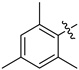
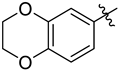
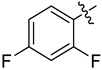
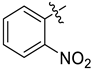
Abstract
Spirocyclic compounds containing heterocyclic moieties represent promising 3D scaffolds for modern drug design. In the search for novel anti-flaviviral agents, we have obtained a series of 3-[N,N-bis(sulfonyl)amino]isoxazolines containing spiro-annulated cyclooctane rings and assessed their antiviral activity against tick-borne encephalitis (TBEV), yellow fever (YFV), and West Nile (WNV) viruses. The structural analogs of spirocyclic compounds with a single sulfonyl group or 1,2-annulated cyclooctane ring were also investigated. Almost all the studied 3-[N,N-bis(sulfonyl)amino]isoxazolines revealed antiviral activity against TBEV and WNV. The most active against TBEV was spiro-isoxazoline derivative containing p-nitrophenyl groups in the sulfonyl part (EC50 2.0 ± 0.5 μM), while the highest potency against WNV was found for the compounds with lipophilic substituents in sulfonyl moiety, naphtyl being the most favorable one (EC50 1.3 ± 0.5 μM). In summary, two novel scaffolds of anti-flaviviral agents based on N,N-bis(sulfonyl)amino]isoxazoline were proposed, and the compounds of this type demonstrated activity against TBEV and WNV.
1. Introduction
In the past two decades, spirocyclic compounds appeared at the focus of research in the field of drug design and discovery as promising 3D scaffolds, enlarging the chemical space, allowing the construction of new types of lead compounds, and improving their potency, selectivity, and pharmacokinetic properties [1]. Spiroheterocyclic motifs are present in a number of approved drugs and natural compounds [1,2,3,4] (Figure 1). Such an interest in spirocyclic structures echoed in the field of synthetic organic chemistry, giving rise to a number of elegant preparative approaches to compounds of this type [5,6,7,8,9,10,11].
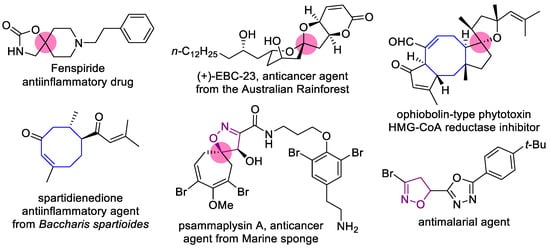
Figure 1.
Examples of approved drugs, natural and bioactive compounds with spirocyclic motifs, medium rings, isoxazoline moieties, and their combinations.
On the other hand, medium-sized rings (8–11 atoms) are rarely used for the construction of spirocyclic structures, reflecting the general tendency concerning medium rings. Although they possess appealing properties, such as a unique balance between rigidity and flexibility and diverse geometry, improve binding affinity to target enzymes, and are present in both bioactive and natural compounds (Figure 1) [12,13,14], application of medium rings in drug design is limited by the lack of synthetic approaches. Successful efforts to obtain the compounds containing medium rings, in most cases with heteroatoms, have been recently reported, illustrating the arising interest in such structures and the need for synthetic methods for them [15,16,17].
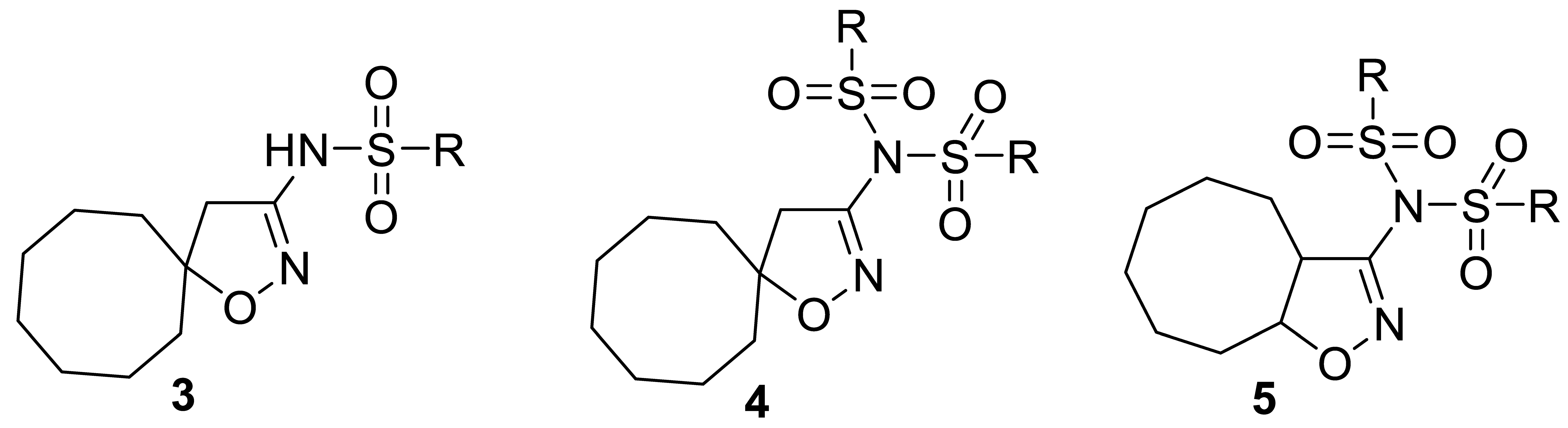
Isoxazoles and isoxazolines reveal a broad range of biological activities, including antitumor, antibacterial, antimalarial, antisclerotic, antidiabetic, and others (Figure 1), and a number of examples exist of how the insertion of an isoxazoline moiety increases the potency of the bioactive compounds [18,19,20,21,22,23]. The most general approach to isoxazoline derivatives is 1,3-dipolar cycloaddition, though other methods, taking advantage of metal-mediated and radical processes, are constantly gaining more significance [24,25]. Recently we have elaborated the preparative approach to spiro- and 1,2-annulated 3-aminoisoxazoles 1,2, containing an 8-membered ring, and demonstrated their potent antiviral activity against influenza A virus (Figure 2) [26]. A series of 3-(N-sulfonylamino)isoxazolines 3 was also obtained and found to possess low-to-no cytotoxicity, making them attractive for the search of other applications in lead discovery [26].
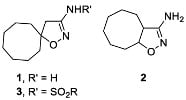
Figure 2.
Previously obtained aminoisoxazoline derivatives 1–3 [26].
Flaviviruses are arthropod-borne viruses that are mainly transmitted between birds and wild animals by the bite of infected blood-sucking arthropods, such as mosquitoes or ticks. Unfortunately, humans could be bitten and infected too, resulting in fevers and encephalitides of different severity, from mild to even lethal, therefore representing a constant epidemic threat [27]. Currently, there are no specific medications for the treatment of flavivirus-borne diseases, so patient care is usually symptomatic, and the main way of preventing infection is vaccination [28]. Nevertheless, vaccines do not provide complete protectivity [29], and there is still no human vaccine against West Nile virus [30]. Thus, the development of specific anti-flaviviral drugs is still very important. Previously we have found several structural types of compounds showing antiviral activity against tick-borne encephalitis (TBEV), yellow fever (YFV), and West Nile (WNV) viruses [28,31,32,33,34,35,36,37,38]. As can be seen in examples (Figure 3), they possess quite a diverse structure.
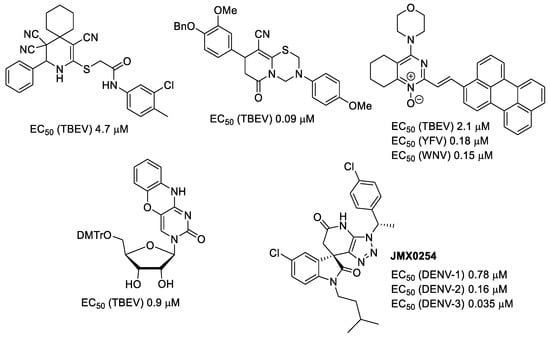
Figure 3.
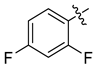
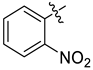
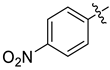
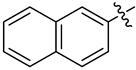
Examples of compounds with antiviral activity against TBEV, YFV, WNV, and DENV [31,32,33,35,39].
A heterocyclic core, spiro-annulated or 1,2-annulated to carbocycle and furnished with lipophilic substituents, may be distinguished as a peculiar pattern. For example, spiropyrazolopyridone derivatives were identified as dengue virus type 2 (DENV-2) inhibitors [40], while substituted 4,6-dihydrospiro[[1,2,3]triazolo[4,5-b]pyridine-7,3′-indoline]-2′,5(3H)-dione analogs such as JMX0254 (Figure 3) effectively inhibit NS4B protein activity of dengue virus type 1 (DENV-1) [39]. That inspired us to synthesize a series of novel 3-[N,N-bis(sulfonyl)amino]isoxazolines 4,5, containing spiro-annulated or 1,2-annulated cyclooctane rings (Figure 4), and probe them as novel anti-flaviviral agents along with the previously obtained series of 3-(N-sulfonylamino)isoxazolines 3.
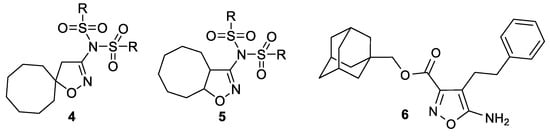
Figure 4.
Structures of 3-[N,N-bis(sulfonyl)amino]isoxazolines 4,5, investigated in the present work, and previously studied isoxazole derivative 6 that was used as a positive control [41].
2. Results and Discussion
2.1. Chemistry
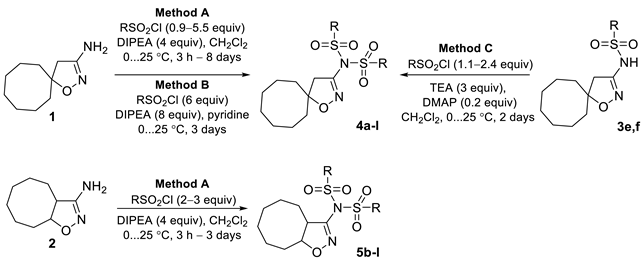
Target 3-[N,N-bis(sulfonyl)amino]isoxazolines were obtained via sulfonylation of 3-aminoisoxazolines 1,2 or, in some cases, 3-(sulfonylamino)isoxazolines 3 upon treatment with corresponding sulfonyl chlorides (Table 1). Depending on the starting amine and sulfonyl chloride, the chosen method, reaction time, and reagent ratio were varied in order to improve the preparative yield (see Section 3.2).

Table 1.
Synthesis of 3-[N,N-bis(sulfonyl)amino]isoxazolines 4a–l and 5b–l.
The interaction of amines 1 and 2 with mesyl chloride and benzyl sulfonyl chloride in the presence of N,N-diisopropylethylamine (DIPEA) (Method A) gave hardly separable mixtures of the products of one- and two-fold sulfonylation, even when an excess of sulfonyl chloride was used. That resulted in lowering the yields of compounds 4a,b, and 5b, and despite all the efforts, we were not able to obtain compound 5a in analytically pure form.
Spirocyclic isoxazoline 1 interacted with aryl sulfonyl chlorides in the presence of DIPEA (Method A), affording the products of two-fold sulfonylation, generally in moderate yields. Nevertheless, bis(sulfonyl)amines 4e,h could not be obtained in these conditions. Compound 4e was successfully obtained via sulfonylation of 3-(sulfonylamino)isoxazoline 3e using the mixture of triethylamine (TEA) and 4-dimethylaminopyridine (DMAP) as the bases (Method C); this method was also probed for the synthesis of bis(sulfonyl)amine 4f more the doubling the yield of the target compound. 2-Nitrobenzenesulfonyl chloride did not give products of two-fold sulfonylation in the presence of N-bases (DIPEA, TEA, DMAP). We managed to obtain compound 4h, containing o-nitrophenyl substituents, only using pyridine as a solvent (Method B).
The reactions of isoxazoline 2 with both EWG- and EDG-substituted aryl sulfonyl chlorides in the presence of DIPEA (Method A) in most cases proceeded smoothly, giving 3-[N,N-bis(sulfonyl)amino]isoxazolines 5c–l in the yields up to quantitative. The exceptions were sterically hindered o-substituted aryl sulfonyl and naphtyl sulfonyl chlorides—the reactions of compound 2 with the latter afforded target heterocycles 5e,h,j in moderate yields.
Such a difference in reactivity between spirocyclic and bicyclic amines 1,2 could be explained according to their geometry. The spiro-annulated carbocycle in compound 1 hinders both sides of the isoxazoline ring, while 1,2-annulated cyclooctane leaves one side of the ring unhindered, thus not preventing the amino group from serving as a nucleophile.
2.2. Antiviral Activity Assessment
For two series of 3-[N,N-bis(sulfonyl)amino]isoxazolines 4a–l and 5b–l, antiviral activity was investigated against TBEV on porcine embryo kidney (PEK) cells using plaque reduction assay based on the estimation of the plaque-forming efficiency of a virus in the presence of different concentrations of a compound. Acute and chronic cytotoxicity of these compounds was studied using resazurin cell viability assay—viable cells turn resazurin into fluorescent resorufin (Table 2; see also Supplementary Materials). In order to compare the bioactivity of mono- and bissulfonylated derivatives, the same tests were performed for the series of previously obtained [26] 3-(N-sulfonylamino)isoxazolines 3a–h,j–l. Previously studied isoxazole derivative 6 (Figure 4) that showed anti-TBEV activity [41] was used as a positive control. Despite it not being structurally similar to the compounds studied in this work, it allowed us to confirm the validity of the obtained EC50 values.

Table 2.
Anti-TBEV activity and chronic cytotoxicity of compounds 3–5.
Most of bis(sulfonyl)amines 4 and 5 and several sulfonylamines 3 revealed anti-TBEV activity in micromolar concentrations. The most favorable substituents in sulfonyl moiety were found to be trimethylphenyl (3–5e), 2- or 4-nitrophenyl (3–5h, 4–5i) and naphtyl (3–5j)—all the types of isoxazolines containing these groups demonstrated antiviral activity.
Comparing spiro-annulated sulfonyl- and bis(sulfonyl)amines 3c–h,j–l and 4c–h,j–l, containing aromatic substituents, one can observe that bis(sulfonyl) derivatives 4 systematically show higher potency than their mono-substituted analogs 3 (3e,h,j vs. 4e,h,j) or demonstrate antiviral activity in the cases where compounds 3 are inactive (3c,d,f,l vs. 4c,d,f,l). On the contrary, mono-benzyl substituted sulfonylamine 3b was more potent than its bis-substituted congener 4b.
Compounds 4b–l and 5b–l, containing spiro-annulated or 1,2-annulated cyclooctane moieties, did not show a clear relation between antiviral activity and the configuration of the two-ring system. Spiro-isoxazolines 4c,d,f,i,l were more potent than 1,2-annulated analogs, while for compounds 4b,g,h,j,k, the relation is opposite. Nevertheless, the most potent compounds of all the investigated species, namely 4c and 4i, belong to the spiro-isoxazoline series.
For all the compounds 3–5, we have also determined the antiviral activity against WNV on African green monkey kidney (Vero) cells using the plaque reduction assay. The acute and chronic cytotoxicity of these compounds was studied using the resazurin cell viability assay (Table 3). Favipiravir was used as a positive control in this screening [42].

Table 3.
Anti-WNV activity and chronic cytotoxicity of compounds 3–5.
Almost all the tested compounds, except 5l, inhibited WNV reproduction. In sulfonylamine series 3, the most active were benzyl- and thiophenyl-substituted derivatives 3b and 3k. All the bis(sulfonyl)amines 4 were active in micromolar concentrations. Bis(sulfonyl)amines 4c–l bearing aromatic or heteroaromatic substituents demonstrated higher potency than methyl or benzyl substituted compounds 4a,b. The highest potency was found for compounds 4c–e,j with lipophilic substituents in sulfonyl moiety, naphtyl being the most favorable one. Isoxazolines 5 containing the 1,2-annulated cyclooctane moiety generally demonstrated lesser anti-WNV activity than their spiro-annulated analogs 4, except benzyl derivative 5b, that was more active than its analog 4b, and almost equally active compounds 4e and 5e. It is also interesting that the addition of the second pyridyl group to the sulfonylamine 3l, resulting in the compound 4l, did not affect the EC50 value, while a change in the position of cyclooctane moiety from spiro-annulated to 1,2-annulated one (compounds 4l and 5l, respectively) resulted in the complete loss of anti-WNV activity.
Finally, all compounds 3–5 have been tested for anti-YFV activity. Spiro-isoxazoline 3b, bearing benzylsulfonyl moiety, was found to be active with EC50 24 ± 11 μM, while other isoxazolines demonstrated no activity in concentrations up to 50 μM. Compound 6 was used as a positive control.
It should be noted that all the tested compounds demonstrated no acute cytotoxicity against PEK cells (see Supplementary Materials), and only compound 5i was slightly toxic during 7 days of incubation. In the case of Vero cells, compounds 3j, 4i, and 5k showed both acute and chronic cytotoxicity, while 4h was only acute toxic (Table 3; see also Supplementary Materials). Thus, the compounds studied here represent a substantially novel class of non-toxic antivirals, deserving further detailed studies of the mechanism of action and antiviral efficiency in vivo.
3. Materials and Methods
3.1. General Remarks
1H and 13C NMR spectra were recorded on a 400 MHz spectrometer Agilent 400-MR (400.0 and 100.6 MHz for 1H and 13C, respectively; Agilent Technologies, Santa Clara, CA, USA) at r.t. in CDCl3; chemical shifts δ were measured with reference to the solvent (CDCl3, δH = 7.26 ppm, δC = 77.16 ppm). When necessary, assignments of signals in NMR spectra were made using 2D techniques. Accurate mass measurements (HRMS) were obtained on Bruker micrOTOF II (Bruker Daltonik GmbH, Bremen, Gemany) with electrospray ionization (ESI). Analytical thin-layer chromatography was carried out with silica gel plates supported on aluminum (ALUGRAM® Xtra SIL G/UV254, Macherey-Nagel, Duren, Germany); the detection was performed by a UV lamp (254 nm). Column chromatography was performed on silica gel (Silica 60, 0.015–0.04 mm, Macherey-Nagel, Duren, Germany). 3-Aminoisoxazolines 1,2 and compounds 3a–l were obtained via described methods [26]. All other starting materials were commercially available. All reagents except commercial products of satisfactory quality were purified according to literature procedures prior to use.
Stock solutions of the compounds with a concentration of 5 mM were prepared in DMSO (Amresco, Cleveland, OH, USA).
3.2. Synthesis of Compounds 4,5 (General Methods)
Method A. To the solution of 3-aminoisoxazoline 1 or 2 (0.18 mmol) in dry DCM (2 mL), DIPEA (4 equiv, 0.72 mmol, 93 mg, 125 μL) was added under argon. The reaction mixture was cooled down to 0 °C, and sulfonyl chloride (0.9–5.5 equiv, 0.16–1.0 mmol) was added. Then the mixture was allowed to warm up to r.t., stirred for 3 h–8 days, and quenched with water (6 mL). The organic layer was separated, and the water layer was extracted with DCM (3 × 6 mL). Combined organic layers were washed with saturated aqueous NaHCO3 (9 mL) and brine (9 mL) and dried over MgSO4. The solvent was evaporated under reduced pressure. The product was isolated via preparative column chromatography (SiO2).
Method B. To the solution of 3-aminoisoxazoline 1 (0.28 mmol, 50 mg) in dry pyridine (1 mL), DIPEA (7.9 equiv, 2.2 mmol, 284 mg, 0.38 mL) was added under argon. The reaction mixture was cooled down to 0 °C, and 2-nitrobenzenesulfonyl chloride (5.9 equiv, 1.65 mmol, 365 mg) in dry pyridine (0.5 mL) was added. Then the mixture was allowed to warm up to r.t., stirred for 3 days, and quenched with 1M HCl (6 mL). The organic layer was separated, and the water layer was extracted with DCM (3 × 6 mL). Combined organic layers were washed with saturated aqueous NaHCO3 (9 mL) and brine (9 mL) and dried over MgSO4. The solvent was evaporated under reduced pressure. The product was isolated via preparative column chromatography (SiO2).
Method C. To the solution of 3-(sulfonylamino)isoxazoline 3e or 3f (0.1 mmol) in dry DCM (1.2 mL), TEA (3 equiv, 0.3 mmol, 0.04 mL, 30 mg) and DMAP (0.2 equiv, 0.02 mmol, 2.5 mg) were added under argon. The reaction mixture was cooled down to 0 °C, and sulfonyl chloride (1.1–2.4 equiv, 0.11–0.24 mmol) was added. Then the mixture was allowed to warm up to the r.t., stirred for 1–2 days, and quenched with water (5 mL). The organic layer was separated, and the water layer was extracted with DCM (3 × 5 mL). Combined organic layers were washed with brine (9 mL) and dried over MgSO4. The solvent was evaporated under reduced pressure. The product was isolated via preparative column chromatography (SiO2).
3.2.1. N-(Methylsulfonyl)-N-(1-oxa-2-azaspiro[4.7]dodec-2-en-3-yl)methanesulfonamide (4a)
Yield 14 mg (23%), method A; reaction time 2 days; 1 mmol of sulfonyl chloride was used. White crystals, m.p. 139–141 °C. Rf = 0.20 (petroleum ether:EtOAc = 8:1).
1H NMR (δ, ppm): 1.40–1.85 (m, 12H, 7CH2); 2.09–2.17 (m, 2H, 2CH2); 2.81 (s, 2H, CH2, Isox); 3.46 (s, 6H, 2CH3).
13C NMR (δ, ppm): 22.0 (2CH2), 24.4 (CH2), 28.0 (2CH2), 35.0 (2CH2), 43.8 (CH2, Isox), 45.6 (2CH3), 95.3 (Cspiro), 149.4 (C, Isox).
HRMS (ESI+, m/z): calculated for C12H22N2O5S2 [M+H]+, 339.1043; found, 339.1042.
3.2.2. N-(Benzylsulfonyl)-1-phenyl-N-(1-oxa-2-azaspiro[4.7]dodec-2-en-3-yl)methanesulfonamide (4b)
Yield 15 mg (19%), method A; reaction time 3 days; 0.16 mmol of sulfonyl chloride was used. White crystals, m.p. 176–177 °C. Rf = 0.28 (petroleum ether:DCM = 1:2).
1H NMR (δ, ppm): 1.19–1.30 (m, 2H, 2CH2); 1.30–1.44 (m, 2H, 2CH2); 1.44–1.66 (m, 8H, 7CH2); 1.49 (s, 2H, CH2, Isox); 1.85–1.96 (m, 2H, 2CH2); 4.87 (s, 4H, 2CH2, Bn); 7.36–7.46 (m, 6H, 6CH, Ph); 7.47–7.57 (m, 4H, 4CH, Ph).
13C NMR (δ, ppm): 21.9 (2CH2), 24.3 (CH2), 27.9 (2CH2), 34.7 (2CH2), 43.5 (CH2, Isox), 61.8 (2CH2, Bn), 94.8 (Cspiro), 126.8 (C, Isox), 129.2 (4CH, Ph), 129.7 (2CH, Ph), 131.8 (4CH, Ph), 150.4 (2C, Ph).
HRMS (ESI+, m/z): calculated for C24H30N2O5S2 [M+H]+, 491.1669; found, 491.1667.
3.2.3. N-(Phenylsulfonyl)-N-(1-oxa-2-azaspiro[4.7]dodec-2-en-3-yl)benzenesulfonamide (4c)
Yield 59 mg (71%), method A; reaction time 2 days; 1 mmol of sulfonyl chloride was used. White crystals, m.p. 139–141 °C. Rf = 0.21 (petroleum ether:EtOAc = 3:1).
1H NMR (δ, ppm): 1.34–1.65 (m, 5H, 5CH2); 1.66–1.81 (m, 3H, 3CH2); 1.84–2.00 (m, 4H, 4CH2); 2.44–2.58 (m, 2H, 2CH2); 3.03 (s, 2H, CH2, Isox); 7.30–7.41 (m, 4H, 4CH, Ph); 7.51–7.60 (m, 2H, 2CH, Ph); 7.69–7.77 (m, 4H, 4CH, Ph).
13C NMR (δ, ppm): 22.7 (2CH2), 25.0 (CH2), 28.0 (2CH2), 28.4 (CH2, Isox), 33.2 (2CH2), 93.1 (Cspiro), 118.0 (C, Isox), 128.9 (4CH, Ph), 129.6 (4CH, Ph), 134.5 (2CH, Ph), 135.3 (2C(SO2)).
HRMS (ESI+, m/z): calculated for C22H26N2O5S2 [M+NH4]+, 480.1621; found, 480.1622.
3.2.4. 4-Methyl-N-(1-oxa-2-azaspiro[4.7]dodec-2-en-3-yl)-N-tosylbenzenesulfonamide (4d)
Yield 49 mg (56%), method A; reaction time 2 days; 1 mmol of sulfonyl chloride was used. White crystals, m.p. 151–153 °C. Rf = 0.41 (petroleum ether:DCM = 1:3).
1H NMR (δ, ppm): 1.34–1.65 (m, 5H, 5CH2); 1.66–1.81 (m, 3H, 3CH2); 1.84–2.00 (m, 4H, 4CH2); 2.40 (s, 6H, 2CH3); 2.43–2.56 (m, 2H, 2CH2); 3.02 (s, 2H, CH2, Isox); 7.08–7.19 (m, 4H, 4CH, Ar); 7.57–7.67 (m, 4H, 4CH, Ar).
13C NMR (δ, ppm): 21.9 (2CH3), 22.7 (2CH2), 25.0 (CH2), 28.0 (2CH2), 28.3 (CH2, Isox), 33.2 (2CH2), 92.8 (Cspiro), 118.1 (C, Isox), 129.4 (4CH, Ar), 129.7 (4CH, Ar), 132.4 (2C(SO2)), 145.8 (2C, Ar).
HRMS (ESI+, m/z): calculated for C24H30N2O5S2 [M+NH4]+, 508.1934; found, 508.1939.
3.2.5. N-(Mesitylsulfonyl)-2,4,6-trimethyl-N-(1-oxa-2-azaspiro[4.7]dodec-2-en-3-yl)benzenesulfonamide (4e)
Yield 33 mg (60%), method C; 0.11 mmol of sulfonyl chloride was used. White crystals, m.p. 180–182 °C. Rf = 0.21 (petroleum ether:EtOAc = 7:1).
1H NMR (δ, ppm): 1.20–1.73 (m, 14H, 7CH2); 2.33 (s, 6H, 2CH3); 2.61 (s, 2H, CH2, Isox); 2.74 (s, 12H, 4CH3); 7.01 (s, 4H, 4CH, Ar).
13C NMR (δ, ppm): 21.4 (2CH3), 22.2 (2CH2), 23.6 (4CH3), 24.1 (CH2), 27.8 (2CH2, cy-Oct + CH2, Isox), 33.3 (2CH2), 91.4 (Cspiro), 117.3 (C, Isox), 132.3 (4CH, Ar + 2C(SO2)), 142.8 (4C, Ar), 145.2 (2C, Ar).
HRMS (ESI+, m/z): calculated for C28H38N2O5S2 [M+NH4]+, 546.2560; found, 546.2553.
3.2.6. N-((2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)sulfonyl)-N-(1-oxa-2-azaspiro[4.7]dodec-2-en-3-yl)-2,3-dihydrobenzo[b][1,4]dioxine-6-sulfonamide (4f)
Yield 38 mg (36%), method A; reaction time 2 days, 1 mmol of sulfonyl chloride was used; 35 mg (61%), method C; 0.24 mmol of sulfonyl chloride was used. White crystals, m.p. 191–193 °C. Rf = 0.32 (petroleum ether:EtOAc = 1:1).
1H NMR (δ, ppm; J, Hz): 1.34–1.65 (m, 5H, 5CH2); 1.66–1.81 (m, 3H, 3CH2); 1.84–2.00 (m, 4H, 4CH2); 2.46–2.58 (m, 2H, 2CH2); 3.03 (s, 2H, CH2, Isox); 4.20–4.36 (m, 8H, 4CH2O); 6.77 (br.d, 3J 8.6, 1H, CH, Ar); 7.20 (br.d, 4J 2.3, 1H, CH, Ar); 7.12–7.20 (m, 2H, 2CH, Ar); 7.23 (dd, 3J 8.6, 4J 2.3, 1H, CH, Ar).
13C NMR (δ, ppm): 22.2 (2CH2), 25.0 (CH2), 28.0 (2CH2), 28.4 (CH2, Isox), 33.1 (2CH2), 64.1 (2CH2O), 64.8 (2CH2O), 92.9 (Cspiro), 117.3 (2CH, Ar), 118.2 (C, Isox), 119.2 (2CH, Ar), 123.5 (2CH, Ar), 127.1 (2C, Ar), 143.1 (2C, Ar), 149.0 (2C, Ar).
HRMS (ESI+, m/z): calculated for C26H30N2O9S2 [M+Na]+, 601.1285; found, 601.1280.
3.2.7. N-((2,4-Difluorophenyl)sulfonyl)-2,4-difluoro-N-(1-oxa-2-azaspiro[4.7]dodec-2-en-3-yl)benzenesulfonamide (4g)
Yield 45 mg (47%), method A; reaction time 4 days, 0.54 mmol of sulfonyl chloride was used. White crystals, m.p. 61–62 °C. Rf = 0.56 (DCM).
1H NMR (δ, ppm; J, Hz): 1.32–1.53 (m, 3H, 3CH2); 1.54–1.66 (m, 2H, 2CH2); 1.68–1.78 (m, 3H, 3CH2); 1.78–1.90 (m, 2H, 2CH2); 1.95 (ddd, 2JHH 15.2, 3JHH 8.1, 3JHH 2.2, 2H, 2CH2); 2.45 (ddd, 2JHH 15.2, 3JHH 10.6, 3JHH 2.1, 2H, 2CH2); 3.00 (s, 2H, CH2, Isox); 6.98 (ddd, 3JHF 10.2, 3JHF 8.5, 4JHH 4.0, 2H, 2CH, Ar); 7.08 (dddd, 3JHF 7.4, 5JHF 1.1, 3JHH 9.0, 4JHH 2.5, 2H, 2CH, Ar); 8.02 (ddd, 4JHF 7.8, 4JHF 6.0, 3JHH 9.0, 2H, 2CH, Ar).
13C NMR (δ, ppm; J, Hz): 22.7 (2CH2); 25.1 (CH2); 28.0 (2CH2); 28.2 (CH2, Isox); 33.1 (2CH2); 93.5 (Cspiro); 106.2 (dd, 2JCF 26, 2JCF 26, 2CH, Ar); 112.4 (dd, 2JCF 22, 4JCF 4, 2CH, Ar); 117.7 (C, Isox); 120.0 (dd, 2JCF 13, 2JCF 4, 2C(SO2)); 135.5 (d, 3JCF 11, 2CH, Ar); 161.2 (dd, 1JCF 265, 3JCF 14, 2CF, Ar); 167.7 (dd, 1JCF 262, 3JCF 12, 2CF, Ar).
19F NMR (δ, ppm; J, Hz): −98.03 (dddd, 4JFF 15.3, 3JHF 10.2, 4JHF 7.8, 5JHF 1.1, 2F, CF, Ar); −95.27 (dddd, 4JFF 15.3, 3JHF 8.5, 3JHF 7.4, 4JHF 6.0, 2F, CF, Ar).
HRMS (ESI+, m/z): calculated for C22H22F4N2O5S2 [M+Na]+, 557.0798; found, 557.0795.
3.2.8. 2-Nitro-N-((2-nitrophenyl)sulfonyl)-N-(1-oxa-2-azaspiro[4.7]dodec-2-en-3-yl)benzenesulfonamide (4h)
Yield 30 mg (20%), method B. Light-yellow crystals, m.p. 155–157 °C. Rf = 0.23 (petroleum ether:EtOAc = 2:1).
1H NMR (δ, ppm): 1.33–1.95 (m, 12H, 7CH2); 2.33–2.42 (m, 2H, 2CH2); 2.95 (s, 2H, CH2, Isox); 7.61–7.66 (m, 2H, 2CH, Ar); 7.71–7.79 (m, 4H, 4CH, Ar); 8.31–8.37 (m, 2H, 2CH, Ar).
13C NMR (δ, ppm): 22.6 (2CH2), 25.1 (CH2), 27.9 (2CH2), 28.3 (CH2, Isox), 32.9 (2CH2), 94.6 (Cspiro), 117.5 (C, Isox), 124.5 (2CH), 130.1 (2C(SO2)), 132.1 (2CH), 132.5 (2CH), 135.7 (2CH), 148.4 (2C(NO2)).
HRMS (ESI+, m/z): calculated for C22H24N4O9S2 [M+NH4]+, 570.1323; found, 570.1322.
3.2.9. 4-Nitro-N-((4-nitrophenyl)sulfonyl)-N-(1-oxa-2-azaspiro[4.7]dodec-2-en-3-yl)benzenesulfonamide (4i)
Yield 19 mg (57%), method A; reaction time 3 h, 0.63 mmol of sulfonyl chloride was used. White crystals, m.p. 178–180 °C. Rf = 0.33 (petroleum ether:EtOAc = 4:1).
1H NMR (δ, ppm): 1.33–1.46 (m, 3H, 3CH2); 1.51–1.64 (m, 2H, 2CH2); 1.68–1.90 (m, 7H, 7CH2); 2.36–2.49 (m, 2H, 2CH2); 2.97 (s, 2H, CH2, Isox); 8.21–8.29 (m, 4H, 4CH); 8.35–8.43 (m, 4H, 4CH).
13C NMR (δ, ppm): 22.7 (2CH2), 25.2 (CH2), 27.9 (2CH2), 28.1 (CH2, Isox), 33.3 (2CH2), 94.0 (Cspiro), 117.7 (C, Isox), 124.2 (4CH), 131.6 (4CH), 141.2 (2C(SO2)), 151.5 (2C(NO2)).
HRMS (ESI+, m/z): calculated for C22H24N4O9S2 [M+Na]+, 575.0877; found, 575.0876.
3.2.10. N-(Naphthalen-2-ylsulfonyl)-N-(1-oxa-2-azaspiro[4.7]dodec-2-en-3-yl)naphthalene-2-sulfonamide (4j)
Yield 73 mg (72%), method A; reaction time 8 days, 0.54 mmol of sulfonyl chloride was used. White crystals, m.p. 160–161 °C. Rf = 0.39 (petroleum ether:DCM = 1:4).
1H NMR (δ, ppm; J, Hz): 1.36–1.88 (m, 8H, 5CH2); 1.92–2.15 (m, 4H, 4CH2); 2.61–2.75 (m, 2H, 2CH2); 3.14 (s, 2H, CH2, Isox); 7.36 (d, 3J 8.7, 2H, 2CH); 7.38–7.42 (m, 2H, 2CH); 7.45–7.50 (m, 2H, 2CH); 7.50–7.53 (m, 4H, 4CH); 7.55 (dd, 3J 8.7, 4J 1.9, 2H, 2CH); 8.09 (d, 4J 1.9, 2H, 2CH).
13C NMR (δ, ppm): 22.7 (2CH2), 25.0 (CH2), 28.1 (2CH2), 28.6 (CH2, Isox), 33.2 (2CH2), 93.6 (Cspiro), 118.2 (C, Isox), 123.2 (2CH), 127.65 (2CH), 127.67 (2CH), 128.7 (2CH), 129.3 (2CH), 129.7 (2CH), 131.31 (2C), 131.38 (2C), 131.40 (2CH), 135.2 (2C).
HRMS (ESI+, m/z): calculated for C30H30N2O5S2 [M+Na]+, 585.1488; found, 585.1477.
3.2.11. N-(1-Oxa-2-azaspiro[4.7]dodec-2-en-3-yl)-N-(thiophen-2-ylsulfonyl)thiophene-2-sulfonamide (4k)
Yield 27 mg (32%), method A; reaction time 2 days; 1 mmol of sulfonyl chloride was used. White crystals, m.p. 147–147 °C. Rf = 0.2 (petroleum ether:DCM = 1:3)
1H NMR (δ, ppm; J, Hz): 1.34–2.03 (m, 12H, 7CH2); 2.48–2.61 (m, 2H, CH2); 3.01 (s, 2H, CH2, Isox); 6.95–6.99 (m, 2H, 2CH, Tioph); 7.57–7.60 (m, 2H, 2CH, Tioph); 7.65–7.68 (m, 2H, 2CH, Tioph).
13C NMR (δ, ppm): 22.8 (2CH2), 25.1 (CH2), 28.1 (2CH2), 28.5 (CH2, Isox), 33.2 (2CH2), 93.6 (Cspiro), 117.8 (C, Isox), 127.2 (2CH, Tioph), 133.6 (2C(SO2)), 136.3 (2CH, Tioph), 137.2(2CH, Tioph).
HRMS (ESI+, m/z): calculated for C18H22N2O5S4 [M+Na]+, 497.0304; found, 497.0300.
3.2.12. N-(Pyridin-3-ylsulfonyl)-N-(1-oxa-2-azaspiro[4.7]dodec-2-en-3-yl)pyridine-3-sulfonamide (4l)
Yield 77 mg (92%), method A; reaction time 4 days, 0.54 mmol of sulfonyl chloride was used. White crystals, m.p. 151–152 °C. Rf = 0.41 (DCM:MeOH = 25:1).
1H NMR (δ, ppm; J, Hz): 1.34–1.52 (m, 3H, 3CH2); 1.54–1.66 (m, 2H, 2CH2); 1.69–1.97 (m, 7H, 7CH2); 2.43–2.55 (m, 2H, 2CH2); 3.00 (s, 2H, CH2, Isox); 7.45 (ddd, 3JHH 8.2, 3JHH 4.9, 5JHH 0.8, 2H, 2CH, Py); 8.25 (ddd, 3JHH 8.2, 4JHH 2.4, 4JHH 1.6, 2H, 2CH, Py); 8.86 (dd, 3JHH 4.9, 4JHH 1.6, 2H, 2CH, Py); 9.02 (dd, 4JHH 2.4, 5JHH 0.8, 2H, 2CH, Py).
13C NMR (δ, ppm): 22.7 (2CH2), 25.1 (CH2), 27.9 (2CH2), 28.2 (CH2, Isox), 33.2 (2CH2), 93.9 (Cspiro), 117.8 (C, Isox), 123.8 (2CH, Py), 132.3 (2C(SO2)), 137.6 (2CH, Py), 150.1 (2CH, Py), 155.2 (2CH, Py).
HRMS (ESI+, m/z): calculated for C20H24N4O5S2 [M+H]+, 465.1261; found, 465.1256.
3.2.13. N-(Benzylsulfonyl)-N-(3a,4,5,6,7,8,9,9a-octahydrocycloocta[d]isoxazol-3-yl)-1-phenylmethanesulfonamide (5b)
Yield 25 mg (29%), method A; reaction time 1 day, 0.54 mmol of sulfonyl chloride was used. White crystals, m.p. 198–199 °C. Rf = 0.45 (petroleum etherl:EtOAc = 8:1).
1H NMR (δ, ppm; J, Hz): 0.30–0.48 (m, 1H, CH2); 0.92–1.03 (m, 2H, CH2); 1.10–1.62 (m, 7H, 4CH2); 1.82–1.95 (m, 2H, CH2); 2.43–2.55 (m, 1H, CH, cy-Oct); 4.53–4.64 (m, 1H, CH-O, cy-Oct); 4.89 (d, 3J 13.9, 2H, 2CH2, Bn); 5.09 (d, 3J 13.9, 2H, 2CH2, Bn); 7.37–7.47 (m, 6H, 6CH, Ph); 7.47–7.55 (m, 4H, 4CH, Ph).
13C NMR (δ, ppm): 22.7 (CH2), 25.1 (CH2), 25.2 (CH2), 25.6 (CH2), 26.1 (CH2), 29.8 (CH2), 49.5 (CH, cy-Oct), 61.7 (2CH2, Bn), 88.1 (CH-O, cy-Oct), 126.7 (C, Isox), 129.2 (4CH, Ph), 129.7 (2CH, Ph), 131.6 (4CH, Ph), 154.9 (2C, Ph).
HRMS (ESI+, m/z): calculated for C23H28N2O5S2 [M+H]+, 477.1512; found, 477.1502.
3.2.14. N-(3a,4,5,6,7,8,9,9a-Octahydrocycloocta[d]isoxazol-3-yl)-N-(phenylsulfonyl)benzenesulfonamide (5c)
Yield 63 mg (78%), method A; reaction time 6 h, 0.45 mmol of sulfonyl chloride was used. White crystals, m.p. 107–108 °C. Rf = 0.56 (DCM).
1H NMR (δ, ppm): 1.36–2.16 (m, 11H, 6CH2); 2.17–2.31 (m, 1H, CH2); 3.76–3.87 (m, 1H, CH, cy-Oct); 4.62–4.73 (m, 1H, CH-O, cy-Oct); 7.22–7.41 (m, 4H, 4CH, Ph); 7.47–7.61 (m, 2H, 2CH, Ph); 7.61–7.73 (m, 4H, 4CH, Ph).
13C NMR (δ, ppm): 23.4 (CH2), 24.2 (CH2), 25.2 (CH2), 27.4 (CH2), 27.9 (CH2), 29.7 (CH2), 34.6 (CH, cy-Oct), 89.9 (CH-O, cy-Oct), 120.4 (C, Isox), 128.8 (2CH, Ph), 129.0 (2CH, Ph), 129.4 (2CH, Ph), 129.6 (2CH, Ph), 134.1 (2C(SO2)), 134.6 (CH, Ph), 134.7 (CH, Ph).
HRMS (ESI+, m/z): calculated for C21H24N2O5S2 [M+H]+, 449.1199; found, 449.1198.
3.2.15. 4-Methyl-N-(3a,4,5,6,7,8,9,9a-octahydrocycloocta[d]isoxazol-3-yl)-N-tosylbenzenesulfonamide (5d)
Yield 65 mg (76%), method A; reaction time 1 day, 0.54 mmol of sulfonyl chloride was used. White crystals, m.p. 113–114 °C. Rf = 0.21 (petroleum ether:EtOAc = 2:1).
1H NMR (δ, ppm): 1.36–1.84 (m, 8H, 4CH2); 1.86–2.15 (m, 3H, 2CH2); 2.16–2.30 (m, 1H, CH2); 2.39 (s, 3H, CH3); 2.40 (s, 3H, CH3); 3.75–3.86 (m, 1H, CH, cy-Oct); 4.58–4.68 (m, 1H, CH-O, cy-Oct); 7.00–7.19 (m, 4H, 4CH, Ar); 7.52–7.60 (m, 4H, 4CH, Ar).
13C NMR (δ, ppm): 21.8 (2CH3), 23.4 (CH2), 24.2 (CH2), 25.2 (CH2), 27.5 (CH2), 28.0 (CH2), 29.7 (CH2), 34.6 (CH, cy-Oct), 89.6 (CH-O, cy-Oct), 120.4 (C, Isox), 129.3 (2CH, Ar), 129.5 (4CH, Ar), 129.8 (2CH, Ar), 131.3 (C(SO2)), 132.0 (C(SO2)), 145.9 (C, Ar), 146.1 (C, Ar).
HRMS (ESI+, m/z): calculated for C23H28N2O5S2 [M+H]+, 477.1512; found, 477.1505.
3.2.16. N-(Mesitylsulfonyl)-2,4,6-trimethyl-N-(3a,4,5,6,7,8,9,9a-octahydrocycloocta[d]isoxazol-3-yl)benzenesulfonamide (5e)
Yield 33 mg (34%), method A; reaction time 3 days, 0.54 mmol of sulfonyl chloride was used. White crystals, m.p. 148–149 °C. Rf = 0.47 (DCM).
1H NMR (δ, ppm): 0.97–1.17 (m, 2H, 2CH2); 1.20–1.67 (m, 8H, 6CH2); 1.82–1.95 (m, 2H, 2CH2); 2.33 (s, 6H, 2CH3); 2.74 (br.s, 12H, 4CH3); 3.13–3.20 (m, 1H, CH, cy-Oct); 3.68–3.78 (m, 1H, CH-O, cy-Oct); 7.03 (br.s, 4H, 4CH, Ar).
13C NMR (δ, ppm): 21.3 (2CH3), 22.9 (CH2), 23.4 (br.s, 4CH3 + CH2), 25.2 (CH2), 27.3 (CH2), 29.3 (CH2), 29.4 (CH2), 34.7 (CH, cy-Oct), 87.3 (CH-O, cy-Oct), 119.4 (C, Isox), 132.4 (4CH, Ar + 2C(SO2)), 142.7 (4C, Ar), 145.3 (2C, Ar).
HRMS (ESI+, m/z): calculated for C27H36N2O5S2 [M+Na]+, 555.1958; found, 555.1958.
3.2.17. N-((2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)sulfonyl)-N-(3a,4,5,6,7,8,9,9a-octahydrocycloocta[d]isoxazol-3-yl)-2,3-dihydrobenzo[b][1,4]dioxine-6-sulfonamide (5f)
Yield 60 mg (59%), method A; reaction time 1 day, 0.54 mmol of sulfonyl chloride was used. White crystals, m.p. 129–130 °C. Rf = 0.22 (DCM).
1H NMR (δ, ppm; J, Hz): 1.35–2.16 (m, 11H, 2CH2); 2.17–2.32 (m, 1H, CH2); 3.76–3.91 (m, 1H, CH, cy-Oct); 4.15–4.44 (m, 8H, 4CH2O); 4.63–4.70 (m, 1H, CH-O, cy-Oct); 6.76 (d, 3J 8.7, 1H, CH, Ar); 6.77 (d, 3J 8.7, 1H, CH, Ar); 7.09 (br.d, 4J 1.2, 1H, CH, Ar); 7.12–7.20 (m, 2H, 2CH, Ar); 7.23 (br.dd, 3J 8.7, 4J 1.2, 1H, CH, Ar).
13C NMR (δ, ppm): 23.4 (CH2), 24.2 (CH2), 25.2 (CH2), 27.5 (CH2), 28.0 (CH2), 29.9 (CH2), 34.6 (CH, cy-Oct), 64.0 (2CH2O), 64.8 (2CH2O), 89.9 (CH-O, cy-Oct), 117.3 (CH, Ar), 117.4 (CH, Ar), 119.0 (CH, Ar), 119.1 (CH, Ar), 120.5 (C, Isox), 123.4 (CH, Ar), 123.8 (CH, Ar), 126.0 (C(SO2)), 126.7 (C(SO2)), 143.0 (C, Ar), 143.2 (C, Ar), 149.1 (C, Ar), 149.3 (C, Ar).
HRMS (ESI+, m/z): calculated for C25H28N2O9S2 [M+Na]+, 587.1128; found, 587.1128.
3.2.18. N-((2,4-difluorophenyl)sulfonyl)-2,4-difluoro-N-(3a,4,5,6,7,8,9,9a-octahydrocycloocta[d]isoxazol-3-yl)benzenesulfonamide (5g)
Yield 92 mg (98%), method A; reaction time 1 day, 0.54 mmol of sulfonyl chloride was used. White crystals, m.p. 129–131 °C. Rf = 0.56 (DCM).
1H NMR (δ, ppm; J, Hz): 1.34–2.18 (m, 11H, 6CH2); 2.19–2.34 (m, 1H, CH2); 3.62–3.79 (m, 1H, CH, cy-Oct); 4.62–4.82 (m, 1H, CH-O, cy-Oct); 7.00 (ddd, 3JHF 9.9, 3JHF 8.6, 4JHH 2.4, 2H, 2CH, Ar); 7.04–7.16 (m, 2H, 2CH, Ar); 8.02 (br.s, 2H, 2CH, Ar).
13C NMR (δ, ppm; J, Hz): 23.3 (CH2), 24.0 (CH2), 25.3 (CH2), 27.6 (CH2), 28.7 (CH2), 29.3 (CH2), 34.5 (CH, cy-Oct), 89.0 (CH-O, cy-Oct), 106.2 (dd, 2JCF 26, 2JCF 26, 2CH, Ar), 112.5 (dd, 2JCF 22, 4JCF 3, 2CH, Ar), 119.1–119.6 (m, C(SO2)), 119.8 (C, Isox), 120.0–120.3 (m, C(SO2)), 134.9–135.8 (m, 2CH, Ar), 161.3 (m, 1JCF 267, 2CF, Ar), 167.7 (dd, 1JCF 262, 3JCF 12, 2CF, Ar).
NMR 19F (δ, ppm): −99.65 (br.s, 1F, CF, Ar); −98.32 (br.s, 1F, CF, Ar); −94.96 (br.s, 1F, CF, Ar); −94.87 (br.s, 1F, CF, Ar).
HRMS (ESI+, m/z): calculated for C21H20F4N2O5S2 [M+Na]+, 543.0642; found, 543.0642.
3.2.19. 2-Nitro-N-((2-nitrophenyl)sulfonyl)-N-(3a,4,5,6,7,8,9,9a-octahydrocycloocta[d]isoxazol-3-yl)benzenesulfonamide (5h)
Yield 47 mg (49%), method A; reaction time 9 h, 0.45 mmol of sulfonyl chloride was used. White crystals, m.p. 153–154 °C. Rf = 0.58 (petroleum ether:EtOAc = 1:1).
1H NMR (δ, ppm; J, Hz): 1.42–2.14 (m, 11H, 6CH2); 2.16–2.28 (m, 1H, CH2); 3.62–3.73 (m, 1H, CH, cy-Oct); 4.70–4.77 (m, 1H, CH-O, cy-Oct); 7.74 (br.d, 3J 7.7, 2H, 2CH, Ar); 7.78–7.93 (m, 4H, 4CH, Ar); 8.27–8.44 (m, 2H, 2CH, Ar).
13C NMR (δ, ppm): 23.3 (CH2), 24.4 (CH2), 25.1 (CH2), 27.4 (CH2), 27.8 (CH2), 29.2 (CH2), 35.1 (CH, cy-Oct), 89.0 (CH-O, cy-Oct), 120.2 (C, Isox), 124.5 (2CH, Ar), 128.1 (2C(SO2)), 132.3 (2CH, Ar), 134.0 (2CH, Ar), 136.5 (2CH, Ar), 149.0 (2C(NO2)).
HRMS (ESI+, m/z): calculated for C21H22N4O9S2 [M+Na]+, 561.0720; found, 561.0713.
3.2.20. 4-Nitro-N-((4-nitrophenyl)sulfonyl)-N-(3a,4,5,6,7,8,9,9a-octahydrocycloocta[d]isoxazol-3-yl)benzenesulfonamide (5i)
Yield 95 mg (98%), method A; reaction time 3 h, 0.36 mmol of sulfonyl chloride was used. White crystals, m.p. 139–140 °C. Rf = 0.32 (petroleum ether:EtOAc = 4:1).
1H NMR (δ, ppm): 1.38–2.18 (m, 11H, 6CH2); 2.18–2.31 (m, 1H, CH2); 3.61–3.68 (m, 1H, CH, cy-Oct); 4.57–4.69 (m, 1H, CH-O, cy-Oct); 8.04–8.23 (m, 4H, 4CH, Ar); 8.27–8.44 (m, 4H, 4CH, Ar).
13C NMR (δ, ppm): 23.7 (CH2), 24.4 (CH2), 25.2 (CH2), 27.35 (CH2), 27.40 (CH2), 29.2 (CH2), 34.7 (CH, cy-Oct), 90.0 (CH-O, cy-Oct), 120.0 (C, Isox), 124.3 (4CH, Ar), 131.4 (2CH, Ar), 131.6 (2CH, Ar), 139.9 (C(SO2)), 140.7 (C(SO2)), 151.5 (2C(NO2), Ar).
HRMS (ESI+, m/z): calculated for C21H22N4O9S2 [M+Na]+, 561.0720; found, 561.0713.
3.2.21. N-(Naphthalen-2-ylsulfonyl)-N-(3a,4,5,6,7,8,9,9a-octahydrocycloocta[d]isoxazol-3-yl)naphthalene-2-sulfonamide (5j)
Yield 51 mg (52%), method A; reaction time 9 h, 0.45 mmol of sulfonyl chloride was used. White crystals, m.p. 160–162 °C. Rf = 0.44 (DCM).
1H NMR (δ, ppm): 1.42–2.23 (m, 11H, 6CH2); 2.35–2.45 (m, 1H, CH2); 3.93–3.99 (m, 1H, CH, cy-Oct); 4.79–4.86 (m, 1H, CH-O, cy-Oct); 7.30–7.41 (m, 4H, 4CH, Ar); 7.42–7.62 (m, 8H, 8CH, Ar); 7.97 (br.s, 1H, CH, Ar); 8.14 (br.s, 1H, CH, Ar).
13C NMR (δ, ppm): 23.6 (CH2), 24.3 (CH2), 25.3 (CH2), 27.5 (CH2), 28.0 (CH2), 29.9 (CH2), 34.8 (CH, cy-Oct), 90.4 (CH-O, cy-Oct), 120.6 (C, Isox), 123.1 (CH, Ar), 123.5 (CH, Ar), 127.6 (2CH, Ar), 127.8 (CH, Ar), 127.9 (CH, Ar), 128.6 (CH, Ar), 128.9 (CH, Ar), 129.4 (2CH, Ar), 129.7 (CH, Ar), 129.8 (CH, Ar), 130.5 (C, Ar), 131.1 (C, Ar), 131.3 (C, Ar), 131.4 (CH, Ar + C, Ar), 131.5 (CH, Ar), 135.3 (C(SO2)), 135.4 (C(SO2)).
HRMS (ESI+, m/z): calculated for C29H28N2O5S2 [M+H]+, 549.1512; found, 549.1504.
3.2.22. N-(3a,4,5,6,7,8,9,9a-Octahydrocycloocta[d]isoxazol-3-yl)-N-(thiophen-2-ylsulfonyl)thiophene-2-sulfonamide (5k)
Yield 54 mg (65%), method A; reaction time 1 day, 0.54 mmol of sulfonyl chloride was used. White crystals, m.p. 131–132 °C. Rf = 0.47 (petroleum ether:EtOAc = 1:1).
1H NMR (δ, ppm): 1.41–2.16 (m, 11H, 6CH2); 2.24–2.36 (m, 1H, CH2); 3.77–3.84 (m, 1H, CH, cy-Oct); 4.70–4.77 (m, 1H, CH-O, cy-Oct); 6.92–7.05 (m, 2H, 2CH, Tioph); 7.54–7.60 (m, 2H, 2CH, Tioph); 7.62–7.70 (m, 2H, 2CH, Tioph).
13C NMR (δ, ppm): 23.5 (CH2), 24.1 (CH2), 25.2 (CH2), 27.5 (CH2), 28.1 (CH2), 29.8 (CH2), 34.8 (CH, cy-Oct), 90.1 (CH-O, cy-Oct), 120.2 (C, Isox), 127.3 (2CH, Tioph), 132.6 (C(SO2)), 133.1 (C(SO2)), 136.4 (CH, Tioph), 136.5 (CH, Tioph), 137.0 (CH, Tioph), 137.2 (CH, Tioph).
HRMS (ESI+, m/z): calculated for C17H20N2O5S4 [M+Na]+, 483.0147; found, 483.0153.
3.2.23. N-(3a,4,5,6,7,8,9,9a-Octahydrocycloocta[d]isoxazol-3-yl)-N-(pyridin-3-ylsulfonyl)pyridine-3-sulfonamide (5l)
Yield 63 mg (78%), method A; reaction time 9 h, 0.45 mmol of sulfonyl chloride was used. White crystals, m.p. 150–151 °C. Rf = 0.46 (DCM:MeOH = 1:0.05).
1H NMR (δ, ppm): 1.40–2.18 (m, 11H, 6CH2); 2.18–2.31 (m, 1H, CH2); 3.71–3.78 (m, 1H, CH, cy-Oct); 4.67–4.75 (m, 1H, CH-O, cy-Oct); 7.35–7.48 (m, 2H, 2CH, Py); 8.05–8.25 (m, 2H, 2CH, Py); 8.74–8.95 (m, 4H, 4CH, Py).
13C NMR (δ, ppm): 23.6 (CH2), 24.3 (CH2), 25.2 (CH2), 27.4 (CH2), 27.7 (CH2), 29.5 (CH2), 34.7 (CH, cy-Oct), 90.3 (CH-O, cy-Oct), 120.1 (C, Isox), 123.8 (2CH, Py), 130.9 (C(SO2)), 131.6 (C(SO2)), 137.3 (CH, Py), 137.8 (CH, Py), 149.7 (2CH, Py), 155.3 (2CH, Py).
HRMS (ESI+, m/z): calculated for C19H22N4O5S2 [M+H]+, 451.1104; found, 451.1105.
3.3. Biology
3.3.1. Cells and Viruses
Porcine embryo kidney (PEK) cells and African green monkey kidney (Vero) cells were obtained from the collection of FSASI “Chumakov FSC R&D IBP RAS” (Institute of Poliomyelitis), Moscow, Russia. PEK cells were grown in the mixture of medium 199 with Earl’s salts and medium 199 with Hanks’ salts (FSASI “Chumakov FSC R&D IBP RAS” (Institute of Poliomyelitis), Moscow, Russia), supplemented with 5% of heat-inactivated fetal bovine serum (Gibco, Grand Island, NY, USA) and penicillin-streptomycin (Paneco-ltd, Moscow, Russia). Vero cells were grown in DMEM with L-glutamine (FSASI “Chumakov FSC R&D IBP RAS” (Institute of Poliomyelitis)) supplemented with 5% of heat-inactivated fetal bovine serum (Gibco, Grand Island, NY, USA) and gentamicin (Paneco-ltd, Moscow, Russia).
Tick-borne encephalitis virus strain Absettarov (GenBank access no. KU885457.1), yellow fever virus strain 17D (Genbank access no. JN628279.1), and West Nile virus strain Strix nebulosa-12 (GenBank access no. OP868929) were taken from the collection of FSASI “Chumakov FSC R&D IBP RAS” (Institute of Poliomyelitis), Moscow, Russia.
3.3.2. Antiviral Activity and Cytotoxicity Assays
Antiviral activity and cytotoxicity of the tested compounds were studied using plaque reduction assay and resazurin cell viability assay, respectively, as described previously [33]. Each compound was tested in at least two independent experiments.
4. Conclusions
Two series of novel 3-[N,N-bis(sulfonyl)amino]isoxazolines, containing spiro-annulated or 1,2-annulated cyclooctane rings, were synthesized via sulfonylation of corresponding 3-amino- or 3-(sulfonylamino)isoxazolines. Almost all the obtained compounds revealed antiviral activity against tick-borne encephalitis and West Nile viruses. The best results were shown by spirocyclic compounds 4i (TBEV EC50 2.0 ± 0.5 μM) and 4j (WNV EC50 1.3 ± 0.5 μM), respectively. It was also found that spiro-isoxazoline 3b is active against the yellow fever virus with EC50 24 ± 11 μM. On this basis, we can conclude that spirocyclic and bicyclic scaffolds, combining isoxazoline and cyclooctane rings, represent promising structural motifs for the further design of anti-flaviviral agents. The mechanism of action and molecular target of the compounds described above still need to be established and will be the subject of further research.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms241310758/s1.
Author Contributions
Conceptualization, D.I.O. and E.B.A.; investigation, A.S.S., D.A.V., K.S.A., M.G.E., E.V.K., A.S.G., V.I.U. and Y.K.G.; data curation, K.N.S., E.V.K., A.S.G., and D.I.O.; writing—original draft preparation, K.N.S. and A.S.G.; writing—review and editing, D.I.O. and E.B.A.; visualization, K.N.S. and Y.K.G.; supervision, A.A.I., D.I.O. and E.B.A.; project administration, D.I.O. and E.B.A.; funding acquisition, A.A.I. and E.B.A. All authors have read and agreed to the published version of the manuscript.
Funding
Synthetic work was supported by the Ministry of Science and Higher Education of the Russian Federation (Agreement with Zelinsky Institute of Organic Chemistry RAS No 075-15-2020-803). Antiviral activity studies were supported by the State Research Funding for FSASI “Chumakov FSC R&D IBP RAS” (Institute of Poliomyelitis) from the Ministry of Science and Higher Education of the Russian Federation (project No. FNZG-2022-0002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was fulfilled using the NMR spectrometer Agilent 400-MR, purchased by the MSU Development Program. The authors thank Marina S. Merkulova, Nadezhda S. Borisenko, and Daria O. Yatsenko for their assistance with biological experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hiesinger, K.; Dar’in, D.; Proschak, E.; Krasavin, M. Spirocyclic Scaffolds in Medicinal Chemistry. J. Med. Chem. 2021, 64, 150–183. [Google Scholar] [CrossRef] [PubMed]
- Chupakhin, E.; Babich, O.; Prosekov, A.; Asyakina, L.; Krasavin, M. Spirocyclic Motifs in Natural Products. Molecules 2019, 24, 4165. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hsu, C.-S. Enantioselective Total Synthesis of (+)-EBC-23, a Potent Anticancer Agent from the Australian Rainforest. J. Org. Chem. 2021, 86, 6351–6360. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, W.; Shen, L.; Hao, X.; Al, A.; Weaam, H.; Lin, S.; Li, H.; Gao, W.; Wang, J.; et al. Bipolaricins A–I, ophiobolin-type tetracyclic sesterterpenes from a phytopathogenic Bipolaris sp. fungus. J. Nat. Prod. 2019, 82, 2897–2906. [Google Scholar] [CrossRef] [PubMed]
- Kotha, S.; Panguluri, N.R.; Ali, R. Design and Synthesis of Spirocycles. Eur. J. Org. Chem. 2017, 2017, 5316–5342. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Zeng, R.; Zhang, Y.; Dai, Q.-S.; Leng, H.-J.; Gou, X.-J.; Li, J.-L. Recent Advances in the Synthesis of Spiroheterocycles via N-Heterocyclic Carbene Organocatalysis. Molecules 2017, 22, 1882. [Google Scholar] [CrossRef]
- Schneider, A.; Ruppert, A.J.; Lystbæk, T.B.; Bastian, S.; Hauer, B. Expanding the Cation Cage: Squalene-Hopene Cyclase-Mediated Enantioselective Semipinacol Rearrangement. ACS Catal. 2023, 13, 1946–1951. [Google Scholar] [CrossRef]
- Salami, S.A.; Smith, V.J.; Krause, R.W.M. Aqua/Mechanochemical Mediated Synthesis of Novel Spiro [Indole–Pyrrolidine] Derivatives. Int. J. Mol. Sci. 2023, 24, 2307. [Google Scholar] [CrossRef]
- Iazzetti, A.; Allevi, D.; Calcaterra, A.; Fabrizi, G.; Goggiamani, A.; Mazzoccanti, G.; Sferrazza, A.; Verdiglione, R.; Vergine, V. Highly Efficient and Mild Gold (I) Catalyzed Synthesis of 3,8-Diarylidene-2,7-dioxaspiro[4.4]nonane-1,6-diones. Molecules 2023, 28, 300. [Google Scholar] [CrossRef]
- Abell, J.C.; Bold, C.P.; Vicens, L.; Jentsch, T.; Velasco, N.; Tyler, J.L.; Straker, R.N.; Noble, A.; Aggarwal, V.K. Synthesis of Dihydropyridine Spirocycles by Semi-Pinacol-Driven Dearomatization of Pyridines. Org. Lett. 2023, 25, 400–404. [Google Scholar] [CrossRef]
- Yokoe, H.; Kiriyama, A.; Shimoda, M.; Nakajima, S.; Hashizume, Y.; Endo, Y.; Iwamoto, R.; Tsubuki, M.; Kanoh, N. Cis-Selective Double Spirocyclization via Dearomatization and Isomerization under Thermodynamic Control. J. Org. Chem. 2023, 88, 1803–1814. [Google Scholar] [CrossRef]
- Clarke, A.K.; Unsworth, W.P. A happy medium: The synthesis of medicinally important medium-sized rings via ring expansion. Chem. Sci. 2020, 11, 2876–2881. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ye, Z.; Ma, Z.-x.; Wildman, S.A.; Blaszczyk, S.A.; Hu, L.; Guizei, I.A.; Tang, W. A general strategy for diversifying complex natural products to polycyclic scaffolds with medium-sized rings. Nat. Commun. 2019, 10, 4015. [Google Scholar] [CrossRef]
- Hussain, A.; Yousuf, S.K.; Mukherjee, D. Importance and synthesis of benzannulated medium-sized and macrocyclic rings (BMRs). RSC Adv. 2014, 4, 43241–43257. [Google Scholar] [CrossRef]
- Reyes, R.L.; Iwai, T.; Sawamura, M. Construction of Medium-Sized Rings by Gold Catalysis. Chem. Rev. 2021, 121, 8926–8947. [Google Scholar] [CrossRef] [PubMed]
- Donald, J.R.; Unsworth, W.P. Ring-Expansion Reactions in the Synthesis of Macrocycles and Medium-Sized Rings. Chem. Eur. J. 2017, 23, 8780–8799. [Google Scholar] [CrossRef]
- Choury, M.; Basilio Lopes, A.; Blond, G.; Gulea, M. Synthesis of Medium-Sized Heterocycles by Transition-Metal-Catalyzed Intramolecular Cyclization. Molecules 2020, 25, 3147. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Q.; Tang, H.; Pan, X. Isoxazole/Isoxazoline Skeleton in the Structural Modification of Natural Products: A Review. Pharmaceuticals 2023, 16, 228. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, A.; Zana, A.; Coser, C.; Tamborini, L.; Basilico, N.; Parapini, S.; Taramelli, D.; Conti, P. Development of Potent 3-Br-isoxazoline-Based Antimalarial and Antileishmanial Compounds. ACS Med. Chem. Lett. 2021, 12, 1726–1732. [Google Scholar] [CrossRef]
- Ikeda, Z.; Kakegawa, K.; Kikuchi, F.; Itono, S.; Oki, H.; Yashiro, H.; Hiyoshi, H.; Tsuchimori, K.; Hamagami, K.; Watanabe, M.; et al. Design, Synthesis, and Biological Evaluation of a Novel Series of 4-Guanidinobenzoate Derivatives as Enteropeptidase Inhibitors with Low Systemic Exposure for the Treatment of Obesity. J. Med. Chem. 2022, 65, 8456–8477. [Google Scholar] [CrossRef]
- Park, S.J.; Yeon, S.K.; Kim, Y.; Kim, H.J.; Kim, S.; Kim, J.; Choi, J.W.; Kim, B.; Lee, E.H.; Kim, R.; et al. Discovery of Novel Sphingosine-1-Phosphate-1 Receptor Agonists for the Treatment of Multiple Sclerosis. J. Med. Chem. 2022, 65, 3539–3562. [Google Scholar] [CrossRef]
- Chalkha, M.; Nour, H.; Chebbac, K.; Nakkabi, A.; Bahsis, L.; Bakhouch, M.; Akhazzane, M.; Bourass, M.; Chtita, S.; Bin Jardan, Y.A.; et al. Synthesis, Characterization, DFT Mechanistic Study, Antimicrobial Activity, Molecular Modeling, and ADMET Properties of Novel Pyrazole-isoxazoline Hybrids. ACS Omega 2022, 7, 46731–46744. [Google Scholar] [CrossRef]
- Paciorek, J.; Höfler, D.; Sokol, K.R.; Wurst, K.; Magauer, T. Total Synthesis of the Dihydrooxepine-Spiroisoxazoline Natural Product Psammaplysin, A. J. Am. Chem. Soc. 2022, 144, 19704–19708. [Google Scholar] [CrossRef] [PubMed]
- Krompiec, S.; Lodowski, P.; Kurpanik-Wójcik, A.; Gołek, B.; Mieszczanin, A.; Fijołek, A.; Matussek, M.; Kaszuba, K. Nitrile Oxide, Alkenes, Dipolar Cycloaddition, Isomerization and Metathesis Involved in the Syntheses of 2-Isoxazolines. Molecules 2023, 28, 2547. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Shankar, R. 2-Isoxazolines: A Synthetic and Medicinal Overview. ChemMedChem 2021, 16, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Sedenkova, K.N.; Andriasov, K.S.; Eremenko, M.G.; Grishin, Y.K.; Alferova, V.A.; Baranova, A.A.; Zefirov, N.A.; Zefirova, O.N.; Zarubaev, V.V.; Gracheva, Y.A.; et al. Bicyclic Isoxazoline Derivatives: Synthesis and Evaluation of Biological Activity. Molecules 2022, 27, 3546. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- Goryashchenko, A.S.; Uvarova, V.I.; Osolodkin, D.I.; Ishmukhametov, A.A. Discovery of small molecule antivirals targeting tick-borne encephalitis virus. In Annual Reports in Medicinal Chemistry; Osolodkin, D.I., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 58, pp. 1–54. [Google Scholar]
- Bologheanu, R.; Schubert, L.; Thurnher, M.; Schiefer, J.; Santonja, I.; Holzmann, H.; Oester-reicher, Z.; Tobudic, S.; Winkler, S.; Faybik, P.; et al. Unexpected complete recovery of a patient with severe tick-borne encephalitis treated with favipiravir. Antivir. Res. 2020, 184, 104952. [Google Scholar] [CrossRef]
- Saiz, J.-C. Animal and Human Vaccines against West Nile Virus. Pathogens 2020, 9, 1073. [Google Scholar] [CrossRef]
- Orlov, A.A.; Eletskaya, A.A.; Frolov, K.A.; Golinets, A.D.; Palyulin, V.A.; Krivokolysko, S.G.; Kozlovskaya, L.I.; Dotsenko, V.V.; Osolodkin, D.I. Probing chemical space of tick-borne encephalitis virus reproduction inhibitors with organoselenium compounds. Arch. Pharm. 2018, 351, e1700353. [Google Scholar] [CrossRef]
- Osolodkin, D.I.; Kozlovskaya, L.I.; Dueva, E.V.; Dotsenko, V.V.; Rogova, Y.V.; Frolov, K.A.; Krivokolysko, S.G.; Romanova, E.G.; Morozov, A.S.; Karganova, G.G.; et al. Inhibitors of Tick-Borne Flavivirus Reproduction from Structure-Based Virtual Screening. ACS Med. Chem. Lett. 2013, 4, 869–874. [Google Scholar] [CrossRef]
- Sedenkova, K.N.; Uvarova, V.I.; Nazarova, A.A.; Peisikova, A.V.; Khvatov, E.V.; Sukhorukov, M.V.; Frolenko, V.S.; Goryashchenko, A.S.; Kholodilov, I.S.; Grishin, Y.K.; et al. Tetrahydroquinazoline N-oxide derivatives inhibit reproduction of tick-borne and mosquito-borne flaviviruses. Arch. Pharm. 2023, 356, e2300027. [Google Scholar] [CrossRef] [PubMed]
- Sedenkova, K.N.; Kolodyazhnaya, J.V.; Vasilenko, D.A.; Gracheva, Y.A.; Kharitonoshvili, E.V.; Grishin, Y.K.; Chistov, A.A.; Rybakov, V.B.; Holt, T.; Kutateladze, A.G.; et al. Novel π-conjugated systems based on pyrimidine N-Oxide. Dye. Pigment. 2019, 164, 72–81. [Google Scholar] [CrossRef]
- Kozlovskaya, L.I.; Andrei, G.; Orlov, A.A.; Khvatov, E.V.; Koruchekov, A.A.; Belyaev, E.S.; Nikolaev, E.N.; Korshun, V.A.; Snoeck, R.; Osolodkin, D.I.; et al. Antiviral activity spectrum of phenoxazine nucleoside derivatives. Antivir. Res. 2019, 163, 117–124. [Google Scholar] [CrossRef]
- Dueva, E.V.; Tuchynskaya, K.K.; Kozlovskaya, L.I.; Osolodkin, D.I.; Sedenkova, K.N.; Averina, E.B.; Palyulin, V.A.; Karganova, G.G. Spectrum of antiviral activity of 4-aminopyrimidine N-oxides against a broad panel of tick-borne encephalitis virus strains. Antivir. Chem. Chemotherapy 2020, 28, 1–10. [Google Scholar] [CrossRef]
- Sedenkova, K.N.; Dueva, E.V.; Averina, E.B.; Grishin, Y.K.; Osolodkin, D.I.; Kozlovskaya, L.I.; Palyulin, V.A.; Savelyev, E.N.; Orlinson, B.S.; Novakov, I.A.; et al. Synthesis and assessment of 4-aminotetrahydroquinazoline derivatives as tick-borne encephalitis virus reproduction inhibitors. Org. Biomol. Chem. 2015, 13, 3406–3415. [Google Scholar] [CrossRef]
- Sedenkova, K.N.; Nazarova, A.A.; Khatov, E.V.; Dueva, E.V.; Orlov, A.A.; Osolodkin, D.I.; Grishin, Y.K.; Kuznetsova, T.S.; Palyulin, V.A.; Averina, E.B. A facile metal-free approach to N,N’-bis(1-oxidopyrimidin-4-yl)diamines with promising biological activity. Mendeleev Commun. 2018, 28, 592–594. [Google Scholar] [CrossRef]
- Xu, J.; Xie, X.; Ye, N.; Zou, J.; Chen, H.; White, M.A.; Shi, P.Y.; Zhou, J. Design, Synthesis, and Biological Evaluation of Substituted 4,6-Dihydrospiro[[1,2,3]triazolo[4,5-b]pyridine-7,3′-indoline]-2′,5(3H)-dione Analogues as Potent NS4B Inhibitors for the Treatment of Dengue Virus Infection. J. Med. Chem. 2019, 62, 7941–7960. [Google Scholar] [CrossRef]
- Zou, B.; Chan, W.L.; Ding, M.; Leong, S.Y.; Nilar, S.; Seah, P.G.; Liu, W.; Karuna, R.; Blasco, F.; Yip, A.; et al. Lead optimization of spiropyrazolopyridones: A new and potent class of dengue virus inhibitors. ACS Med. Chem. Lett. 2015, 6, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Vasilenko, D.A.; Dueva, E.V.; Kozlovskaya, L.I.; Zefirov, N.A.; Grishin, Y.K.; Butov, G.M.; Palyulin, V.A.; Kuznetsova, T.S.; Karganova, G.G.; Zefirova, O.N.; et al. Tick-borne flavivirus reproduction inhibitors based on isoxazole core linked with adamantane. Bioorg. Chem. 2019, 87, 629–637. [Google Scholar] [CrossRef]
- Escribano-Romero, E.; Jiménez de Oya, N.; Domingo, E.; Saiz, J.C. Extinction of West Nile Virus by Favipiravir through Lethal Mutagenesis. Antimicrob. Agents Chemother. 2017, 61, e01400-17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).