Control of the Electroporation Efficiency of Nanosecond Pulses by Swinging the Electric Field Vector Direction
Abstract
1. Introduction
2. Results
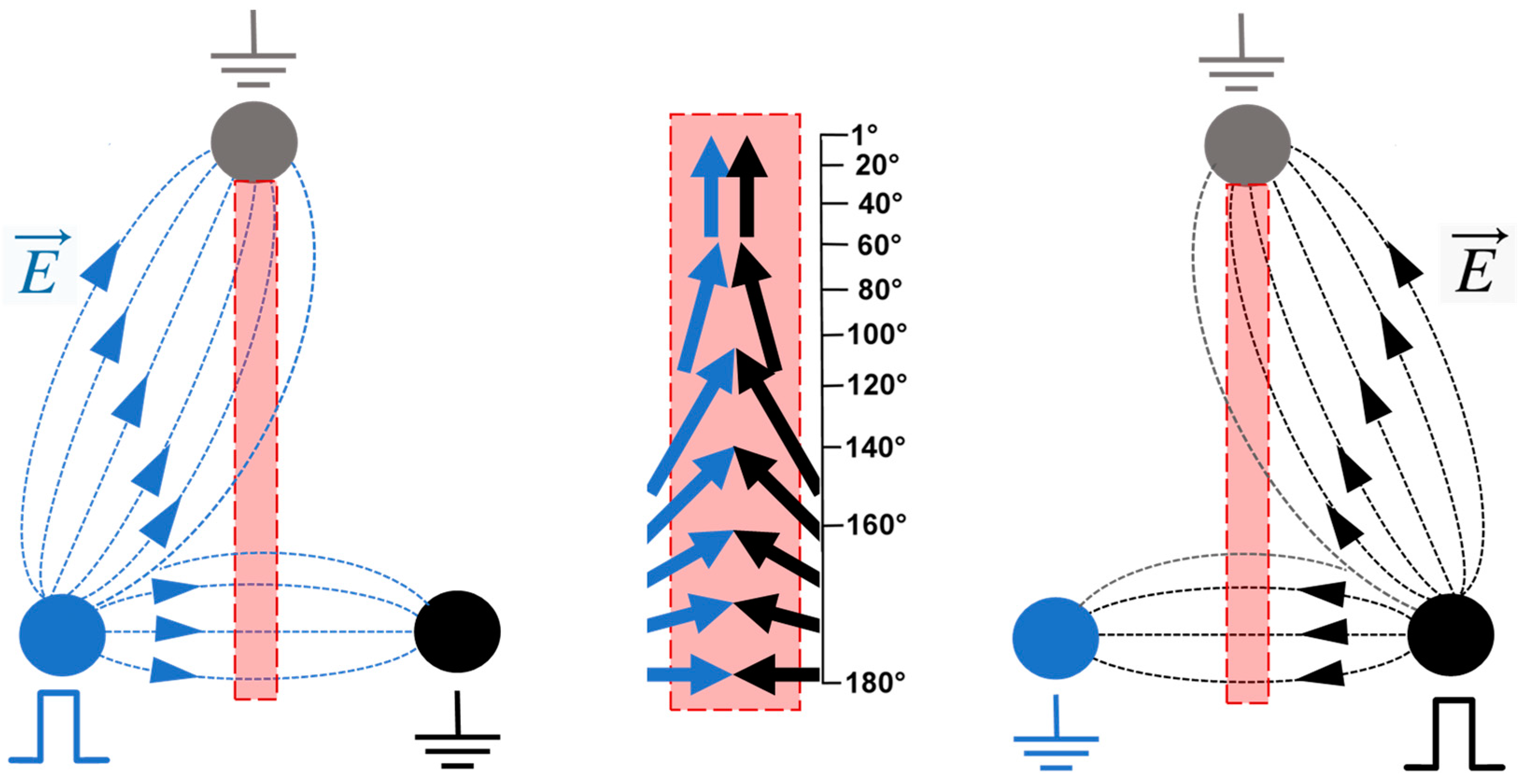
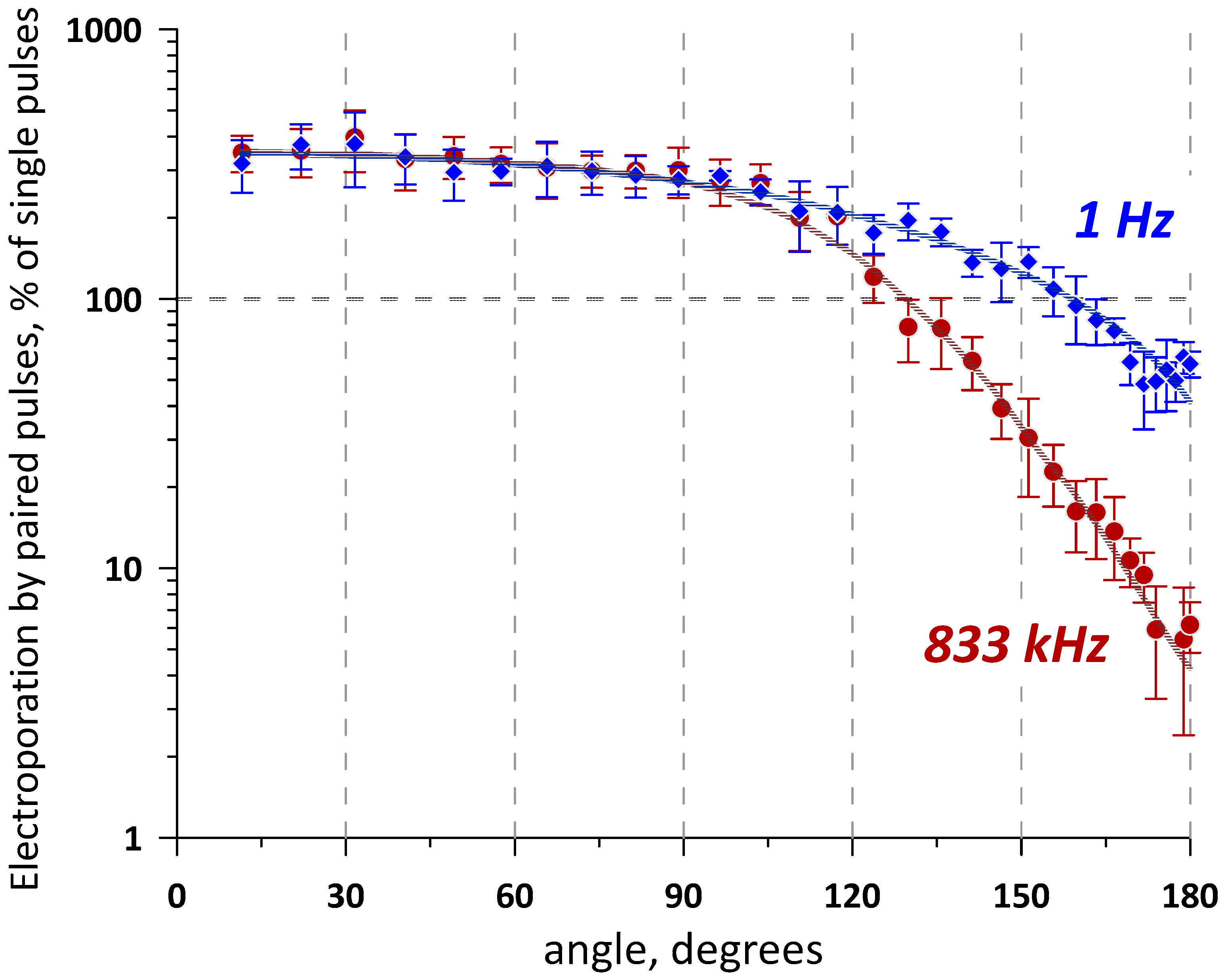
2.1. Applying nsEPs from Different Directions Can Inhibit or Facilitate Electroporation
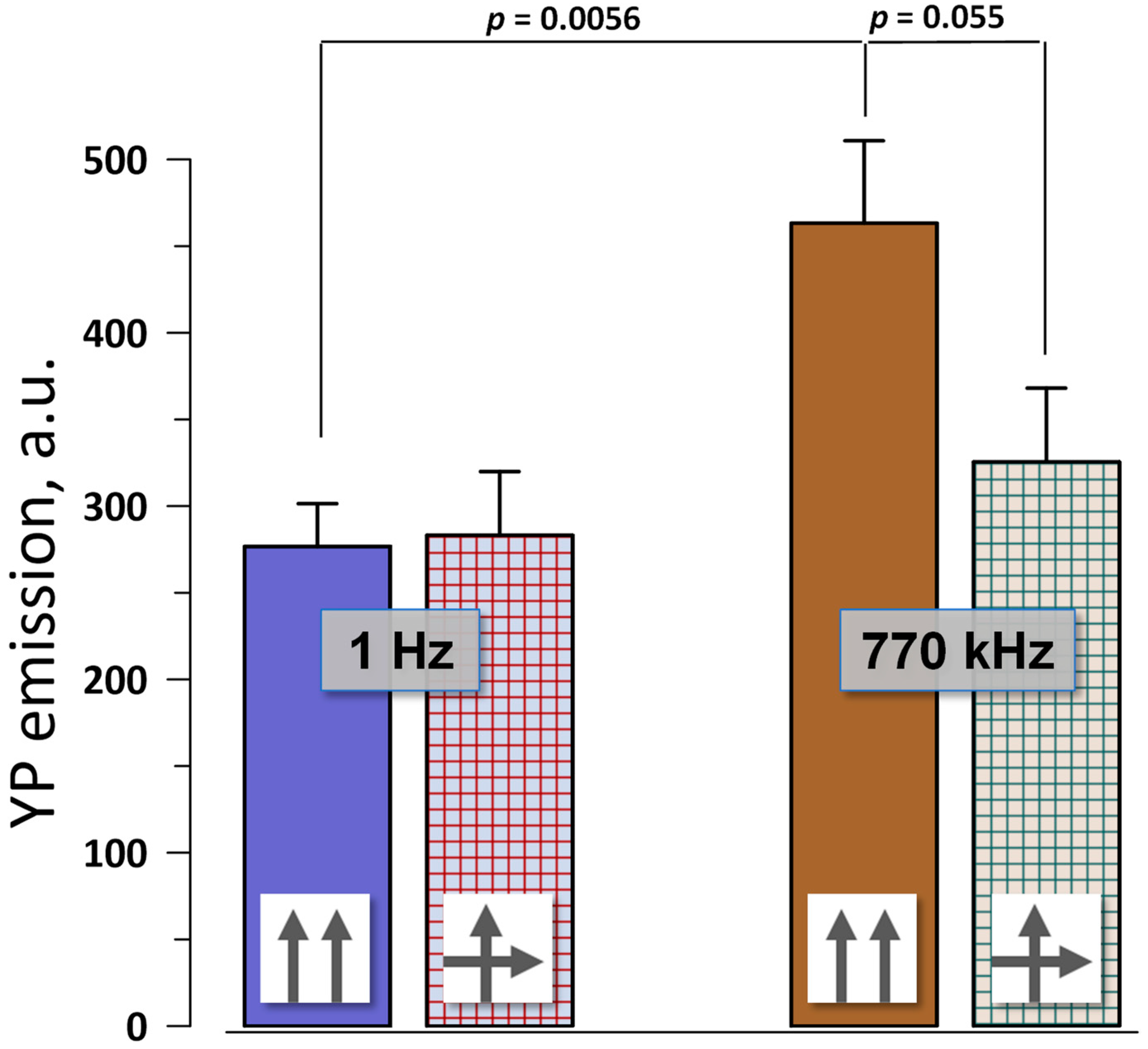
2.2. No Change in Electroporation When the Electric Field Direction Is Rotated by 90°
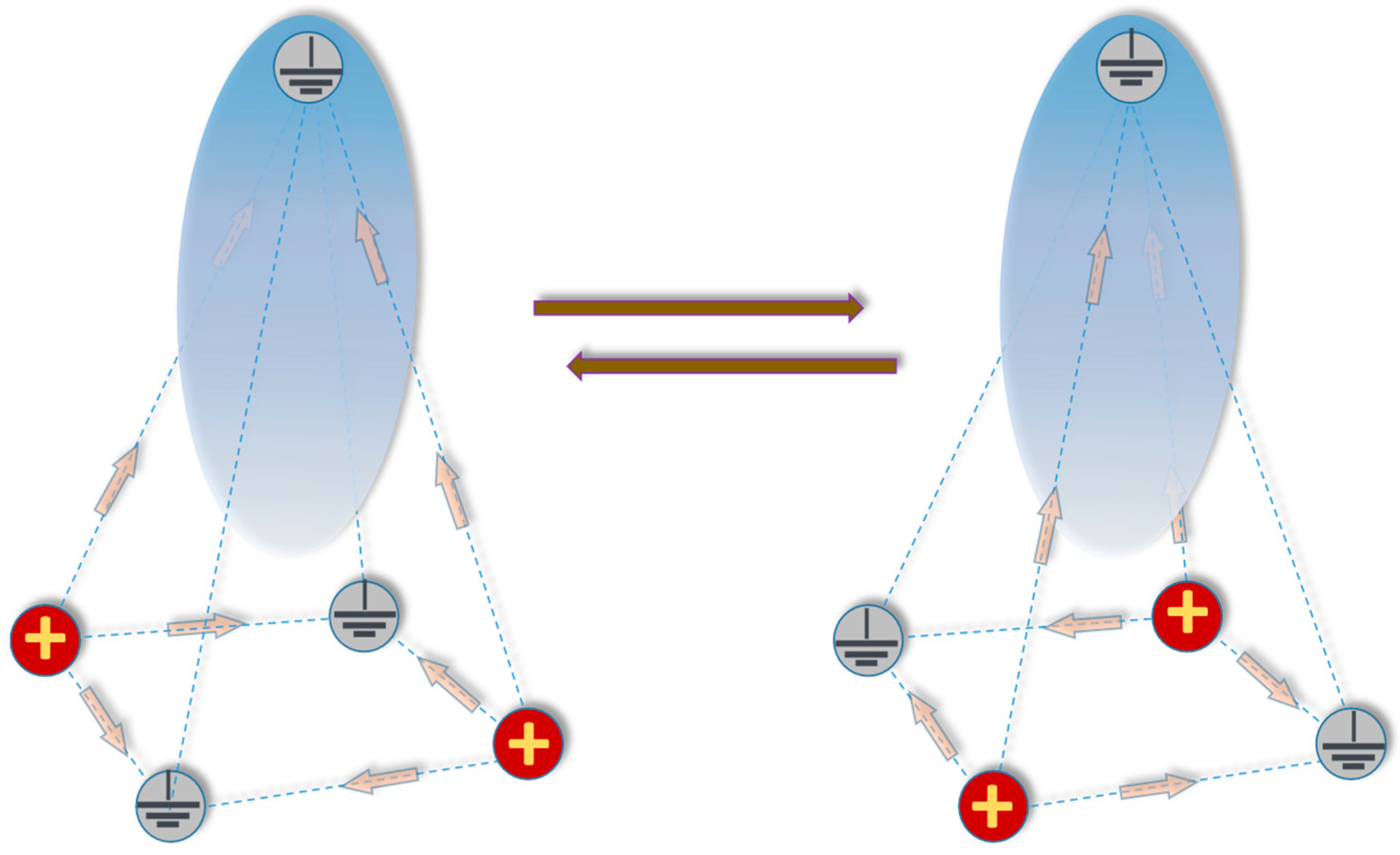
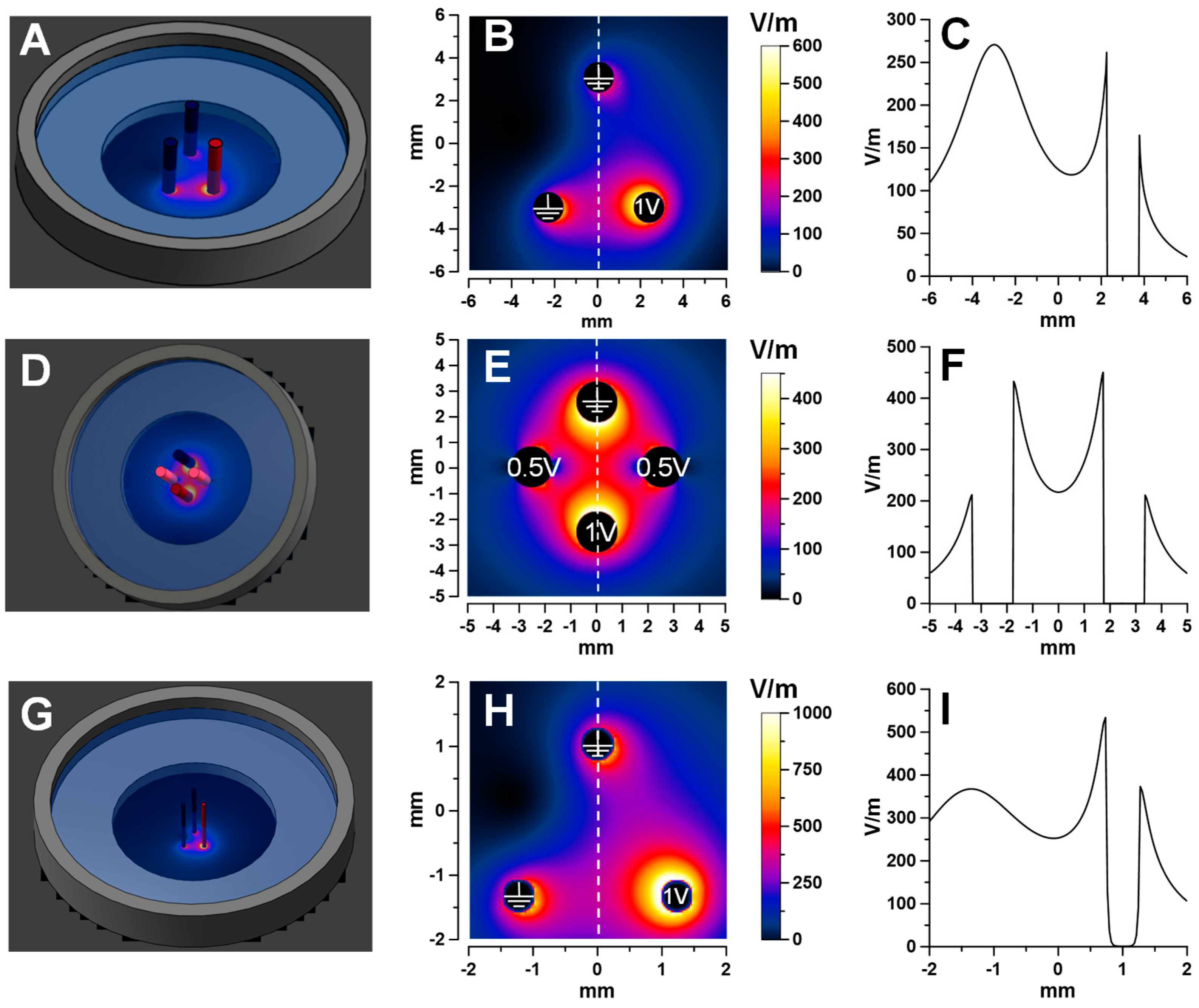
2.3. Focal Electroporation in the Triangular Array
2.4. Cell Membrane Charging by Co- and Counter-Directional nsEPs
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Electric Pulse Treatments
4.3. Numerical Simulation of the Electric Field Distribution
4.4. Protocols and Quantitation of Electroporation in Cell Monolayers
4.5. Strobe Imaging of Cell Membrane Charging by Electric Pulses
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Virgilio, A.; Ralli, M.; Longo, L.; Mancini, P.; Attanasio, G.; Atturo, F.; De Vincentiis, M.; Greco, A. Electrochemotherapy in head and neck cancer: A review of an emerging cancer treatment. Oncol. Lett. 2018, 16, 3415–3423. [Google Scholar] [CrossRef] [PubMed]
- Batista Napotnik, T.; Rebersek, M.; Vernier, P.T.; Mali, B.; Miklavcic, D. Effects of high voltage nanosecond electric pulses on eukaryotic cells (in vitro): A systematic review. Bioelectrochemistry 2016, 110, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chopinet, L.; Rols, M.P. Nanosecond electric pulses: A mini-review of the present state of the art. Bioelectrochemistry 2015, 103, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Teissie, J.; Golzio, M.; Rols, M.P. Mechanisms of cell membrane electropermeabilization: A minireview of our present (lack of ?) knowledge. Biochim. Biophys. Acta 2005, 1724, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Latouche, E.L.; Arena, C.B.; Ivey, J.W.; Garcia, P.A.; Pancotto, T.E.; Pavlisko, N.; Verbridge, S.S.; Davalos, R.V.; Rossmeisl, J.H. High-Frequency Irreversible Electroporation for Intracranial Meningioma: A Feasibility Study in a Spontaneous Canine Tumor Model. Technol. Cancer Res. Treat. 2018, 17, 1533033818785285. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, D.C.; Rebersek, M.; Dermol, J.; Rems, L.; Miklavcic, D.; Davalos, R.V. Quantification of cell membrane permeability induced by monopolar and high-frequency bipolar bursts of electrical pulses. Biochim. Biophys. Acta 2016, 1858, 2689–2698. [Google Scholar] [CrossRef]
- Neumann, E.; Sowers, A.E.; Jordan, C.A. Electroporation and Electrofusion in Cell Biology; Plenum: New York, NY, USA, 1989. [Google Scholar]
- Zimmermann, U.; Neil, G.A. Electromanipulation of Cells; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Pakhomov, A.G.; Miklavcic, D.; Markov, M.S. Advanced Electroporation Techniques in Biology in Medicine; CRC Press: Boca Raton, FL, USA, 2010; p. 528. [Google Scholar]
- Faurie, C.; Phez, E.; Golzio, M.; Vossen, C.; Lesbordes, J.C.; Delteil, C.; Teissie, J.; Rols, M.P. Effect of electric field vectoriality on electrically mediated gene delivery in mammalian cells. Biochim. Biophys. Acta 2004, 1665, 92–100. [Google Scholar] [CrossRef]
- Sersa, G.; Cemazar, M.; Semrov, D.; Miklavcic, D. Changing electrode orientation improves the efficacy of electrochemotherapy of solid tumors in mice. Bioelectrochem. Bioenerg. 1996, 39, 61–66. [Google Scholar]
- Todorovic, V.; Kamensek, U.; Sersa, G.; Cemazar, M. Changing electrode orientation, but not pulse polarity, increases the efficacy of gene electrotransfer to tumors in vivo. Bioelectrochemistry 2014, 100, 119–127. [Google Scholar] [CrossRef]
- Gilbert, R.A.; Jaroszeski, M.J.; Heller, R. Novel electrode designs for electrochemotherapy. Biochim. Biophys. Acta 1997, 1334, 9–14. [Google Scholar] [CrossRef]
- Heller, R.; Cruz, Y.; Heller, L.C.; Gilbert, R.A.; Jaroszeski, M.J. Electrically mediated delivery of plasmid DNA to the skin, using a multielectrode array. Hum. Gene Ther. 2010, 21, 357–362. [Google Scholar] [CrossRef]
- Ferraro, B.; Heller, L.C.; Cruz, Y.L.; Guo, S.; Donate, A.; Heller, R. Evaluation of delivery conditions for cutaneous plasmid electrotransfer using a multielectrode array. Gene Ther. 2011, 18, 496–500. [Google Scholar] [CrossRef]
- Zheng, M.D.; Shan, J.W.; Lin, H.; Shreiber, D.I.; Zahn, J.D. Hydrodynamically controlled cell rotation in an electroporation microchip to circumferentially deliver molecules into single cells. Microfluid. Nanofluid. 2016, 20, 16. [Google Scholar] [CrossRef]
- Kim, V.; Semenov, I.; Kiester, A.S.; Keppler, M.A.; Ibey, B.L.; Bixler, J.N.; Pakhomov, A.G. Action spectra and mechanisms of (in) efficiency of bipolar electric pulses at electroporation. Bioelectrochemistry 2023, 149, 108319. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Grigoryev, S.; Semenov, I.; Casciola, M.; Jiang, C.; Xiao, S. The second phase of bipolar, nanosecond-range electric pulses determines the electroporation efficiency. Bioelectrochemistry 2018, 122, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Semenov, I.; Xiao, S.; Pakhomova, O.N.; Gregory, B.; Schoenbach, K.H.; Ullery, J.C.; Beier, H.T.; Rajulapati, S.R.; Ibey, B.L. Cancellation of cellular responses to nanoelectroporation by reversing the stimulus polarity. Cell Mol. Life Sci. 2014, 71, 4431–4441. [Google Scholar] [CrossRef]
- Valdez, C.M.; Barnes, R., Jr.; Roth, C.C.; Moen, E.; Ibey, B. The interphase interval within a bipolar nanosecond electric pulse modulates bipolar cancellation. Bioelectromagnetics 2018, 39, 441–450. [Google Scholar] [CrossRef]
- Zaklit, J.; Craviso, G.L.; Leblanc, N.; Vernier, P.T.; Sozer, E.B. 2-ns Electrostimulation of Ca(2+) Influx into Chromaffin Cells: Rapid Modulation by Field Reversal. Biophys. J. 2021, 120, 556–567. [Google Scholar] [CrossRef]
- Sozer, E.B.; Vernier, P.T. Modulation of biological responses to 2ns electrical stimuli by field reversal. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.; Gudvangen, E.; Kondratiev, O.; Redondo, L.; Xiao, S.; Pakhomov, A.G. Peculiarities of Neurostimulation by Intense Nanosecond Pulsed Electric Fields: How to Avoid Firing in Peripheral Nerve Fibers. Int. J. Mol. Sci. 2021, 22, 7051. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Gudvangen, E.; Mangalanathan, U.; Kondratiev, O.; Redondo, L.; Semenov, I. Next generation CANCAN focusing for remote stimulation by nanosecond electric pulses. Bioelectrochemistry 2023, 152, 108437. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Gudvangen, E.; Xiao, S.; Semenov, I. Interference targeting of bipolar nanosecond electric pulses for spatially focused electroporation, electrostimulation, and tissue ablation. Bioelectrochemistry 2021, 141, 107876. [Google Scholar] [CrossRef] [PubMed]
- Silkunas, M.; Gudvangen, E.; Novickij, V.; Pakhomov, A.G. Sub-MHz bursts of nanosecond pulses excite neurons at paradoxically low electric field thresholds without membrane damage. Biochim. Biophys. Acta Biomembr. 2022, 1864, 184034. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Xiao, S.; Novickij, V.; Casciola, M.; Semenov, I.; Mangalanathan, U.; Kim, V.; Zemlin, C.; Sozer, E.; Muratori, C.; et al. Excitation and electroporation by MHz bursts of nanosecond stimuli. Biochem. Biophys. Res. Commun. 2019, 518, 759–764. [Google Scholar] [CrossRef]
- Novickij, V.; Ruzgys, P.; Grainys, A.; Satkauskas, S. High frequency electroporation efficiency is under control of membrane capacitive charging and voltage potential relaxation. Bioelectrochemistry 2018, 119, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.G.; Gianulis, E.; Vernier, P.T.; Semenov, I.; Xiao, S.; Pakhomova, O.N. Multiple nanosecond electric pulses increase the number but not the size of long-lived nanopores in the cell membrane. Biochim. Biophys. Acta 2015, 1848, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Rols, M.P.; Teissie, J. Electropermeabilization of mammalian cells. Quantitative analysis of the phenomenon. Biophys. J. 1990, 58, 1089–1098. [Google Scholar] [CrossRef]
- Puc, M.; Kotnik, T.; Mir, L.M.; Miklavcic, D. Quantitative model of small molecules uptake after in vitro cell electropermeabilization. Bioelectrochemistry 2003, 60, 1–10. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef]
- Merla, C.; Pakhomov, A.G.; Semenov, I.; Vernier, P.T. Frequency spectrum of induced transmembrane potential and permeabilization efficacy of bipolar electric pulses. Biochim. Biophys. Acta 2017, 1859, 1282–1290. [Google Scholar] [CrossRef]
- Kiester, A.S.; Ibey, B.L.; Coker, Z.N.; Pakhomov, A.G.; Bixler, J.N. Strobe photography mapping of cell membrane potential with nanosecond resolution. Bioelectrochemistry 2021, 142, 107929. [Google Scholar] [CrossRef] [PubMed]
- Kandratsyeu, A.; Sabaleuski, U.; Redondo, L.; Pakhomov, A.G. Four Channel 6.5 kV, 65 A, 100 ns—100 micros Generator with Advanced Control of Pulse and Burst Protocols for Biomedical and Biotechnological Applications. Appl. Sci. 2021, 11, 11782. [Google Scholar] [CrossRef]
- Ibey, B.L.; Xiao, S.; Schoenbach, K.H.; Murphy, M.R.; Pakhomov, A.G. Plasma membrane permeabilization by 60- and 600-ns electric pulses is determined by the absorbed dose. Bioelectromagnetics 2009, 30, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Gudvangen, E.; Kim, V.; Novickij, V.; Battista, F.; Pakhomov, A.G. Electroporation and cell killing by milli- to nanosecond pulses and avoiding neuromuscular stimulation in cancer ablation. Sci. Rep. 2022, 12, 1763. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, V.; Semenov, I.; Kiester, A.S.; Keppler, M.A.; Ibey, B.L.; Bixler, J.N.; Colunga Biancatelli, R.M.L.; Pakhomov, A.G. Control of the Electroporation Efficiency of Nanosecond Pulses by Swinging the Electric Field Vector Direction. Int. J. Mol. Sci. 2023, 24, 10921. https://doi.org/10.3390/ijms241310921
Kim V, Semenov I, Kiester AS, Keppler MA, Ibey BL, Bixler JN, Colunga Biancatelli RML, Pakhomov AG. Control of the Electroporation Efficiency of Nanosecond Pulses by Swinging the Electric Field Vector Direction. International Journal of Molecular Sciences. 2023; 24(13):10921. https://doi.org/10.3390/ijms241310921
Chicago/Turabian StyleKim, Vitalii, Iurii Semenov, Allen S. Kiester, Mark A. Keppler, Bennett L. Ibey, Joel N. Bixler, Ruben M. L. Colunga Biancatelli, and Andrei G. Pakhomov. 2023. "Control of the Electroporation Efficiency of Nanosecond Pulses by Swinging the Electric Field Vector Direction" International Journal of Molecular Sciences 24, no. 13: 10921. https://doi.org/10.3390/ijms241310921
APA StyleKim, V., Semenov, I., Kiester, A. S., Keppler, M. A., Ibey, B. L., Bixler, J. N., Colunga Biancatelli, R. M. L., & Pakhomov, A. G. (2023). Control of the Electroporation Efficiency of Nanosecond Pulses by Swinging the Electric Field Vector Direction. International Journal of Molecular Sciences, 24(13), 10921. https://doi.org/10.3390/ijms241310921







