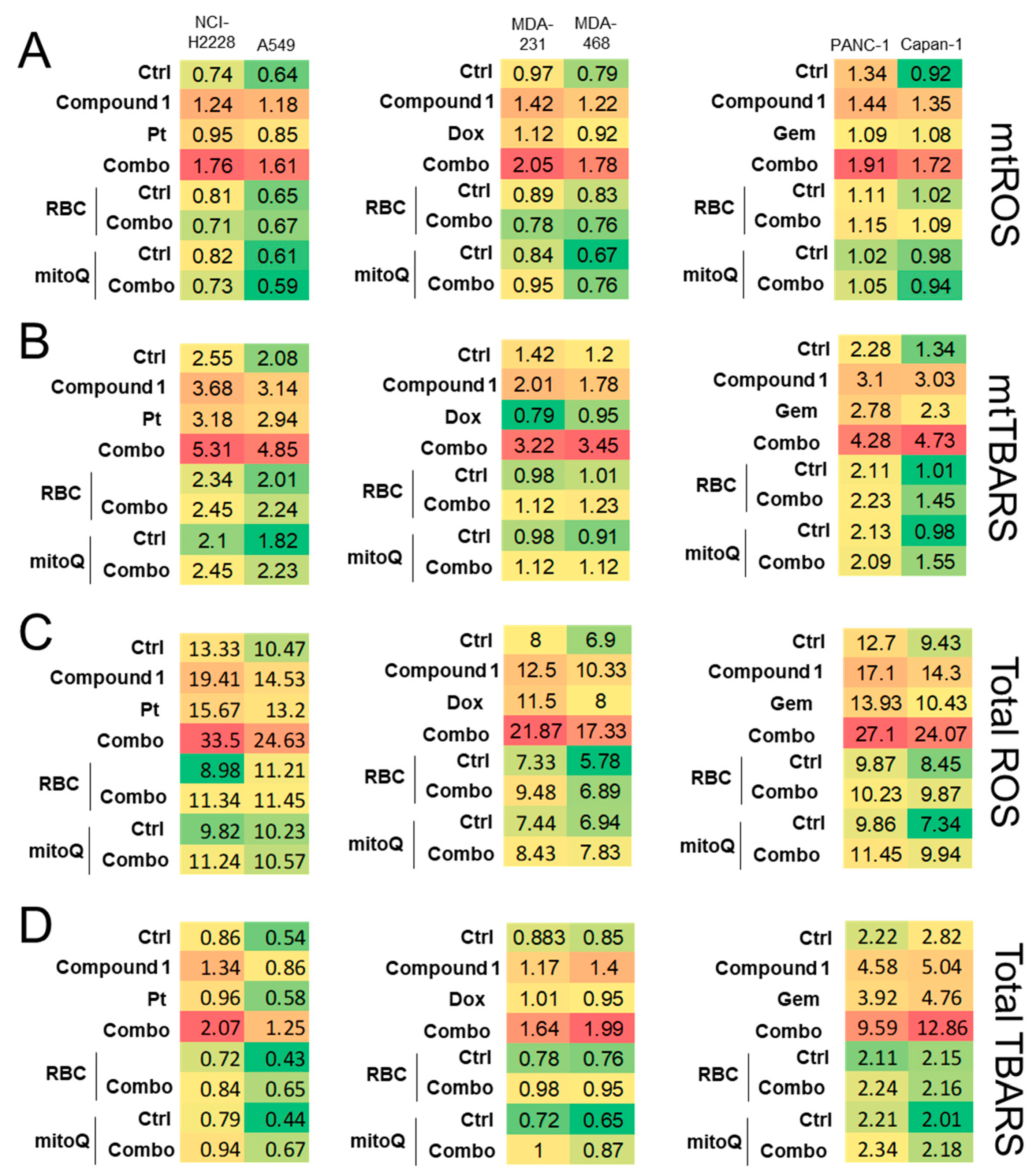
2.3.3. CORM-Induced Chemosensitization Is Caused by Increased Oxidative Stress and CO Release
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. Synthesis
4.1.2. Stability Assay
4.2. Biology
4.2.1. Cell Lines
4.2.2. CORM Intracellular Uptake and CO Release
4.2.3. Cell Viability
4.2.4. ABC Transporters Quantification and Activity
4.2.5. Mitochondrial Extraction, ETC and ATP Measurement
4.2.6. Mitochondrial Depolarization
4.2.7. ROS Measurement
4.2.8. Lipoperoxidation Assay
4.2.9. Caspase-9 and Caspase-3 Activation
4.2.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.K.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Wang, J.Q.; Yang, Y.; Cai, C.Y.; Teng, Q.X.; Cui, Q.; Lin, J.; Assaraf, Y.G.; Chen, Z.S. Multidrug resistance proteins (MRPs): Structure, function and the overcoming of cancer multidrug resistance. Drug. Resist. Updat. 2021, 54, 100743. [Google Scholar] [CrossRef] [PubMed]
- Chegaev, K.; Riganti, C.; Lazzarato, L.; Rolando, B.; Guglielmo, S.; Campia, I.; Fruttero, R.; Bosia, A.; Gasco, A. Nitric oxide donor doxorubicins accumulate into doxorubicin-resistant human colon cancer cells inducing cytotoxicity. ACS Med. Chem. Lett. 2011, 2, 494–497. [Google Scholar] [CrossRef]
- Riganti, C.; Rolando, B.; Kopecka, J.; Campia, I.; Chegaev, K.; Lazzarato, L.; Federico, A.; Fruttero, R.; Ghigo, D. Mitochondrial-targeting nitrooxy-doxorubicin: A new approach to overcome drug resistance. Mol. Pharm. 2013, 10, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Masetto, F.; Chegaev, K.; Gazzano, E.; Mullappilly, N.; Rolando, B.; Arpicco, S.; Fruttero, R.; Riganti, C.; Donadelli, M. MRP5 nitration by NO-releasing gemcitabine encapsulated in liposomes confers sensitivity in chemoresistant pancreatic adenocarcinoma cells. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118824. [Google Scholar] [CrossRef] [PubMed]
- Chegaev, K.; Rolando, B.; Cortese, D.; Gazzano, E.; Buondonno, I.; Lazzarato, L.; Fanelli, M.; Hattinger, C.M.; Serra, M.; Riganti, C.; et al. H2S-Donating Doxorubicins May Overcome Cardiotoxicity and Multidrug Resistance. J. Med. Chem. 2016, 59, 4881–4889. [Google Scholar] [CrossRef]
- Buondonno, I.; Gazzano, E.; Tavanti, E.; Chegaev, K.; Kopecka, J.; Fanelli, M.; Rolando, B.; Fruttero, R.; Gasco, A.; Hattinger, C.; et al. Endoplasmic reticulum-targeting doxorubicin: A new tool effective against doxorubicin-resistant osteosarcoma. Cell. Mol. Life Sci. 2019, 76, 609–625. [Google Scholar] [CrossRef]
- Gazzano, E.; Rolando, B.; Chegaev, K.; Salaroglio, I.C.; Kopecka, J.; Pedrini, I.; Saponara, S.; Sorge, M.; Buondonno, I.; Stella, B.; et al. Folate-targeted liposomal nitrooxy-doxorubicin: An effective tool against P-glycoprotein-positive and folate receptor-positive tumors. J. Control. Release 2018, 270, 37–52. [Google Scholar] [CrossRef]
- Gazzano, E.; Buondonno, I.; Marengo, A.; Rolando, B.; Chegaev, K.; Kopecka, J.; Saponara, S.; Sorge, M.; Hattinger, C.M.; Gasco, A.; et al. Hyaluronated liposomes containing H2S-releasing doxorubicin are effective against P-glycoprotein-positive/doxorubicin-resistant osteosarcoma cells and xenografts. Cancer Lett. 2019, 456, 29–39. [Google Scholar] [CrossRef]
- Wu, L.; Wang, R. Carbon monoxide: Endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 2005, 57, 585–630. [Google Scholar] [CrossRef]
- Gullotta, F.; di Masi, A.; Ascenzi, P. Carbon monoxide: An unusual drug. IUBMB Life 2012, 64, 378–386. [Google Scholar] [CrossRef]
- Yao, X.X.; Yang, P.; Jin, Z.K.; Jiang, Q.; Guo, R.R.; Xie, R.H.; He, Q.J.; Yang, W.L. Multifunctional Nanoplatform for Photooacoustic Imaging-Guided Combined Therapy Enhanced by CO Induced Ferroptosis. Biomaterials 2019, 197, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Ferreira, M.; Albuquerque, I.S.; Dijana, M.V.; Coelho, A.C.; Carvalho, S.M.; Saraiva, L.M.; Romao, C.C.; Bernardes, G.J.L. Spontaneous CO release from Ru(II)(CO)2-protein complexes in aqueous solution, cells and mice. Angew. Chem. 2015, 127, 1188–1191. [Google Scholar] [CrossRef]
- He, Q.; Kiesewetter, D.O.; Qu, Y.; Fu, X.; Fan, J.; Huang, P.; Liu, Y.; Zhu, G.; Qian, Z. NIR-responsive on-demand release of CO from metal carbonyl-caged graphene oxide nanomedicine. Adv. Mater. 2015, 27, 6537. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, G.; Chen, X.; Chen, Z.; Tan, A.Y.; Lin, A.; Zhang, C.; Torres, L.K.; Bajrami, S.; Zhang, T.; et al. Low-dose carbon monoxide suppresses metastatic progression of disseminated cancer cells. Cancer Lett. 2022, 546, 215831. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wang, F.; Nie, X.; Zhang, Z.; Chen, G.; Xia, L.; Wang, L.-H.; Wang, C.-H.; Hao, Z.-Y.; Zhang, W.-J.; et al. Mitochondria-targeted delivery and light controlled release of iron prodrug and CO to enhance cancer therapy by ferroptosis. New J. Chem. 2020, 44, 3478–3486. [Google Scholar] [CrossRef]
- Wegiel, B.; Gallo, D.; Csizmadia, E.; Harris, C.; Belcher, J.; Vercellotti, G.M.; Penacho, N.; Seth, P.; Sukhatme, V.; Ahmed, A.; et al. Carbon Monoxide Expedites Metabolic Exhaustion to Inhibit Tumor Growth. Cancer Res. 2013, 73, 7009–7021. [Google Scholar] [CrossRef]
- Giddings, E.L.; Champagne, D.P.; Wu, M.H.; Laffin, J.M.; Thornton, T.M.; Valenca-Pereira, F.; Culp-Hill, R.; Fortner, K.A.; Romero, N.; East, J.; et al. Mitochondrial ATP fuels ABC transporter-mediated drug efflux in cancer chemoresistance. Nat. Commun. 2021, 12, 2804. [Google Scholar] [CrossRef]
- Zobi, F. CO and CO-releasing molecules in medicinal chemistry. Future Med. Chem. 2013, 5, 175–188. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, T.; Li, M.; Xi, N.; Zheng, Y.; Zhao, Q.; Chen, Y.; Liu, B. Toxicity, biodistribution and metabolism of CO-releasing molecules based on cobalt. Free. Radic. 2016, 97, 362–374. [Google Scholar] [CrossRef]
- Mann, B.E. CO-Releasing Molecules: A Personal View. Organometallics 2012, 31, 5728–5735. [Google Scholar] [CrossRef]
- Ji, X.; Ji, K.; Chittavong, V.; Yu, B.; Pana, Z.; Wang, B. An esterase-activated click and release approach to metal-free CO-prodrugs. Chem. Commun. 2017, 53, 8296. [Google Scholar] [CrossRef] [PubMed]
- Salaroglio, I.C.; Belisario, D.C.; Akman, M.; La Vecchia, S.; Godel, M.; Anobile, D.P.; Ortone, G.; Digiovanni, S.; Fontana, S.; Costamagna, C.; et al. Mitochondrial ROS drive resistance to chemotherapy and immune-killing in hypoxic non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2022, 41, 243. [Google Scholar] [CrossRef] [PubMed]
- Salaroglio, I.C.; Gazzano, E.; Abdullrahman, A.; Mungo, E.; Castella, B.; Abd-Elrahman, G.; Massaia, M.; Donadelli, M.; Rubinstein, M.; Riganti, C.; et al. Increasing intratumor C/EBP-β LIP and nitric oxide levels overcome resistance to doxorubicin in triple negative breast cancer. J. Exp. Clin. Cancer Res. 2018, 37, 286. [Google Scholar] [CrossRef] [PubMed]
- Dávila-González, D.; Choi, D.S.; Rosato, R.R.; Granados-Principal, S.M.; Kuhn, J.G.; Li, W.F.; Qian, W.; Chen, W.; Kozielski, A.J.; Wong, H.; et al. Pharmacological inhibition of NOS activates ASK1/JNK pathway augmenting docetaxel-mediated apoptosis in triple-negative breast cancer. Clin. Cancer Res. 2018, 24, 1152–1162. [Google Scholar] [CrossRef]
- Higashihara, T.; Yoshitomi, H.; Nakata, Y.; Kagawa, S.; Takano, S.; Shimizu, H.; Kato, A.; Furukawa, K.; Ohtsuka, M.; Miyazaki, M. Sex determining region Y box 9 induces chemoresistance in pancreatic cancer cells by induction of putative cancer stem cell characteristics and its high expression predicts poor prognosis. Pancreas 2017, 46, 1296–1304. [Google Scholar] [CrossRef]
- Samoylova, N.A.; Gureev, A.P.; Popov, V.N. Methylene blue induces antioxidant defense and reparation of mitochondrial DNA in a Nrf2-dependent manner during cisplatin-induced renal toxicity. Int. J. Mol. Sci. 2023, 24, 6118. [Google Scholar] [CrossRef]
- She, G.; Du, J.C.; Wu, W.; Pu, T.T.; Zhang, Y.; Bai, R.Y.; Zhang, Y.; Pang, Z.D.; Wang, H.F.; Ren, Y.J.; et al. Hippo pathway activation mediates chemotherapy-induced anti-cancer effect and cardiomyopathy through causing mitochondrial damage and dysfunction. Theranostics 2023, 13, 560–577. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Li, Y.; Chen, Z.; Xie, L.; Li, W.; Zhu, Y.; Xue, H.; Koeffler, H.P.; Wu, W.; et al. PARK2 promotes mitochondrial pathway of apoptosis and antimicrotubule drugs chemosensitivity via degradation of phospho-BCL-2. Theranostics 2020, 10, 9984–10000. [Google Scholar] [CrossRef]
- Inamura, A.; Muraoka-Hirayama, S.; Sakurai, K. Loss of mitochondrial DNA by gemcitabine triggers mitophagy and cell death. Biol. Pharm. Bull. 2019, 42, 1977–1987. [Google Scholar] [CrossRef]
- Al-Shahat, A.; Hulail, M.A.E.; Soliman, N.M.M.; Khamis, T.; Fericean, L.M.; Arisha, A.H.; Moawad, R.S. Melatonin mitigates cisplatin-induced ovarian dysfunction via altering steroidogenesis, inflammation, apoptosis, oxidative stress, and PTEN/PI3K/Akt/mTOR/AMPK signaling pathway in female rats. Pharmaceutics 2022, 14, 2769. [Google Scholar] [CrossRef]
- Riganti, C.; Gazzano, E.; Gulino, G.R.; Volante, M.; Ghigo, D.; Kopecka, J. Two repeated low doses of doxorubicin are more effective than a single high dose against tumors overexpressing P-glycoprotein. Cancer Lett. 2015, 360, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Motafeghi, F.; Mortazavi, P.; Salman Mahiny, A.H.; Abtahi, M.M.; Shokrzadeh, M. The role of ginger’s extract and N-acetylcysteine against docetaxel-induced oxidative stress and genetic disorder. Drug Chem. Toxicol. 2022, 46, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Behera, C.; Sandha, K.K.; Banjare, N.; Malik, S.B.; Tabassum, M.; Kumar, R.; Kumar, A.; Mondhe, D.M.; Gupta, P.N. Implication of methylselenocysteine in combination chemotherapy with gemcitabine for improved anticancer efficacy. Eur. J. Pharm. Sci. 2022, 176, 106238. [Google Scholar] [CrossRef] [PubMed]
- Kopecka, J.; Trouillas, P.; Gašparović, A.Č.; Gazzano, E.; Assaraf, Y.G.; Riganti, C. Phospholipids and cholesterol: Inducers of cancer multidrug resistance and therapeutic targets. Drug. Resist. Updat. 2020, 49, 100670. [Google Scholar] [CrossRef] [PubMed]
- Alexa-Stratulat, T.; Pešić, M.; Gašparović, A.Č.; Trougakos, I.P.; Riganti, C. What sustains the multidrug resistance phenotype beyond ABC efflux transporters? Looking beyond the tip of the iceberg. Drug. Resist. Updates 2019, 46, 100643. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Gazzano, E.; Lazzarato, L.; Rolando, B.; Kopecka, J.; Guglielmo, S.; Costamagna, C.; Chegaev, K.; Riganti, C. Mitochondrial delivery of phenol substructure triggers mitochondrial depolarization and apoptosis of cancer cells. Front. Pharmacol. 2018, 9, 580. [Google Scholar] [CrossRef]
- Buondonno, I.; Gazzano, E.; Jean, S.R.; Audrito, V.; Kopecka, J.; Fanelli, M.; Salaroglio, I.C.; Costamagna, C.; Roato, I.; Mungo, E.; et al. Mitochondria-targeted doxorubicin: A new therapeutic strategy against doxorubicin-resistant osteosarcoma. Mol. Cancer Ther. 2016, 15, 2640–2652. [Google Scholar] [CrossRef]
- Riganti, C.; Kopecka, J.; Panada, E.; Barak, S.; Rubinstein, M. The role of C/EBP-β LIP in multidrug resistance. J. Natl. Cancer Inst. 2015, 107, djv046. [Google Scholar] [CrossRef]
- Retamal, M.A. Carbon monoxide modulates connexin function through a lipid peroxidation-dependent process: A hypothesis. Front. Physiol. 2016, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Xing, N.; Du, Q.; Guo, S.; Xiang, G.; Zhang, Y.; Meng, X.; Xiang, L.; Wang, S. Ferroptosis in lung cancer: A novel pathway regulating cell death and a promising target for drug therapy. Cell Death Discov. 2023, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Guan, X.Y. Ferroptosis: Promising approach for cancer and cancer immunotherapy. Cancer Lett. 2023, 561, 216152. [Google Scholar] [CrossRef] [PubMed]
- Marini, E.; Rolando, B.; Sodano, F.; Blua, F.; Concina, G.; Guglielmo, S.; Lazzarato, L.; Chegaev, K. Comparative study of different H2S donors as vasodilators and attenuators of superoxide-induced endothelial damage. Antioxidants 2023, 12, 344. [Google Scholar] [CrossRef] [PubMed]
- Kopecka, J.; Salzano, G.; Campia, I.; Lusa, S.; Ghigo, D.; De Rosa, G.; Riganti, C. Insights in the chemical components of liposomes responsible for P-glycoprotein inhibition. Nanomedicine 2014, 10, 77–87. [Google Scholar] [CrossRef]


| Tumor Type | Cell Line | Drug Resistance to: | ABCB1 (% Positive Cells) | ABCC1 (% Positive Cells) | ABCC5 (% Positive Cells) |
|---|---|---|---|---|---|
| NSCLC | NCI-H2228 | Cisplatin [23] | 14.6 ± 1.5% | 76.6 ± 8.6% | 9.9 ± 0.7% |
| NSCLC | A549 | Cisplatin [23] | 37.8 ± 2.3% | 89.6 ± 8.5% | 10.1 ± 1.2% |
| TNBC | MDA-MB-231 | Doxorubicin, Docetaxel [24] | 55.9 ± 5.4% | 23.2 ± 3.7% | 34.8 ± 3.8% |
| TNBC | MDA-MB-468 | Doxorubicin, Docetaxel [25] | 64.0 ± 5.0% | 14.9 ± 2.0% | 28.7 ± 2.3% |
| PDAC | PANC-01 | Gemcitabine [26] | 10.7 ± 1.1% | 5.7 ± 2.0% | 71.3 ± 8.5% |
| PDAC | Capan-1 | Gemcitabine [26] | 9.2 ± 1.0% | 4.6 ± 1.1% | 64.0 ± 4.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sodano, F.; Rolando, B.; Lazzarato, L.; Costamagna, C.; Failla, M.; Riganti, C.; Chegaev, K. Use of Enzymatically Activated Carbon Monoxide Donors for Sensitizing Drug-Resistant Tumor Cells. Int. J. Mol. Sci. 2023, 24, 11258. https://doi.org/10.3390/ijms241411258
Sodano F, Rolando B, Lazzarato L, Costamagna C, Failla M, Riganti C, Chegaev K. Use of Enzymatically Activated Carbon Monoxide Donors for Sensitizing Drug-Resistant Tumor Cells. International Journal of Molecular Sciences. 2023; 24(14):11258. https://doi.org/10.3390/ijms241411258
Chicago/Turabian StyleSodano, Federica, Barbara Rolando, Loretta Lazzarato, Costanzo Costamagna, Mariacristina Failla, Chiara Riganti, and Konstantin Chegaev. 2023. "Use of Enzymatically Activated Carbon Monoxide Donors for Sensitizing Drug-Resistant Tumor Cells" International Journal of Molecular Sciences 24, no. 14: 11258. https://doi.org/10.3390/ijms241411258
APA StyleSodano, F., Rolando, B., Lazzarato, L., Costamagna, C., Failla, M., Riganti, C., & Chegaev, K. (2023). Use of Enzymatically Activated Carbon Monoxide Donors for Sensitizing Drug-Resistant Tumor Cells. International Journal of Molecular Sciences, 24(14), 11258. https://doi.org/10.3390/ijms241411258







