The Role of Selenium and Manganese in the Formation, Diagnosis and Treatment of Cervical, Endometrial and Ovarian Cancer
Abstract
1. Introduction
2. Selenium
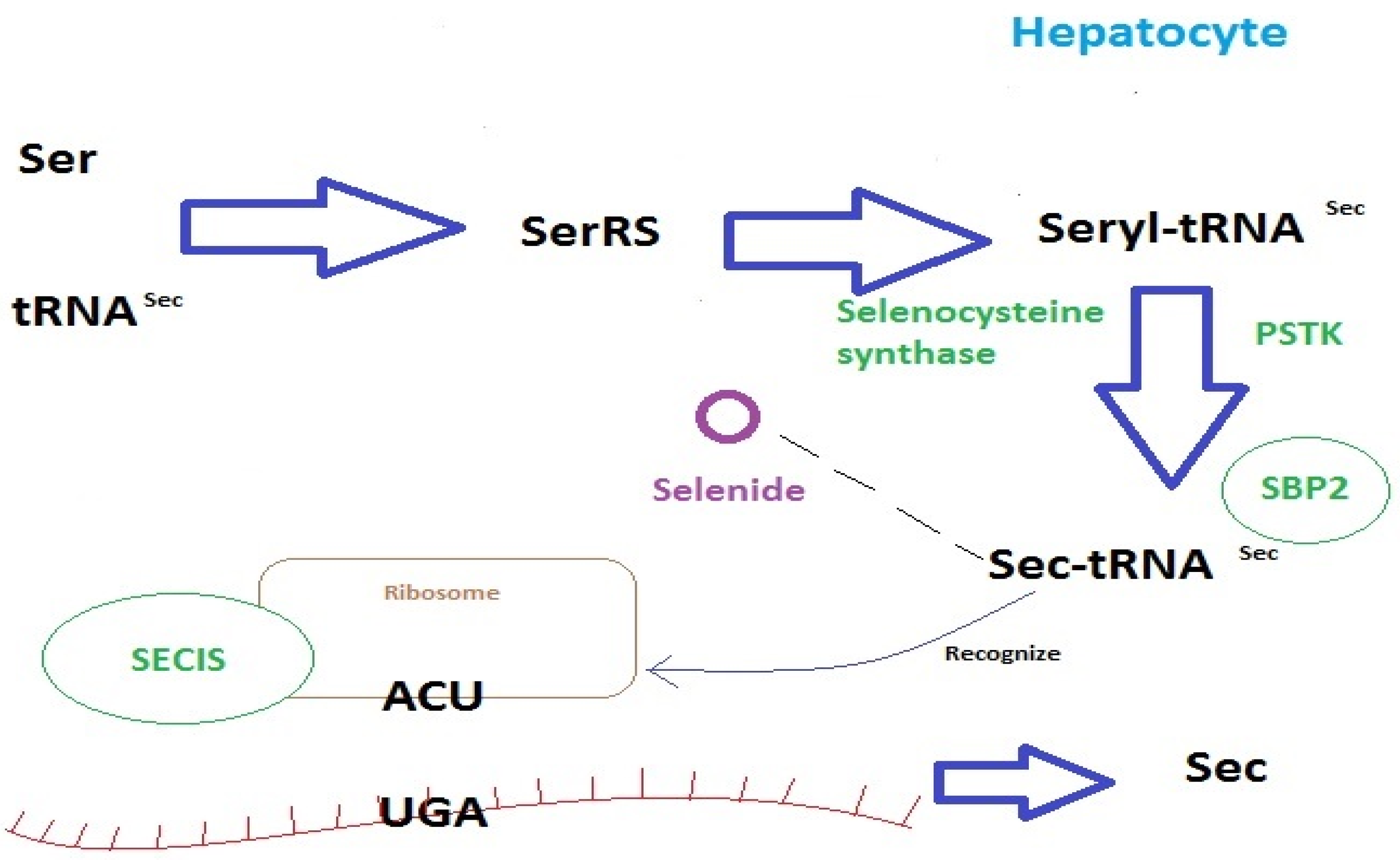
2.1. Selenium Homeostasis
2.2. Glutathione Peroxidase
2.3. Antioxidant Properties
2.4. Selenium and Oncogenesis
2.5. Selenium Supplementation
2.6. The Importance of Selenium Supplementation for Cancer Treatment
3. Manganese
3.1. Manganese Homeostasis
3.2. Manganese and Oncogenesis
3.3. Manganese Supplementation
3.4. Importance of Manganese Supplementation for Cancer Treatment
4. Cervical Cancer
4.1. Se and Cervical Cancer
4.2. Mn and Cervical Cancer
5. Endometrial Cancer
5.1. Se and Endometrial Cancer
5.2. Mn and Endometrial Cancer
6. Ovarian Cancer
6.1. Se and Ovarian Cancer
6.2. Mn and Ovarian Cancer
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kieliszek, M. Selenium–fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [PubMed]
- Erikson, K.M.; Aschner, M. Manganese: Its Role in Disease and Health. Met. Ions Life Sci. 2019, 19, 253–266. [Google Scholar] [CrossRef]
- Chen, P.; Bornhorst, J.; Aschner, M. Manganese metabolism in humans. Front. Biosci. 2018, 23, 1655–1679. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M. Selenium. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2021; Volume 96, pp. 417–429. ISBN 9780128206485. [Google Scholar]
- Kielczykowska, M.; Kocot, J.; Pazdzior, M.; Musik, I. Selenium—A fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. 2018, 27, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, X. The essential element manganese, oxidative stress, and metabolic diseases: Links and interactions. Oxid. Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Wu, Q.; Rayman, M.P.; Lv, H.; Schomburg, L.; Cui, B.; Gao, C.; Chen, P.; Zhuang, G.; Zhang, Z.; Peng, X.; et al. Low population selenium status is associated with increased prevalence of thyroid disease. J. Clin. Endocrinol. Metab. 2015, 100, 4037–4047. [Google Scholar] [CrossRef]
- Lin, J.C.; Kuo, W.R.; Chiang, F.Y.; Hsiao, P.J.; Lee, K.W.; Wu, C.W.; Hank Juo, S.H. Glutathione peroxidase 3 gene polymorphisms and risk of differentiated thyroid cancer. Surgery 2009, 145, 508–513. [Google Scholar] [CrossRef]
- Bülow Pedersen, I.; Knudsen, N.; Carlé, A.; Schomburg, L.; Köhrle, J.; Jørgensen, T.; Rasmussen, L.B.; Ovesen, L.; Laurberg, P. Serum selenium is low in newly diagnosed Graves’ disease: A population-based study. Clin. Endocrinol. 2013, 79, 584–590. [Google Scholar] [CrossRef]
- Rayman, M.P.; Leonidas; Duntas, H. Selenium Deficiency and Thyroid Disease. In The Thyroid and Its Diseases; Springer: New York, NY, USA, 2018. [Google Scholar]
- Sayehmiri, K.; Azami, M.; Mohammadi, Y.; Soleymani, A.; Tardeh, Z. The association between selenium and prostate cancer: A systematic review and meta-analysis. Asian Pacific J. Cancer Prev. 2018, 19, 1431–1437. [Google Scholar] [CrossRef]
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium Exposure and Cancer Risk: An Updated Meta-analysis and Meta-regression. Sci. Rep. 2016, 6, 1–18. [Google Scholar] [CrossRef]
- Kim, J.; Chung, H.S.; Choi, M.K.; Roh, Y.K.; Yoo, H.J.; Park, J.H.; Kim, D.S.; Yu, J.M.; Moon, S. Association between serum selenium level and the presence of diabetes mellitus: A meta-analysis of observational studies. Diabetes Metab. J. 2019, 43, 447–460. [Google Scholar] [CrossRef]
- Kohler, L.N.; Foote, J.; Kelley, C.P.; Florea, A.; Shelly, C.; Chow, H.H.S.; Hsu, P.; Batai, K.; Ellis, N.; Saboda, K.; et al. Selenium and type 2 diabetes: Systematic review. Nutrients 2018, 10, 1924. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Zeng, H.; Combs, G.F. Selenium as an anticancer nutrient: Roles in cell proliferation and tumor cell invasion. J. Nutr. Biochem. 2008, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Minich, W.B. Selenium Metabolism and Biosynthesis of Selenoproteins in the Human Body. Biochem. 2022, 87, S168–S177. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Arthur, J. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Flohé, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef]
- Saito, Y. Selenoprotein P as an in vivo redox regulator:disorders related to its deficiency and excess. J. Clin. Biochem. Nutr. 2019, 66, 1–7. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Ogra, Y.; Ishiwata, K.; Takayama, H.; Aimi, N.; Suzuki, K.T. Selenosugars are key and urinary metabolites for selenium excretion within the required to low-toxic range. Proc. Natl. Acad. Sci. USA 2002, 99, 15932–15936. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Flohé, L.; Toppo, S.; Orian, L. The glutathione peroxidase family: Discoveries and mechanism. Free Radic. Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Loschen, G.; Günzler, W.A.; Eichele, E. Glutathione Peroxidase, V. The kinetic mechanism. Biol. Chem. 1972, 353, 987–1000. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, Y.; Guo, S.; Wang, G. Glutathione peroxidases as oncotargets. Oncotarget 2017, 8, 80093–80102. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.J.; Banu, L.; Harney, J.W.; Larsen, P.R. Functional characterization of the eukaryotic SECIS elements which direct selenocysteine insertion at UGA codons. EMBO J. 1993, 12, 3315–3322. [Google Scholar] [CrossRef] [PubMed]
- Hubert, N.; Walczak, R.; Sturchler, C.; Myslinski, E.; Schuster, C.; Westhof, E.; Carbon, P.; Krol, A. RNAs mediating cotranslational insertion of selenocysteine in eukaryotic selenoproteins. Biochimie 1996, 78, 590–596. [Google Scholar] [CrossRef]
- Jablonska, E.; Gromadzinska, J.; Peplonska, B.; Fendler, W.; Reszka, E.; Krol, M.B.; Wieczorek, E.; Bukowska, A.; Gresner, P.; Galicki, M.; et al. Lipid peroxidation and glutathione peroxidase activity relationship in breast cancer depends on functional polymorphism of GPX1. BMC Cancer 2015, 15, 657. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, E.; Gromadzinska, J.; Reszka, E.; Wasowicz, W.; Sobala, W.; Szeszenia-Dabrowska, N.; Boffetta, P. Association between GPx1 Pro198Leu polymorphism, GPx1 activity and plasma selenium concentration in humans. Eur. J. Nutr. 2009, 48, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, R.; Chen, N.; Yang, L.; Wang, Y.; Sun, Y.; Huang, L.; Zhu, M.; Ji, Y.; Li, W. Association between glutathione peroxidase-1 (GPX1) Rs1050450 polymorphisms and cancer risk. Int. J. Clin. Exp. Pathol. 2017, 10, 9527–9540. [Google Scholar]
- Men, T.; Zhang, X.; Yang, J.; Shen, B.; Li, X.; Chen, D.; Wang, J. The rs1050450 C > T polymorphism of GPX1 is associated with the risk of bladder but not prostate cancer: Evidence from a meta-analysis. Tumor Biol. 2014, 35, 269–275. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, G.W.; Wang, N.; Wang, Y.J. GPX1 Pro198Leu polymorphism and breast cancer risk: A meta-analysis. Breast Cancer Res. Treat. 2010, 124, 425–431. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Sørensen, M.; Hansen, R.D.; Frederiksen, K.; Tjønneland, A.; Overvad, K.; Vogel, U. GPX1 Pro198Leu polymorphism, interactions with smoking and alcohol consumption, and risk for lung cancer. Cancer Lett. 2007, 247, 293–300. [Google Scholar] [CrossRef]
- Arsova-Sarafinovska, Z.; Matevska, N.; Eken, A.; Petrovski, D.; Banev, S.; Dzikova, S.; Georgiev, V.; Sikole, A.; Erdem, O.; Sayal, A.; et al. Glutathione peroxidase 1 (GPX1) genetic polymorphism, erythrocyte GPX activity, and prostate cancer risk. Int. Urol. Nephrol. 2009, 41, 63–70. [Google Scholar] [CrossRef]
- Hansen, R.; Sæbø, M.; Skjelbred, C.F.; Nexø, B.A.; Hagen, P.C.; Bock, G.; Bowitz Lothe, I.M.; Johnson, E.; Aase, S.; Hansteen, I.L.; et al. GPX Pro198Leu and OGG1 Ser326Cys polymorphisms and risk of development of colorectal adenomas and colorectal cancer. Cancer Lett. 2005, 229, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Bənescu, C.; Trifa, A.P.; Voidəzan, S.; Moldovan, V.G.; Macarie, I.; Benedek Lazar, E.; Dima, D.; Duicu, C.; Dobreanu, M. CAT, GPX1, MnSOD, GSTM1, GSTT1, and GSTP1 Genetic polymorphisms in chronic myeloid leukemia: A case-control study. Oxid. Med. Cell. Longev. 2014, 2014, 875861. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, Q.; Qin, C.; Shao, P.; Wu, Y.; Wang, M.; Zhang, Z.; Yin, C. GPx-1 polymorphism (rs1050450) contributes to tumor susceptibility: Evidence from meta-analysis. J. Cancer Res. Clin. Oncol. 2011, 137, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Tian, C.; Zhang, X. GPX1 gene Pro200Leu polymorphism, erythrocyte GPX activity, and cancer risk. Mol. Biol. Rep. 2013, 40, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Qiu, H.; Xu, J.; Mo, J.; Liu, Y.; Gui, Y.; Huang, G.; Zhang, S.; Yao, H.; Huang, X.; et al. Expression and prognostic potential of GPX1 in human cancers based on data mining. Ann. Transl. Med. 2020, 8, 124. [Google Scholar] [CrossRef]
- Gouazé, V.; Mirault, M.E.; Carpentier, S.; Salvayre, R.; Levade, T.; Andrieu-Abadie, N. Glutathione peroxidase-1 overexpression prevents ceramide production and partially inhibits apoptosis in doxorubicin-treated human breast carcinoma cells. Mol. Pharmacol. 2001, 60, 488–496. [Google Scholar]
- Lee, S.; Lee, E.K.; Kang, D.H.; Lee, J.; Hong, S.H.; Jeong, W.; Kang, S.W. Glutathione peroxidase-1 regulates ASK1-dependent apoptosis via interaction with TRAF2 in RIPK3-negative cancer cells. Exp. Mol. Med. 2021, 53, 1080–1091. [Google Scholar] [CrossRef]
- Chen, B.; Shen, Z.; Wu, D.; Xie, X.; Xu, X.; Lv, L.; Dai, H.; Chen, J.; Gan, X. Glutathione peroxidase 1 promotes NSCLC resistance to cisplatin via ROS-induced activation of PI3K/AKT pathway. Biomed. Res. Int. 2019, 2019, 7640547. [Google Scholar] [CrossRef]
- Chaudiere, J.; Wilhelmsen, E.C.; Tappel, A.L. Mechanism of selenium-glutathione peroxidase and its inhibition by mercaptocarboxylic acids and other mercaptans. J. Biol. Chem. 1984, 259, 1043–1050. [Google Scholar] [CrossRef]
- Behnisch-Cornwell, S.; Bandaru, S.S.M.; Napierkowski, M.; Wolff, L.; Zubair, M.; Urbainsky, C.; Lillig, C.; Schulzke, C.; Bednarski, P.J. Pentathiepins: A Novel Class of Glutathione Peroxidase 1 Inhibitors that Induce Oxidative Stress, Loss of Mitochondrial Membrane Potential and Apoptosis in Human Cancer Cells. ChemMedChem 2020, 15, 1515–1528. [Google Scholar] [CrossRef]
- Lange, C.; Bednarski, P.J. In vitro assessment of synergistic effects in combinations of a temoporfin-based photodynamic therapy with glutathione peroxidase 1 inhibitors. Photodiagnosis Photodyn. Ther. 2021, 36, 102478. [Google Scholar] [CrossRef]
- Chu, F.F.; Doroshow, J.H.; Esworthy, R.S. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J. Biol. Chem. 1993, 268, 2571–2576. [Google Scholar] [CrossRef]
- Florian, S.; Wingler, K.; Schmehl, K.; Jacobasch, G.; Kreuzer, O.J.; Meyerhof, W. Cellular and Subcellular Localization of Gastrointestinal Glutathione Peroxidase in Normal and Malignant Human. Intestinal Tissue. Free. Radic. Res. 2001, 35, 655–663. [Google Scholar] [CrossRef]
- Thomas, J.P.; Geiger, P.G.; Maiorino, M.; Ursini, F.; Girotti, A.W. Enzymatic reduction of phospholipid and cholesterol hydroperoxides in artificial bilayers and lipoproteins. Biochim. Biophys. Acta 1990, 1045, 252–260. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Flohé, L.; Toppo, S.; Cozza, G.; Ursini, F. A comparison of thiol peroxidase mechanisms. Antioxid. Redox Signal. 2010, 15, 763–780. [Google Scholar] [CrossRef]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef]
- Lee, K.H.; Jeong, D. Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: The selenium paradox (Review). Mol. Med. Rep. 2012, 5, 299–304. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Enqvist, M.; Nilsonne, G.; Hammarfjord, O.; Wallin, R.P.A.; Björkström, N.K.; Björnstedt, M.; Hjerpe, A.; Ljunggren, H.-G.; Dobra, K.; Malmberg, K.-J.; et al. Selenite Induces Posttranscriptional Blockade of HLA-E Expression and Sensitizes Tumor Cells to CD94/NKG2A-Positive NK Cells. J. Immunol. 2011, 187, 3546–3554. [Google Scholar] [CrossRef]
- Hagemann-Jensen, M.; Uhlenbrock, F.; Kehlet, S.; Andresen, L.; Gabel-Jensen, C.; Ellgaard, L.; Gammelgaard, B.; Skov, S. The selenium metabolite methylselenol regulates the expression of ligands that trigger immune activation through the lymphocyte receptor NKG2D. J. Biol. Chem. 2014, 289, 31576–31590. [Google Scholar] [CrossRef]
- Panel, E.; Nda, A. Scientific Opinion on Dietary Reference Values for selenium. EFSA J. 2014, 12, 1–67. [Google Scholar] [CrossRef]
- Cunzhi, H.; Jiexian, J.; Xianwen, Z.; Jingang, G.; Shumin, Z.; Lili, D.U. Serum and Tissue Levels of Six. Trace Elements and Copper/Zinc Ratio in Patients with Cervical Cancer and Uterine Myoma. Biol. Trace Elem. Res. 2003, 94, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Obhielo, E.; Ezeanochie, M.; Olokor O, O.; Okonkwo, A.; Gharoro, E. The Relationship between the Serum Level of Selenium and Cervical Intraepithelial Neoplasia: A Comparative Study in a Population of Nigerian Women. Asian Pacific J. Cancer Prev. 2019, 20, 1433–1436. [Google Scholar] [CrossRef]
- He, D.; Wang, Z.; Huang, C.; Fang, X.; Chen, D. Serum Selenium Levels and Cervical Cancer: Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2017, 179, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Okunade, K.S.; Dawodu, O.O.; Salako, O.; Osanyin, G.E.; Okunowo, A.A.; Anorlu, R.I. Comparative analysis of serum trace element levels in women with invasive cervical cancer in Lagos, Nigeria. Pan Afr. Med. J. 2018, 31, 194. [Google Scholar] [CrossRef] [PubMed]
- Karamali, M.; Nourgostar, S.; Zamani, A.; Vahedpoor, Z.; Asemi, Z. The favourable effects of long-term selenium supplementation on regression of cervical tissues and metabolic profiles of patients with cervical intraepithelial neoplasia: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2015, 114, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Muecke, R.; Schomburg, L.; Glatzel, M.; Berndt-Skorka, R.; Baaske, D.; Reichl, B.; Buentzel, J.; Kundt, G.; Prott, F.J.; Devries, A.; et al. Multicenter, phase 3 trial comparing selenium supplementation with observation in gynecologic radiation oncology. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Muecke, R.; Micke, O.; Schomburg, L.; Buentzel, J.; Glatzel, M.; Baaske, D.; Berndt-Skorka, R.; Prott, F.J.; Reichl, B.; Kisters, K.; et al. Impact of treatment planning target volumen (PTV) size on radiation induced diarrhoea following selenium supplementation in gynecologic radiation oncology—a subgroup analysis of a multicenter, phase III trial. Radiat. Oncol. 2013, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Janowska, M.; Potocka, N.; Paszek, S.; Skrzypa, M.; Wróbel, A.; Kluz, M.; Baszuk, P.; Marciniak, W.; Gronwald, J.; Lubiński, J.; et al. An Assessment of Serum Selenium Concentration in Women with Endometrial Cancer. Nutrients 2022, 14, 958. [Google Scholar] [CrossRef]
- Kho, P.F.; Glubb, D.M.; Thompson, D.J.; Spurdle, A.B.; O’Mara, T.A. Assessing the role of selenium in endometrial cancer risk: A mendelian randomization study. Front. Oncol. 2019, 9, 182. [Google Scholar] [CrossRef]
- Wadhwa, S.K.; Kazi, T.G.; Afridi, H.I.; Talpur, F.N. Interaction between carcinogenic and anti-carcinogenic trace elements in the scalp hair samples of different types of Pakistani female cancer patients. Clin. Chim. Acta 2015, 439, 178–184. [Google Scholar] [CrossRef]
- Caglayan, A.; Katlan, D.C.; Tuncer, Z.S.; Yüce, K. Evaluation of trace elements associated with antioxidant enzymes in blood of primary epithelial ovarian cancer patients. J. Trace Elem. Med. Biol. 2019, 52, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.D.; Qin, B.; Camacho, F.; Moorman, P.G.; Alberg, A.J.; Barnholtz-Sloan, J.S.; Bondy, M.; Cote, M.L.; Funkhouser, E.; Guertin, K.A.; et al. Supplemental selenium may decrease ovarian cancer risk in African-American women. J. Nutr. 2017, 147, 621–627. [Google Scholar] [CrossRef]
- Dziaman, T.; Huzarski, T.; Gackowski, D.; Rozalski, R.; Siomek, A.; Szpila, A.; Guz, J.; Lubinski, J.; Wasowicz, W.; Roszkowski, K.; et al. Selenium supplementation reduced oxidative DNA damage in adnexectomized BRCA1 mutations carriers. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 2923–2928. [Google Scholar] [CrossRef]
- Sieja, K.; Talerczyk, M. Selenium as an element in the treatment of ovarian cancer in women receiving chemotherapy. Gynecol. Oncol. 2004, 93, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Erikson, K.M.; Aschner, M. Manganese neurotoxicity and glutamate-GABA interaction. Neurochem. Int. 2003, 43, 475–480. [Google Scholar] [CrossRef]
- Keen, C.L.; Ensunsa, J.L.; Watson, M.H.; Baly, D.L.; Donovan, S.M.; Monaco, M.H.; Clegg, M.S. Nutritional aspects of manganese from experimental studies. Neurotoxicology 1999, 20, 213–223. [Google Scholar]
- Tuschl, K.; Clayton, P.T.; Gospe, S.M.; Gulab, S.; Ibrahim, S.; Singhi, P.; Aulakh, R.; Ribeiro, R.T.; Barsottini, O.G.; Zaki, M.S.; et al. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am. J. Hum. Genet. 2012, 90, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zheng, W. Cardiovascular toxicities upon manganese exposure. Cardiovasc. Toxicol. 2005, 5, 345–354. Available online: http://www.cardiotox.com (accessed on 29 May 2023). [CrossRef]
- Frisbie, S.H.; Ortega, R.; Maynard, D.M.; Sarkar, B. Environmental Health Perspectives • VOLUME 110 | NUMBER 11,”. 2002. Available online: http://ehpnet1.niehs.nih.gov/docs/2002/110p1147-1153frisbie/abstract.html (accessed on 29 May 2023).
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Aspects Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- Finley, J.W.; Johnson, P.E.; Johnson, L.K. Sex affects manganese absorption and retention by humans from a diet adequate in manganese. Am. J. Clin. Nutr. 1994, 60, 949–955. Available online: https://academic.oup.com/ajcn/article-abstract/60/6/949/4732139 (accessed on 29 May 2023). [CrossRef] [PubMed]
- Leblondel, G.; Allain, P. Manganese Transport by Caco-2 Cells. Biol. Trace Elem. Res. 1999, 67, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Mena, I.; Marin, O.; Fuenzalida, S.; Cotzias, G.C. Chronic manganese poisoning. Clinical picture and manganese turnover. Neurology 1967, 17, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Lonnerdal, B.; Keen, C.L.; Hurley, L.S. Manganese binding proteins in human and cow’s miIk. Am. J. Clin. Nutr. 1985, 41, 550–559. Available online: https://academic.oup.com/ajcn/article-abstract/41/3/550/4691495 (accessed on 29 May 2023). [CrossRef]
- Davidsson, L.; Cederbiad, A.; Lönnerdal, B.; Sandström, B. The effect of individual dietary components on manganese absorption in humans. Am. J. Clin. Nutr. 1991, 54, 1065–1070. [Google Scholar] [CrossRef]
- Keen, C.L.; Bell, J.G.; Lã-Nnerdal, A. The Effect of Age on Manganese Uptake and Retention from Milk. and Infant Formulas in Rats. J. Nutr. 1986, 116, 395–402. Available online: https://academic.oup.com/jn/article-abstract/116/3/395/4763115 (accessed on 29 May 2023).
- Schroeder, H.A.; Balassa, J.J.; Tipton, I.H.; Schroeder, H.A. Essential Trace Metals in Man: Manganese a Study in Homeostasis. J. Chronic Dis. 1966, 19, 545–571. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Xiong, Z.; Shen, M.; Conti, P.S.; Shi, X.; Chen, K. Polyethyleneimine-Coated Manganese Oxide Nanoparticles for Targeted Tumor PET/MR Imaging. ACS Appl. Mater. Interfaces 2018, 10, 34954–34964. [Google Scholar] [CrossRef]
- Abdurhman, A.A.M.; Zhang, Y.; Zhang, G.; Wang, S. Hierarchical nanostructured noble metal/metal oxide/graphene-coated carbon fiber: In situ electrochemical synthesis and use as microelectrode for real-time molecular detection of cancer cells. Anal. Bioanal. Chem. 2015, 407, 8129–8136. [Google Scholar] [CrossRef]
- Hu, H.; Dai, A.; Sun, J.; Li, X.; Gao, F.; Wu, L.; Fang, Y.; Yang, H.; An, L.; Wu, H.; et al. Aptamer-conjugated Mn3O4@SiO2 core-shell nanoprobes for targeted magnetic resonance imaging. Nanoscale 2013, 5, 10447–10454. [Google Scholar] [CrossRef]
- Panel, E.; Nda, A. Scientific Opinion on Dietary Reference Values for manganese. EFSA J. 2013, 11, 1–44. [Google Scholar] [CrossRef]
- Zhang, C.; Qi, X.; Shi, Y.; Sun, Y.; Li, S.; Gao, X.; Yu, H. Estimation of trace elements in mace (Myristica fragrans Houtt) and their effect on uterine cervix cancer induced by methylcholanthrene. Biol. Trace Elem. Res. 2012, 149, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Shu Chen, S.S. Manganese Deficiency Promotes the Progression of Invasive Adenocarcinoma of the Cervix by Enhancing the Inflammatory Response. Ann. Clin. Lab. Sci. 2022, 52, 269–277. [Google Scholar]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, C.; Li, L.; Han, C.; Jing, J.; Zhu, Q. The relationship of cervical cancer with pathogen infectious, cytokine and Se. Zhonghua Shi Yan He Lin. Chuang Bing. Du. Xue Za Zhi 2002, 16, 179–183. [Google Scholar]
- Qi, L.; Wang, Y.; Su, S.; Wang, M.; Jablonska, E.; Jia, Y.; Wang, R.; Hao, S.; Feng, C.; Li, G.; et al. Sodium selenite inhibits cervical cancer growth via ROS mediated AMPK/FOXO3a /GADD45a axis. Chem. Biol. Interact. 2022, 367, 110171. [Google Scholar] [CrossRef]
- Martínez-Esquivias, F.; Gutiérrez-Angulo, M.; Pérez-Larios, A.; Sánchez-Burgos, J.A.; Becerra-Ruiz, J.S.; Guzmán-Flores, J.M. Anticancer Activity of Selenium Nanoparticles In Vitro Studies. Anticancer. Agents Med. Chem. 2022, 22, 1658–1673. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, D.; Li, S.; Xue, C. Synthesis and cytotoxicity of selenium nanoparticles stabilized by α-D-glucan from Castanea mollissima Blume. Int. J. Biol. Macromol. 2019, 129, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xiao, M.; Zhao, M.; Xu, T.; Guo, M.; Wang, C.; Li, Y.; Zhu, B.; Liu, H. Doxorubicin-loaded functionalized selenium nanoparticles for enhanced antitumor efficacy in cervical carcinoma therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qu, G.; Zhang, C.; Zhang, S.; He, J.; Sang, N.; Liu, S. Quantum dots (QDs) restrain human cervical carcinoma HeLa cell proliferation through inhibition of the ROCK-c-Myc signaling. Integr. Biol. 2013, 5, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Krakowiak, A.; Czernek, L.; Pichlak, M.; Kaczmarek, R. Intracellular HINT1-Assisted Hydrolysis of Nucleoside 5′-O-Selenophosphate Leads to the Release of Hydrogen Selenide That Exhibits Toxic Effects in Human Cervical Cancer Cells. Int. J. Mol. Sci. 2022, 23, 607. [Google Scholar] [CrossRef]
- Zhao, C.; Zeng, H.; Wu, R.T.Y.; Cheng, W.H. Loss of selenium-binding protein 1 decreases sensitivity to clastogens and intracellular selenium content in HeLa cells. PLoS ONE 2016, 11, e0158650. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuan, Z.; Tang, Z.; Chen, Q.; Huang, J.; He, L.; Chen, T. Selenium-driven enhancement of synergistic cancer chemo-/radiotherapy by targeting nanotherapeutics. Biomater. Sci. 2021, 9, 4691–4700. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Fu, Y.; Liu, X.; Zhang, Z.; Wang, J.; Mei, Q.; Wang, X.; Deng, G.; Lua, J.; Hu, J. Tumor microenvironment responsive self-cascade catalysis for synergistic chemo/chemodynamic therapy by multifunctional biomimetic nanozymes. J. Mater. Chem. B 2022, 10, 637–645. [Google Scholar] [CrossRef]
- Xia, Y.; Tang, G.; Wang, C.; Zhong, J.; Chen, Y.; Hua, L.; Li, Y.; Liu, H.; Zhu, B. Functionalized selenium nanoparticles for targeted siRNA delivery silence Derlin1 and promote antitumor efficacy against cervical cancer. Drug Deliv. 2020, 27, 15–25. [Google Scholar] [CrossRef]
- Palazzotti, B.; Pani, G.; Colavitti, R.; De Leo, M.E.; Bedogni, B.; Borrello, S.; Galeotti, T. Increased growth capacity of cervical-carcinoma cells over-expressing manganous superoxide dismutase. Int. J. Cancer 1999, 82, 145–150. [Google Scholar] [CrossRef]
- Tong, S.Y.; Lee, J.M.; Song, E.S.; Lee, K.B.; Kim, M.K.; Lee, J.K.; Son, S.K.; Lee, J.P.; Kim, J.H.; Kwon, Y., II. Functional polymorphism in manganese superoxide dismutase and antioxidant status: Their interactions on the risk of cervical intraepithelial neoplasia and cervical cancer. Gynecol. Oncol. 2009, 115, 272–276. [Google Scholar] [CrossRef]
- Attatippaholkun, W.; Wikainapakul, K. Predominant genotypes and alleles of two functional polymorphisms in the manganese superoxide dismutase gene are not associated with thai cervical or breast cancer. Asian Pac. J. Cancer Prev. 2013, 14, 3955–3961. [Google Scholar] [CrossRef]
- Helena, S.; Santos, R.-; Termini, L.; Boccardo, E.; Derchain, S.; Longatto-Filho, A.; Andreoli, M.A.; Cecília Costa, M.; Almeida, R.; Nunes, L.; et al. Strong SOD2 expression and HPV-16/18 positivity are independent events in cervical cancer. Oncotarget 2018, 9, 21630–21640. [Google Scholar]
- Nakano, T.; Oka, K.; Taniguchi, N. Manganese superoxide dismutase expression correlates with p53 status and local recurrence of cervical carcinoma treated with radiation therapy. Cancer Res. 1996, 56, 2771–2775. [Google Scholar]
- Li, Y.; Zhao, J.; He, C.C.; Zhang, L.; Sun, S.R.; Xu, G.C. Synthesis, crystal structure and biological activity of two Mn complexes with 4-acyl pyrazolone derivatives. J. Inorg. Biochem. 2015, 150, 28–37. [Google Scholar] [CrossRef]
- Narayanan, S.; Dutta, D.; Arora, N.; Sahoo, L.; Ghosh, S.S. Phytaspase-loaded, Mn-doped ZnS quantum dots when embedded into chitosan nanoparticles leads to improved chemotherapy of HeLa cells using in cisplatin. Biotechnol. Lett. 2017, 39, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Q.; Zou, X.; Chen, J.; Hu, L.; Dong, Z.; Zhou, J.; Chen, Y.; Liu, Z.; Cheng, L. Intelligent protein-coated bismuth sulfide and manganese oxide nanocomposites obtained by biomineralization for multimodal imaging-guided enhanced tumor therapy. J. Mater. Chem. B 2019, 7, 5170–5181. [Google Scholar] [CrossRef]
- Fang, J.; Wang, Q.; Yang, G.; Xiao, X.; Li, L.; Yu, T. Albumin-MnO 2 gated hollow mesoporous silica nanosystem for modulating tumor hypoxia and synergetic therapy of cervical carcinoma. Colloids Surf. B Biointerfaces 2019, 179, 250–259. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, L.; Wu, Q.; Yang, E.; Zhang, G.; Sun, H.; Wang, F. A recombinant trans-membrane protein hMnSOD-R9 inhibits the proliferation of cervical cancer cells in vitro. Mol. Cell. Biochem. 2014, 385, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial cancer. Lancet 2022, 399, P1412–P1428. [Google Scholar] [CrossRef]
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D. Endometrial cancer. Nat. Rev. Dis. Prim. 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, K.F.; Karlan, B.Y.; Barbuto, D.A.; Leuchter, R.S.; Lagasse, L.D.; Judd, H.L. Development of endometrial cancer in women on estrogen and progestin hormone replacement therapy. Gynecol. Oncol. 1994, 55, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Dossus, L.; Lukanova, A.; Rinaldi, S.; Allen, N.; Cust, A.E.; Becker, S.; Tjonneland, A.; Hansen, L.; Overvad, K.; Chabbert-Buffet, N.; et al. Hormonal, metabolic, and inflammatory profiles and endometrial cancer risk within the EPIC cohort—A factor analysis. Am. J. Epidemiol. 2013, 177, 787–799. [Google Scholar] [CrossRef]
- Błasiak, J.; Kadłubek, M.; Kowalik, J.; Romanowicz-Makowska, H.; Pertyński, T. Inhibition of Telomerase Activity in Endometrial Cancer Cells by Selenium-Cisplatin Conjugate Despite Suppression of its DNA-Damaging Activity by Sodium Ascorbate. Teratog. Carcinog. Mutagen. 2002, 22, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Cebecioglu, R.; Yildirim, M.; Akagunduz, D.; Korkmaz, I.; Tekin, H.O.; Atasever-Arslan, B.; Catal, T. Synergistic effects of quercetin and selenium on oxidative stress in endometrial adenocarcinoma cells. Bratislava Med. J. 2019, 120, 449–455. [Google Scholar] [CrossRef]
- Shah, Y.M.; Al-Dhaheri, M.; Dong, Y.; Ip, C.; Jones, F.E.; Rowan, B.G. Selenium disrupts estrogen receptors α signaling and potentiates tamoxifen antagonism in endometrial cancer cells and tamoxifen-resistant breast cancer cells. Mol. Cancer Ther. 2005, 4, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Galazis, N.; Pang, Y.L.; Galazi, M.; Haoula, Z.; Layfield, R.; Atiomo, W. Proteomic biomarkers of endometrial cancer risk in women with polycystic ovary syndrome: A systematic review and biomarker database integration. Gynecol. Endocrinol. 2013, 29, 638–644. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, S.; Zhu, J.; Yin, L.; Liu, S.; Huang, X.; Ke, G. The Promotional Effect of Hollow MnO2with Brucea Javanica Oil Emulsion (BJOE) on Endometrial Cancer Apoptosis. Biomed. Res. Int. 2021, 2021, 6631533. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, A.; Schiattarella, A.; Musella, A.; Mancini, R.; Capasso, C.; De Luca, V.; Carginale, V.; Sanseverino, M.; Tornesello, A.L.; Gori, E.; et al. A Molecular Carrier to Transport and Deliver Cisplatin into Endometrial Cancer Cells. Chem. Biol. Drug Des. 2012, 80, 9–16. [Google Scholar] [CrossRef]
- Kujawa, K.A.; Lisowska, K.M. Rak jajnika—Od biologii do kliniki. Postepy Hig. Med. Dosw. 2015, 69, 1275–1290. [Google Scholar] [CrossRef]
- Murphy, S.K. Targeting Ovarian Cancer-Initiating Cells. Anticancer. Agents Med. Chem. 2012, 10, 157–163. [Google Scholar] [CrossRef]
- La Vecchia, C. Ovarian cancer: Epidemiology and risk factors. Eur. J. Cancer Prev. 2017, 26, 55–62. [Google Scholar] [CrossRef]
- Ramus, S.J.; Gayther, S.A. The Contribution of BRCA1 and BRCA2 to Ovarian Cancer. Mol. Oncol. 2009, 3, 138–150. [Google Scholar] [CrossRef]
- Dunneram, Y.; Greenwood, D.C.; Cade, J.E. Diet, menopause and the risk of ovarian, endometrial and breast cancer. Proc. Nutr. Soc. 2019, 78, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Slomovitz, B.; De Haydu, C.; Taub, M.; Coleman, R.L.; Monk, B.J. Asbestos and ovarian cancer: Examining the historical evidence. Int. J. Gynecol. Cancer 2021, 31, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Savant, S.S.; Sriramkumar, S.; O’hagan, H.M. The role of inflammation and inflammatory mediators in the development, progression, metastasis, and chemoresistance of epithelial ovarian cancer. Cancers 2018, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Brinton, L.A.; Lamb, E.J.; Moghissi, K.S.; Scoccia, B.; Althuis, M.D.; Mabie, J.E.; Westhoff, C.L. Ovarian cancer risk associated with varying causes of infertility. Fertil. Steril. 2004, 82, 405–414. [Google Scholar] [CrossRef]
- Jensen, A.; Sharif, H.; Olsen, J.H.; Kjær, S.K. Risk of breast cancer and gynecologic cancers in a large population of nearly 50,000 infertile Danish women. Am. J. Epidemiol. 2008, 168, 49–57. [Google Scholar] [CrossRef]
- Prowse, A.H.; Manek, S.; Varma, R.; Liu, J.; Godwin, A.K.; Maher, E.R.; Tomlinson, I.P.M.; Kennedy, S.H. Molecular genetic evidence that endometriosis is a precursor of ovarian cancer. Int. J. Cancer 2006, 119, 556–562. [Google Scholar] [CrossRef]
- Munksgaard, P.S.; Blaakaer, J. The association between endometriosis and ovarian cancer: A review of histological, genetic and molecular alterations. Gynecol. Oncol. 2012, 124, 164–169. [Google Scholar] [CrossRef]
- Lee, A.W.; Templeman, C.; Stram, D.A.; Beesley, J.; Tyrer, J.; Berchuck, A.; Pharoah, P.P.; Chenevix-Trench, G.; Pearce, C.L.; Ness, R.B.; et al. Evidence of a genetic link between endometriosis and ovarian cancer. Fertil. Steril. 2016, 105, 35–43. [Google Scholar] [CrossRef]
- Yu-Rice, Y.; Edassery, S.L.; Urban, N.; Hellstrom, I.; Hellstrom, K.E.; Deng, Y.; Li, Y.; Luborsky, J.L. Selenium-Binding Protein 1 (SBP1) autoantibodies in ovarian disorders and ovarian cancer. Reproduction 2017, 153, 277–284. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.E.; Zhang, P.; Liu, F.; Sung, C.J.; Steinhoff, M.M.; Quddus, M.R.; Lawrence, W.D. Progressive loss of selenium-binding protein 1 expression correlates with increasing epithelial proliferation and papillary complexity in ovarian serous borderline tumor and low-grade serous carcinoma. Hum. Pathol. 2010, 41, 255–261. [Google Scholar] [CrossRef]
- Sanmartín, C.; Plano, D.; Palop, J.A. Selenium Compounds and Apoptotic Modulation: A New. Perspective in Cancer Therapy. Mini Rev. Med. Chem. 2008, 8, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.; Rådestad, E.; Khalkar, P.; Diaz-Argelich, N.; Schröder, A.; Klynning, C.; Ungerstedt, J.; Uhlin, M.; Fernandes, A.P. Methylseleninic acid sensitizes ovarian cancer cells to T-cell mediated killing by decreasing PDL1 and VEGF levels. Front. Oncol. 2018, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, P.B.; Frenkel, G.D. Prevention of Carboplatin-induced Resistance inHuman Ovarian Tumor Xenografts by Selenite. Anticancer Res. 2013, 33, 4249–4254. [Google Scholar]
- Frenkel, G.D.; Caffrey, P.B. A Prevention Strategy for Circumventing Drug Resistance in Cancer Chemotherapy. Curr. Pharm. Des. 2001, 7, 1595–1614. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, P.B.; Frenkel, G.D. Selenium compounds prevent the induction of drug resistance by cisplatin in human ovarian tumor xenografts in vivo. Cancer Chemother. Pharmacol. 2000, 46, 74–78. [Google Scholar] [CrossRef]
- Song, M.; Kumaran, M.N.; Gounder, M.; Gibbon, D.G.; Nieves-Neira, W.; Vaidya, A.; Hellmann, M.; Kane, M.P.; Buckley, B.; Shih, W.; et al. Phase I trial of selenium plus chemotherapy in gynecologic cancers. Gynecol. Oncol. 2018, 150, 478–486. [Google Scholar] [CrossRef]
- Raza, A.; Singh, A.; Amin, S.; Spallholz, J.E.; Sharma, A.K. Identification and biotin receptor-mediated activity of a novel seleno-biotin compound that inhibits viability of and induces apoptosis in ovarian cancer cells. Chem. Biol. Interact. 2022, 365, 110071. [Google Scholar] [CrossRef]
- Toubhans, B.; Gazze, S.A.; Bissardon, C.; Bohic, S.; Gourlan, A.T.; Gonzalez, D.; Charlet, L.; Conlan, R.S.; Francis, L.W. Selenium nanoparticles trigger alterations in ovarian cancer cell biomechanics. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102258. [Google Scholar] [CrossRef]
- Nasrolahi Shirazi, A.; Tiwari, R.K.; Oh, D.; Sullivan, B.; Kumar, A.; Beni, Y.A.; Parang, K. Cyclic peptide-selenium nanoparticles as drug transporters. Mol. Pharm. 2014, 11, 3631–3641. [Google Scholar] [CrossRef]
- Wang, C.; Xia, Y.; Huo, S.; Shou, D.; Mei, Q.; Tang, W.; Li, Y.; Liu, H.; Zhou, Y.; Zhu, B. Silencing of mef2d by sirna loaded selenium nanoparticles for ovarian cancer therapy. Int. J. Nanomed. 2020, 15, 9759–9770. [Google Scholar] [CrossRef]
- Santos, I.; Ramos, C.; Mendes, C.; Sequeira, C.O.; Tomé, C.S.; Fernandes, D.G.H.; Mota, P.; Pires, R.F.; Urso, D.; Hipólito, A.; et al. Targeting glutathione and cystathionine β-synthase in ovarian cancer treatment by selenium–chrysin polyurea dendrimer nanoformulation. Nutrients 2019, 11, 2523. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.L.; Charneira, C.; Gandin, V.; Ferreira Da Silva, J.L.; Justino, G.C.; Telo, J.P.; Vieira, A.J.S.C.; Marzano, C.; Antunes, A.M.M. Selenium-containing chrysin and quercetin derivatives: Attractive scaffolds for cancer therapy. J. Med. Chem. 2015, 58, 4250–4265. [Google Scholar] [CrossRef] [PubMed]
- Palakurthi, S.; Yellepeddi, V.K.; Vangara, K.K. Recent trends in cancer drug resistance reversal strategies using nanoparticles. Expert Opin. Drug Deliv. 2012, 9, 287–301. [Google Scholar] [CrossRef]
- Huang, S.; Sheng, X.; Bian, M.; Yang, Z.; Lu, Y.; Liu, W. Synthesis and in vitro anticancer activities of selenium N-heterocyclic carbene compounds. Chem. Biol. Drug. Des. 2021, 98, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Koketsu, M.; Yang, E.M.; Kim, Y.M.; Ishihara, H.; Yang, H.O. 2-(4-Methylphenyl)-1,3-selenazol-4-one induces apoptosis by different mechanisms in SKOV3 and HL 60 cells. J. Cell. Biochem. 2006, 99, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Gewandter, J.S.; Freeman, R.; Kitt, R.A.; Cavaletti, G.; Gauthier, L.R.; McDermott, M.P.; Mohile, N.A.; Mohlie, S.G.; Smith, A.G.; Tejani, M.A.; et al. Chemotherapy-induced peripheral neuropathy clinical trials. Neurology 2017, 89, 859–869. [Google Scholar] [CrossRef]
- Park, S.J.; Yim, G.W.; Paik, H.; Lee, N.; Lee, S.; Lee, M.; Kim, H.S. Efficacy and safety of intravenous administration of high-dose selenium for preventing chemotherapy-induced peripheral neuropathy in platinum-sensitive recurrent ovarian, fallopian or primary peritoneal cancer: Study protocol for a phase III, double-blind, randomized study. J. Ginecol. Oncol. 2021, 32, e73. [Google Scholar]
- Murdoch, W.J. Carcinogenic potential of ovulatory genotoxicity. Biol. Reprod. 2005, 73, 586–590. [Google Scholar] [CrossRef]
- Bag, A.; Bag, N. Human manganese superoxide dismutase target sequence polymorphism and ovarian cancer. Ann. Med. Health Sci. Res. 2014, 4, 69. [Google Scholar] [CrossRef]
- Hu, Y.; Rosen, D.G.; Zhou, Y.; Feng, L.; Yang, G.; Liu, J.; Huang, P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: Role in cell proliferation and response to oxidative stress. J. Biol. Chem. 2005, 280, 39485–39492. [Google Scholar] [CrossRef]
- Caglayan, A.; Katlan, D.C.; Selçuk Tuncer, Z.; Yüce, K.; Sayal, H.B.; Coşkun Salman, M.; Kocer-Gumusel, B. Impaired antioxidant enzyme functions with increased lipid peroxidation in epithelial ovarian cancer. IUBMB Life 2017, 69, 802–813. [Google Scholar] [CrossRef]
- Kim, Y.S.; Gupta Vallur, P.; Jones, V.M.; Worley, B.L.; Shimko, S.; Shin, D.H.; Crawford, L.T.C.; Chen, C.W.; Aird, K.M.; Abraham, T.; et al. Context-dependent activation of SIRT3 is necessary for anchorage-independent survival and metastasis of ovarian cancer cells. Oncogene 2020, 39, 1619–1633. [Google Scholar] [CrossRef]
- Hasegawa, S.; Saito, S.; Koshikawa-Yano, M.; Furukawa, T.; Aoki, I.; Saga, T. Tumor Enhancement EŠect of Overexpressed Manganese-superoxide Dismutase in Manganese-enhanced MR Imaging. Magn. Reson. Med. Sci. 2011, 10, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Xie, T.; Wang, K.; Jin, S.; Li, K.; Dou, P.; Yu, N.; Xu, K. Development of fluorescence/MR dual-modal manganese-nitrogen-doped carbon nanosheets as an efficient contrast agent for targeted ovarian carcinoma imaging. J. Nanobiotechnol. 2020, 18, 175. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, X.; Zhao, B.; Zhang, R.; Xie, Z.; He, Y.; Chen, A.; Xie, X.; Yao, K.; Zhong, M.; et al. CuS-MnS2 nano-flowers for magnetic resonance imaging guided photothermal/photodynamic therapy of ovarian cancer through necroptosis. Nanoscale 2019, 11, 12983–12989. [Google Scholar] [CrossRef] [PubMed]
- Yeung, B.H.Y.; Wong, K.Y.; Lin, M.C.; Wong, C.K.C.; Mashima, T.; Tsuruo, T.; Wong, A.S.T. Chemosensitisation by manganese superoxide dismutase inhibition is caspase-9 dependent and involves extracellular signal-regulated kinase 1/2. Br. J. Cancer 2008, 99, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shu, J.; Chen, L.; Chen, X.; Zhao, J.; Li, S.; Mou, X.; Tong, X. Synergistic suppression effect on tumor growth of ovarian cancer by combining cisplatin with a manganese superoxide dismutase-armed oncolytic adenovirus. Onco. Targets Ther. 2016, 9, 6381–6388. [Google Scholar] [CrossRef]
- Guo, T.; Zhu, Y.; Yue, M.; Wang, F.; Li, Z.; Lin, M. The Therapeutic Effects of DDP/CD44-shRNA Nanoliposomes in AMF on Ovarian Cancer. Front. Oncol. 2022, 12, 811783. [Google Scholar] [CrossRef]
- Wang, H.; Jia, D.; Yuan, D.; Yin, X.; Yuan, F.; Wang, F.; Shi, W.; Li, H.; Zhu, L.M.; Fan, Q. Dimeric Her2-specific affibody mediated cisplatin-loaded nanoparticles for tumor enhanced chemo-radiotherapy. J. Nanobiotechnol. 2021, 19, 138. [Google Scholar] [CrossRef]
- Lenis-Rojas, O.A.; Carvalho, B.; Cabral, R.; Silva, M.; Friães, S.; Roma-Rodrigues, C.; Meireles, M.S.H.; Gomes, C.S.B.; Fernández, J.A.A.; Vila, S.F.; et al. Manganese(I) tricarbonyl complexes as potential anticancer agents. J. Biol. Inorg. Chem. 2022, 27, 49–64. [Google Scholar] [CrossRef]
- Slator, C.; Molphy, Z.; McKee, V.; Kellett, A. Triggering autophagic cell death with a di-manganese(II) developmental therapeutic. Redox Biol. 2017, 12, 150–161. [Google Scholar] [CrossRef] [PubMed]

| Function | |
|---|---|
| Glutathione peroxidase | |
| GPx1 | Antioxidative defence |
| GPx2 | Protection against lipid peroxides resulting from lipid peroxidation |
| GPx3 | Reduction of peroxides in plasma and other body fluids |
| GPx4 | Reduce H2O2 and small hydroperoxides in complex lipids such as phospholipid, cholesterol and cholesterolester hydroperoxides |
| Iodothyronine deiodinase | |
| DIO1 | Conversion T4 to T3 |
| DIO2 | Local production (intracellular) of T3 from T4 |
| DIO3 | Production of rT3 from T4, and T2 from T3 |
| Thioredoxin reductases | |
| TrxR1 | Main antioxidant “weapon” at the cellular level |
| TrxR2 | Regulates cell proliferation |
| Selenoprotein | |
| Selenoprotein W | Antioxidant role; maintaining homeostasis of Ca2+ in neurons; control of expression of genes responsible for synthesis of glutathione de novo |
| Selenoprotein H | Antioxidant role; maintaining homeostasis of Ca2+ in neurons; control of expression of genes responsible for synthesis of glutathione de novo |
| Selenoprotein T | Antioxidant role; maintaining homeostasis of Ca2+ in neurons; control of expression of genes responsible for synthesis of glutathione de novo |
| Selenoprotein P | Selenium transporter; cooperates with the ApoER2 receptor in the transport of selenium to the brain; control of the redox potential in the cell |
| Selenoprotein M | Reduce isomerisation of disulphide bridges; protection of neurons against oxidative stress |
| Selenoprotein N | Degradation of H2O2; involved in the development of muscle tissue at an early stage of development organism; regeneration of skeletal muscle tissue |
| Selenoprotein K | Building protein-protein complexes; degradation of misfolded proteins on the endoplasmic reticulum (ERAD machinery); regulate the anti-inflammatory properties of selenium and its importance in the immune response |
| Selenoprotein S | Building protein-protein complexes; degradation of misfolded proteins on the endoplasmic reticulum (ERAD machinery); regulate the anti-inflammatory properties of selenium and its importance in the immune response |
| Selenoprotein V | Testes-specific expression |
| Selenoprotein I | Function unknown |
| Selenoprotein O | Function unknown |
| Cancer | Selenium Form | Result | Reference |
|---|---|---|---|
| Cervical cancer | Sodium selenite (SS) | SS is involved in inducing cell cycle arrest and enhancing cell apoptosis caused by ROS-dependent activation of the AMPK/FOXO3a/GADD45a axis. | Qi et al. [100] |
| Cervical cancer | Water-soluble and dispersed selenium nanoparticles (CPA-SeNPs) | The CPA-SeNPs can induce cell apoptosis HeLa cell apoptosis via an intrinsic pathway. | Li et al. [102] |
| Cervical cancer | Selenium nanoparticles HA-Se@DOX | HA-Se@DOX exhibits more potent anti-tumor activity than free DOX and Se@DOX in vitro and in vivo. | Xia et al. [103] |
| Cervical cancer | CdSe quantum dots (QDs) | QD treatment limits the growth of HeLa cells by reducing the ability of c-Myc to drive cell proliferation and decreasing levels of HSPC111, which is involved in regulating cell growth. | Chen et al. [104] |
| Cervical cancer | Selenium derivative, 2′-deoxyguanosine-5′-O-selenophosphate (dGMPSe) | It causes the release of H2Se in HeLa cells, but requires a different metabolic pathway involving the enzyme HINT1 (histine nucleotide triad-binding protein1) for conversion to H2Se. | Krakowiak et al. [105] |
| Cervical cancer | Selenomethionine | The treatment of HeLa cer-vical cancer cells with selenomethionine results in increased expression of SBP1 protein. | Zhao et al. [106] |
| Cervical cancer | SeD@MSNs-FA nanosystem | The compound is able to target cervical cancer cells and enhance their radiosensitization in vivo and in vitro. This creates the possibility of simul-taneous treatment with chemotherapy and radiotherapy for cervical cancer. | Liu et al. [107] |
| Cervical cancer | Multifunctional Se@SiO2—Mn@Au/DOX biomimetic nanozyme (named SSMA/DOX) | It undergoes self-destruction catalysis responding to the tumour microenvironment, thereby improving anti-cancer therapy. | Zheng et al. [108] |
| Cervical cancer | RGDfC-Se @ siRNA | It silences genes in HeLa cells, inhibits HeLa cell invasion, migration and proliferation, induces HeLa cell apoptosis and induces mitochondrial membrane potential disruption and increases reactive oxygen species (ROS) production in HeLa cells. | Xia et al. [109] |
| Endometrial Cancer | Selenium and cisplatin conjugate (NH3)2Pt(SeO3) | It inhibits telomerase in tumour cells obtained from endometrial tumours. | Blasiak et al. [124] |
| Endometrial Cancer | Combination of quercetin and sodium selenite | It increases cell viability, reduces malondialdehyde (MDA) levels, decreases BAD and p53 gene expression and has synergistic effects in terms of gene expression | Cebecioglu et al. [125] |
| Endometrial Cancer | Methylselenic acid (MSA) | The combination of 4-hydroxytamoxifen with MSA results in even stronger growth inhibition of tamoxifen-resistant breast cancer cell lines MCF-7-LCC2 and MCF7-H2Delta16, as well as endometrial-derived HEC1A and Ishikawa cells. | Shah et al. [126] |
| Ovarian Cancer | Methylselenic acid | It may sensitise ovarian cancer cells to destruction by T cells, which reduce PDL1 and VEGF levels. | Nair et al. [145] |
| Ovarian Cancer | Selenite | It may prevent the induction of resistance to cisplatin or carboplatin. | Caffrey et al. [146] |
| Ovarian Cancer | Selenobiotin analogs containing a selenocyanate group | They reduce ovarian cancer cell viability and induce apoptosis in a dose-dependent manner. | Raza et al. [150] |
| Ovarian Cancer | Inorganic selenium nanoparticles (SeNPs) | They inhibit the growth of SKOV-3 and OVCAR-3 ovarian cancer cells. | Toubhans et al. [151] |
| Ovarian Cancer | Cyclic peptide-selenium nanoparticles | They can be effective transporters of drugs, such as paclitaxel, in the treatment of ovarian cancer. | Shirazi et al. [152] |
| Ovarian Cancer | R-Se@MEF2D-siRNA | It effectively silences MEF2D gene expression in SKOV3 cells, triggers their apoptosis, has the ability to disrupt mitochondrial membrane potential (MMP) in SKOV3 cells, and causes overproduction of reactive oxygen species (ROS). | Wang et al. [153] |
| Ovarian Cancer | Encapsulated formulation of SeChry (SeChry@PURE G4-FA) | It delivers SeChry to ovarian cancer cells (ES2, OVCAR3 and OVCAR8), reducing toxicity in non-cancerous cells. | Palakurthi et al. [156] |
| Ovarian Cancer | Selenium N-heterocyclic carbene (Se-NHC) | The most active compound 2b has twice the cytotoxicity against A2780 cells than against normal ovarian epithelial IOSE80 cells. | Huang et al. [157] |
| Ovarian Cancer | Synthetic selenium compound, 2-(4-methylphenyl)-1,3-selenazol-4-one | It can also be used to induce apoptosis in a human ovarian cancer cell line (SKOV3) through translocation of AIF, a novel proapoptotic protein [158]. | Ahn et al. [158] |
| Cancer | Manganese Form | Result | Reference |
|---|---|---|---|
| Cervical cancer | Mn complexes [Mn(HLa)(La)]-(CH3CN)1,5-H2O and [Mn2(Lb)2(μ -EtO)2(EtOH)2] | They have DNA and protein binding capacity and anti-cancer activity. They inhibit the growth of Eca-109 oesophageal cancer cells and HeLa cervical cancer cells. | Li et al. [115] |
| Cervical cancer | Phytaspase-loaded, Mn-doped ZnS quantum dots embedded in chitosan nanoparticles | In cisplatin chemotherapy, HeLa cells can increase treatment efficacy. | Narayanan et al. [116] |
| Cervical cancer | Protein-coated nanocomposites of bismuth sulfide and manga-nese oxide (Bi2S3-MnO2) | They can be used to modulate hypoxic TME and increase the efficacy of RT in cervical cancer. | Zhang et al. [117] |
| Cervical cancer | Manganese dioxide nanoparticles integrated with bovine serum albumin (BSA-MnO2) and anchored on the surface of doxorubicin (DOX) and chlorine e6 photo-sensitizer (Ce6) co-loaded with hollow mesoporous silica nanospheres (BSA-MnO2@HMSNs-DOX-Ce6, BMHDC) | BSA-MnO2 prevents premature charge release, and is an oxygen generator, through the breakdown of endogenous H2O2, which effectively deals with tumor resistance to photodynamic therapy (PDT) associated with hypoxia. | Fang et al. [118] |
| Cervical cancer | hMnSOD-R9, a human manganese superoxide dismutase (hMnSOD) and arginine nonamer (R9) | He compound induces apoptosis of HeLa cells through up-regulation of cleaved caspase-3 and down-regulation of the phospho-STAT3 pathway in a dose-dependent manner. It causes an increase in the expression of Bax, JNK, TBK1 genes and a progressive decrease in STAT3 a gene expression in HeLa cells. | Zhang et al. [119] |
| Endometrial Cancer | Highly monodisperse hollow MnO2 with a mesoporous envelope | The combination of H-MnO2—PEG/BJOE (Brucea javanica oil emulsion) shows the killing effect of BJOE on cancer cells and tumor inhibition. | Hu et al. [128] |
| Endometrial Cancer | The leader peptide of recombinant manganese superoxide dismutase (rMnSOD-Lp) | It can be used as a molecular carrier to transport cisplatin directly to cancer cells, as it delivers approximately four times more cisplatin to HTB-112 cells. | Borrelli et al. [129] |
| Ovarian Cancer | Small interfering RNA (siRNA) on MnSOD | It can sensitise ovarian cancer cells to doxorubicin and paclitaxel. | Yeung et al. [169] |
| Ovarian Cancer | ZD55-manganese superoxide dismutase (MnSOD) | It can sensitise human ovarian cancer cells to cisplatin-induced apoptosis. | Wang et al. [170] |
| Ovarian Cancer | The combi-nation of zinc-manganese ferrite nanoparticles (PEG-MZF-NP) with CD44-shRNA, DDP (cisplatin) and magnetic fluid hyperthermia (MFH) | Promising alternative treatment for ovarian cancer. | Guo et al. [171] |
| Ovarian Cancer | Mesoporous polydopamine/MnO2/polydopamine nanopar-ticles (Pt@mPDA/MnO2/PDA-Z Her2) loaded with cisplatin | Promising alternative treatment for ovarian cancer. | Wang et al. [172] |
| Ovarian Cancer | Di-manganese(II) therapeutic [Mn2(μ-oda)(phen)4(H2O)2][Mn2(μ-oda)(phen)4(ode)2]- 4H2O (Mn-Oda) | It induces autophagy-promoted apopto-sis in ovarian cancer cells (SKOV3). | Slator et al. [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golara, A.; Kozłowski, M.; Guzik, P.; Kwiatkowski, S.; Cymbaluk-Płoska, A. The Role of Selenium and Manganese in the Formation, Diagnosis and Treatment of Cervical, Endometrial and Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 10887. https://doi.org/10.3390/ijms241310887
Golara A, Kozłowski M, Guzik P, Kwiatkowski S, Cymbaluk-Płoska A. The Role of Selenium and Manganese in the Formation, Diagnosis and Treatment of Cervical, Endometrial and Ovarian Cancer. International Journal of Molecular Sciences. 2023; 24(13):10887. https://doi.org/10.3390/ijms241310887
Chicago/Turabian StyleGolara, Anna, Mateusz Kozłowski, Paweł Guzik, Sebastian Kwiatkowski, and Aneta Cymbaluk-Płoska. 2023. "The Role of Selenium and Manganese in the Formation, Diagnosis and Treatment of Cervical, Endometrial and Ovarian Cancer" International Journal of Molecular Sciences 24, no. 13: 10887. https://doi.org/10.3390/ijms241310887
APA StyleGolara, A., Kozłowski, M., Guzik, P., Kwiatkowski, S., & Cymbaluk-Płoska, A. (2023). The Role of Selenium and Manganese in the Formation, Diagnosis and Treatment of Cervical, Endometrial and Ovarian Cancer. International Journal of Molecular Sciences, 24(13), 10887. https://doi.org/10.3390/ijms241310887





