Optimization of Protocols for the Induction of Callus and Plant Regeneration in White Clover (Trifolium repens L.)
Abstract
1. Introduction
2. Results
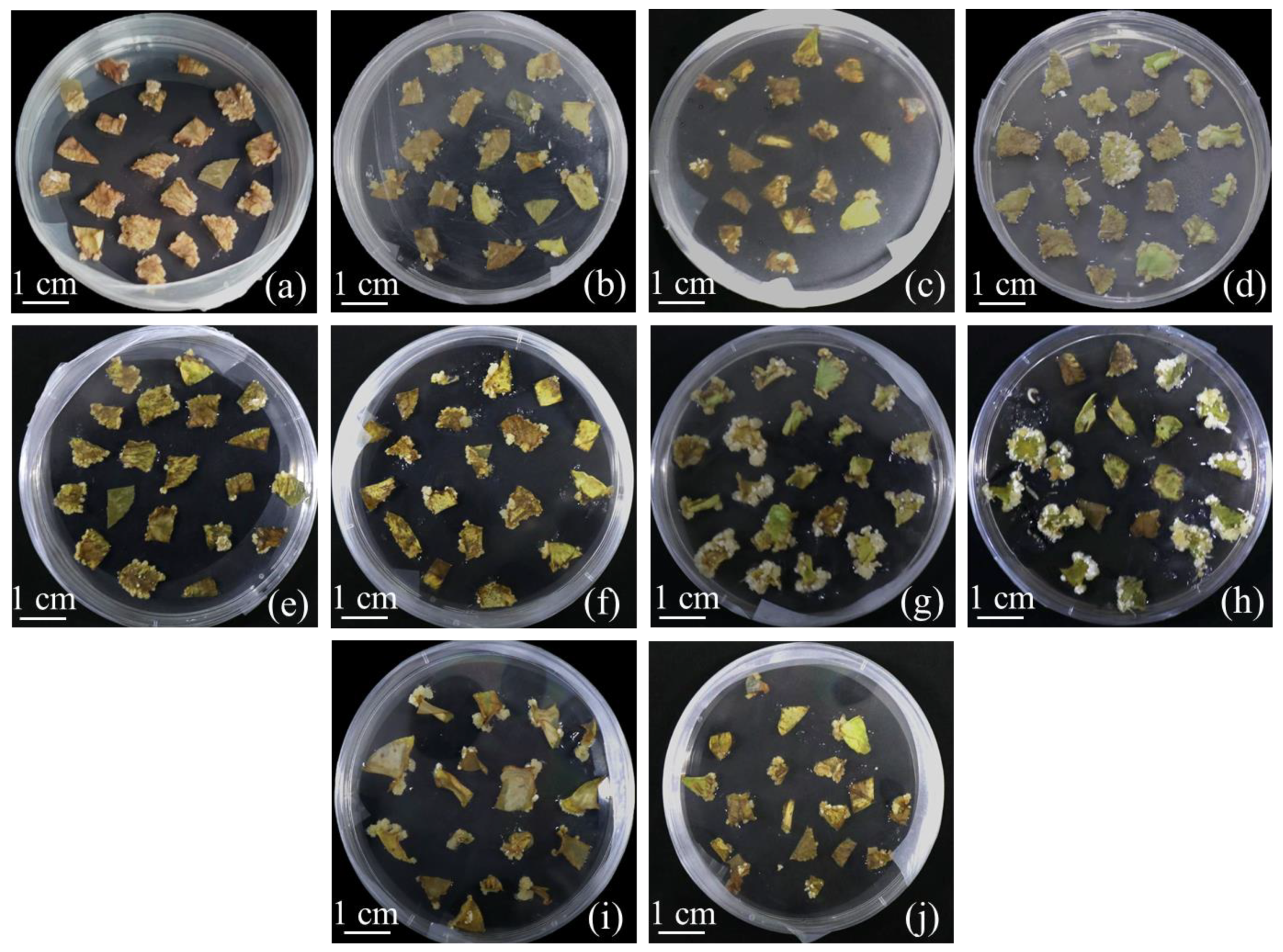
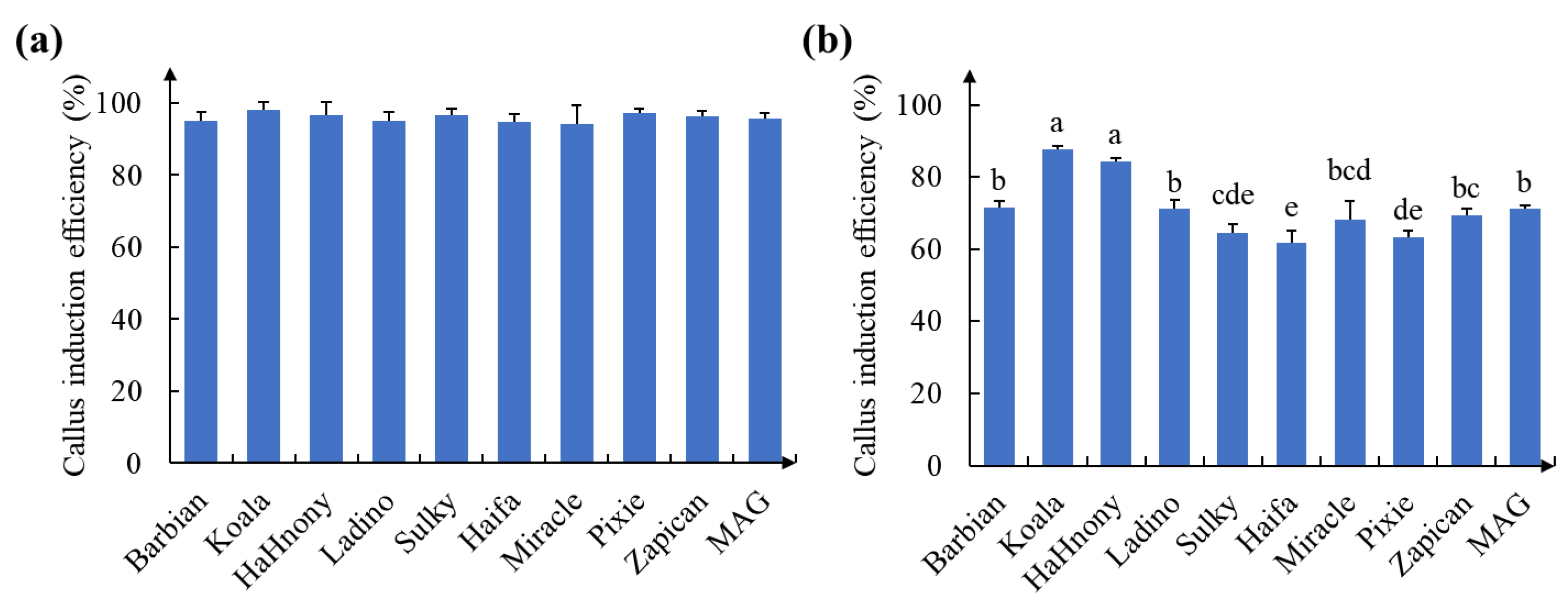
2.1. Formation of Calluses on White Clover
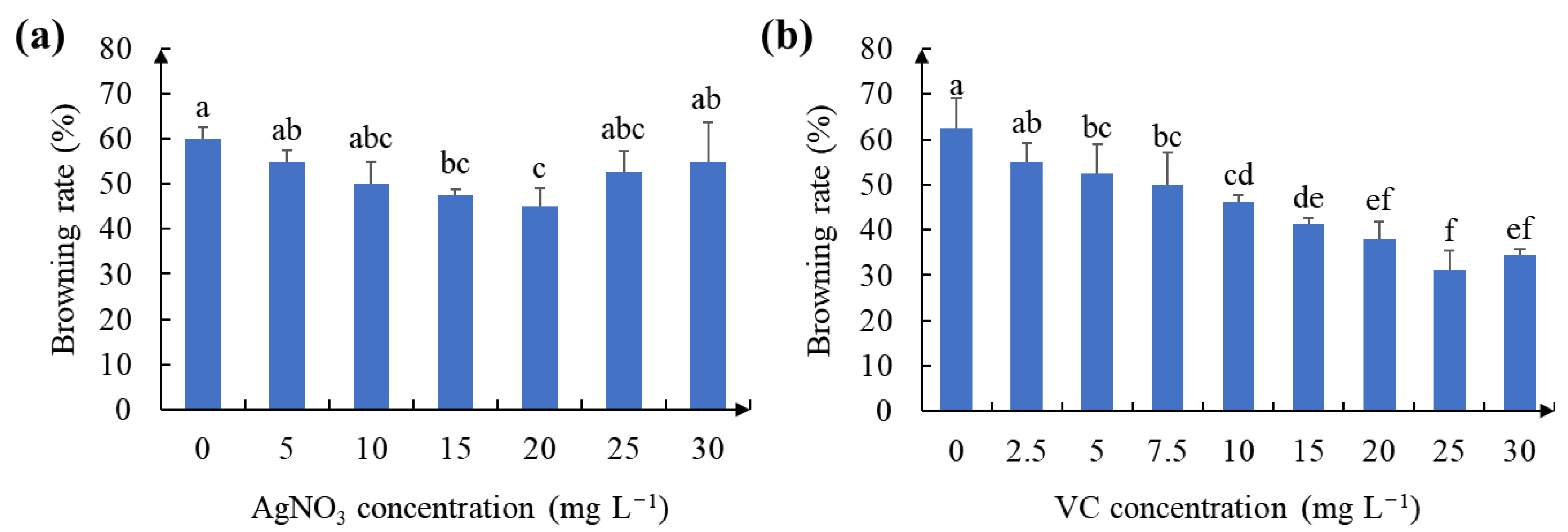
2.2. Effect of Antioxidant on Callus Browning
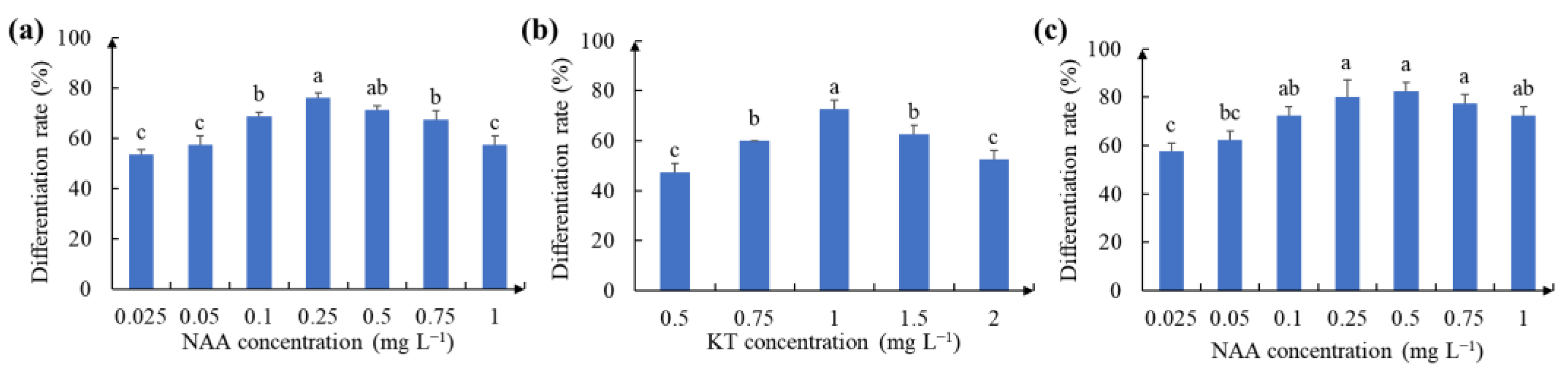
2.3. Effect of Hormone Concentrations on the Redifferentiation of White Clover Calluses
2.4. Agrobacterium tumefaciens-Mediated Transformation
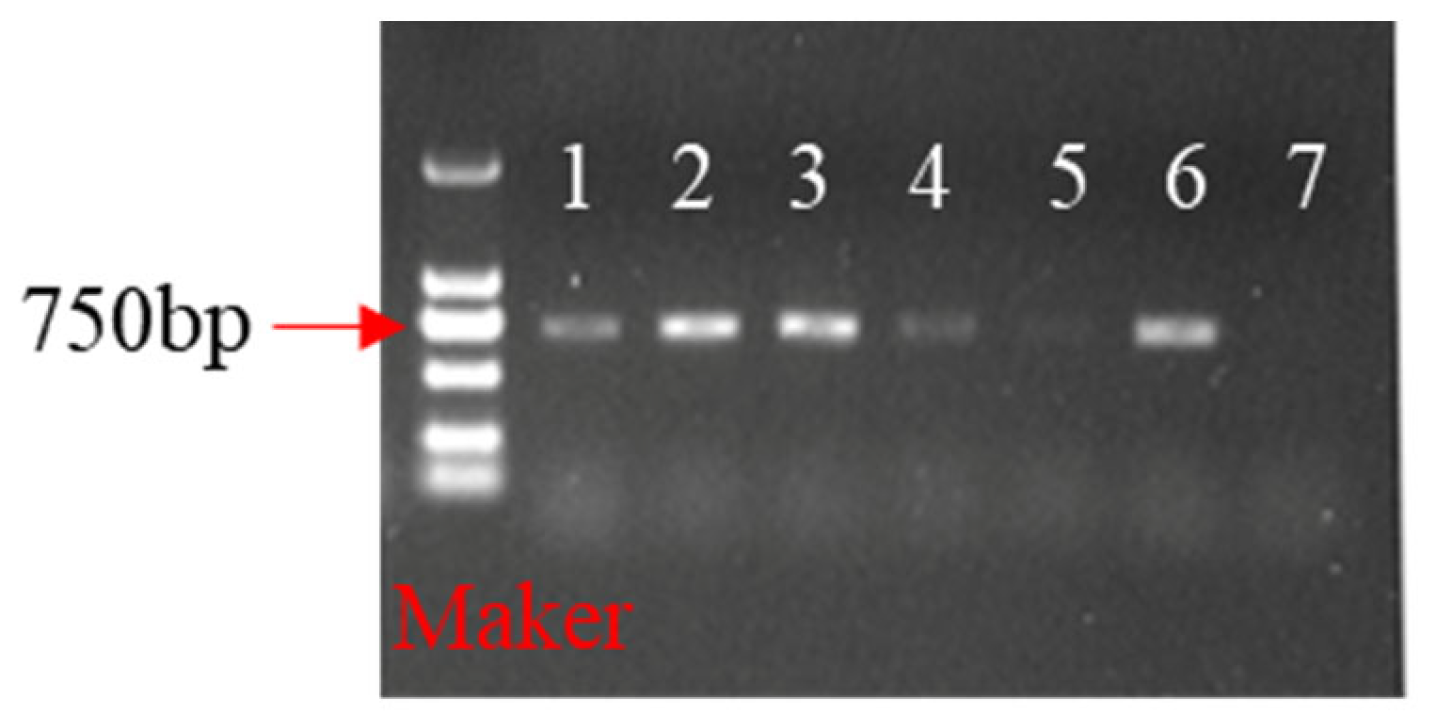
2.5. Identification of Transgenic Plants
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Callus Induction
4.3. Antioxidant Concentration Test
4.4. Effect of Hormones on the Differentiation of Calluses
4.5. Agrobacterium tumefaciens-Mediated Leaf Transformation
4.6. Identification of Transformed Explants and Plants
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forster, J.W.; Panter, S.; Mouradov, A.; Mason, J.; Spangenberg, G.C. Transgenic technologies for enhanced molecular breeding of white clover (Trifolium repens L.). Crop Pasture Sci. 2013, 64, 26–38. [Google Scholar] [CrossRef]
- Mather, R.D.J.; Melhuish, D.T.; Herlihy, M. Trends in the global marketing of white clover cultivars. NZGA Res. Pract. Ser. 1995, 6, 7–14. [Google Scholar] [CrossRef]
- Brummer, E.C.; Moore, K.J. Persistence of perennial cool-season grass and legume cultivars under continuous grazing by beef cattle. Agron. J. 2000, 92, 466–471. [Google Scholar] [CrossRef]
- Zhao, X.; Bushman, B.S.; Zhang, X.; Robbins, M.D.; Larson, S.R.; Robins, J.G.; Thomas, A. Association of candidate genes with heading date in a diverse Dactylis glomerata population. Plant Sci. 2017, 265, 146–153. [Google Scholar] [CrossRef]
- Yang, Z.; Nie, G.; Feng, G.; Xu, X.; Li, D.; Wang, X. Genome-wide identification of MADS-box gene family in orchardgrass and the positive role of DgMADS114 and DgMADS115 under different abiotic stress. Int. J. Biol. Macromol. 2022, 223, 129–142. [Google Scholar] [CrossRef]
- Brink, G.E.; Pederson, G.A.; Alison, M.W.; Ball, D.M.; Bouton, J.H. Growth of white clover ecotypes, cultivars, and germplasms in the southeastern USA. Crop Sci. 1999, 39, 1809–1814. [Google Scholar] [CrossRef]
- Barrett, B.; Griffiths, A.; Schreiber, M.; Ellison, N.; Mercer, C. A microsatellite map of white clover. Theor. Appl. Genet. 2004, 109, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Ma, S.; Zhou, J.; Han, C.; Hu, R.; Yang, X. Genetic diversity and population structure analysis in a large collection of white clover (Trifolium repens L.) germplasm worldwide. PeerJ 2021, 9, e11325. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Hou, J.; Iqbal, M.Z.; Zhang, Y.; Cheng, B.; Feng, H.; Peng, Y. Overexpression of the white clover TrSAMDC1 gene enhanced salt and drought resistance in Arabidopsis thaliana. Plant Physiol. Biochem. 2021, 165, 147–160. [Google Scholar] [CrossRef]
- Cao, H.; Xie, L.S.; Wu, Y.M.; Liu, S.; Huang, D.G.; Lei, J.L.; Wang, D. Transformation of a novel drought-response transcription factor gene PeDREB2b into white clover via soaking seeds with Agrobacterium tumefaciens. Congr. Sci. Hortic. 2010, 929, 343–350. [Google Scholar] [CrossRef]
- Williams, W.M.; Verry, I.M.; Ansari, H.A.; Hussain, S.W.; Ullah, I.; Ellison, N.W. A Eurasia-wide polyploid species complex involving 6× Trifolium ambiguum, 2× T. occidentale and 4× T. repens produces interspecific hybrids with significance for clover breeding. BMC Plant Biol. 2019, 19, 438. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Woodfield, D.; White, D.W. Allelic composition and genetic background effects on transgene expression and inheritance in white clover. Mol. Breed. 1998, 4, 479–490. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.J.; Yan, R.; Han, J.G.; Hong, F.; Wang, J.H.; Cao, K. Genetic variation of white clover (Trifolium repens L.) collections from China detected by morphological traits, RAPD and SSR. Afr. J. Biotechnol. 2010, 9, 3032–3041. [Google Scholar]
- Roldan, M.B.; Cousins, G.; Muetzel, S.; Zeller, W.E.; Fraser, K.; Salminen, J.-P.; Blanc, A.; Kaur, R.; Richardson, K.; Maher, D.; et al. Condensed tannins in white clover (Trifolium repens) foliar tissues expressing the transcription factor TaMYB14-1 bind to forage protein and reduce ammonia and methane emissions in vitro. Front. Plant Sci. 2022, 12, 777354. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.A.; Maher, D.A.; Jones, C.S.; Bryan, G. Genetic transformation of western clover (Trifolium occidentale DE Coombe.) as a model for functional genomics and transgene introgression in clonal pasture legume species. Plant Methods 2013, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Nakamura, Y.; Asamizu, E.; Isobe, S.; Tabata, S. Genome sequencing and genome resources in model legumes. Plant Physiol. 2007, 144, 588–593. [Google Scholar] [CrossRef]
- Faville, M.J.; Griffiths, A.G.; Baten, A.; Cao, M.; Ashby, R.; Ghamkhar, K.; Webber, Z. Genomic assessment of white clover and perennial ryegrass genetic resources. J. N. Z. Grassl. 2020, 82, 27–34. [Google Scholar] [CrossRef]
- Griffiths, A.G.; Moraga, R.; Tausen, M.; Gupta, V.; Bilton, T.P.; Campbell, M.A. Breaking free: The genomics of allopolyploidy-facilitated niche expansion in white clover. Plant Cell 2019, 31, 1466–1487. [Google Scholar] [CrossRef]
- Aasmo-Finne, M.; Rognli, O.A.; Schjelderup, I. Genetic variation in a Norwegian germplasm collection of white clover (Trifolium repens L.). Euphytica 2000, 112, 57–68. [Google Scholar] [CrossRef]
- Yamada, T.; Spangenberg, G.; Williams, W.M.; Griffiths, A.G. Development of Trifolium occidentale as a plant model system for perennial clonal species. In Molecular Breeding of Forage and Turf; Springer: New York, NY, USA, 2009; pp. 45–53. [Google Scholar] [CrossRef]
- Maheswaran, G.; Williams, E.G. Direct somatic embryoid formation on immature embryos of Trifolium repens, T. pratense and Medicago sativa, and rapid clonal propagation of T. repens. Ann. Bot. 1984, 54, 201–212. [Google Scholar] [CrossRef]
- Baker, J.M. Vegetative propagation of kura clover: A field-scale test. Can. J. Plant Sci. 2012, 92, 1245–1251. [Google Scholar] [CrossRef]
- Pindel, A. Vegetative Propagation of White Clover (Trifolium repens L.) by Callus Culture; Scientific Papers of the Agricultural University in Krakow; University of Agriculture in Krakow: Krakow, Poland, 1990. [Google Scholar]
- White, D.W.; Bhojwani, S.S. Callus formation from Trifolium arvense protoplast-derived cells plated at low densities. Z. Pflanzenphysiol. 1981, 102, 257–261. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Gresshoff, P.M. Ecotypic variation of in vitro plantlet formation in white clover (Trifolium repens). Plant Cell Rep. 1982, 1, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T. Selection of a highly-regenerative genotype of white clover (Trifolium repens L.) and plant regeneration from protoplasts derived from this genotype. Euphytica 1989, 44, 181–186. [Google Scholar] [CrossRef]
- White, D.W.; Greenwood, D. Transformation of the forage legume Trifolium repens L. using binary Agrobacterium vectors. Plant Mol. Biol. 1987, 8, 461–469. [Google Scholar] [CrossRef]
- Ahuja, P.S.; Lu, D.Y.; Cocking, E.C.; Davey, M.R. An assessment of the cultural capabilities of Trifolium repens L.(white clover) and Onobrychis viciifolia Scop.(sainfoin) mesophyll protoplasts. Plant Cell Rep. 1983, 2, 269–272. [Google Scholar] [CrossRef]
- Altpeter, F.; Springer, N.M.; Bartley, L.E.; Blechl, A.E.; Brutnell, T.P. Advancing crop transformation in the era of genome editing. Plant Cell 2016, 28, 1510–1520. [Google Scholar] [CrossRef]
- Nagle, M.; Déjardin, A.; Pilate, G.; Strauss, S.H. Opportunities for innovation in genetic transformation of forest trees. Front. Plant Sci. 2018, 9, 1443. [Google Scholar] [CrossRef]
- Gordon-Kamm, B.; Sardesai, N.; Arling, M.; Lowe, K.; Hoerster, G. Using morphogenic genes to improve recovery and regeneration of transgenic plants. Plants 2019, 8, 38. [Google Scholar] [CrossRef]
- Maren, N.A.; Duan, H.; Da, K.; Yencho, G.C.; Ranney, T.G.; Liu, W. Genotype-independent plant transformation. Hortic. Res. 2022, 9, uhac047. [Google Scholar] [CrossRef]
- Smith, E.F.; Townsend, C.O. A plant-tumor of bacterial origin. Science 1907, 25, 671–673. [Google Scholar] [CrossRef]
- Gelvin, S.B. Agrobacterium-mediated plant transformation: The biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 2003, 67, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Nester, E.W. Agrobacterium: Nature’s genetic engineer. Front. Plant Sci. 2015, 5, 730. [Google Scholar] [CrossRef] [PubMed]
- Rojo, F.P.; Seth, S.; Erskine, W.; Kaur, P. An improved protocol for Agrobacterium-mediated transformation in subterranean Clover (Trifolium subterraneum L.). Int. J. Mol. Sci. 2021, 22, 4181. [Google Scholar] [CrossRef]
- Voisey, C.R.; White, D.W.; Dudas, B.; Appleby, R.D.; Ealing, P.M.; Scott, A.G. Agrobacterium-mediated transformation of white clover using direct shoot organogenesis. Plant Cell Rep. 1994, 13, 309–314. [Google Scholar] [CrossRef]
- Christiansen, P.; Gibson, J.; Moore, A.; Pedersen, C.; Tabe, L.; Larkin, P. Transgenic Trifolium repens with foliage accumulating the high sulphur protein, sunfower seed albumin. Transgenic Res. 2000, 9, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Rahimi Ashtiani, S. Biosynthesis of Proanthocyanidins in White Clover (Trifolium repens L.): Single Cell ‘Omics’ for Designing Pathway Re-Programming. Ph.D. Thesis, La Trobe University, Bundoora, VIC, Australia, 2015. [Google Scholar]
- Narancio, R.; Ding, Y.L.; Lin, Y.H.; Sahab, S.; Panter, S.; Hayes, M.; John, U.; Anderson, H.; Mason, J.; Spangenberg, G. Application of linked and unlinked co-transformation to generate triple stack, marker-free, transgenic white clover (Trifolium repens L.). Plant Cell Tissue Organ Cult. 2020, 142, 635–646. [Google Scholar] [CrossRef]
- Gresshoff, P.M. In vitro culture of white clover: Callus, suspension, protoplast culture, and plant regeneration. Bot. Gaz. 1980, 141, 157–164. [Google Scholar] [CrossRef]
- Jia, T.; Cheng, B.; Feng, H.; Hassan, M.J.; Iqbal, M.Z.; Peng, Y. Two Agrobacterium-mediated transformation protocols of white Clover (Trifolium repens) through the callus system. Preprint 2022. [Google Scholar] [CrossRef]
- Bezirganoglu, İ. Promoting effects of melatonin supplements on the embryogenic callus maintainance in alfalfa (Medicago sativa L.). J. Inst. Sci. Technol. 2021, 11, 927–932. [Google Scholar] [CrossRef]
- Balakrishnamurthy, G.; Rao, V.M. Changes in phenols during rhizogenesis in rose (Rosa bourboniana Desp). Curr. Sci. 1988, 57, 960–962. [Google Scholar]
- Perl, A.; Kless, H.; Blumenthal, A.; Galili, G.; Galun, E. Improvement of plant regeneration and GUS expression in scutellar wheat calli by optimization of culture conditions and DNA-microprojectile delivery procedures. Mol. Gen. Genet. 1992, 235, 279–284. [Google Scholar] [CrossRef]
- Songstad, D.D.; Armstrong, C.L.; Petersen, W.L. AgNO3 increases type II callus production from immature embryos of maize inbred B73 and its derivatives. Plant Cell Rep. 1991, 9, 699–702. [Google Scholar] [CrossRef]
- Irshad, M.; He, B.; Liu, S.; Mitra, S.; Debnath, B.; Li, M. In vitro regeneration of Abelmoschus esculentus L. Cv. Wufu: Influence of anti-browning additives on phenolic secretion and callus formation frequency in explants. Hortic. Environ. Biotechnol. 2017, 58, 503–513. [Google Scholar] [CrossRef]
- Liang, S.; He, Y.; Zheng, H.; Yuan, Q.; Zhang, F.; Sun, B. Effects of sucrose and browning inhibitors on callus proliferation and anti-browning of chinese kale. IOP Conf. Ser. Earth Environ. Sci. 2019, 252, 022018. [Google Scholar] [CrossRef]
- Bond, J.E.; Webb, K.J. Regeneration and analysis of plants from stolon segments of Trifolium repens (white clover). Plant Sci. 1989, 61, 119–125. [Google Scholar] [CrossRef]
- Siddique, A.B.; Islam, S.M. Effect of light and dark on callus induction and regeneration in tobacco (Nicotiana tabacum L.). Bangl J. Bot. 2015, 44, 643–651. [Google Scholar] [CrossRef]
- Long, Y.; Yang, Y.; Pan, G.; Shen, Y. New insights into tissue culture plant-regeneration mechanisms. Front. Plant Sci. 2022, 13, 926752. [Google Scholar] [CrossRef]
- Morinaka, H.; Coleman, D.; Sugimoto, K.; Iwase, A. Molecular mechanisms of plant regeneration from differentiated cells: Approaches from historical tissue culture systems. Plant Cell Physiol. 2023, 64, 297–304. [Google Scholar] [CrossRef]
- Konieczny, R.; Sliwinska, E.; Pilarska, M.; Tuleja, M. Morphohistological and flow cytometric analyses of somatic embryogenesis in Trifolium nigrescens Viv. Plant Cell Tissue Organ Cult. 2012, 109, 131–141. [Google Scholar] [CrossRef]
- Larkin, P.J.; Gibson, J.M.; Mathesius, U.; Weinman, J.J.; Gartner, E.; Hall, E.; Tanner, G.J.; Rolfe, B.G.; Djordjevic, M.A. Transgenic white clover. Studies with the auxin-responsive promoter, GH3, in root gravitropism and lateral root development. Transgenic Res. 1996, 5, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Palomo-Ríos, E.; Cerezo, S.; Mercado, J.A.; Pliego-Alfaro, F. Agrobacterium-mediated transformation of avocado (Persea americana Mill.) somatic embryos with fluorescent marker genes and optimization of transgenic plant recovery. Plant Cell Tissue Organ Cult. 2017, 128, 447–455. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, T.; Tang, T.; Zhou, Q.; Yang, W.; Hassan, M.J.; Cheng, B.; Nie, G.; Li, Z.; Peng, Y. Optimization of Protocols for the Induction of Callus and Plant Regeneration in White Clover (Trifolium repens L.). Int. J. Mol. Sci. 2023, 24, 11260. https://doi.org/10.3390/ijms241411260
Qi T, Tang T, Zhou Q, Yang W, Hassan MJ, Cheng B, Nie G, Li Z, Peng Y. Optimization of Protocols for the Induction of Callus and Plant Regeneration in White Clover (Trifolium repens L.). International Journal of Molecular Sciences. 2023; 24(14):11260. https://doi.org/10.3390/ijms241411260
Chicago/Turabian StyleQi, Tiangang, Tao Tang, Qinyu Zhou, Weiqiang Yang, Muhammad Jawad Hassan, Bizhen Cheng, Gang Nie, Zhou Li, and Yan Peng. 2023. "Optimization of Protocols for the Induction of Callus and Plant Regeneration in White Clover (Trifolium repens L.)" International Journal of Molecular Sciences 24, no. 14: 11260. https://doi.org/10.3390/ijms241411260
APA StyleQi, T., Tang, T., Zhou, Q., Yang, W., Hassan, M. J., Cheng, B., Nie, G., Li, Z., & Peng, Y. (2023). Optimization of Protocols for the Induction of Callus and Plant Regeneration in White Clover (Trifolium repens L.). International Journal of Molecular Sciences, 24(14), 11260. https://doi.org/10.3390/ijms241411260







