Abstract
This review article offers an outlook on the use of opioids as therapeutics for treating several diseases, including cancer and non-cancer pain, and focuses the analysis on the opportunity to target opioid receptors for treating opioid use disorder (OUD), drug withdrawal, and addiction. Unfortunately, as has been well established, the use of opioids presents a plethora of side effects, such as tolerance and physical and physiological dependence. Accordingly, considering the great pharmacological potential in targeting opioid receptors, the identification of opioid receptor ligands devoid of most of the adverse effects exhibited by current therapeutic agents is highly necessary. To this end, herein, we analyze some interesting molecules that could potentially be useful for treating OUD, with an in-depth analysis regarding in vivo studies and clinical trials.
1. Introduction
Considering the last update provided in January 2023, opioid addiction and opioid use disorder (OUD) represent a global epidemic [1]. OUD is defined by physical and/or psychological reliance on legal (prescribed drugs such as oxymorphone or hydrocodone) and illegal opioids (heroin or fentanyl, and the derivatives from the opium resin obtained from Papaver somniferum) [2,3]. It has been established that in the U.S., about three million citizens have had or currently suffer from OUD, while worldwide, about 16 million individuals suffer from this disorder [1,4]. The diagnosis of OUD is based on the guidelines presented in the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5) [5,6], in which it has been reported that OUD is defined as a “problematic pattern of opioid use leading to clinically significant impairment or distress” [2]. Opioid medications are employed for the treatment of moderate-to-severe chronic cancer and non-cancer pain [7,8,9,10,11,12], although some opioids can be used for treating diarrhea [13,14,15] and cough [16,17]. Moreover, opioids can be used by patients suffering from chronic backaches [18,19] and headaches [20,21], by people recovering from surgery [22,23,24], and by both adults and children who have suffered serious injuries in falls, while playing sports, or in auto accidents, etc. [25]. Finally, opioids can be prescribed as palliative care or for limiting the suffering of patients at the end of life [26,27,28]. Despite the possible therapeutic role of opioid medications, the treatment of the above-mentioned diseases with opioids is still under debate for several reasons. In fact, in long-term therapy, the well-established phenomenon of psychological addiction occurs [29,30,31]. Furthermore, as these drugs become more widely available, there are increasing problems of abuse and diversion, which undermine the clinical efficacy of these therapeutic agents and pose a public health concern. Last but not least, the fact that these powerful analgesic agents are linked to significant adverse effects and problems has an impact on the use of opioid derivatives for treating chronic pain [29]. Accordingly, the main adverse effects of opioid prescription treatment include drowsiness, constipation, vomiting, dizziness, nausea, respiratory depression, tolerance, and, as mentioned, physical dependence. Delays in stomach emptying, hyperalgesia, hormonal and immunological alterations, muscular rigidity, and myoclonus are some less frequently observed adverse effects. Constipation and nausea are the side effects of opioid use that are most frequently reported and extremely difficult to manage [29,32,33,34,35,36]. The mentioned undesired effects could be so severe as to require the interruption of opioid use, which also increases the risk of underdosing and insufficient analgesia [37]. In particular, physical dependence and addiction are the main and most severe clinical issues that may prevent proper prescribing, and in turn, may cause insufficient pain management; they represent the most prominent outcomes of OUD [38]. In fact, people who become dependent on an opioid prescription and quit taking it may experience significant withdrawal symptoms following the last dose. For these reasons, many people encounter severe difficulties in stopping opioid use, either legally prescribed or illicit, and it can be incredibly painful (there may be severe pain in the muscles and bones, diarrhea and vomiting, cold flashes with goosebumps, involuntary movement of legs, severe problems in sleeping, severe craving, and compulsive drug-seeking behavior) [39,40,41]. Together, pain and the use of opioids have enormous societal costs. It has been estimated that pain-related healthcare and lost productivity have together cost more than $635 billion, while opioid abuse-related healthcare, criminal justice, reduced quality of life, and life lost due to overdose cost more than $1.03 trillion per year [42,43,44]. Accordingly, the next generation of opioid therapeutics is highly necessary in order to maintain the known efficacy of opioids, but engender a dramatic reduction in side effects [45]. In this review article, we analyze the main advances in the discovery of novel opioid agents, and how they can be useful in replacing the existing opioid agents, in order to treat OUD.
2. Opioid Receptor Functioning and Common Approved Drugs for Treating OUD
Opioids exert their pharmacological effects by targeting opioid receptors. These transmembrane proteins are members of the superfamily of class A G-protein-coupled receptors, and they are responsive toward both endogenous (such as enkephalins, dynorphins, endomorphins and endorphins) and exogenous opioids. Opioid receptors, which are found in the central nervous system and peripheral tissues, are divided into three main classes: mu (μ), kappa (κ) and delta (δ), with different subtypes (Table 1). Moreover, another member of this class, opioid receptor-like 1 (ORL1), has been identified, but there is debate about its inclusion in the opioid receptor family [46,47,48,49,50,51]. These seven-transmembrane helix group members are coupled to inhibitory Gi/o proteins. G protein (Gαi and Gβγ) signaling pathways, mitogen-activated protein kinase (MAPK), and arrestin signaling pathways are used by these receptors to transduce extracellular information [52,53,54]. In response to the significant agonist activation provided by endogenous or exogenous opioids, Gα and Gβγ subunits separate from one another to affect a number of intracellular pathways. In particular, opioid receptor activation can lead to (i) the inhibition of adenylyl cyclase, which leads to a depletion of cyclic adenosine monophosphate (cAMP); (ii) the modulation of the calcium and potassium ion channels, which modifies the concentration outward shift of K+, thus reducing intracellular influx of Ca2+; and (iii) an altered gene expression [53,55,56,57]. Considering that differential expression of opioid receptors and related endogenous ligands occurs throughout the brain, their activation could culminate in different effects [58]. For instance, the activation of opioid receptors located in the presynaptic terminals of nociceptive fibers prevents the release of the neurotransmitters implicated in pain transduction, such as glutamate, substance P, and calcitonin gene-related peptide [57]. Likewise, the activation of opioid receptors on GABAergic neurons inhibits γ-aminobutyric acid (GABA) release in the ventral tegmental area, and this event can allow dopaminergic neurons to release more dopamine into the nucleus accumbens [40,57]. Accordingly, since opioid receptors are widely expressed, they are implicated in a variety of physiological and behavioral functions, such as nociception, fear learning, social memory, drug reward and consumptive behavior, immune activation, stress and emotion, and a number of physiological functions, including gastrointestinal tract motility and respiration (Table 1) [59,60,61,62,63].

Table 1.
Opioid receptor types and their main roles in physiological and non-physiological functions.
As previously mentioned, tolerance and dependence are undesired complications related to the use of legal and illegal opioids. Morphine and other opioids generate higher tolerance and dependence than any other class of therapeutic agents when used repeatedly. As a result of tolerance, higher opioid doses are necessary to provide some effects. Once the level of tolerance is high, the maximum response to the opioid is similarly diminished. The primary cause of tolerance is the functional desensitization of opioid receptors caused by their functional dissociation from specific G-proteins, which uncouple the receptors from their effector components [64,65]. In particular, specific kinases have the ability to phosphorylate opioid receptors after activation, which causes G-protein disconnection, with the consequent binding of β-arrestin. Desensitized receptors are made inactive at the plasma membrane via β-arrestin pathway signaling, which also makes it easier for them to be endocytosed and subsequently degraded or recycled. As a result, these cellular pathways play a crucial role in promoting tolerance development at various levels [66,67,68].
Although the terms dependence and tolerance are frequently considered synonyms, they indicate separate phenomena. For example, if opioid drug consumption is stopped, or an opioid receptor antagonist such as naloxone (Figure 1) is administered, dependence is canceled. The result is a withdrawal or abstinence reaction. The withdrawal response is an extremely intricate process involving numerous areas of the brain. In humans, buprenorphine-precipitated withdrawal can be observed after a single dose of morphine, because dependence develops much more quickly than tolerance. The central noradrenergic cell group known as the locus ceruleus, which is thought to play a significant role in opioid withdrawal, shows enhanced adenylate cyclase activity after chronic morphine therapy. This finding supports the long-standing theory that adenylate cyclase is involved in opioid withdrawal. However, the exact mechanism of its involvement is still unclear [69]. Furthermore, dysregulation of other neurotransmitters has been reported to be crucial in opioid withdrawal symptoms. In particular, the brain ceases making dopamine on its own after prolonged opioid usage, and becomes dependent on opioids for these effects. After becoming addicted to opioids, people who quit using them or are prescribed opioid receptor antagonists (such as naloxone) suddenly experience symptoms such as anxiety and depression, because their brains create less dopamine [69]. The administration of naloxone or another opioid antagonist is correlated with an increment in norepinephrine and dopamine release, and triggers systemic withdrawal symptoms [70,71]. Moreover, it has been demonstrated that serotonin levels also increase during opioid withdrawal [72]. Finally, other neurotransmitters, such as glutamate, are involved in opioid addiction and withdrawal. This neurotransmitter, acting on its receptors, can influence the release of dopamine in opioid addiction and withdrawal. Accordingly, the activation of the glutamatergic system in particular brain areas (i.e., nucleus accumbens) during morphine withdrawal may be crucial regarding negative effects [73]. Acute opioid withdrawal causes stress and activates the hypothalamic–pituitary–adrenal (HPA) axis, increasing the release of adrenal cortisol, adrenocorticotropic hormone (ACTH), and pituitary pro-opiomelanocortin mRNA [74,75,76].
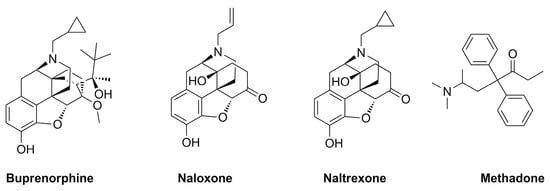
Figure 1.
Chemical structures of the main drugs used in opioid replacement therapy (ORT).
Dependence is one of the main hallmarks of OUD that is characterized by physical and physiological dependence on prescription and/or illicit opioids. Most patients suffering from OUD and individuals with abstinence induced by medically supervised withdrawal may require long-term care to avoid relapse. The most common first-line treatment for people with OUD consists of the administration of an opioid receptor agonist or antagonist, as well as adjuvant psychosocial counseling. Buprenorphine, methadone, and naltrexone (Figure 1) are FDA-approved drugs for treating OUD, whereas naloxone is not considered a therapeutic agent for the treatment of OUD per se, being approved by the FDA for the diagnosis or treatment of the respiratory depressive symptoms of opioid use that can lead to fatal opioid overdose. Furthermore, when promptly delivered, this medication has been shown to decrease the morbidity and mortality related to opioid overdoses [77].
Buprenorphine is a ligand of opioid receptors with relevant affinity. It behaves as an antagonist of the δ- and κ-opioid receptors, and as a partial agonist of the µ-opioid receptor and opioid receptor-like 1 (Table 1). Buprenorphine is just a partial agonist, so it cannot completely replace other opioids (heroin, codeine, and oxycodone) acting on the µ-opioid receptors. Similar to methadone, buprenorphine can ease a patient going through opioid withdrawal [78]. Notably, if the patient uses opioid derivatives while taking buprenorphine, the rewarding effect is significantly reduced due to its partial agonist effect. Taking advantage of this effect, buprenorphine shows a limited risk of overdose with respect to methadone and other opioids, and has a limited impact on respiratory depression [79,80]. Currently, buprenorphine is available alone or in combination with the opioid receptor antagonist naloxone. Although some studies indicate that patients who taper off buprenorphine experience higher rates of relapse than those maintained on the medication for a longer period, both formulations of buprenorphine are still helpful for treating OUD [81]. In a Swedish trial, patients who received 16 mg/day of buprenorphine were compared to a control group who received the drug for detoxification for 6 days before receiving a placebo. Psychosocial support was provided to all patients. In this trial, buprenorphine showed a treatment failure rate of 25% compared to 100% for placebo. Treatment discontinuation occurred when there were more than two opioid-positive urine tests within three months; hence, relapse was directly associated with treatment retention. Notably, a 20% death rate was detected for patients who were not maintained with treatment [82]. According to a meta-analysis, buprenorphine diminished the amount of opioid-positive drug tests by 14.2%, and increased the likelihood of patients staying in treatment by 1.82 times compared to placebo-treated patients [83]. Accordingly, buprenorphine must be administered at a dose high enough to be effective (typically, 16 mg/day or more). Buprenorphine treatment failure and the false belief that the drug is not effective result from certain treatment providers who were reluctant to use opioids prescribing lower doses for shorter treatment durations [84]. Interestingly, both methadone and buprenorphine work equally well in cutting down opioid consumption. There were no differences in opioid-positive drug tests or self-reported heroin usage while patients were treated with methadone or buprenorphine at medium-to-high doses, according to a thorough review comparing methadone, buprenorphine, and placebo. Buprenorphine doses of 6 mg or less and flexible dosing regimens are particularly ineffective in keeping patients in treatment compared to methadone, emphasizing the importance of using evidence-based dosing regimens for these drugs [83].
Methadone is a synthetic, long-acting opioid agonist. The µ-opioid receptors in the brain are totally activated by methadone in the same way that they are by prescription and illegal opioids. In therapeutic doses, in people suffering from OUD, methadone reduces the euphoric “highs” of shorter-acting opioids (heroin, oxycodone, and codeine) and the unpleasant “lows” of opioid withdrawal. Nevertheless, it may take days to weeks to obtain a therapeutic dose, and this dose must be customized for each patient to limit cravings and discourage the further use of opioids [85,86]. Interestingly, the effectiveness of methadone treatment and psychosocial support in comparison with placebo was detailed in a recent study. The research proved beyond doubt that methadone was successful in lowering opioid usage, the spread of infectious diseases linked to opioid use, and criminality. Methadone users, compared to controls, had 33% fewer opioid-positive drug tests and were 4.44 times more likely to complete their treatment. Long-term (more than six months) outcomes were better in groups receiving methadone, independent of the frequency of counseling received. Methadone treatment considerably improves outcomes, even when administered in the absence of frequent counseling sessions [87,88].
Naltrexone is a full antagonist of the µ-opioid receptor. Its pharmacological behavior is relevant in preventing the effects of all opioid derivatives, such as euphoria and analgesia [78]. Naltrexone does not result in physical dependence or any of the pleasurable benefits of opioids. In theory, extended-release naltrexone patients quickly learn to abstain from the opioids that led to their addictive behaviors, and following regular usage of the drug, their cravings subside [89,90]. Naltrexone, formulated for oral administration, has received approval for treating OUD. Although the drug does not produce tolerance or withdrawal, the efficacy of this formulation has been principally constrained by poor treatment adherence [91]. Based on the available research, the effectiveness of oral naltrexone as a treatment for OUD is therefore not sufficiently demonstrated [92]. On the contrary, there is no need for daily dosing with extended-release injectable naltrexone, because it is given monthly. Even though this formulation is the most recent type of OUD treatment, evidence to date indicates its effectiveness [93].
The double-blind, placebo-controlled experiment that had the biggest impact on the FDA’s decision to approve naltrexone for treating OUD in 2010 also demonstrated that the drug significantly boosted opioid abstinence. Briefly, 90% of the confirmed abstinence weeks were in the naltrexone group, versus 35% in the placebo group. The naltrexone-treated group also had a greater rate of treatment completion (58% vs. 42%), as well as lower rates of subjective drug craving and relapse (0.8 vs. 13.7%). The naltrexone group continued to improve over the course of a 76-week open label period. To determine its effectiveness in a larger population, more research is necessary [89,94,95].
According to a National Institute on Drug Abuse (NIDA) clinical trial, extended-release naltrexone and a buprenorphine/naloxone combination showed equal effectiveness in treating OUD after treatment began. Starting therapy with naltrexone among active opioid users was more challenging, because this opioid requires total detoxification. The naltrexone formulation, however, was just as effective as the buprenorphine/naloxone combination once detoxification was complete [96].
Buprenorphine, like methadone, maintains opioid tolerance and physical dependence in patients; thus, discontinuing its use can result in withdrawal, even if buprenorphine withdrawal symptoms can be milder. The beginning of non-life-threatening opioid withdrawal following the first dose of buprenorphine is the most significant side effect for OUD patients. Furthermore, people with OUD who start buprenorphine treatment immediately have a lower risk of passing away from an opioid overdose [97]. Notably, complications related to the use of buprenorphine, such as hypogonadotropic effects, QT prolongation, or cardiac arrhythmias, are limited with respect to the use of methadone [98]. It is crucial to remember that since methadone and buprenorphine are opioids, overuse is possible. Buprenorphine and methadone, like other opioids, can cause physical dependence and a diagnosable OUD, necessitating their secure storage and exclusive use by the person to whom they are prescribed.
It is significant to remember that first-line therapy for OUD is not devoid of possible undesired effects due to drug–drug interaction issue. Recently, in an interesting work, Berger and colleagues reported a retrospective study in which the potential for drug–drug interaction in patients with OUD was assessed. Starting from the fact that some treatments for OUD show common metabolic pathways, the use of these drugs could be related to the insurgence of drug–drug interactions. For example, buprenorphine and methadone are metabolized by CYP3A4, so other drugs able to both inhibit or induce CYP3A4 enzymes could be relevant in drug–drug interactions, and could modify the duration and intensity of their effects. In addition, some of the drugs used for treating OUD, such as methadone, show an increased risk of QT prolongation, so the use of other drugs with similar effects could act synergistically, leading to fatal arrhythmias.
In fact, in the aforementioned work, it was reported that QT prolongation (24.2% inpatient, 45.8% discharge) and additive central nervous system effects/respiratory depression (68.8% inpatient, 50.6% discharge) were the two most prevalent categories of drug–drug interactions. Instead of being considered contraindicated, many drug–drug interactions were labeled as requiring strict monitoring. Opioids, benzodiazepines, antipsychotics, and anti-infective agents were the four drug classes with a risk of drug–drug interactions in the inpatient setting. Furthermore, due to the increase in the use of drugs to treat OUD, specialists (i.e., physicians, pharmacists) will require the ability to identify and manage potential drug–drug interactions [99].
Considering that the use of the aforementioned drugs is associated with an increase in undesired side effects, to exploit their pharmacological potential, it is necessary to identify novel opioid receptor ligands devoid of most of the adverse effects exhibited by current therapeutic agents. To this end, in this review article, in the following sections, we introduce some interesting molecules that could be potentially useful for treating OUD, including their pharmacological activities observed in in vivo studies and in clinical trials.
4. Mechanisms of Drug Withdrawal and Novel Therapeutic Options for Treating OUD
Due to the need to restrain the OUD crisis, which is a public health issue worldwide, research has focused on finding novel drugs, either synthetic or naturally derived, that can effectively treat opioid addiction, avoiding the limitations of drugs used for treating OUD [127,128]. Indeed, existing treatments for OUD, namely methadone, buprenorphine, and naloxone, target the disease at the opioid receptor: ORT, also known as OAT or opioid substitution therapy (OST), reduces overdoses and the damaging effects of substance abuse, although it does maintain tolerance and physical dependence on opioids [102,129].
Despite the fact that the opioid crisis was caused by overprescription of opioid drugs, research into new treatments for OUD takes into account, on one hand, the discovery of partial agonists or mixed agonist/antagonists which work through the same receptors; this could allow for antinociceptive effects during the withdrawal process and OUD treatment, thereby avoiding some of the main complications related to the opioid treatment. On the other hand, designing and synthesizing innovative antinociceptive agents with reduced undesired effects may be pivotal in resolving and preventing the opioid crisis [127,128,130,131,132].
Before deepening our analysis of the novel strategies of interaction concerning opioid receptors, it is worth mentioning that although it is not the focus of this article, much of the research carried out in the last few years focuses on looking beyond the opioid receptor [127,133,134]. Indeed, progress in neuroimaging has allowed for the discovery of possible therapeutic targets in addiction, including the prefrontal cortical reward network, as well as the development of interventional psychiatric therapeutic strategies that act via the modulation of the higher order neural circuitry involved in behavior [127]. Transcranial magnetic stimulation (TMS) [135], transcranial direct current stimulation (tDCS) [136], deep brain stimulation (DBS) [137] (NCT04354077; NCT03950492), vagus nerve stimulation (VNS) [138], and focused ultrasound (FUS) [139] are potential interventional treatments for OUD, currently FDA-approved for treating depression, anxiety, epilepsy, Parkinson’s disease, tremor, alcohol and cigarette use, and cocaine or methamphetamine use [140]. The potential benefit of these treatments is that they do not continue to rely on opioids and can be used as a stand-alone, non-drug treatment for OUD or as an additional treatment to the currently existing OAT. However, they are invasive because they require the implantation of a device in the brain or neck, which carries risks associated with surgery. Moreover, since some of these interventions are presently FDA-approved for treating resistant anxiety and depression, which are often linked to OUD, the treatment of these comorbidities with interventional approaches could improve medication experience and adherence [140].
The therapy of depression, anxiety, and addiction to tobacco and alcohol is also based on preclinical evidence of psychedelic agents. Serotonergic hallucinogens such as lysergic acid diethylamide (LSD) act as agonists of the 5-HT2A receptor, specifically those located on the dendrites of cortical pyramidal cells, while at the anatomic level, they increase structural neuroplasticity and the synthesis of neurites, dendritic spines, and synapses in the brain areas related to emotion processing and social cognition [141,142,143]. These data suggest a possible use of psychedelic agents in substance abuse, because several of these drugs are not related to physical dependence and are usually tolerated at significantly high doses with no associated permanent adverse effects [127].
Further possible innovative therapeutic strategies indicated for treating OUD comprise genetic and gene product approaches. According to genetic epidemiology research, genes account for around 50% of the risk of developing substance use disorders, including OUD. Even though no particular genes have been found that could be used as OUD biomarkers, a series of genes appear related to the cause of opioid addiction. Among them are the gene that encodes the μ-opioid receptor, OPRM1, the gene that modulates the trafficking and gating properties of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid) receptors, CNIH3, the gene that encodes a voltage-gated potassium channel, KCNJ6, the dopamine receptor D2 gene (DRD2), and the brain-derived neurotrophic factor (BDNF) gene [101]. Alongside the classic genetic research, the epigenetic approach is gaining attention. In response to both internal and external stimuli, hundreds of genes can have their expression influenced by microRNAs (miRNAs), which are pleiotropic epigenetic regulators of gene expression. In fact, it has been established that numerous miRNAs could play a crucial role in synaptic plasticity and drug addiction. Neuron-specific miRNAs have been detected in the bloodstream in response to at least one drug of abuse (tobacco), implying that these could represent biomarkers for addiction and potential drug targets for developing innovative therapeutics [144]. One of the latest tools that is under investigation for preventing and treating opioid addiction is gene editing, with novel clustered regularly interspaced short palindromic repeats (CRISPR) [134].
Another possible therapeutic strategy to treat OUD is immunotherapy. This approach has been demonstrated to improve adherence and reduce the risk of relapse. Among its advantages, it is necessary to mention the use of selective drugs of abuse, which is unlike the use of the non-selective orally administered μ-opioid receptor antagonists currently available. Unlike orally administered μ-opioid receptor antagonists, vaccines for the treatment of OUD do not require prior detoxification or monitoring of treatment compliance [145]. On the other hand, one of the limitations of immunotherapies for substance use disorders is that antibodies do not improve drug cravings, and can precipitate withdrawal symptoms [145,146]. Finally, a crucial feature of the design process is finding haptens with significant structural congruence with the target drug; failures to meet this requirement are likely to blame for failure of first-generation conjugate vaccines against cocaine and nicotine in clinical trials [145,146]. Meanwhile, the second generation of vaccines demonstrated better outcomes in preclinical settings, and we expect corresponding success in clinical studies. Although some encouraging results have been obtained, additional studies are necessary to obtain clinically useful products [127,145,146].
One last mention must be made regarding the use of cannabis or Δ-9-tetrahydrocannabinol (THC) as a possible therapeutic approach to alleviate opioid withdrawal. Despite preliminary evidence in this sense, further clinical studies are necessary to establish the real risk/benefit ratio of this possible therapeutic approach [133].
Lastly, public health measures and behavioral interventions at the individual level are fundamental strategies to treat OUD [101,127].
4.1. Promising Opioid Receptor Modulators for the Treatment and Prevention of OUD
4.1.1. Methocinnamox (MCAM)
Methocinnamox, chemically 14β-(4′-methylcinnamoylamido)-7,8-dihydro-N-cyclopropylmethyl-normorphinone, (MCAM), is a potent, long-lasting, pseudo-irreversible μ-opioid receptor antagonist [130,147,148]. First described by Broadbear and colleagues [149], MCAM is structurally similar to buprenorphine; it reversibly binds μ- and δ-opioid receptors without interacting with other nociceptors [130,147,148]. The mechanism of action of MCAM was well described by Zamora and colleagues, who tested the time-dependent nature of the antagonism, including its non-surmountability by agonists and its lack of reversibility binding at μ-opioid receptors, both in vitro and in vivo [147]. Indeed, in HEK cells expressing the human μ-opioid receptor, MCAM pretreatment diminished the maximal response to the μ-opioid receptor agonist, DAMGO ([D-Ala2, N-MePhe4, Gly-ol5]-enkephalin), in a time-dependent and non-washable manner. Similarly, this effect was shared by the irreversible antagonist, β-FNA (β-funaltrexamine). In contrast, the competitive antagonist naloxone was completely surmountable by DAMGO, and its antagonism was not dependent on time and totally reversible upon washout. Conversely, the binding of MCAM was found to be reversible or pseudo-irreversible. The idea that MCAM may have both a (pseudo)irreversible orthosteric action to diminish the agonist’s maximal response and an allosteric action to change the µ-opioid receptor agonist’s potency offers one explanation for the slow off-rate of MCAM from the orthosteric location. Interestingly, pretreatment of cells with naloxone, at a concentration to completely occupy the μ-opioid receptor orthosteric site, before the MCAM administration, followed by a significant washing process for removing naloxone and the unbound MCAM, prevented the MCAM-induced reduction in the maximal response to DAMGO but did not block the dextral shift in the DAMGO concentration–response curve. This result is consistent with the presence of a naloxone-insensitive (allosteric) site targeted by MCAM that could mediate a reduction in the potency of DAMGO, as demonstrated by the shift in the DAMGO concentration–response curve and by a differential modulation of opioid agonist responses. The pseudo-irreversible binding incapacitated the μ-opioid receptors; thus, cells should synthesize nascent μ-opioid receptors to reestablish previous functionality. For this reason, MCAM showed an exclusively long duration of action (DOA) [130].
Additionally, in cells expressing the human δ- or κ-opioid receptors, pretreatment with MCAM shifted the concentration–response curves to the δ-agonist, DPDPE (D-Pen2,5]-enkephalin), and the κ-agonist, U50488, to the right in a surmountable, time-independent and fully washable manner, suggesting that MCAM could act as a reversible competitive antagonist in δ- and k-opioid receptors [147].
Although not tested in human trials yet, data obtained from animal studies showed a medication that could exert an important positive influence on the treatment of opioid overdose as well as OUD [150,151,152,153]. As a matter of fact, the administration of MCAM (0.32 mg/kg) to Rhesus monkeys before and after injection of heroin prevents and reverses respiratory depression in a similar manner to naltrexone (0.032 mg/kg) and naloxone (0.0032–0.1 mg/kg), respectively. However, the persistent effects of MCAM represent the most impressive difference; in the prevention study, MCAM could attenuate the respiratory depressant effects of heroin for at least 4 days, whereas the antagonist effects of naltrexone disappeared in 1 day. Likewise, in the reversal study, MCAM protects against the respiratory depressant effects of heroin on the day of administration as well as on subsequent days when additional doses of heroin are given, according to the heroin dose–effect curve that did not return fully to control values within 8 days; however, the administration of the largest dose of naloxone caused a return to baseline values within 45 min [152]. Additionally, the impact of MCAM on the abuse-related effects of opioids was characterized by examining its capability to attenuate opioid self-administration in Rhesus monkeys. In one experiment, intravenous infusions of heroin (0.0032 mg/kg) or cocaine (0.032 mg/kg) were delivered according to a fixed-ratio schedule of reinforcement; in a second trial, monkeys were given the option of eating or receiving 3.2 mg/kg of the µ-opioid receptor agonist remifentanil intravenously. In a third trial, the direct effects of MCAM (0.32 mg/kg) were studied by monitoring responses to food and physiologic variables (heart rate, blood pressure, temperature, and activity). In the heroin self-administration experiment, naltrexone (0.032 mg/kg) as well as MCAM dose-dependently decreased infusions obtained on the day of treatment; however, in naltrexone-treated monkeys, the reduction in opioid intake was significant on the day of treatment and response, returning to baseline levels the next day, while MCAM significantly decreased heroin consumption on the first day of therapy and for several days subsequently, with decreases persisting, on average, for 10 days. Neither naltrexone nor MCAM could alter the response sustained by cocaine on the day of treatment or for several days thereafter, establishing the selectivity of MCAM for attenuating opioid-maintained behavior. In monkeys responding to a food/drug choice procedure, the choice of food decreased as the dose of remifentanil increased (0.32 and 1.0 μg/kg). The injection of 0.032 mg/kg naltrexone immediately before, but not the same dose of naltrexone administered 24 h before, decreased the choice of remifentanil and increased the choice of food, shifting the remifentanil dose–effect curve rightward, before it returned to control levels the next day. The remifentanil dose–effect curve was shifted rightward and downward in a dose-dependent manner by MCAM, and the time it took for the curve to recover was inversely proportional to the dose of MCAM, with the curve fully recovering 4 days after 0.32 mg/kg MCAM administration, 8 days after 1.0 mg/kg, and 12 days after 3.2 mg/kg. The last group of monkeys were treated with MCAM 1 h before the activity session, and doses of MCAM that relevantly reduced opioid intake did not alter the rate of response, the number of pellets earned, or physiological measures such as blood pressure, heart rate, and body temperature, indicating a favorable safety profile of MCAM for these physiological parameters [153].
The same research team also evaluated the effects of acute and repeated MCAM administration on the self-administration of fentanyl, a μ-opioid receptor agonist [150]. Cocaine (0.032 mg/kg/infusion) or fentanyl (0.00032 mg/kg/infusion) were delivered intravenously to four Rhesus monkeys, and MCAM (0.1–0.32 mg/kg) or the opioid receptor antagonist naltrexone (0.001–0.032 mg/kg) were injected before test sessions to assess acute effects. To test the efficacy of repeated therapy, 0.32 mg/kg MCAM was injected once every 12 days for a total of five injections. Following acute injection, MCAM and naltrexone both reduced the amount of fentanyl that patients self-administered on the treatment day. This attenuation persisted about 2 weeks after the higher MCAM dose and for only one day after naltrexone. Repeated MCAM administration diminished cocaine self-administration but did not affect fentanyl self-administration for more than two months [150].
MCAM’s ability to revert ventilatory depression induced by fentanyl was also evaluated considering the route of administration (intravenous and subcutaneous) and compared to naloxone, the only FDA-approved medication available to treat opioid overdose. MCAM and naloxone were able to revert the ventilatory depressant effects of 0.178 mg/kg fentanyl injected i.v. in male Sprague Dawley rats, in a dose-dependent manner. MCAM, but not naloxone, decreased the ventilatory depressant effects of fentanyl the day following antagonist delivery. After intravenous and subcutaneous treatment, the duration of the effect of MCAM extended up to 3 days and at least 2 weeks, respectively. Additionally, MCAM reduced fentanyl’s antinociceptive effects, with antagonism persisting for up to 5 days and more than 2 weeks following intravenous and subcutaneous treatment, respectively [151].
Taken together, these findings emphasize MCAM’s long-lasting effects, which are likely due to its pseudo-irreversible binding to µ-opioid receptors, its capacity to safely and effectively reduce opioid self-administration for extended periods of time after a single administration, and its ability to remain effective with repeated administration, even at very low plasma concentrations. These findings support the idea that pharmacodynamic factors are important in its long-lasting effects, the quick recovery from opioid-induced ventilatory depression, and the subsequent protection from respiratory depression brought on by the re-emergence of effects of an agonist with an action duration longer than naloxone. Accordingly, MCAM may be a medication recommended for those who co-use alcohol or benzodiazepines, because it has a positive safety profile and may not cause any adverse drug reactions [150,153]. By increasing the effectiveness of the treatment and patient compliance, a drug that addresses all these issues could have a substantial positive influence on the treatment of opioid overdose and OUD. Although MCAM is being researched for its potential as a long-term OUD treatment for the opioid crisis, it is relevant to note that no testing has been performed on humans.
4.1.2. Mitragynine (Kratom)
Given its wild availability and fast spread worldwide for both recreational and medicinal purposes, we believe that the Mitragyna speciosa, a Southeast Asian evergreen tree in the coffee family commonly known as kratom, deserved to be mentioned in this review, notwithstanding the deficiency of human data available.
According to several anonymous online surveys, kratom is primarily used by white, middle-aged Americans for several health-related purposes, such as pain relief, reduced fatigue, increased energy and focus, reduction of anxiety and depression, and as an alternative to alcohol, opioids, and/or other drugs to manage withdrawal and maintain abstinence [154,155,156].
Because of its dose-related mild stimulant- and opioid-like effects [154,156], its use is raising concerns with regulatory agencies, and has resulted in scheduling actions in a number of countries [157,158]. In fact, kratom contains more than 30 different indole alkaloids, including its main alkaloid mitragynine, which accounts for roughly 66% of all the alkaloids found in kratom leaves. Among the minor alkaloids, its naturally oxidized derivative 7-hydroxymitragynine (7-OH) is of particular interest [128,159,160,161]. In particular, these alkaloids represent a novel class of opioid receptor modulators with distinctive pharmacological characteristics: mitragynine and its oxidized analog, 7-OH, are partial agonists of the human μ-opioid receptor and competitive antagonists at the κ- and δ-opioid receptors, respectively. Additionally, 7-OH and mitragynine are G-protein-biased agonists of μ-opioid receptors that do not recruit β-arrestin after receptor activation, which has been linked to many undesirable effects of opioids, including constipation, respiratory depression, and dependence [128,161]. As a result, mitragynine did not lead to dependence or increased self-administration in animal models, and it even decreased the preceding administration of morphine. However, the results of the study presented with a dependence liability of 7-OH, which is readily self-administered, and prior exposure increased subsequent morphine intake, which is ascribable to its reinforcing effects, mediated in part by μ- and δ-opioid receptors. Pretreatment with nalxonaxine, a μ-opioid receptor antagonist, and naltrindole, a δ-opioid receptor antagonist, on 7-OH and morphine revealed a reduction in 7-OH self-administration, whereas only nalxonaxine decreased morphine intake. The results showed that 7-OH should be regarded as a kratom component with strong abuse potential that could also raise the intake of other opioids, while mitragynine does not have abuse potential and decreases morphine consumption [162]. Notably, Kruegel and colleagues highlighted that since 7-OH could not be present in all plant extracts, its potential contribution to the actions of Mitragyna speciosa is not universal, unless 7-OH is produced as a metabolite. Although they attributed competitive antagonist activity at µ-opioid receptors to a variety of different main alkaloids isolated from the plant, this results in a complicated interplay of antagonist and agonist actions that compete at the opioid receptors, which then translate into the strong psychoactive effects of the crude plant material [161]. In order to fully analyze the abuse potential of mitragynine, rats trained to self-administer methamphetamine had their own self-administration assessed and compared to that of heroin. Unlike heroin, mitragynine did not continue to provide reaction rates that were higher than those obtained using saline injections. While having little influence on the rates of response sustained by methamphetamine across the same range of mitragynine doses, mitragynine dose-dependently reduced the rates of response maintained by heroin. These findings collectively imply a low risk of abuse of mitragynine and the possibility of using mitragynine therapy to minimize opioid abuse in particular [163]. To deepen the characterization of kratom alkaloids, using a radiant heat tail flick assay on mice, the antinociceptive profile of mitragynine and its derivatives was assessed. Mitragynine induced antinociception that was 66 times less strong than morphine following subcutaneous injection, although 7-OH was around five times more potent [128]. Of particular interest, mitragynine pseudoindoxyl, a rearrangement product of 7-OH with a spiro-pseudoindoxyl nucleus, was found to be 1.5-fold more potent than morphine after intracerebroventricular administration and 3-fold more potent following subcutaneous administration; it showed a shorter duration of antinociceptive effect than morphine, with a peak effect at 15 min, and also displayed activity following oral administration [128].
Since only one study was conducted in Thailand in 2015, and no other controlled clinical trials using kratom or its alkaloids have been undertaken, the behavioral pharmacology and abuse potential of kratom have not been fully described in a large human population to date [155]. The mentioned prospective study enrolled ten chronic, regular, healthy kratom consumers. By administering a known dose of kratom tea (60 mL) daily for seven days before the experiment, the steady state was modified in each subject. On day 8, a loading dose of mitragynine tea was administered, and blood concentrations of the drug were assessed at 17 time points. Additionally, urine concentrations during the 24 h period were collected and quantified using the liquid chromatography–tandem mass spectrometry technique. According to the outcomes, the authors proposed linear pharmacokinetics, which followed an oral two-compartment model, hypothesizing a hepatic metabolism. No severe undesired effects were detected by kratom users during the trial, while some mild events included tongue numbness after having finished drinking kratom tea, and an increment in blood pressure and heart rate, although the onset occurred 8 h after consuming kratom tea [164].
Given the lack of controlled human studies, surveys represent one effective way, although not fully reliable, to provide initial insights and understand more about outcomes following kratom exposure, and to inform prospective evaluations of kratom for diverse indications as well as regulatory decisions regarding scheduling. Data collected from surveys conducted in the U.S. mostly agree that the primary reasons for kratom use include increased energy and focus, alleviating pain, anxiety, or depression, cutting down on or quitting the use of prescription opioids or heroin, and relieving withdrawal symptoms. Respondents evidenced dose-dependent detrimental effects including constipation, nausea, drowsiness, and dizziness as the most commonly detected adverse reactions, generally perceived as mild and less than 24 h in duration [154,155,156,165]. The self-selected convenience sample is the main drawback of internet-based surveys of kratom users, because it is likely to exhibit selection bias in favor of people who are younger and more likely to be positive about kratom, which understates the use of the preparation by older participants [155,156].
In conclusion, mitragynine (kratom), one of the main alkaloids of Mitragyna speciosa, deserves further research, not only as a possible pharmacotherapy for OUD with low abuse potential, but also as an effective new medication with minimal abuse liability to manage acute and chronic pain, especially due to its public health impact. Since history and its patterns of use and effects confirm its benefits to consumers, some authors suggest that making kratom illegal could lead to greater opioid consumption among those who are now using it to stop taking opioids, and to the establishment of a black market [154,165].
4.2. Safer and More Tolerable Therapeutics for Treating Severe Pain: Oliceridine, the Opioid of the 21st Century?
As previously mentioned, the misuse and diversion of opioid analgesics were made easier by their overprescription, and patients with chronic pain were put at risk of addiction and overdose without necessarily experiencing any relief from their agony. Postoperative pain treatment continues to be a prevalent difficulty in contemporary medicine, despite the implementation of prescription drug monitoring programs in all states, alongside better physician education in opioid prescriptions and pain management aiming to prevent inappropriate dosing [102,166]. As a result, the development of new and safer pain medications that warrant effective pain management is needed.
Oliceridine (formerly known as TRV130, C22H30N2O2S·C4H4O4) represents a novel class of opioid medicines able to target μ-opioid receptors acting as biased ligands. Opioid analgesics are full agonists at the µ-opioid receptors, which after receptor activation, engage two independent transduction pathways (the G-protein-coupled signaling and the β-arrestin pathways) with totally different pharmacological effects. G-protein signaling is predominantly involved in analgesia, reward, and liking, whereas the β-arrestin pathway is implicated in negative effects such as respiratory depression and gastrointestinal symptoms, as well as the attenuation of analgesic benefits [166,167,168,169,170,171]. By downregulating the β-arrestin pathway and selectively activating the G-protein-coupled signaling pathway, olceridine has the potential to limit the occurrence of opioid-related adverse events and increase the therapeutic window, thereby satisfying the need for an analgesic with the effectiveness of a conventional opioid but with a more manageable side effect profile [166,167,168,172].
Oliceridine formulated as a fumarate salt [166] was first discussed for potential approval at the FDA’s meeting regarding anesthetic and analgesic drug products in October 2018 [173]; recently, it has been approved by the FDA under the brand name Olinvyk for intravenous use for treating severe acute pain when alternative therapeutic options are inadequate [166]. According to the prescribing information, the maximum cumulative daily dose should be 27 mg, and the period should be no longer than 48 h, because the sole approved use for oliceridine is to relieve postoperative pain through patient-controlled analgesia (PCA), i.v. infusion, or bolus. A 1.5 mg first dose given by a healthcare professional followed by 0.35 mg demand doses with a 6 min lockout could be used with supplemental doses of 0.75 mg starting 1 h after the original dose and continuing as needed after that. The patient is expected to gain pain relief in 2–5 min [167,174].
Preclinical studies have shown a higher potency of oliceridine than of morphine in achieving analgesia [132,175]. Notably, even at doses several times the maximum daily exposure of 40 mg/day, no oliceridine-induced toxicity other than the standard opioid side effects (such as decreased activity, lower blood pressure, and body temperature) and generally fewer adverse events were detected [132]. According to Liang and colleagues, the level of physical dependence produced by oliceridine is comparable to or no different from that produced by morphine, and compared to morphine, tolerance is less likely to develop during long-term oliceridine treatment. Additionally, the degree of sensitization or opioid-induced hyperalgesia produced by oliceridine is not as severe as that produced by morphine [175,176].
Ascending TRV130 doses were used in the first TRV130 in-human clinical trial to study the drug’s pharmacokinetics, pharmacodynamics, and tolerability in healthy volunteers. Nausea and vomiting were reported at the 7 mg dose, which prevented further dose escalation. Doses between 0.15 and 7 mg administered intravenously over 1 h were well tolerated [177].
Based on these findings, Soergel and colleagues conducted a randomized, double-blind, placebo-controlled, crossover study (NCT02083315) enrolling 30 healthy male volunteers. During the 11-day/10-night sequestration, the volunteers randomly received single doses of TRV130, a placebo, or morphine intravenously on days 1, 3, 5, 7, and 9. TRV130 plasma concentration measurements reached their peak within 10 min of infusion and then decreased biphasically, indicating a fast distribution followed by an elimination phase. When compared to placebo and morphine at 10 and 30 min, TRV130 at all dosages (1.5, 3, and 4.5 mg) caused a rapid and significant increase in CPT hand removal latency from baseline. This resulted in a temporary decrease in respiratory drive at all doses tested, which peaked at 30 min and was comparable in magnitude to that after morphine administration; however, unlike the transitory effect of TRV130, the effect of morphine on respiratory drive persisted through the final ventilatory response to the hypercapnia measurement at 4 h post dose. The drug TRV130 was generally well tolerated; however, more patients reported experiencing severe nausea after taking morphine than after TRV130. These adverse effects included nausea, dizziness, vomiting, headache, pruritus/flushing, and somnolence [131].
This evidence showed that oliceridine had a safer safety profile than morphine, which led to oliceridine having a clinical advantage over morphine within clinical concentration ranges; it is evident that at clinically relevant doses, oliceridine appears to separate analgesia from respiratory depression more effectively than morphine. However, the authors note that predicting respiratory depression occurrences based on the utility function should presently be regarded as exploratory, and requires additional research to support their findings [168].
Sixty healthy, non-dependent, recreational opioid users participated in a single-dose, randomized, double-blind crossover study to compare the abuse potential of intravenous oliceridine to that of intravenous morphine and placebo. The blinded treatments were given as 1 min intravenous infusions of oliceridine 1 mg, 2 mg, or 4 mg, morphine 10 mg or 20 mg, or placebo. Standard abuse liability endpoints were evaluated during each period. Based on the findings of a previous dose escalation trial (Phase A of trial 1011), it was anticipated that the effects of oliceridine 2 mg and 4 mg would be comparable to those of morphine 10 mg and 20 mg, respectively. The study found that oliceridine and morphine showed the same propensity for misuse at equianalgesic doses. For this reason, the Controlled Substances Act requires that oliceridine be classified as a Schedule II drug, which would provide the same regulations and safeguards as those now in place for traditional i.v. opioid drugs given in a hospital or controlled settings [173].
The pharmacokinetics of oliceridine was also investigated in a special population of patients. Simons and colleagues conducted a four-arm double-blind, randomized, crossover study that evaluated the respiratory effects of intravenous 0.5 or 2 mg oliceridine and 2 or 8 mg morphine in an elderly population (18 healthy male and female volunteers, aged 55 to 89), on four separate occasions. The results demonstrated that while at low doses of oliceridine, no significant respiratory effects were observed, the peak effects of respiratory depression from high doses of oliceridine and both doses of morphine occurred 0.5 to 1 h after opioid administration. However, oliceridine-induced respiratory depression recovered to baseline more quickly than morphine-induced respiratory depression. Moreover, the magnitude of the respiratory depression induced by oliceridine appeared smaller over time compared with that induced by morphine [178]. The assessment of oliceridine’s pharmacokinetics, tolerability, and safety in patients showing hepatic or renal impairment was performed in a phase I, open-label, single-dose clinical trial. The researchers compared the pharmacokinetics and safety of 0.5 mg of intravenous oliceridine to 1 mg in participants with end-stage renal disease (ESRD) or hepatic impairment. According to the results, neither oliceridine clearance nor AUC was impacted by hepatic impairment, nor was there a difference in clearance between persons with normal renal function and ESRD patients [179].
Phase II clinical trials have assessed the effectiveness and safety of several oliceridine doses and dosing regimens in comparison to placebo and a traditional intravenous opioid in managing moderate-to-severe pain after surgery.
A phase II, randomized, placebo- and active-controlled trial was conducted to examine the efficacy and tolerability of TRV130 for the treatment of acute pain after bunion surgery. This study was divided into two phases: stage A, or the pilot phase, in which 144 patients with moderate-to-severe acute pain following bunion surgery were randomized to receive double-blind TRV130 (1 mg, 2 mg, 3 mg, or 4 mg every 4 h), a placebo, or morphine (4 mg every 4 h); and stage B, in which 195 patients were randomized to receive double-dummy TRV130 (0.5 mg, 1 mg, 2 mg, or 3 mg every 3 h), placebo, or morphine 4 mg every 4 h intravenously. The oliceridine dosing interval was reduced and the doses were changed in accordance with the stage A results, since the dose-related decrease in pain intensity that was visible early in the oliceridine dosing interval was essentially absent by the conclusion of the dosing interval. The results showed that TRV130 at 2 and 3 mg produced significantly greater categorical pain relief than morphine after the first dose, with meaningful pain relief occurring in 5 min, and that morphine at 4 mg produced significant reductions in pain intensity than placebo over the course of 48 h. Additionally, it has been determined that after a single dose, oliceridine is almost five times more potent than morphine. No significant side effects were observed with TRV130, and it was tolerated similarly to morphine [180].
Another phase IIb, randomized, double-blind, patient-controlled analgesia trial (NCT02335294) compared oliceridine to morphine and placebo in terms of effectiveness, safety, and tolerability in patients experiencing moderate-to-severe pain after abdominal surgery (NCT02335294). A total of 200 patients were randomly assigned to one of three treatment regimens in the first stage of the study, which was divided into two phases. These treatment regimens were placebo, oliceridine (1.5 mg loading dose and 0.1 mg demand doses with a 6 min lockout interval), or morphine (4 mg loading dose and 1 mg demand doses). The interim analysis led to an increase in the oliceridine demand dose from 0.10 to 0.35 mg. According to efficacy assessments, oliceridine treatment resulted in a statistically significant analgesia when compared to placebo, and the analgesia it caused for the full 24 h of treatment was comparable to morphine. A notable difference was the speed at which the pain was relieved, which was statistically different from both the placebo and morphine. All treatments were generally well tolerated, showing a limited incidence of reported undesired effects, mainly nausea, vomiting, and headache, asserting a favorable safety and tolerability profile [181].
APOLLO-1 (NCT02815709) was a phase III, double-blind, randomized trial for individuals who had undergone bunion surgery and were experiencing moderate-to-severe pain. Similar to the prior study, PCA was used to administer the medication. The study enrolled 389 patients who were randomly assigned to one of five group medications: placebo; oliceridine 1.5 mg loading dose/0.1 mg on demand; oliceridine 1.5 mg loading dose/0.35 mg on demand; oliceridine 1.5 mg loading dose/0.5 mg on demand; and morphine 4 mg loading dose/1 mg on demand. All oliceridine dose regimens were shown to have considerably greater responder percentages than placebo as the primary endpoint, and the 0.35 and 0.5 mg dosing regimens were comparable to morphine. The prevalent side effects of oliceridine were similar to those of conventional opioids, and became stronger with increasing doses. While the highest demand dose regimen of oliceridine (0.5 mg) was not statistically distinct from morphine, patients in the 0.1 and 0.35 mg regimens had a considerably decreased risk of experiencing a respiratory safety event compared with morphine. The incidence of gastrointestinal side effects was also similar to morphine in the 0.5 mg dose schedule, but lower than morphine in the 0.1 and 0.35 mg treatment regimens [182].
The effectiveness and safety of oliceridine for acute pain after abdominoplasty were assessed in the APOLLO-2 (NCT02820324) trial. With respect to APOLLO-1, patients were given a loading dosage of either a placebo, oliceridine (1.5 mg), or morphine (4 mg), and then demanded doses through patient-controlled analgesia (0.1, 0.35, or 0.5 mg oliceridine, 1 mg morphine, or placebo) with a 6 min lockout interval. The investigated drug was administered to a total of 401 subjects. All oliceridine dosing regimens showed statistically superior analgesia to placebo, with a higher number of treatment responders in the 0.35 and 0.5 mg demand dose regimens across the 24 h treatment period, which is consistent with earlier findings in both phase II and phase III trials. The oliceridine 0.35 and 0.5 mg demand dose regimens were consistently superior to placebo in terms of the proportion of treatment responders over time, the amount and timing of self-reported pain relief, the proportion of patients receiving rescue pain medication over time, the timing of the first use of rescue pain medication, and clinician and patient-reported satisfaction. In comparison to morphine, oliceridine (0.35 mg regimen) had a better safety and acceptability profile in relation to respiratory and gastrointestinal side effects. Similar to earlier trials, the oliceridine 0.35 and 0.5 mg dose regimens seemed to reduce the respiratory safety burden (a measure to assess the risk of opioid-induced respiratory depression (OIRD)), albeit this effect was not statistically significant [183].
ATHENA was a phase III, multicenter, open-label clinical trial (NCT02656875) that aimed to represent intravenous opioid use in a large, “real world” setting by being less restrictive in terms of patient eligibility criteria, treatment protocol requirements, patient population, and mode of administration. Patients 18 years of age or older with moderate-to-severe acute pain after surgery or with a non-surgical painful medical condition were included. In total, 768 patients who had been enrolled received i.v. oliceridine treatment through PCA and/or bolus dosage provided by physicians. A loading dose of 1 to 2 mg for intravenous bolus dosing was given, and if necessary, a supplemental dose of 1 mg was given within 15 min. Every 1 to 3 h, as needed, additional doses of 1 to 3 mg were given. In the case of PCA, a 6 min lockout interval was used to provide a 1.5 mg loading dosage and a 0.5 mg demand dose. Supplementary dosages of 1 mg were permitted as soon as 15 min following the original dose, if clinically necessary. 76% of patients received local anesthetics, 69% received non-steroidal anti-inflammatory medicines (NSAIDs), and 48% received oral opioids prior to the first dosage of oliceridine. The findings of this study showed that oliceridine was generally safe and well tolerated in a large population whether delivered alone or as a component of multimodal analgesia to adult patients experiencing moderate-to-severe pain due to surgical procedures or medical disorders. In line with previous investigations, oliceridine was linked to a strong analgesic effect and quick onset of action. Although not free from side effects (64% of patients experienced adverse events while receiving oliceridine treatment), the side effects were generally mild and consistent with previous data. Notably, individuals receiving oliceridine had a much lower incidence of OIRD events compared with those receiving conventional opioids despite the inclusion of patients with risk factors for ORAEs, such as advanced age, obesity, and sleep apnea. The absence of a concurrent control group, however, is the study’s primary flaw [184].
Brzezinski and colleagues conducted an exploratory and retrospective examination of ATHENA data to learn more about the prevalence of OIRD in aged and/or obese surgical subjects. They discovered that using oliceridine for postsurgical analgesia in patients with advanced age and/or higher body mass index (BMI) who were experiencing moderate-to-severe pain was not linked to an increased risk of respiratory depression, and may even be clinically appropriate for this group of patients, who are at a high risk of OIRD [185].
A phase IV clinical trial (VOLITION, NCT04979247) which is presently enrolling 200 patients and is expected to be completed by July 2025 is investigating the effects of oliceridine. The trial’s design specifications state that patients will receive a bolus of 1.5 mg oliceridine near the conclusion of the operation. After surgery, PCA will be initiated with 0.35 mg demand doses, a 6 min lock-out, and no background infusion. Following the initial 1.5 mg loading dosage, additional boluses (1 mg) of oliceridine will be administered as soon as 15 min later, depending on the NRS score and clinical evaluation of the patient. A dose of 0.5 mg oliceridine can be added to the PCA demand dose in addition to additional 1 mg bolus doses. The primary goal of the clinical trial was to assess the percentage of participants who experienced respiratory compromise 24 h after receiving the first dose. Oliceridine currently has only one approved use, that is, relieving postoperative pain through PCA, IV infusion, or bolus. Evidence suggests that this drug has the potential to be used for additional therapeutic purposes in addition to serving as an important part of a multimodal analgesic regimen, particularly for people who have risk factors such as advanced age, obesity, and sleep apnea. Table 2 provides a thorough summary of the clinical trials involving oliceridine.

Table 2.
Clinical studies on oliceridine: an overview and findings.
5. Conclusions and Future Perspectives
In summary, in this article, we have reviewed the current status of the use of opioids as therapeutic agents employed to treat several diseases, including cancer and non-cancer pain. We paid special attention to the analysis of the opportunity to target opioid receptors for treating opioid use disorder (OUD), drug withdrawal, and addiction. Although there is great pharmacological potential in targeting opioid receptors, the clinical use of opioids is severely limited by several adverse effects such as tolerance and physical and physiological dependence. Finally, we investigated some drugs targeting opioid receptors that seem to hold great promise for treating severe pain but could also be indicated for individuals suffering from OUD, such as mitragynine, MCAM, and oliceridine, analyzing experimental and clinical evidence. We also considered the significant improvements made in the last decade in biophysical techniques such as cryo-EM, which allowed us to obtain experimental three-dimensional structures of several members of the G-protein-coupled receptor family, including opioid receptors. In the future, it is expected that this structural information will be useful for designing a novel generation of opioid receptor ligands [186,187]. In fact, understanding the binding mode of agonists and antagonists related to reduced side effects could pave the way for the rational design of drugs able to target opioid receptors with improved efficacy and selectivity. Accordingly, in few years, it is expected that innovative opioid receptor-targeting drugs will be entered into clinical trials to provide clinically usable molecules for treating OUD, and that these drugs will later be employed to improve the quality of life of patients with chronic pain and related disorders.
Author Contributions
Conceptualization, R.T., S.B. and V.C.; investigation, R.T. and S.B.; data curation, R.T., S.B. and V.C.; writing—original draft preparation, R.T. and S.B.; writing—review and editing, R.T., S.B. and V.C.; supervision, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Azadfard, M.; Huecker, M.R.; Leaming, J.M. Opioid Addiction [Updated 1 January 2023]; StatPearls: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448203/ (accessed on 10 January 2023).
- Available online: https://www.cdc.gov/opioids/healthcare-professionals/prescribing/opioid-use-disorder.html (accessed on 10 January 2023).
- Strang, J.; Volkow, N.D.; Degenhardt, L.; Hickman, M.; Johnson, K.; Koob, G.F.; Marshall, B.D.L.; Tyndall, M.; Walsh, S.L. Opioid use disorder. Nat. Rev. Dis. Prim. 2020, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Korthuis, P.T.; McCarty, D.; Weimer, M.; Bougatsos, C.; Blazina, I.; Zakher, B.; Grusing, S.; Devine, B.; Chou, R. Primary Care-Based Models for the Treatment of Opioid Use Disorder: A Scoping Review. Ann. Intern. Med. 2017, 166, 268–278. [Google Scholar] [CrossRef]
- Donroe, J.H.; Bhatraju, E.P.; Tsui, J.I.; Edelman, E.J. Identification and Management of Opioid Use Disorder in Primary Care: An Update. Curr. Psychiatry Rep. 2020, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, K.A.; Ponce Terashima, J.; McCarty, D. Opioid use disorder and treatment: Challenges and opportunities. BMC Health Serv. Res. 2019, 19, 884. [Google Scholar] [CrossRef] [PubMed]
- Busse, J.W.; Wang, L.; Kamaleldin, M.; Craigie, S.; Riva, J.J.; Montoya, L.; Mulla, S.M.; Lopes, L.C.; Vogel, N.; Chen, E.; et al. Opioids for Chronic Noncancer Pain: A Systematic Review and Meta-analysis. J. Am. Med. Assoc. 2018, 320, 2448–2460. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Wee, B.; Derry, S.; Bell, R.F.; Moore, R.A. Opioids for cancer pain—An overview of Cochrane reviews. Cochrane Database Syst. Rev. 2017, 7, CD012592. [Google Scholar] [CrossRef]
- Quigley, C. Opioids in people with cancer-related pain. BMJ Clin. Evid. 2008, 2008, 2408. [Google Scholar]
- Busse, J.W.; Craigie, S.; Juurlink, D.N.; Buckley, D.N.; Wang, L.; Couban, R.J.; Agoritsas, T.; Akl, E.A.; Carrasco-Labra, A.; Cooper, L.; et al. Guideline for opioid therapy and chronic noncancer pain. Can. Med. Assoc. J. 2017, 189, E659–E666. [Google Scholar] [CrossRef]
- Nadeau, S.E.; Lawhern, R.A. Management of chronic non-cancer pain: A framework. Pain Manag. 2022, 12, 751–777. [Google Scholar] [CrossRef]
- Bertin, C.; Delage, N.; Rolland, B.; Pennel, L.; Fatseas, M.; Trouvin, A.P.; Delorme, J.; Chenaf, C.; Authier, N. Analgesic opioid use disorders in patients with chronic non-cancer pain: A holistic approach for tailored management. Neurosci. Biobehav. Rev. 2021, 121, 160–174. [Google Scholar] [CrossRef]
- Graven-Nielsen, C.S.; Knoph, C.S.; Okdahl, T.; Hoyer, K.L.; Krogh, K.; Hellstrom, P.M.; Drewes, A.M. Opioids in the Treatment of Chronic Idiopathic Diarrhea in Humans-A Systematic Review and Treatment Guideline. J. Clin. Med. 2023, 12, 2488. [Google Scholar] [CrossRef] [PubMed]
- Schiller, L.R.; Pardi, D.S.; Sellin, J.H. Chronic Diarrhea: Diagnosis and Management. Clin. Gastroenterol. Hepatol. 2017, 15, 182–193.e13. [Google Scholar] [CrossRef] [PubMed]
- Ruppin, H. Review: Loperamide—A potent antidiarrhoeal drug with actions along the alimentary tract. Aliment. Pharmacol. Ther. 1987, 1, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Belvisi, M.G.; Geppetti, P. Cough. 7: Current and future drugs for the treatment of chronic cough. Thorax 2004, 59, 438–440. [Google Scholar] [CrossRef] [PubMed]
- On, P.C. Updates in treatment of adults with chronic cough. Am. J. Manag. Care 2020, 26, S239–S245. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Maffulli, N.; Baroncini, A.; Eschweiler, J.; Tingart, M.; Quack, V. Opioids for chronic low back pain management: A Bayesian network meta-analysis. Expert. Rev. Clin. Pharmacol. 2021, 14, 635–641. [Google Scholar] [CrossRef]
- Petzke, F.; Klose, P.; Welsch, P.; Sommer, C.; Hauser, W. Opioids for chronic low back pain: An updated systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks of double-blind duration. Eur. J. Pain 2020, 24, 497–517. [Google Scholar] [CrossRef]
- Derakhshan, I. The diagnosis and treatment of chronic migraine: The case for daily scheduled opioid treatment in chronic headache. Ther. Adv. Chronic. Dis. 2015, 6, 389. [Google Scholar] [CrossRef]
- Rothrock, J.F. Opiate and opioid (“narcotic”) therapy for acute migraine headache. Headache 2010, 50, 1255–1256. [Google Scholar] [CrossRef]
- Srivastava, D.; Hill, S.; Carty, S.; Rockett, M.; Bastable, R.; Knaggs, R.; Lambert, D.; Levy, N.; Hughes, J.; Wilkinson, P. Surgery and opioids: Evidence-based expert consensus guidelines on the perioperative use of opioids in the United Kingdom. Br. J. Anaesth. 2021, 126, 1208–1216. [Google Scholar] [CrossRef]
- Kelley-Quon, L.I.; Kirkpatrick, M.G.; Ricca, R.L.; Baird, R.; Harbaugh, C.M.; Brady, A.; Garrett, P.; Wills, H.; Argo, J.; Diefenbach, K.A.; et al. Guidelines for Opioid Prescribing in Children and Adolescents After Surgery: An Expert Panel Opinion. JAMA Surg. 2021, 156, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Bui, T. Opioid-free analgesia after surgery. Lancet 2022, 399, 2245–2247. [Google Scholar] [CrossRef] [PubMed]
- Berecki-Gisolf, J.; Hassani-Mahmooei, B.; Collie, A.; McClure, R. Prescription Opioid and Benzodiazepine Use After Road Traffic Injury. Pain Med. 2016, 17, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Bemand-Qureshi, L.; Gishen, F.; Tookman, A. Opioid use in palliative care: New developments and guidelines. Prescriber 2019, 30, 25–31. [Google Scholar] [CrossRef]
- Lau, J.; Mazzotta, P.; Whelan, C.; Abdelaal, M.; Clarke, H.; Furlan, A.D.; Smith, A.; Husain, A.; Fainsinger, R.; Hui, D.; et al. Opioid safety recommendations in adult palliative medicine: A North American Delphi expert consensus. BMJ Support. Palliat. Care 2022, 12, 81–90. [Google Scholar] [CrossRef]
- Rome, R.B.; Luminais, H.H.; Bourgeois, D.A.; Blais, C.M. The role of palliative care at the end of life. Ochsner. J. 2011, 11, 348–352. [Google Scholar] [PubMed]
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid complications and side effects. Pain Physician 2008, 11, S105–S120. [Google Scholar] [CrossRef]
- Adverse effects of opioids for non-cancer pain. Drug Ther. Bull. 2018, 56, 15–16. [CrossRef]
- Els, C.; Jackson, T.D.; Kunyk, D.; Lappi, V.G.; Sonnenberg, B.; Hagtvedt, R.; Sharma, S.; Kolahdooz, F.; Straube, S. Adverse events associated with medium- and long-term use of opioids for chronic non-cancer pain: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 10, CD012509. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S. Opioid Analgesics Adverse Effects: The Other Side of the Coin. Curr. Pharm. Des. 2019, 25, 3197–3202. [Google Scholar] [CrossRef]
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid Analgesia and Opioid-Induced Adverse Effects: A Review. Pharmaceuticals 2021, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Corli, O.; Santucci, C.; Corsi, N.; Radrezza, S.; Galli, F.; Bosetti, C. The Burden of Opioid Adverse Events and the Influence on Cancer Patients’ Symptomatology. J. Pain Symptom Manag. 2019, 57, 899–908.e6. [Google Scholar] [CrossRef] [PubMed]
- Schug, S.A.; Zech, D.; Grond, S. Adverse effects of systemic opioid analgesics. Drug Saf. 1992, 7, 200–213. [Google Scholar] [CrossRef]
- Vella-Brincat, J.; MacLeod, A.D. Adverse Effects of Opioids on the Central Nervous Systems of Palliative Care Patients. J. Pain Palliat. Care Pharmacother. 2009, 21, 15–25. [Google Scholar] [CrossRef]
- Pergolizzi, J.V.; Varrassi, G.; Paladini, A.; LeQuang, J. Stopping or Decreasing Opioid Therapy in Patients on Chronic Opioid Therapy. Pain Ther. 2019, 8, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Vallersnes, O.M.; Jacobsen, D.; Ekeberg, O.; Brekke, M. Mortality, morbidity and follow-up after acute poisoning by substances of abuse: A prospective observational cohort study. Scand. J. Public Health 2019, 47, 452–461. [Google Scholar] [CrossRef]
- Kosten, T.R.; Baxter, L.E. Review article: Effective management of opioid withdrawal symptoms: A gateway to opioid dependence treatment. Am. J. Addict. 2019, 28, 55–62. [Google Scholar] [CrossRef]
- Pergolizzi, J.V., Jr.; Raffa, R.B.; Rosenblatt, M.H. Opioid withdrawal symptoms, a consequence of chronic opioid use and opioid use disorder: Current understanding and approaches to management. J. Clin. Pharm. Ther. 2020, 45, 892–903. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Gaskin, D.J.; Richard, P. The economic costs of pain in the United States. J. Pain 2012, 13, 715–724. [Google Scholar] [CrossRef]
- Florence, C.; Luo, F.; Rice, K. The economic burden of opioid use disorder and fatal opioid overdose in the United States, 2017. Drug Alcohol. Depend. 2021, 218, 108350. [Google Scholar] [CrossRef]
- Smith, T.J.; Hillner, B.E. The Cost of Pain. JAMA Netw. Open 2019, 2, e191532. [Google Scholar] [CrossRef] [PubMed]
- Machelska, H.; Celik, M.O. Advances in Achieving Opioid Analgesia Without Side Effects. Front. Pharmacol. 2018, 9, 1388. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.H.; Pasternak, G.W. Historical review: Opioid receptors. Trends Pharmacol. Sci. 2003, 24, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mestek, A.; Liu, J.; Hurley, J.A.; Yu, L. Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol. Pharmacol. 1993, 44, 8–12. [Google Scholar] [CrossRef]
- Kieffer, B.L.; Befort, K.; Gaveriaux-Ruff, C.; Hirth, C.G. The delta-opioid receptor: Isolation of a cDNA by expression cloning and pharmacological characterization. Proc. Natl. Acad. Sci. USA 1992, 89, 12048–12052. [Google Scholar] [CrossRef] [PubMed]
- Minami, M.; Toya, T.; Katao, Y.; Maekawa, K.; Nakamura, S.; Onogi, T.; Kaneko, S.; Satoh, M. Cloning and expression of a cDNA for the rat kappa-opioid receptor. FEBS Lett. 1993, 329, 291–295. [Google Scholar] [CrossRef]
- Cox, B.M. A Concise Review of Concepts in Opioid Pharmacology up to the Discovery of Endogenous Opioids. Mol. Pharmacol. 2020, 98, 392–400. [Google Scholar] [CrossRef]
- Higginbotham, J.A.; Markovic, T.; Massaly, N.; Moron, J.A. Endogenous opioid systems alterations in pain and opioid use disorder. Front. Syst. Neurosci. 2022, 16, 1014768. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Rasmussen, S.G.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef]
- Al-Hasani, R.; Bruchas, M.R. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 2011, 115, 1363–1381. [Google Scholar] [CrossRef] [PubMed]
- Brogi, S.; Tafi, A.; Desaubry, L.; Nebigil, C.G. Discovery of GPCR ligands for probing signal transduction pathways. Front. Pharmacol. 2014, 5, 255. [Google Scholar] [CrossRef] [PubMed]
- Yamada, D.; Takahashi, J.; Iio, K.; Nagase, H.; Saitoh, A. Modulation of glutamatergic synaptic transmission and neuronal excitability in the prelimbic medial prefrontal cortex via delta-opioid receptors in mice. Biochem. Biophys. Res. Commun. 2021, 560, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Freye, E.; Levy, J. Constitutive opioid receptor activation: A prerequisite mechanism involved in acute opioid withdrawal. Addict. Biol. 2005, 10, 131–137. [Google Scholar] [CrossRef]
- Trescot, A.M.; Datta, S.; Lee, M.; Hansen, H. Opioid pharmacology. Pain Physician 2008, 11, S133–S153. [Google Scholar] [CrossRef]
- Le Merrer, J.; Becker, J.A.; Befort, K.; Kieffer, B.L. Reward processing by the opioid system in the brain. Physiol. Rev. 2009, 89, 1379–1412. [Google Scholar] [CrossRef]
- Reeves, K.C.; Shah, N.; Munoz, B.; Atwood, B.K. Opioid Receptor-Mediated Regulation of Neurotransmission in the Brain. Front. Mol. Neurosci. 2022, 15, 919773. [Google Scholar] [CrossRef]
- Eisenstein, T.K. The Role of Opioid Receptors in Immune System Function. Front. Immunol. 2019, 10, 2904. [Google Scholar] [CrossRef]
- Patel, D.; Callaway, J.; Vaezi, M. Opioid-Induced Foregut Dysfunction. Am. J. Gastroenterol. 2019, 114, 1716–1725. [Google Scholar] [CrossRef]
- Drews, E.; Zimmer, A. Modulation of alcohol and nicotine responses through the endogenous opioid system. Prog. Neurobiol. 2010, 90, 1–15. [Google Scholar] [CrossRef]
- Galaj, E.; Xi, Z.X. Progress in opioid reward research: From a canonical two-neuron hypothesis to two neural circuits. Pharmacol. Biochem. Behav. 2021, 200, 173072. [Google Scholar] [CrossRef] [PubMed]
- Reisine, T.; Bell, G.I. Molecular biology of opioid receptors. Trends Neurosci. 1993, 16, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Matsui, A.; Jarvie, B.C.; Robinson, B.G.; Hentges, S.T.; Williams, J.T. Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal. Neuron 2014, 82, 1346–1356. [Google Scholar] [CrossRef] [PubMed]
- Dumas, E.O.; Pollack, G.M. Opioid tolerance development: A pharmacokinetic/pharmacodynamic perspective. AAPS J. 2008, 10, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ma, R.; Jin, Y.; Fang, J.; Du, J.; Shao, X.; Liang, Y.; Fang, J. Molecular mechanisms of opioid tolerance: From opioid receptors to inflammatory mediators (Review). Exp. Ther. Med. 2021, 22, 1004. [Google Scholar] [CrossRef] [PubMed]
- Eidson, L.N.; Murphy, A.Z. Inflammatory mediators of opioid tolerance: Implications for dependency and addiction. Peptides 2019, 115, 51–58. [Google Scholar] [CrossRef]
- Kosten, T.R.; George, T.P. The neurobiology of opioid dependence: Implications for treatment. Sci. Pract. Perspect. 2002, 1, 13–20. [Google Scholar] [CrossRef]
- Bassareo, V.; Tanda, G.; Di Chiara, G. Increase of extracellular dopamine in the medial prefrontal cortex during spontaneous and naloxone-precipitated opiate abstinence. Psychopharmacology 1995, 122, 202–205. [Google Scholar] [CrossRef]
- Rossetti, Z.L.; Longu, G.; Mercuro, G.; Gessa, G.L. Extraneuronal noradrenaline in the prefrontal cortex of morphine-dependent rats: Tolerance and withdrawal mechanisms. Brain Res. 1993, 609, 316–320. [Google Scholar] [CrossRef]
- Espejo, E.F.; Serrano, M.I.; Caille, S.; Stinus, L. Behavioral expression of opiate withdrawal is altered after prefrontocortical dopamine depletion in rats: Monoaminergic correlates. Neuropsychopharmacology 2001, 25, 204–212. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.L.; Xiang, X.H.; Zhao, Y. The role of glutamate and its receptors in mesocorticolimbic dopaminergic regions in opioid addiction. Neurosci. Biobehav. Rev. 2009, 33, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K. Afferent effects on locus coeruleus in opiate withdrawal. Prog. Brain Res. 1991, 88, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Leri, F.; Ho, A.; Kreek, M.J. Suppression of hypothalamic-pituitary-adrenal axis by acute heroin challenge in rats during acute and chronic withdrawal from chronic heroin administration. Neurochem. Res. 2013, 38, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Shokri-Kojori, E.; Wang, G.J.; Volkow, N.D. Naloxone precipitated withdrawal increases dopamine release in the dorsal striatum of opioid dependent men. Transl. Psychiatry 2021, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Coffin, P.O.; Behar, E.; Rowe, C.; Santos, G.M.; Coffa, D.; Bald, M.; Vittinghoff, E. Nonrandomized Intervention Study of Naloxone Coprescription for Primary Care Patients Receiving Long-Term Opioid Therapy for Pain. Ann. Intern. Med. 2016, 165, 245–252. [Google Scholar] [CrossRef]
- Kleber, H.D. Pharmacologic treatments for opioid dependence: Detoxification and maintenance options. Dialogues Clin. Neurosci. 2007, 9, 455–470. [Google Scholar] [CrossRef]
- Dahan, A.; Yassen, A.; Romberg, R.; Sarton, E.; Teppema, L.; Olofsen, E.; Danhof, M. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br. J. Anaesth. 2006, 96, 627–632. [Google Scholar] [CrossRef]
- Connery, H.S. Medication-assisted treatment of opioid use disorder: Review of the evidence and future directions. Harv. Rev. Psychiatry 2015, 23, 63–75. [Google Scholar] [CrossRef]
- Fiellin, D.A.; Schottenfeld, R.S.; Cutter, C.J.; Moore, B.A.; Barry, D.T.; O’Connor, P.G. Primary care-based buprenorphine taper vs maintenance therapy for prescription opioid dependence: A randomized clinical trial. JAMA Intern. Med. 2014, 174, 1947–1954. [Google Scholar] [CrossRef]
- Kakko, J.; Svanborg, K.D.; Kreek, M.J.; Heilig, M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: A randomised, placebo-controlled trial. Lancet 2003, 361, 662–668. [Google Scholar] [CrossRef]
- Mattick, R.P.; Breen, C.; Kimber, J.; Davoli, M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2014, 6, CD002207. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.; Lamb, K.; Thomas, M.L.; Khentigan, W. Buprenorphine Maintenance Treatment of Opiate Dependence: Correlations Between Prescriber Beliefs and Practices. Subst. Use Misuse 2016, 51, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Kreek, M.J. Methadone-related opioid agonist pharmacotherapy for heroin addiction. History, recent molecular and neurochemical research and future in mainstream medicine. Ann. N. Y. Acad. Sci. 2000, 909, 186–216. [Google Scholar] [CrossRef] [PubMed]
- Koehl, J.L.; Zimmerman, D.E.; Bridgeman, P.J. Medications for management of opioid use disorder. Am. J. Health Syst. Pharm. 2019, 76, 1097–1103. [Google Scholar] [CrossRef]
- Mattick, R.P.; Breen, C.; Kimber, J.; Davoli, M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst. Rev. 2009, 2009, CD002209. [Google Scholar] [CrossRef]
- Schwartz, R.P.; Highfield, D.A.; Jaffe, J.H.; Brady, J.V.; Butler, C.B.; Rouse, C.O.; Callaman, J.M.; O’Grady, K.E.; Battjes, R.J. A randomized controlled trial of interim methadone maintenance. Arch. Gen. Psychiatry 2006, 63, 102–109. [Google Scholar] [CrossRef]
- Krupitsky, E.; Nunes, E.V.; Ling, W.; Illeperuma, A.; Gastfriend, D.R.; Silverman, B.L. Injectable extended-release naltrexone for opioid dependence: A double-blind, placebo-controlled, multicentre randomised trial. Lancet 2011, 377, 1506–1513. [Google Scholar] [CrossRef]
- Lee, J.D.; Nunes, E.V., Jr.; Novo, P.; Bachrach, K.; Bailey, G.L.; Bhatt, S.; Farkas, S.; Fishman, M.; Gauthier, P.; Hodgkins, C.C.; et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): A multicentre, open-label, randomised controlled trial. Lancet 2018, 391, 309–318. [Google Scholar] [CrossRef]
- Nunes, E.V.; Krupitsky, E.; Ling, W.; Zummo, J.; Memisoglu, A.; Silverman, B.L.; Gastfriend, D.R. Treating Opioid Dependence With Injectable Extended-Release Naltrexone (XR-NTX): Who Will Respond? J. Addict. Med. 2015, 9, 238–243. [Google Scholar] [CrossRef]
- Minozzi, S.; Amato, L.; Vecchi, S.; Davoli, M.; Kirchmayer, U.; Verster, A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst. Rev. 2011, 2011, CD001333. [Google Scholar] [CrossRef]
- Krupitsky, E.; Nunes, E.V.; Ling, W.; Gastfriend, D.R.; Memisoglu, A.; Silverman, B.L. Injectable extended-release naltrexone (XR-NTX) for opioid dependence: Long-term safety and effectiveness. Addiction 2013, 108, 1628–1637. [Google Scholar] [CrossRef]
- Syed, Y.Y.; Keating, G.M. Extended-release intramuscular naltrexone (VIVITROL(R)): A review of its use in the prevention of relapse to opioid dependence in detoxified patients. CNS Drugs 2013, 27, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Jackson, H.; Mandell, K.; Johnson, K.; Chatterjee, D.; Vanness, D.J. Cost-Effectiveness of Injectable Extended-Release Naltrexone Compared with Methadone Maintenance and Buprenorphine Maintenance Treatment for Opioid Dependence. Subst. Abus. 2023, 36, 226–231. [Google Scholar] [CrossRef]
- Available online: https://nida.nih.gov/publications/research-reports/medications-to-treat-opioid-addiction/efficacy-medications-opioid-use-disorder (accessed on 10 June 2023).
- Sordo, L.; Barrio, G.; Bravo, M.J.; Indave, B.I.; Degenhardt, L.; Wiessing, L.; Ferri, M.; Pastor-Barriuso, R. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. Br. Med. J. 2017, 357, j1550. [Google Scholar] [CrossRef]
- Fareed, A.; Patil, D.; Scheinberg, K.; Blackinton Gale, R.; Vayalapalli, S.; Casarella, J.; Drexler, K. Comparison of QTc interval prolongation for patients in methadone versus buprenorphine maintenance treatment: A 5-year follow-up. J. Addict. Dis. 2013, 32, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Berger, O.; Rector, K.; Meredith, J.; Sebaaly, J. Evaluation of drug-drug interactions in hospitalized patients on medications for OUD. Ment. Health Clin. 2021, 11, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Schuckit, M.A. Treatment of Opioid-Use Disorders. N. Engl. J. Med. 2016, 375, 357–368. [Google Scholar] [CrossRef]
- Blanco, C.; Volkow, N.D. Management of opioid use disorder in the USA: Present status and future directions. Lancet 2019, 393, 1760–1772. [Google Scholar] [CrossRef]
- Volkow, N.D.; Jones, E.B.; Einstein, E.B.; Wargo, E.M. Prevention and Treatment of Opioid Misuse and Addiction: A Review. JAMA Psychiatry 2019, 76, 208–216. [Google Scholar] [CrossRef]
- Marsden, J.; Stillwell, G.; Hellier, J.; Brown, A.M.; Byford, S.; Kelleher, M.; Kelly, J.; Murphy, C.; Shearer, J.; Mitcheson, L. Effectiveness of adjunctive, personalised psychosocial intervention for non-response to opioid agonist treatment: Study protocol for a pragmatic randomised controlled trial. Contemp. Clin. Trials 2017, 53, 36–43. [Google Scholar] [CrossRef]
- McHugh, R.K.; Hearon, B.A.; Otto, M.W. Cognitive behavioral therapy for substance use disorders. Psychiatr Clin. N. Am. 2010, 33, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Kakko, J.; Alho, H.; Baldacchino, A.; Molina, R.; Nava, F.A.; Shaya, G. Craving in Opioid Use Disorder: From Neurobiology to Clinical Practice. Front. Psychiatry 2019, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.C.; Murphy, J.G.; Witkiewitz, K.; Hand, S.B.; Thomas, F.; Johnson, K.C.; Cowan, R.; Harris, M.; Derefinko, K.J. Use of a sequential multiple assignment randomized trial to test contingency management and an integrated behavioral economic and mindfulness intervention for buprenorphine-naloxone medication adherence for opioid use disorder. Trials 2023, 24, 237. [Google Scholar] [CrossRef]
- Petry, N.M.; Martin, B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. J. Consult. Clin. Psychol. 2002, 70, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, M.; Podus, D.; Finney, J.; Greenwell, L.; Roll, J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction 2006, 101, 1546–1560. [Google Scholar] [CrossRef]
- Messina, N.; Farabee, D.; Rawson, R. Treatment responsivity of cocaine-dependent patients with antisocial personality disorder to cognitive-behavioral and contingency management interventions. J. Consult. Clin. Psychol. 2003, 71, 320–329. [Google Scholar] [CrossRef]
- Preston, K.L.; Umbricht, A.; Epstein, D.H. Abstinence reinforcement maintenance contingency and one-year follow-up. Drug Alcohol. Depend. 2002, 67, 125–137. [Google Scholar] [CrossRef]
- Willner-Reid, J.; Whitaker, D.; Epstein, D.H.; Phillips, K.A.; Pulaski, A.R.; Preston, K.L.; Willner, P. Cognitive-behavioural therapy for heroin and cocaine use: Ecological momentary assessment of homework simplification and compliance. Psychol. Psychother 2016, 89, 276–293. [Google Scholar] [CrossRef]
- Dutra, L.; Stathopoulou, G.; Basden, S.L.; Leyro, T.M.; Powers, M.B.; Otto, M.W. A meta-analytic review of psychosocial interventions for substance use disorders. Am. J. Psychiatry 2008, 165, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Bowen, S.; Chawla, N.; Collins, S.E.; Witkiewitz, K.; Hsu, S.; Grow, J.; Clifasefi, S.; Garner, M.; Douglass, A.; Larimer, M.E.; et al. Mindfulness-based relapse prevention for substance use disorders: A pilot efficacy trial. Subst. Abus. 2009, 30, 295–305. [Google Scholar] [CrossRef]
- Price, C.J.; Merrill, J.O.; McCarty, R.L.; Pike, K.C.; Tsui, J.I. A pilot study of mindful body awareness training as an adjunct to office-based medication treatment of opioid use disorder. J. Subst. Abus. Treat 2020, 108, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Brady, K.T.; Killeen, T.; Baker, N.L. Efficacy of mindfulness-based relapse prevention in veterans with substance use disorders: Design and methodology of a randomized clinical trial. Contemp. Clin. Trials 2021, 105, 106393. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Hanley, A.W.; Kline, A.; Cooperman, N.A. Mindfulness-Oriented Recovery Enhancement reduces opioid craving among individuals with opioid use disorder and chronic pain in medication assisted treatment: Ecological momentary assessments from a stage 1 randomized controlled trial. Drug Alcohol. Depend. 2019, 203, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.F.; Kaminer, Y.; Kahler, C.W.; Hoeppner, B.; Yeterian, J.; Cristello, J.V.; Timko, C. A pilot randomized clinical trial testing integrated 12-Step facilitation (iTSF) treatment for adolescent substance use disorder. Addiction 2017, 112, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Attwood, L.O.; McKechnie, M.; Vujovic, O.; Higgs, P.; Lloyd-Jones, M.; Doyle, J.S.; Stewardson, A.J. Review of management priorities for invasive infections in people who inject drugs: Highlighting the need for patient-centred multidisciplinary care. Med. J. Aust. 2022, 217, 102–109. [Google Scholar] [CrossRef]
- Levengood, T.W.; Yoon, G.H.; Davoust, M.J.; Ogden, S.N.; Marshall, B.D.L.; Cahill, S.R.; Bazzi, A.R. Supervised Injection Facilities as Harm Reduction: A Systematic Review. Am. J. Prev. Med. 2021, 61, 738–749. [Google Scholar] [CrossRef]
- Giulini, F.; Keenan, E.; Killeen, N.; Ivers, J.H. A Systematized Review of Drug-checking and Related Considerations for Implementation as A Harm Reduction Intervention. J. Psychoact. Drugs 2023, 55, 85–93. [Google Scholar] [CrossRef]
- Lloyd-Smith, E.; Wood, E.; Zhang, R.; Tyndall, M.W.; Sheps, S.; Montaner, J.S.; Kerr, T. Determinants of hospitalization for a cutaneous injection-related infection among injection drug users: A cohort study. BMC Public Health 2010, 10, 327. [Google Scholar] [CrossRef]
- Madah-Amiri, D.; Skulberg, A.K.; Braarud, A.C.; Dale, O.; Heyerdahl, F.; Lobmaier, P.; Clausen, T. Ambulance-attended opioid overdoses: An examination into overdose locations and the role of a safe injection facility. Subst. Abus. 2019, 40, 383–388. [Google Scholar] [CrossRef]
- Marshall, B.D.; Milloy, M.J.; Wood, E.; Montaner, J.S.; Kerr, T. Reduction in overdose mortality after the opening of North America’s first medically supervised safer injecting facility: A retrospective population-based study. Lancet 2011, 377, 1429–1437. [Google Scholar] [CrossRef]
- Myer, A.J.; Belisle, L. Highs and Lows: An Interrupted Time-Series Evaluation of the Impact of North America’s Only Supervised Injection Facility on Crime. J. Drug Issues 2017, 48, 36–49. [Google Scholar] [CrossRef]
- Salmon, A.M.; van Beek, I.; Amin, J.; Kaldor, J.; Maher, L. The impact of a supervised injecting facility on ambulance call-outs in Sydney, Australia. Addiction 2010, 105, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Stoltz, J.A.; Wood, E.; Small, W.; Li, K.; Tyndall, M.; Montaner, J.; Kerr, T. Changes in injecting practices associated with the use of a medically supervised safer injection facility. J. Public Health 2007, 29, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Gold, M.S.; Fuehrlein, B.S. Looking beyond the opioid receptor: A desperate need for new treatments for opioid use disorder. J. Neurol. Sci. 2022, 432, 120094. [Google Scholar] [CrossRef] [PubMed]
- Varadi, A.; Marrone, G.F.; Palmer, T.C.; Narayan, A.; Szabo, M.R.; Le Rouzic, V.; Grinnell, S.G.; Subrath, J.J.; Warner, E.; Kalra, S.; et al. Mitragynine/Corynantheidine Pseudoindoxyls As Opioid Analgesics with Mu Agonism and Delta Antagonism, Which Do Not Recruit beta-Arrestin-2. J. Med. Chem. 2016, 59, 8381–8397. [Google Scholar] [CrossRef]
- Torres-Lockhart, K.E.; Lu, T.Y.; Weimer, M.B.; Stein, M.R.; Cunningham, C.O. Clinical Management of Opioid Withdrawal. Addiction 2022, 117, 2540–2550. [Google Scholar] [CrossRef]
- Jordan, C.G.; Kennalley, A.L.; Roberts, A.L.; Nemes, K.M.; Dolma, T.; Piper, B.J. The Potential of Methocinnamox as a Future Treatment for Opioid Use Disorder: A Narrative Review. Pharmacy 2022, 10, 48. [Google Scholar] [CrossRef]
- Soergel, D.G.; Subach, R.A.; Burnham, N.; Lark, M.W.; James, I.E.; Sadler, B.M.; Skobieranda, F.; Violin, J.D.; Webster, L.R. Biased agonism of the mu-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: A randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain 2014, 155, 1829–1835. [Google Scholar] [CrossRef]
- DeWire, S.M.; Yamashita, D.S.; Rominger, D.H.; Liu, G.; Cowan, C.L.; Graczyk, T.M.; Chen, X.T.; Pitis, P.M.; Gotchev, D.; Yuan, C.; et al. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J. Pharmacol. Exp. Ther. 2013, 344, 708–717. [Google Scholar] [CrossRef]
- De Aquino, J.P.; Bahji, A.; Gomez, O.; Sofuoglu, M. Alleviation of opioid withdrawal by cannabis and delta-9-tetrahydrocannabinol: A systematic review of observational and experimental human studies. Drug Alcohol. Depend. 2022, 241, 109702. [Google Scholar] [CrossRef]
- Wang, S.C.; Chen, Y.C.; Lee, C.H.; Cheng, C.M. Opioid Addiction, Genetic Susceptibility, and Medical Treatments: A Review. Int. J. Mol. Sci. 2019, 20, 4294. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, C.A.; Kearney-Ramos, T.; Dowdle, L.T.; Hamilton, S.; DeVries, W.; Mithoefer, O.; Austelle, C.; Lench, D.H.; Correia, B.; Canterberry, M.; et al. Developing Repetitive Transcranial Magnetic Stimulation (rTMS) as a Treatment Tool for Cocaine Use Disorder: A Series of Six Translational Studies. Curr. Behav. Neurosci. Rep. 2017, 4, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Razza, L.B.; Palumbo, P.; Moffa, A.H.; Carvalho, A.F.; Solmi, M.; Loo, C.K.; Brunoni, A.R. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress. Anxiety 2020, 37, 594–608. [Google Scholar] [CrossRef]
- Troster, A.I. Neuropsychology of deep brain stimulation in neurology and psychiatry. Front. Biosci. 2009, 14, 1857–1879. [Google Scholar] [CrossRef]
- Bauer, S.; Baier, H.; Baumgartner, C.; Bohlmann, K.; Fauser, S.; Graf, W.; Hillenbrand, B.; Hirsch, M.; Last, C.; Lerche, H.; et al. Transcutaneous Vagus Nerve Stimulation (tVNS) for Treatment of Drug-Resistant Epilepsy: A Randomized, Double-Blind Clinical Trial (cMPsE02). Brain Stimul. 2016, 9, 356–363. [Google Scholar] [CrossRef]
- Bond, A.E.; Shah, B.B.; Huss, D.S.; Dallapiazza, R.F.; Warren, A.; Harrison, M.B.; Sperling, S.A.; Wang, X.Q.; Gwinn, R.; Witt, J.; et al. Safety and Efficacy of Focused Ultrasound Thalamotomy for Patients With Medication-Refractory, Tremor-Dominant Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. 2017, 74, 1412–1418. [Google Scholar] [CrossRef]
- Mahoney, J.J., 3rd; Hanlon, C.A.; Marshalek, P.J.; Rezai, A.R.; Krinke, L. Transcranial magnetic stimulation, deep brain stimulation, and other forms of neuromodulation for substance use disorders: Review of modalities and implications for treatment. J. Neurol. Sci. 2020, 418, 117149. [Google Scholar] [CrossRef]
- Bogenschutz, M.P.; Johnson, M.W. Classic hallucinogens in the treatment of addictions. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 250–258. [Google Scholar] [CrossRef]
- Shao, W.; Kuhn, C.; Mayr, D.; Ditsch, N.; Kailuwait, M.; Wolf, V.; Harbeck, N.; Mahner, S.; Jeschke, U.; Cavailles, V.; et al. Cytoplasmic LXR expression is an independent marker of poor prognosis for patients with early stage primary breast cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 2535–2544. [Google Scholar] [CrossRef]
- Rodrigues, L.S.; Rossi, G.N.; Rocha, J.M.; Osório, F.L.; Bouso, J.C.; Hallak, J.E.C.; Dos Santos, R.G. Effects of ayahuasca and its alkaloids on substance use disorders: An updated (2016–2020) systematic review of preclinical and human studies. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 541–556. [Google Scholar] [CrossRef]
- Smith, A.C.W.; Kenny, P.J. MicroRNAs regulate synaptic plasticity underlying drug addiction. Genes Brain Behav. 2018, 17, e12424. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, O. Current Trends and Perspectives in the Immune Therapy for Substance Use Disorders. Front. Psychiatry 2022, 13, 882491. [Google Scholar] [CrossRef] [PubMed]
- Bremer, P.T.; Janda, K.D. Conjugate Vaccine Immunotherapy for Substance Use Disorder. Pharmacol. Rev. 2017, 69, 298–315. [Google Scholar] [CrossRef]
- Zamora, J.C.; Smith, H.R.; Jennings, E.M.; Chavera, T.S.; Kotipalli, V.; Jay, A.; Husbands, S.M.; Disney, A.; Berg, K.A.; Clarke, W.P. Long-term antagonism and allosteric regulation of mu opioid receptors by the novel ligand, methocinnamox. Pharmacol. Res. Perspect. 2021, 9, e00887. [Google Scholar] [CrossRef] [PubMed]
- Townsend, E.A. The lasting impact of methocinnamox on opioid self-administration. Neuropsychopharmacology 2020, 45, 1963–1964. [Google Scholar] [CrossRef] [PubMed]
- Broadbear, J.H.; Sumpter, T.L.; Burke, T.F.; Husbands, S.M.; Lewis, J.W.; Woods, J.H.; Traynor, J.R. Methocinnamox is a potent, long-lasting, and selective antagonist of morphine-mediated antinociception in the mouse: Comparison with clocinnamox, beta-funaltrexamine, and beta-chlornaltrexamine. J. Pharmacol. Exp. Ther. 2000, 294, 933–940. [Google Scholar]
- Maguire, D.R.; Gerak, L.R.; Sanchez, J.J.; Javors, M.A.; Disney, A.; Husbands, S.M.; France, C.P. Effects of acute and repeated treatment with methocinnamox, a mu opioid receptor antagonist, on fentanyl self-administration in rhesus monkeys. Neuropsychopharmacology 2020, 45, 1986–1993. [Google Scholar] [CrossRef]
- Jimenez, V.M., Jr.; Castaneda, G.; France, C.P. Methocinnamox Reverses and Prevents Fentanyl-Induced Ventilatory Depression in Rats. J. Pharmacol. Exp. Ther. 2021, 377, 29–38. [Google Scholar] [CrossRef]
- Gerak, L.R.; Maguire, D.R.; Woods, J.H.; Husbands, S.M.; Disney, A.; France, C.P. Reversal and Prevention of the Respiratory-Depressant Effects of Heroin by the Novel mu-Opioid Receptor Antagonist Methocinnamox in Rhesus Monkeys. J. Pharmacol. Exp. Ther. 2019, 368, 229–236. [Google Scholar] [CrossRef]
- Maguire, D.R.; Gerak, L.R.; Woods, J.H.; Husbands, S.M.; Disney, A.; France, C.P. Long-Lasting Effects of Methocinnamox on Opioid Self-Administration in Rhesus Monkeys. J. Pharmacol. Exp. Ther. 2019, 368, 88–99. [Google Scholar] [CrossRef]
- Coe, M.A.; Pillitteri, J.L.; Sembower, M.A.; Gerlach, K.K.; Henningfield, J.E. Kratom as a substitute for opioids: Results from an online survey. Drug Alcohol. Depend. 2019, 202, 24–32. [Google Scholar] [CrossRef]
- Garcia-Romeu, A.; Cox, D.J.; Smith, K.E.; Dunn, K.E.; Griffiths, R.R. Kratom (Mitragyna speciosa): User demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol. Depend. 2020, 208, 107849. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, O. Patterns of Kratom use and health impact in the US-Results from an online survey. Drug Alcohol. Depend. 2017, 176, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Veltri, C.; Grundmann, O. Current perspectives on the impact of Kratom use. Subst. Abus. Rehabil. 2019, 10, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Drug Enforcement Administration. Kratom (Mitragynine speciose korth) (Street Names: Thang, Kakuam, Thom, Ketum, Biak). Unites States Department of Justice, Drug Enforcement Administration; 2013; Drug Enforcement Administration. In Drugs of Abuse. A DEA Resource Guide; United States Department of Justice, Drug Enforcement Administration: Washington, DC, USA, 2017; Volume 94. [Google Scholar]
- Kruegel, A.C.; Grundmann, O. The medicinal chemistry and neuropharmacology of kratom: A preliminary discussion of a promising medicinal plant and analysis of its potential for abuse. Neuropharmacology 2018, 134, 108–120. [Google Scholar] [CrossRef]
- Takayama, H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem. Pharm. Bull. 2004, 52, 916–928. [Google Scholar] [CrossRef]
- Kruegel, A.C.; Gassaway, M.M.; Kapoor, A.; Varadi, A.; Majumdar, S.; Filizola, M.; Javitch, J.A.; Sames, D. Synthetic and Receptor Signaling Explorations of the Mitragyna Alkaloids: Mitragynine as an Atypical Molecular Framework for Opioid Receptor Modulators. J. Am. Chem. Soc. 2016, 138, 6754–6764. [Google Scholar] [CrossRef]
- Hemby, S.E.; McIntosh, S.; Leon, F.; Cutler, S.J.; McCurdy, C.R. Abuse liability and therapeutic potential of the Mitragyna speciosa (kratom) alkaloids mitragynine and 7-hydroxymitragynine. Addict. Biol. 2019, 24, 874–885. [Google Scholar] [CrossRef]
- Yue, K.; Kopajtic, T.A.; Katz, J.L. Abuse liability of mitragynine assessed with a self-administration procedure in rats. Psychopharmacology 2018, 235, 2823–2829. [Google Scholar] [CrossRef]
- Trakulsrichai, S.; Sathirakul, K.; Auparakkitanon, S.; Krongvorakul, J.; Sueajai, J.; Noumjad, N.; Sukasem, C.; Wananukul, W. Pharmacokinetics of mitragynine in man. Drug Des. Dev. Ther. 2015, 9, 2421–2429. [Google Scholar] [CrossRef]
- Henningfield, J.E.; Fant, R.V.; Wang, D.W. The abuse potential of kratom according the 8 factors of the controlled substances act: Implications for regulation and research. Psychopharmacology 2018, 235, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Daksla, N.; Wang, A.; Jin, Z.; Gupta, A.; Bergese, S.D. Oliceridine for the Management of Moderate to Severe Acute Postoperative Pain: A Narrative Review. Drug Des. Dev. Ther. 2023, 17, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Goudra, B. Oliceridine-Opioid of the 21(st) Century. Saudi. J. Anaesth. 2022, 16, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; van Dam, C.J.; Niesters, M.; van Velzen, M.; Fossler, M.J.; Demitrack, M.A.; Olofsen, E. Benefit and Risk Evaluation of Biased mu-Receptor Agonist Oliceridine versus Morphine. Anesthesiology 2020, 133, 559–568. [Google Scholar] [CrossRef]
- Bohn, L.M.; Lefkowitz, R.J.; Gainetdinov, R.R.; Peppel, K.; Caron, M.G.; Lin, F.T. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 1999, 286, 2495–2498. [Google Scholar] [CrossRef]
- Raehal, K.M.; Walker, J.K.; Bohn, L.M. Morphine side effects in beta-arrestin 2 knockout mice. J. Pharmacol. Exp. Ther. 2005, 314, 1195–1201. [Google Scholar] [CrossRef]
- Miyano, K.; Manabe, S.; Komatsu, A.; Fujii, Y.; Mizobuchi, Y.; Uezono, E.; Ohshima, K.; Nonaka, M.; Kuroda, Y.; Narita, M.; et al. The G Protein Signal-Biased Compound TRV130; Structures, Its Site of Action and Clinical Studies. Curr. Top. Med. Chem. 2020, 20, 2822–2829. [Google Scholar] [CrossRef]
- Chen, X.T.; Pitis, P.; Liu, G.; Yuan, C.; Gotchev, D.; Cowan, C.L.; Rominger, D.H.; Koblish, M.; Dewire, S.M.; Crombie, A.L.; et al. Structure-activity relationships and discovery of a G protein biased mu opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl](2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan-9-yl]ethyl)amine (TRV130), for the treatment of acute severe pain. J. Med. Chem. 2013, 56, 8019–8031. [Google Scholar] [CrossRef]
- Oliceridine Briefing Document. FDA Advisory Committee Meeting. 11 October 2018. Available online: https://www.fda.gov/media/121230/download (accessed on 10 April 2023).
- Food and Drug Administration (FDA). Highlights of Prescribing Information—Olinvyk (Oliceridine). 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/210730s001lbl.pdf (accessed on 26 March 2023).
- Liang, D.Y.; Li, W.W.; Nwaneshiudu, C.; Irvine, K.A.; Clark, J.D. Pharmacological Characters of Oliceridine, a mu-Opioid Receptor G-Protein-Biased Ligand in Mice. Anesth. Analg. 2019, 129, 1414–1421. [Google Scholar] [CrossRef]
- Altarifi, A.A.; David, B.; Muchhala, K.H.; Blough, B.E.; Akbarali, H.; Negus, S.S. Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J. Psychopharmacol. 2017, 31, 730–739. [Google Scholar] [CrossRef]
- Soergel, D.G.; Subach, R.A.; Sadler, B.; Connell, J.; Marion, A.S.; Cowan, C.L.; Violin, J.D.; Lark, M.W. First clinical experience with TRV130: Pharmacokinetics and pharmacodynamics in healthy volunteers. J. Clin. Pharmacol. 2014, 54, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Simons, P.; van der Schrier, R.; van Lemmen, M.; Jansen, S.; Kuijpers, K.W.K.; van Velzen, M.; Sarton, E.; Nicklas, T.; Michalsky, C.; Demitrack, M.A.; et al. Respiratory Effects of Biased Ligand Oliceridine in Older Volunteers: A Pharmacokinetic-Pharmacodynamic Comparison with Morphine. Anesthesiology 2023, 138, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Nafziger, A.N.; Arscott, K.A.; Cochrane, K.; Skobieranda, F.; Burt, D.A.; Fossler, M.J. The Influence of Renal or Hepatic Impairment on the Pharmacokinetics, Safety, and Tolerability of Oliceridine. Clin. Pharmacol. Drug Dev. 2020, 9, 639–650. [Google Scholar] [CrossRef]
- Viscusi, E.R.; Webster, L.; Kuss, M.; Daniels, S.; Bolognese, J.A.; Zuckerman, S.; Soergel, D.G.; Subach, R.A.; Cook, E.; Skobieranda, F. A randomized, phase 2 study investigating TRV130, a biased ligand of the mu-opioid receptor, for the intravenous treatment of acute pain. Pain 2016, 157, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Minkowitz, H.S.; Soergel, D.G.; Burt, D.A.; Subach, R.A.; Salamea, M.Y.; Fossler, M.J.; Skobieranda, F. A randomized, Phase IIb study investigating oliceridine (TRV130), a novel micro-receptor G-protein pathway selective (mu-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J. Pain Res. 2017, 10, 2413–2424. [Google Scholar] [CrossRef]
- Viscusi, E.R.; Skobieranda, F.; Soergel, D.G.; Cook, E.; Burt, D.A.; Singla, N. APOLLO-1: A randomized placebo and active-controlled phase III study investigating oliceridine (TRV130), a G protein-biased ligand at the micro-opioid receptor, for management of moderate-to-severe acute pain following bunionectomy. J. Pain Res. 2019, 12, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.K.; Skobieranda, F.; Soergel, D.G.; Salamea, M.; Burt, D.A.; Demitrack, M.A.; Viscusi, E.R. APOLLO-2: A Randomized, Placebo and Active-Controlled Phase III Study Investigating Oliceridine (TRV130), a G Protein-Biased Ligand at the mu-Opioid Receptor, for Management of Moderate to Severe Acute Pain Following Abdominoplasty. Pain Pract. 2019, 19, 715–731. [Google Scholar] [CrossRef]
- Bergese, S.D.; Brzezinski, M.; Hammer, G.B.; Beard, T.L.; Pan, P.H.; Mace, S.E.; Berkowitz, R.D.; Cochrane, K.; Wase, L.; Minkowitz, H.S.; et al. ATHENA: A Phase 3, Open-Label Study Of The Safety And Effectiveness Of Oliceridine (TRV130), A G-Protein Selective Agonist At The micro-Opioid Receptor, In Patients With Moderate To Severe Acute Pain Requiring Parenteral Opioid Therapy. J. Pain Res. 2019, 12, 3113–3126. [Google Scholar] [CrossRef]
- Brzezinski, M.; Hammer, G.B.; Candiotti, K.A.; Bergese, S.D.; Pan, P.H.; Bourne, M.H.; Michalsky, C.; Wase, L.; Demitrack, M.A.; Habib, A.S. Low Incidence of Opioid-Induced Respiratory Depression Observed with Oliceridine Regardless of Age or Body Mass Index: Exploratory Analysis from a Phase 3 Open-Label Trial in Postsurgical Pain. Pain Ther. 2021, 10, 457–473. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, Y.; He, B.; He, X.; Zhou, X.E.; Guo, S.; Rao, Q.; Yang, J.; Liu, J.; Zhou, Q.; et al. Molecular recognition of morphine and fentanyl by the human mu-opioid receptor. Cell 2022, 185, 4361–4375. [Google Scholar] [CrossRef]
- Robertson, M.J.; Papasergi-Scott, M.M.; He, F.; Seven, A.B.; Meyerowitz, J.G.; Panova, O.; Peroto, M.C.; Che, T.; Skiniotis, G. Structure determination of inactive-state GPCRs with a universal nanobody. Nat. Struct. Mol. Biol. 2022, 29, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).