Composite Fibrin/Carbon Microfiber Implants for Bridging Spinal Cord Injury: A Translational Approach in Pigs
Abstract
1. Introduction
2. Results
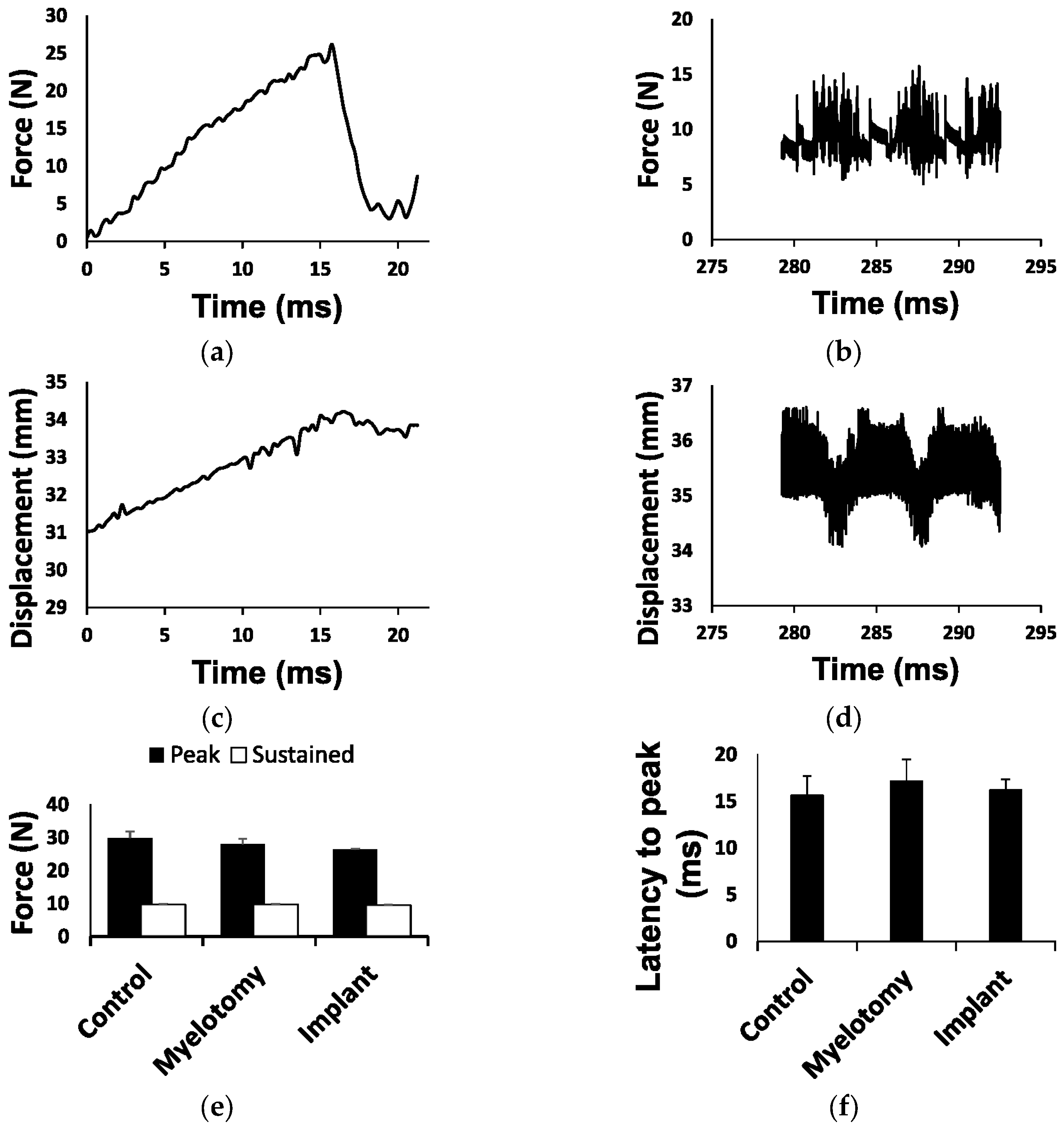
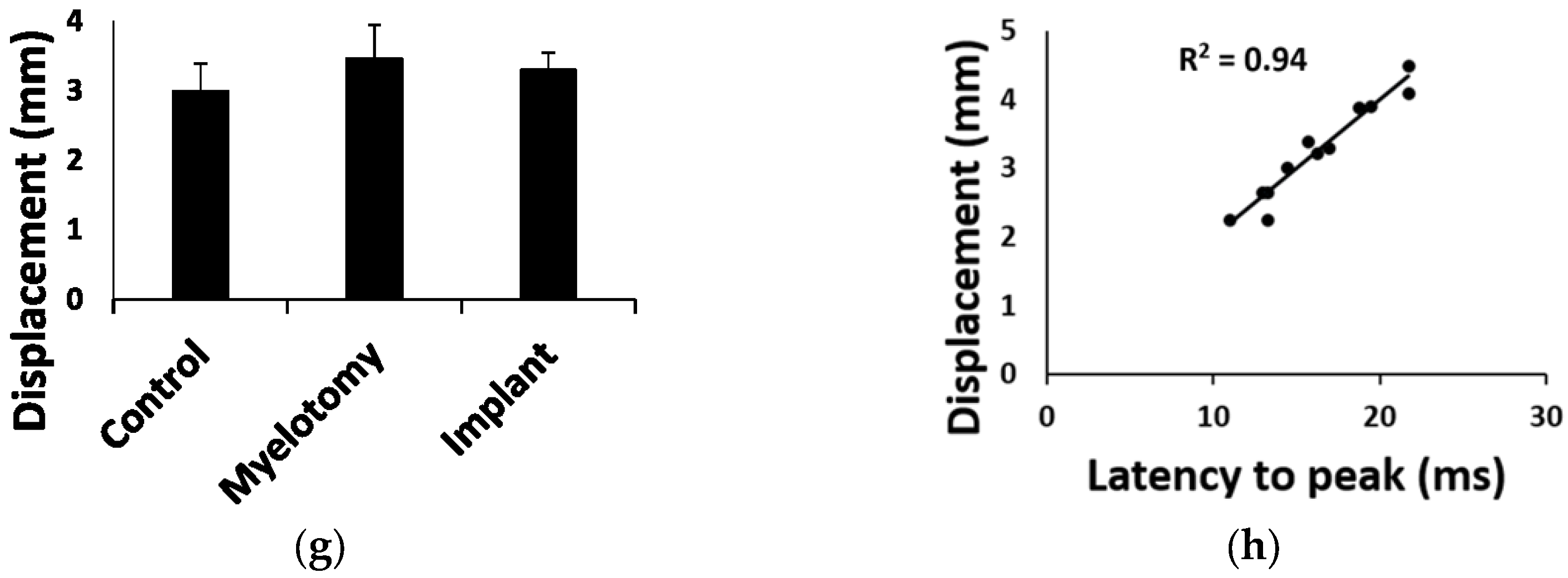
2.1. Biomechanical Parameters of the Injury
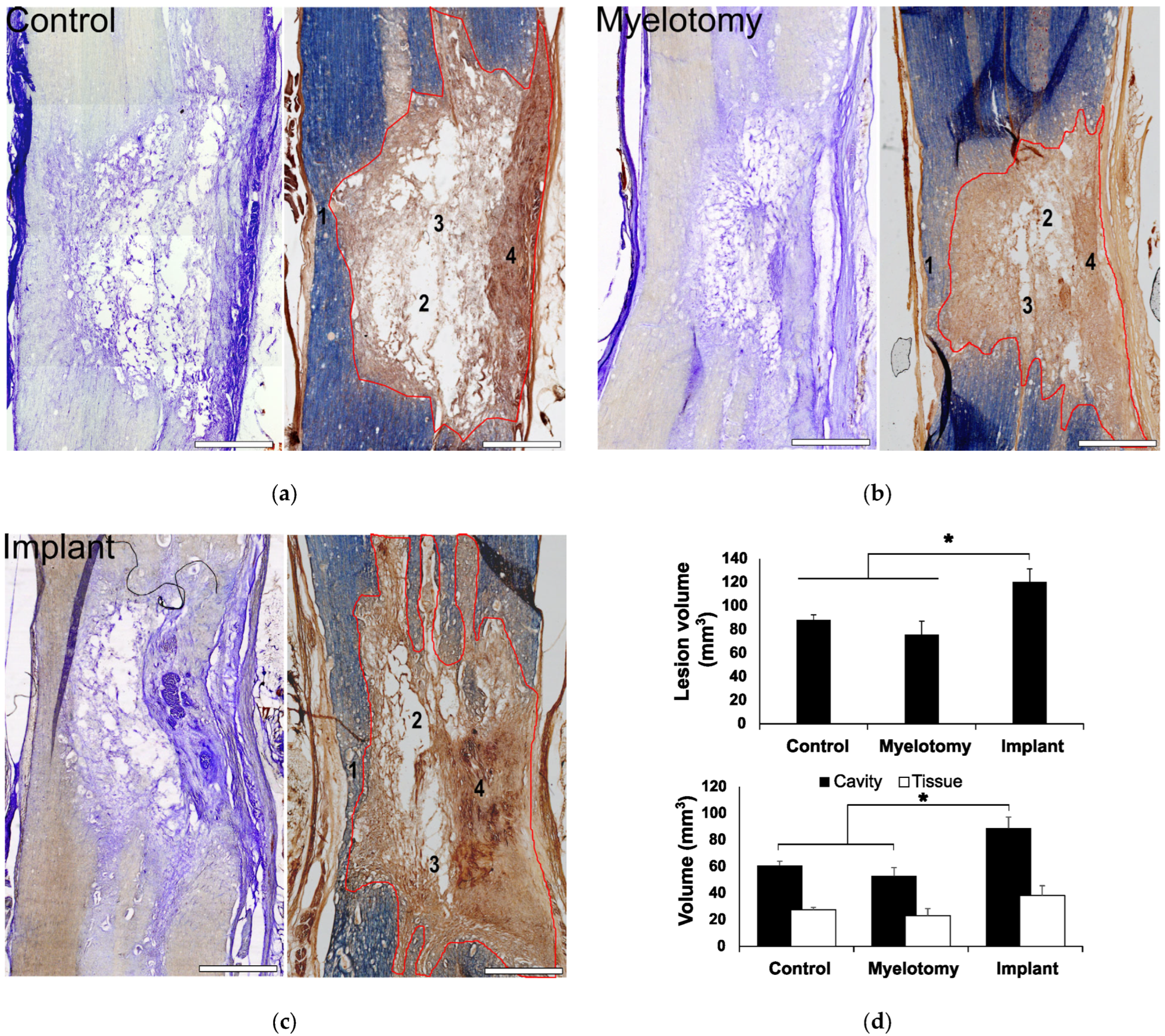
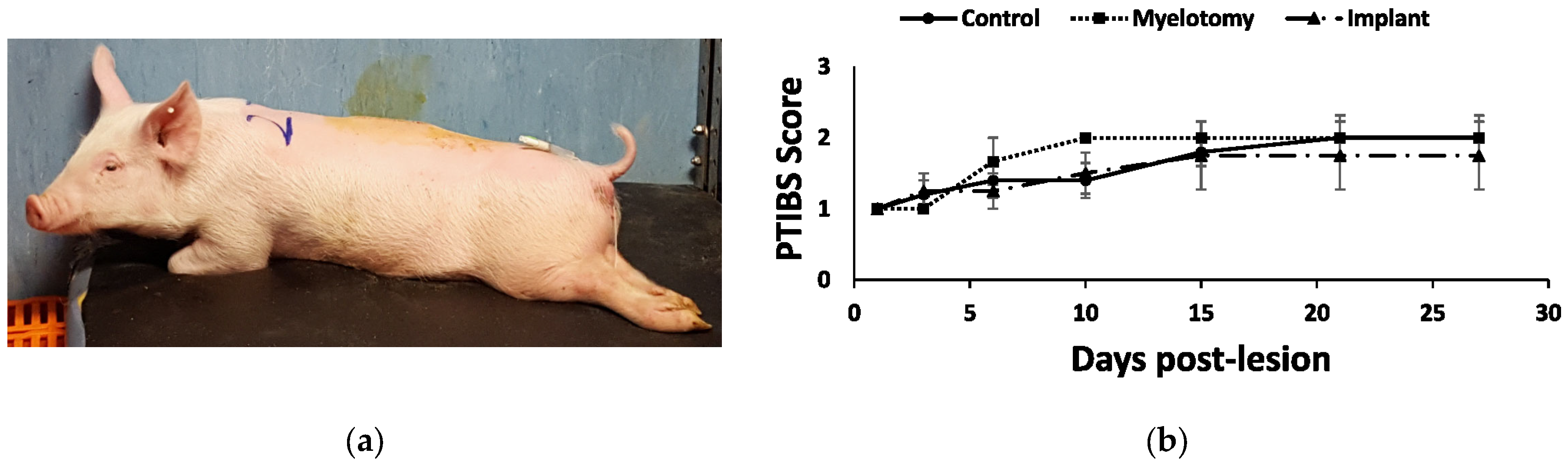
2.2. Gross Histology
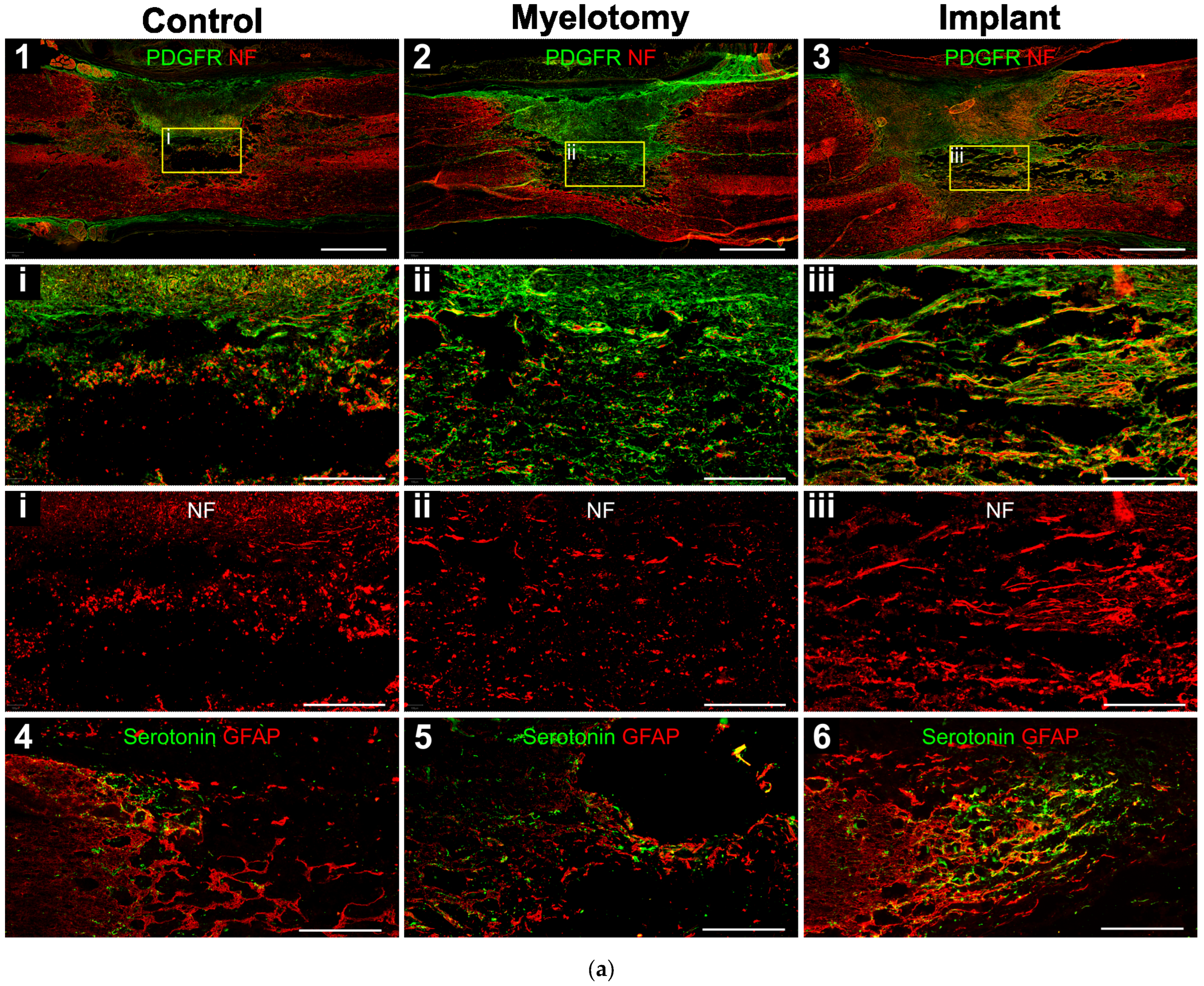
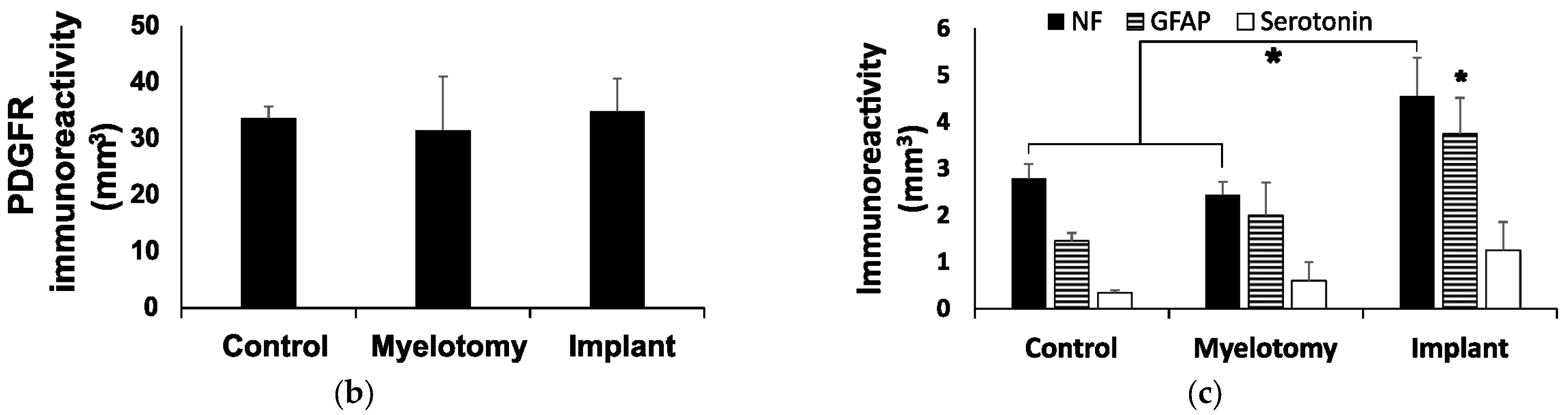
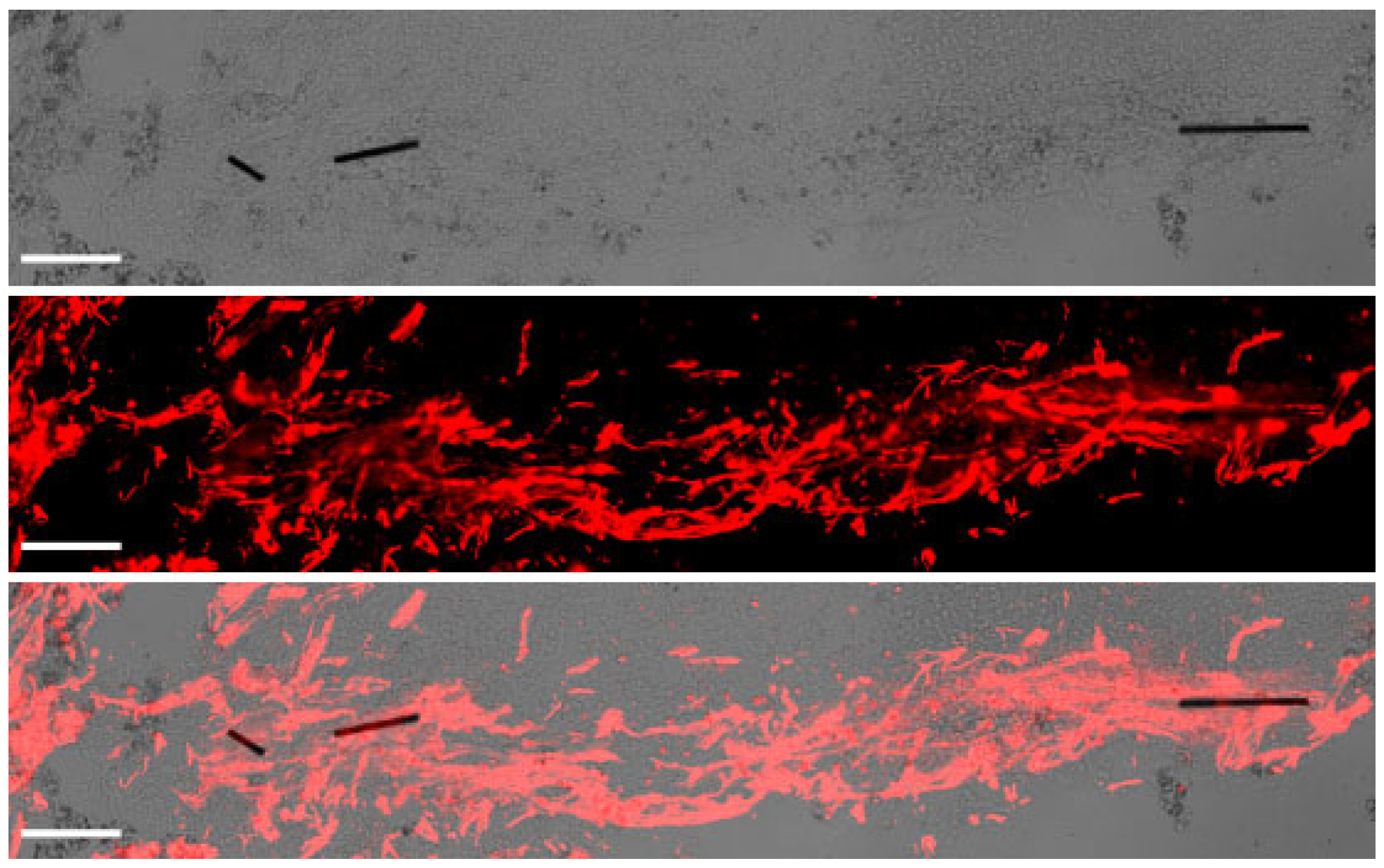
2.3. Immunohistochemistry
2.4. Animal and Behavioral Outcomes
3. Discussion
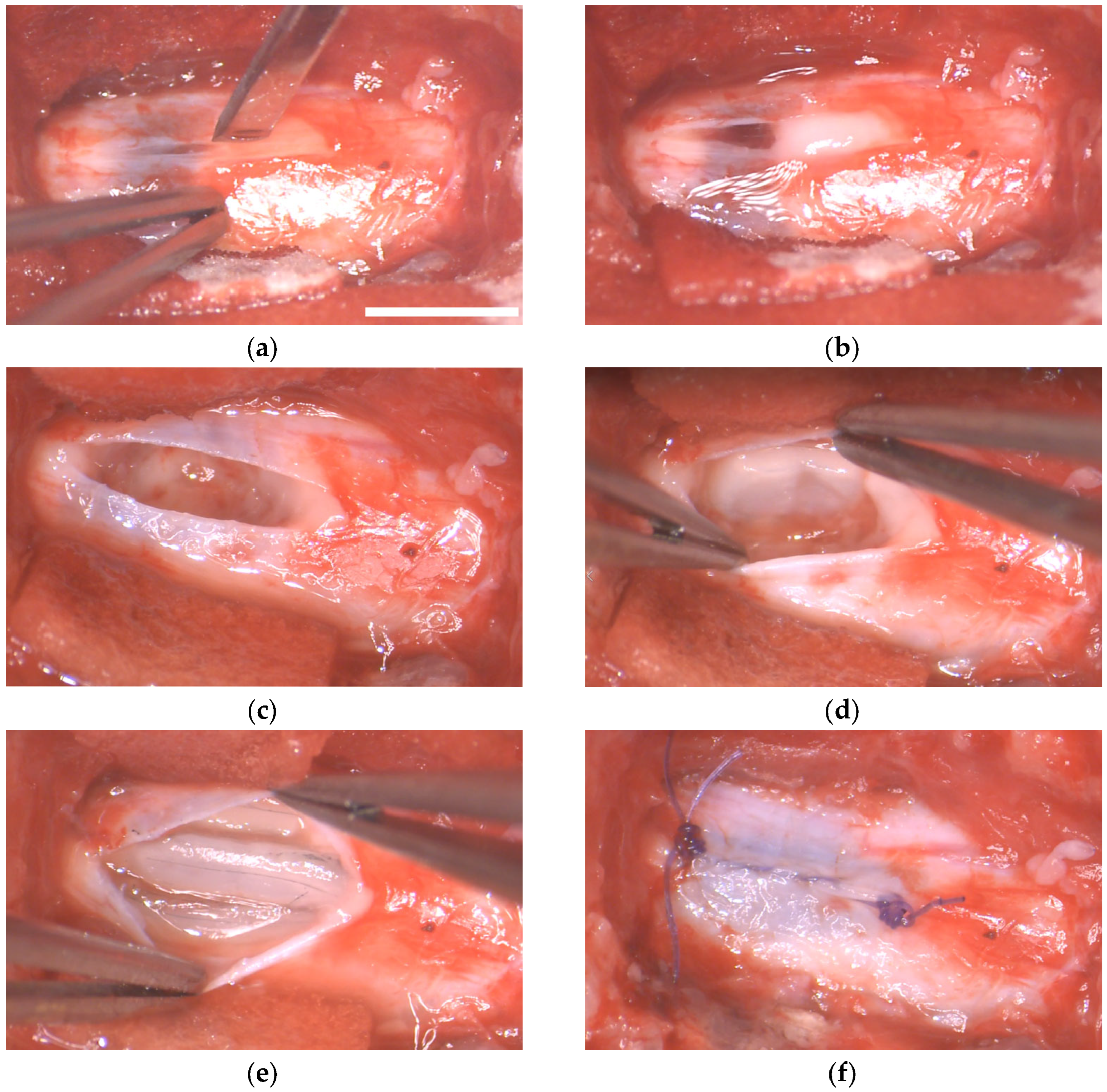
4. Materials and Methods
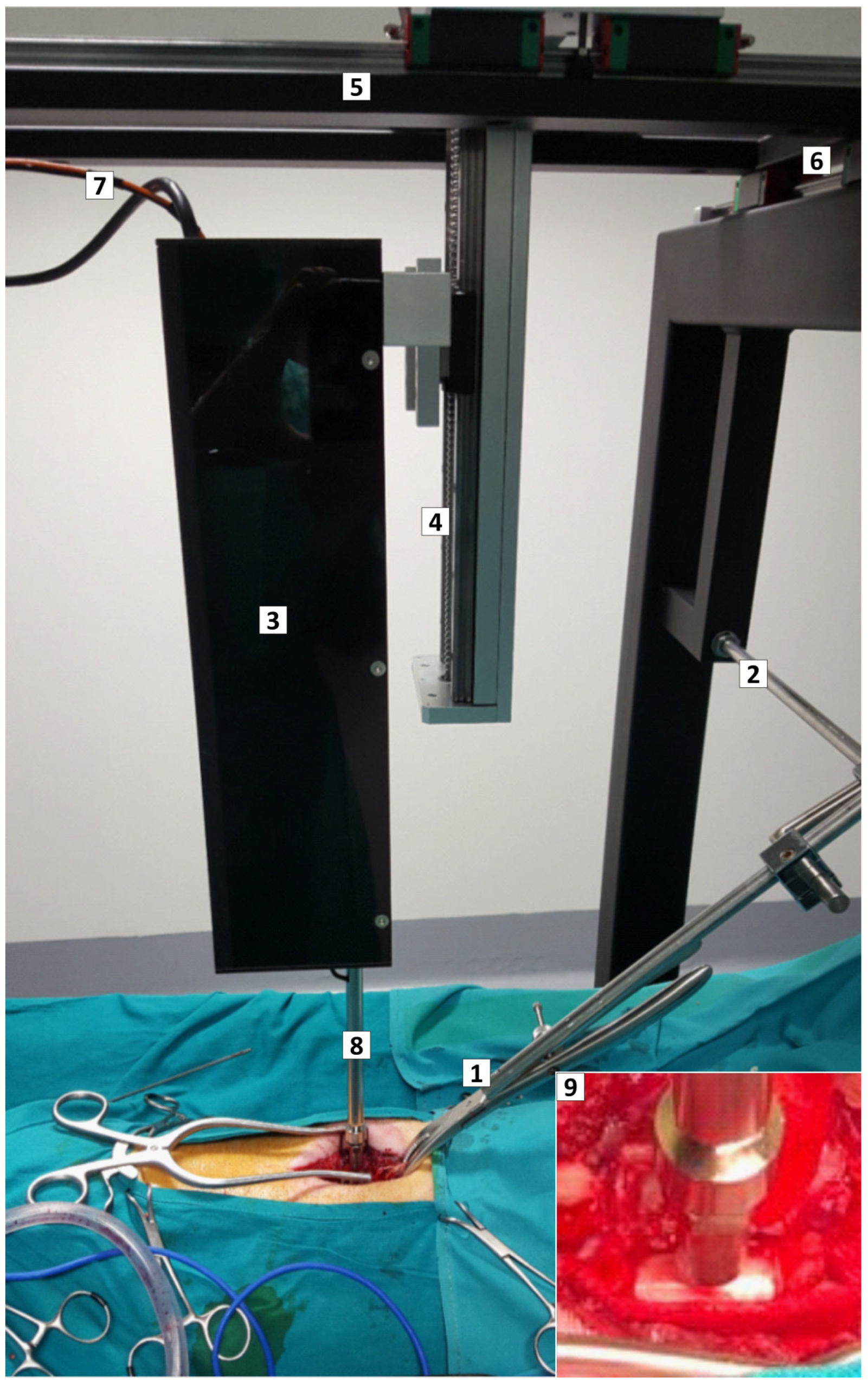
4.1. Impactor Device
4.2. Animals and Experimental Groups
4.3. Anesthesia and Surgical Procedures
4.4. Preparation of MFs/Fibrin Implants
4.5. Animal Care
4.6. Motor Assessment
4.7. Histological Procedures and Analyses
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collazos-Castro, J.E. Biomaterial-based systems as biomimetic agents in the repair of the central nervous system. In Handbook of Innovations in Central Nervous System Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 259–289. [Google Scholar]
- Hurtado, A.; Cregg, J.M.; Wang, H.B.; Wendell, D.F.; Oudega, M.; Gilbert, R.J.; McDonald, J.W. Robust CNS regeneration after complete spinal cord transection using aligned poly-L-lactic acid microfibers. Biomaterials 2011, 32, 6068–6079. [Google Scholar] [CrossRef]
- Alves-Sampaio, A.; García-Rama, C.; Collazos-Castro, J.E. Biofunctionalized PEDOT-coated microfibers for the treatment of spinal cord injury. Biomaterials 2016, 89, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.D.; Moore, S.W.; Aimetti, A.A.; Kutikov, A.B.; Santamaria, A.J.; Hofstetter, C.P.; Ropper, A.E.; Theodore, N.; Ulich, T.R.; Layer, R.T. Internal decompression of the acutely contused spinal cord: Differential effects of irrigation only versus biodegradable scaffold implantation. Biomaterials 2018, 185, 284–300. [Google Scholar] [CrossRef]
- Dumont, C.M.; Carlson, M.A.; Munsell, M.K.; Ciciriello, A.J.; Strnadova, K.; Park, J.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Aligned hydrogel tubes guide regeneration following spinal cord injury. Acta Biomater. 2019, 86, 312–322. [Google Scholar] [CrossRef]
- Koffler, J.; Zhu, W.; Qu, X.; Platoshyn, O.; Dulin, J.N.; Brock, J.; Graham, L.; Lu, P.; Sakamoto, J.; Marsala, M.S.; et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019, 25, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Imajo, Y.; Funaba, M.; Ikeda, H.; Nishida, N.; Sakai, T. Current Concepts of Biomaterial Scaffolds and Regenerative Therapy for Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 2528. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Deng, L.; Yong, Y.Y.; Wu, J.M.; Qin, D.L.; Yu, L.; Zhou, X.G.; Wu, A.G. The Application of Biomaterials in Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 816. [Google Scholar] [CrossRef]
- Shrestha, B.; Coykendall, K.; Li, Y.; Moon, A.; Priyadarshani, P.; Yao, L. Repair of injured spinal cord using biomaterial scaffolds and stem cells. Stem Cell. Res. Ther. 2014, 5, 91. [Google Scholar] [CrossRef]
- Führmann, T.; Anandakumaran, P.N.; Shoichet, M.S. Combinatorial therapies after spinal cord injury: How can biomaterials help? Adv. Healthc. Mater. 2017, 6, 1601130. [Google Scholar] [CrossRef]
- Wang, Z.; Nong, J.; Shultz, R.B.; Zhang, Z.; Kim, T.; Tom, V.J.; Ponnappan, R.K.; Zhong, Y. Local delivery of minocycline from metal ion-assisted self-assembled complexes promotes neuroprotection and functional recovery after spinal cord injury. Biomaterials 2017, 112, 62–71. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, T.; Li, N.; Gao, J. Cell membrane-based biomimetic vehicles for effective central nervous system target delivery: Insights and challenges. J. Control. Release 2023, 23, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Vijayaraghavalu, S.; Stees, M.; Kwon, B.K.; Labhasetwar, V. Evaluating accessibility of intravenously administered nanoparticles at the lesion site in rat and pig contusion models of spinal cord injury. J. Control. Release 2019, 302, 160–168. [Google Scholar] [CrossRef]
- Martin-Granados, C.; McCaig, C.D. Harnessing the Electric Spark of Life to Cure Skin Wounds. Adv. Wound Care 2014, 3, 127–138. [Google Scholar] [CrossRef]
- Collazos-Castro, J.E.; Polo, J.L.; Hernández-Labrado, G.R.; Padial-Cañete, V.; García-Rama, C. Bioelectrochemical control of neural cell development on conducting polymers. Biomaterials 2010, 31, 9244–9255. [Google Scholar] [CrossRef]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef] [PubMed]
- Carmel, J.B.; Martin, J.H. Motor cortex electrical stimulation augments sprouting of the corticospinal tract and promotes recovery of motor function. Front. Integr. Neurosci. 2014, 18, 51. [Google Scholar] [CrossRef]
- Kiyotake, E.A.; Martin, M.D.; Detamore, M.S. Regenerative rehabilitation with conductive biomaterials for spinal cord injury. Acta Biomater. 2022, 139, 43–64. [Google Scholar] [CrossRef]
- Liu, Z.; Wan, X.; Wang, Z.L.; Li, L. Electroactive Biomaterials and Systems for Cell Fate Determination and Tissue Regeneration: Design and Applications. Adv. Mater. 2021, 33, e2007429. [Google Scholar] [CrossRef] [PubMed]
- Collazos-Castro, J.E.; Hernández-Labrado, G.R.; Polo, J.L.; García-Rama, C. N-Cadherin- and L1-functionalised conducting polymers for synergistic stimulation and guidance of neural cell growth. Biomaterials 2013, 34, 3603–3617. [Google Scholar] [CrossRef]
- Collazos-Castro, J.E.; García-Rama, C.; Alves-Sampaio, A. Glial progenitor cell migration promotes CNS axon growth on functionalized electroconducting microfibers. Acta Biomater. 2016, 35, 42–56. [Google Scholar] [CrossRef]
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Sun, G.; Wang, X.; Chen, P. Microfiber devices based on carbon materials. Mater. Today 2015, 18, 215–226. [Google Scholar] [CrossRef]
- El Waly, B.; Escarrat, V.; Perez-Sanchez, J.; Kaur, J.; Pelletier, F.; Collazos-Castro, J.E.; Debarbieux, F. Intravital Assessment of Cells Responses to Conducting Polymer-Coated Carbon Microfibres for Bridging Spinal Cord Injury. Cells 2021, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Escarrat, V.; Perez-Sanchez, J.; El-Waly, B.; Collazos-Castro, J.E.; Debarbieux, F. Composite Fibrin and Carbon Microfibre Implant to Modulate Postraumatic Inflammation after Spinal Cord Injury. Cells 2023, 12, 839. [Google Scholar] [CrossRef] [PubMed]
- Cerro, P.D.; Barriga-Martín, A.; Vara, H.; Romero-Muñoz, L.M.; Rodríguez-De-Lope, Á.; Collazos-Castro, J.E. Neuropathological and Motor Impairments after Incomplete Cervical Spinal Cord Injury in Pigs. J. Neurotrauma 2021, 38, 2956–2977. [Google Scholar] [CrossRef]
- Strauch, J.T.; Lauten, A.; Zhang, N.; Wahlers, T.; Griepp, R.B. Anatomy of spinal cord blood supply in the pig. Ann. Thorac. Surg. 2007, 83, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.F.; Lee, J.H.; Burstyn, U.; Okon, E.B.; Kwon, B.K.; Cripton, P.A. Cerebrospinal fluid pressures resulting from experimental traumatic spinal cord injuries in a pig model. J. Biomech. Eng. 2013, 135, 101005. [Google Scholar] [CrossRef]
- Del Cerro, P.; Rodríguez-De-Lope, Á.; Collazos-Castro, J.E. The Cortical Motor System in the Domestic Pig: Origin and Termination of the Corticospinal Tract and Cortico-Brainstem Projections. Front. Neuroanat. 2021, 15, 748050. [Google Scholar] [CrossRef]
- Calvano, C.J.; Moran, M.E.; Parekh, A.; Desai, P.J.; Cisek, L.J. Laparoscopic augmentation cystoplasty using the novel biomaterial Surgisis: Small-intestinal submucosa. J. Endourol. 2000, 14, 213–217. [Google Scholar] [CrossRef]
- Robotti, F.; Sterner, I.; Bottan, S.; Monné Rodríguez, J.M.; Pellegrini, G.; Schmidt, T.; Falk, V.; Poulikakos, D.; Ferrari, A.; Starck, C. Microengineered biosynthesized cellulose as anti-fibrotic in vivo protection for cardiac implantable electronic devices. Biomaterials 2020, 229, 119583. [Google Scholar] [CrossRef]
- Lee, H.; Chun, W.; Kim, G. Three-Dimensional Artificial Skin Construct Bioprinted with a Marine-Based Biocomposite. Biomacromolecules 2023, 24, 2864–2878. [Google Scholar] [CrossRef]
- Rojas-Canales, D.; Walters, S.N.; Penko, D.; Cultrone, D.; Bailey, J.; Chtanova, T.; Nitschke, J.; Johnston, J.; Kireta, S.; Loudovaris, T.; et al. Intracutaneous Transplantation of Islets Within a Biodegradable Temporizing Matrix as an Alternative Site for Islet Transplantation. Diabetes 2023, 72, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Kuluz, J.; Samdani, A.; Benglis, D.; Gonzalez-Brito, M.; Solano, J.P.; Ramirez, M.A.; Luqman, A.; De Los Santos, R.; Hutchinson, D.; Nares, M.; et al. Pediatric spinal cord injury in infant piglets: Description of a new large animal model and review of the literature. J. Spinal Cord. Med. 2010, 33, 43–57. [Google Scholar] [CrossRef]
- Navarro, R.; Juhas, S.; Keshavarzi, S.; Juhásová, J.; Motlik, J.; Johe, K.; Marsala, S.; Scadeng, M.; Lazar, P.; Tomori, Z.; et al. Chronic spinal compression model in minipigs: A systematic behavioral, qualitative, and quantitative neuropathological study. J. Neurotrauma 2012, 29, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jones, C.F.; Okon, E.B.; Anderson, L.; Tigchelaar, S.; Kooner, P.; Godbey, T.; Chua, B.; Gray, G.; Hildebrandt, R.; et al. A novel porcine model of traumatic thoracic spinal cord injury. J. Neurotrauma 2013, 30, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Lamanna, J.J.; Grin, N.; Hurtig, C.V.; Miller, J.H.; Riley, J.; Urquia, L.; Avalos, P.; Svendsen, C.N.; Federici, T.; et al. Preclinical Validation of Multilevel Intraparenchymal Stem Cell Therapy in the Porcine Spinal Cord. Neurosurgery 2015, 77, 604–612. [Google Scholar] [CrossRef]
- Santamaria, A.J.; Benavides, F.D.; Padgett, K.; Guada, L.; Nunez-Gomez, Y.; Solano, J.; Guest, J. Dichotomous Locomotor Recoveries Are Predicted by Acute Changes in Segmental Blood Flow after Thoracic Spinal Contusion Injuries in Pigs. J. Neurotrauma 2019, 36, 1399–1415. [Google Scholar] [CrossRef]
- Kutikov, A.B.; Moore, S.W.; Layer, R.T.; Podell, P.; Sridhar, N.; Santamaria, A.; Aimetti, A.; Hofstetter, C.; Ulich, T.; Guest, J.; et al. Method and Apparatus for the Automated Delivery of Continuous Neural Stem Cell Trails Into the Spinal Cord of Small and Large Animals. Neurosurgery 2019, 85, 560–573. [Google Scholar] [CrossRef]
- Züchner, M.; Escalona, M.J.; Teige, L.H.; Balafas, E.; Zhang, L.; Kostomitsopoulos, N.; Boulland, J.-L. How to generate graded spinal cord injuries in swine-tools and procedures. Dis. Model. Mech. 2021, 14, dmm049053. [Google Scholar] [CrossRef]
- Weber-Levine, C.; Hersh, A.M.; Jiang, K.; Routkevitch, D.; Tsehay, Y.; Perdomo-Pantoja, A.; Judy, B.F.; Kerensky, M.; Liu, A.; Adams, M.; et al. Porcine Model of Spinal Cord Injury: A Systematic Review. Neurotrauma Rep. 2022, 3, 352–368. [Google Scholar] [CrossRef]
- Bunge, R.P.; Puckett, W.R.; Hiester, E.D. Observations on the pathology of several types of human spinal cord injury, with emphasis on the astrocyte response to penetrating injuries. Adv. Neurol. 1997, 72, 305–315. [Google Scholar]
- Layer, R.T.; Ulich, T.R.; Coric, D.; Arnold, P.M.; Guest, J.D.; Heary, R.H.; Hsieh, P.C.; Jenkins, A.L.; Kim, K.D.; Lee, K.S.; et al. New Clinical-Pathological Classification of Intraspinal Injury Following Traumatic Acute Complete Thoracic Spinal Cord Injury: Postdurotomy/Myelotomy Observations From the INSPIRE Trial. Neurosurgery 2017, 64, 105–109. [Google Scholar] [CrossRef]
- Allen, A.R. Remarks on the histopathological changes in the spinal cord due to impact. An experimental study. J. Nerv. Ment. Dis. 1914, 41, 141–147. [Google Scholar] [CrossRef]
- Khaing, Z.Z.; Cates, L.N.; Dewees, D.M.; Hyde, J.E.; Gaing, A.; Birjandian, Z.; Hofstetter, C.P. Effect of Durotomy versus Myelotomy on Tissue Sparing and Functional Outcome after Spinal Cord Injury. J. Neurotrauma 2021, 38, 746–755. [Google Scholar] [CrossRef]
- Yang, C.H.; Quan, Z.X.; Wang, G.J.; He, T.; Chen, Z.Y.; Li, Q.C.; Yang, J.; Wang, Q. Elevated intraspinal pressure in traumatic spinal cord injury is a promising therapeutic target. Neural Regen. Res. 2022, 17, 1703–1710. [Google Scholar] [CrossRef]
- Kim, K.D.; Lee, K.S.; Coric, D.; Harrop, J.S.; Theodore, N.; Toselli, R.M. Acute Implantation of a Bioresorbable Polymer Scaffold in Patients With Complete Thoracic Spinal Cord Injury: 24-Month Follow-up From the INSPIRE Study. Neurosurgery 2022, 90, 668–675. [Google Scholar] [CrossRef]
- Riew, T.R.; Choi, J.H.; Kim, H.L.; Jin, X.; Lee, M.Y. PDGFR-β-Positive Perivascular Adventitial Cells Expressing Nestin Contribute to Fibrotic Scar Formation in the Striatum of 3-NP Intoxicated Rats. Front. Mol. Neurosci. 2018, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Vara, H.; Collazos-Castro, J.E. Biofunctionalized Conducting Polymer/Carbon Microfiber Electrodes for Ultrasensitive Neural Recordings. ACS Appl. Mater. Interfaces 2015, 7, 27016–27026. [Google Scholar] [CrossRef] [PubMed]
- Vara, H.; Collazos-Castro, J.E. Enhanced spinal cord microstimulation using conducting polymer-coated carbon microfibers. Acta Biomater. 2019, 90, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Tuszynski, M.H.; Gabriel, K.; Gerhardt, K.; Szollar, S. Human spinal cord retains substantial structural mass in chronic stages after injury. J. Neurotrauma 1999, 16, 523–531. [Google Scholar] [CrossRef]
- Metz, G.A.; Curt, A.; van de Meent, H.; Klusman, I.; Schwab, M.E.; Dietz, V. Validation of the weight-drop contusion model in rats: A comparative study of human spinal cord injury. J. Neurotrauma 2000, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, H.; Xia, P.; Kong, W.; Chang, Y.; Fu, C.; Wang, K.; Yang, X.; Qi, Z. Application of fibrin-based hydrogels for nerve protection and regeneration after spinal cord injury. J. Biol. Eng. 2020, 14, 22. [Google Scholar] [CrossRef]
- Bayer, I.S. Advances in Fibrin-Based Materials in Wound Repair: A Review. Molecules 2022, 27, 4504. [Google Scholar] [CrossRef]
- Song, F.; Yin, H.; Zhang, Z.; Zhang, J.; Dai, X.; Wang, Z.; Ge, Y.; Liu, Y.; Chang, Y.; Huan, Y. Monitoring of acute axonal injury in the swine spinal cord with EAE by diffusion tensor imaging. J. Magn. Reson. Imaging 2009, 30, 277–285. [Google Scholar] [CrossRef]
- Fenrich, K.K.; Rose, P.K. Spinal interneuron axons spontaneously regenerate after spinal cord injury in the adult feline. J. Neurosci. 2009, 29, 12145–12158. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; O’Shea, T.M.; Burda, J.E.; Ao, Y.; Barlatey, S.L.; Bernstein, A.M.; Kim, J.H.; James, N.D.; Rogers, A.; Kato, B.; et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 2018, 561, 396–400. [Google Scholar] [CrossRef]
- Ghosh, M.; Pearse, D.D. The role of the serotonergic system in locomotor recovery after spinal cord injury. Front. Neural Circuits 2015, 8, 151. [Google Scholar] [CrossRef]
- Perrin, F.E.; Noristani, H.N. Serotonergic mechanisms in spinal cord injury. Exp. Neurol. 2019, 318, 174–191. [Google Scholar] [CrossRef]
- Hawthorne, A.L.; Hu, H.; Kundu, B.; Steinmetz, M.P.; Wylie, C.J.; Deneris, E.S.; Silver, J. The Unusual Response of Serotonergic Neurons after CNS Injury: Lack of Axonal Dieback and Enhanced Sprouting within the Inhibitory Environment of the Glial Scar. J. Neurosci. 2011, 31, 5605–5616. [Google Scholar] [CrossRef]
- Yiu, G.; He, Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006, 7, 617–627. [Google Scholar] [CrossRef]
- Sofroniew, M.W. Dissecting spinal cord regeneration. Nature 2018, 557, 343–350. [Google Scholar] [CrossRef]
- Okada, S.; Nakamura, M.; Katoh, H.; Miyao, T.; Shimazaki, T.; Ishii, K.; Yamane, J.; Yoshimura, A.; Iwamoto, Y.; Toyama, Y.; et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 2006, 12, 829–834. [Google Scholar] [CrossRef]
- Liu, K.; Lu, Y.; Lee, J.K.; Samara, R.; Willenberg, R.; Sears-Kraxberger, I.; Tedeschi, A.; Park, K.K.; Jin, D.; Cai, B.; et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci. 2010, 13, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Zheng, S.; Zhang, Q.; Li, S.; Gao, X.; Wang, J.; Jiang, L.; Liu, K. Pten deletion promotes regrowth of corticospinal tract axons 1 year after spinal cord injury. J. Neurosci. 2015, 35, 9754–9763. [Google Scholar] [CrossRef] [PubMed]
- Collazos-Castro, J.E.; López-Dolado, E.; Nieto-Sampedro, M. Locomotor deficits and adaptive mechanisms after thoracic spinal cord contusion in the adult rat. J. Neurotrauma 2006, 23, 1–17. [Google Scholar] [CrossRef]
- Collazos-Castro, J.E.; Soto, V.M.; Gutiérrez-Dávila, M.; Nieto-Sampedro, M. Motoneuron loss associated with chronic locomotion impairments after spinal cord contusion in the rat. J. Neurotrauma 2005, 22, 544–558. [Google Scholar] [CrossRef]
- Holstege, G.; van Neerven, J.; Evertse, F. Spinal cord location of the motoneurons innervating the abdominal, cutaneous maximus, latissimus dorsi and longissimus dorsi muscles in the cat. Exp. Brain Res. 1987, 67, 179–194. [Google Scholar] [CrossRef]
- Toossi, A.; Everaert, D.G.; Perlmutter, S.I.; Mushahwar, V.K. Functional organization of motor networks in the lumbosacral spinal cord of non-human primates. Sci. Rep. 2019, 19, 13539. [Google Scholar] [CrossRef] [PubMed]
- Falgairolle, M.; de Seze, M.; Juvin, L.; Morin, D.; Cazalets, J.R. Coordinated network functioning in the spinal cord: An evolutionary perspective. J. Physiol. Paris 2006, 100, 304–316. [Google Scholar] [CrossRef]
- Saadoun, S.; Papadopoulos, M.C. Spinal cord injury: Is monitoring from the injury site the future? Crit. Care 2016, 20, 308. [Google Scholar] [CrossRef]
- Göritz, C.; Dias, D.O.; Tomilin, N.; Barbacid, M.; Shupliakov, O.; Frisén, J. A pericyte origin of spinal cord scar tissue. Science 2011, 333, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Soderblom, C.; Luo, X.; Blumenthal, E.; Bray, E.; Lyapichev, K.; Ramos, J.; Krishnan, V.; Lai-Hsu, C.; Park, K.K.; Tsoulfas, P.; et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J. Neurosci. 2013, 33, 13882–13887. [Google Scholar] [CrossRef]
- Fernandez, E.; Pallini, E. Connective tissue scarring in experimental spinal cord lesions: Significance of dural continuity and role of epidural tissues. Acta Neurochir. 1985, 76, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Zukor, K.; Belin, S.; Wang, C.; Keelan, N.; Wang, X.; He, Z. Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. J. Neurosci. 2013, 33, 15350–15361. [Google Scholar] [CrossRef] [PubMed]
- Zareen, N.; Dodson, S.; Armada, K.; Awad, R.; Sultana, N.; Hara, E.; Alexander, H.; Martin, J.H. Stimulation-dependent remodeling of the corticospinal tract requires reactivation of growth-promoting developmental signaling pathways. Exp. Neurol. 2018, 307, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.H.; Johnson, F.B.; Whiting, J.; Roller, P.P. Formaldehyde fixation. J. Histochem. Cytochem. 1985, 33, 845–853. [Google Scholar] [CrossRef]
- Metz, B.; Kersten, G.F.; Hoogerhout, P.; Brugghe, H.F.; Timmermans, H.A.; de Jong, A.; Meiring, H.; ten Hove, J.; Hennink, W.E.; Crommelin, D.J.; et al. Identification of formaldehyde-induced modifications in proteins: Reactions with model peptides. J. Biol. Chem. 2004, 20, 6235–6243. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves-Sampaio, A.; Del-Cerro, P.; Collazos-Castro, J.E. Composite Fibrin/Carbon Microfiber Implants for Bridging Spinal Cord Injury: A Translational Approach in Pigs. Int. J. Mol. Sci. 2023, 24, 11102. https://doi.org/10.3390/ijms241311102
Alves-Sampaio A, Del-Cerro P, Collazos-Castro JE. Composite Fibrin/Carbon Microfiber Implants for Bridging Spinal Cord Injury: A Translational Approach in Pigs. International Journal of Molecular Sciences. 2023; 24(13):11102. https://doi.org/10.3390/ijms241311102
Chicago/Turabian StyleAlves-Sampaio, Alexandra, Patricia Del-Cerro, and Jorge E. Collazos-Castro. 2023. "Composite Fibrin/Carbon Microfiber Implants for Bridging Spinal Cord Injury: A Translational Approach in Pigs" International Journal of Molecular Sciences 24, no. 13: 11102. https://doi.org/10.3390/ijms241311102
APA StyleAlves-Sampaio, A., Del-Cerro, P., & Collazos-Castro, J. E. (2023). Composite Fibrin/Carbon Microfiber Implants for Bridging Spinal Cord Injury: A Translational Approach in Pigs. International Journal of Molecular Sciences, 24(13), 11102. https://doi.org/10.3390/ijms241311102




