Characterising the Effect of Wnt/β-Catenin Signalling on Melanocyte Development and Patterning: Insights from Zebrafish (Danio rerio)
Abstract
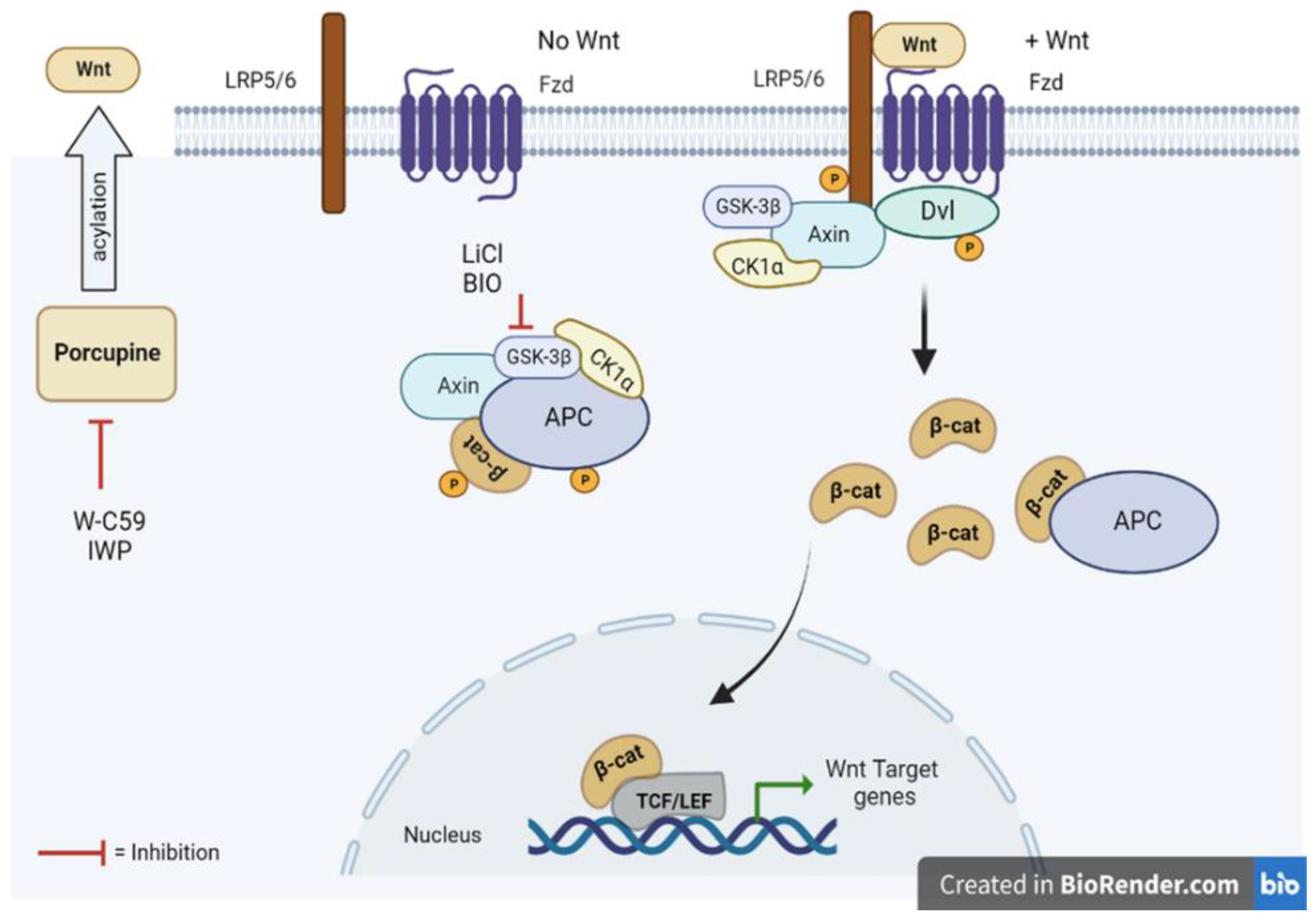
1. Introduction
2. Results
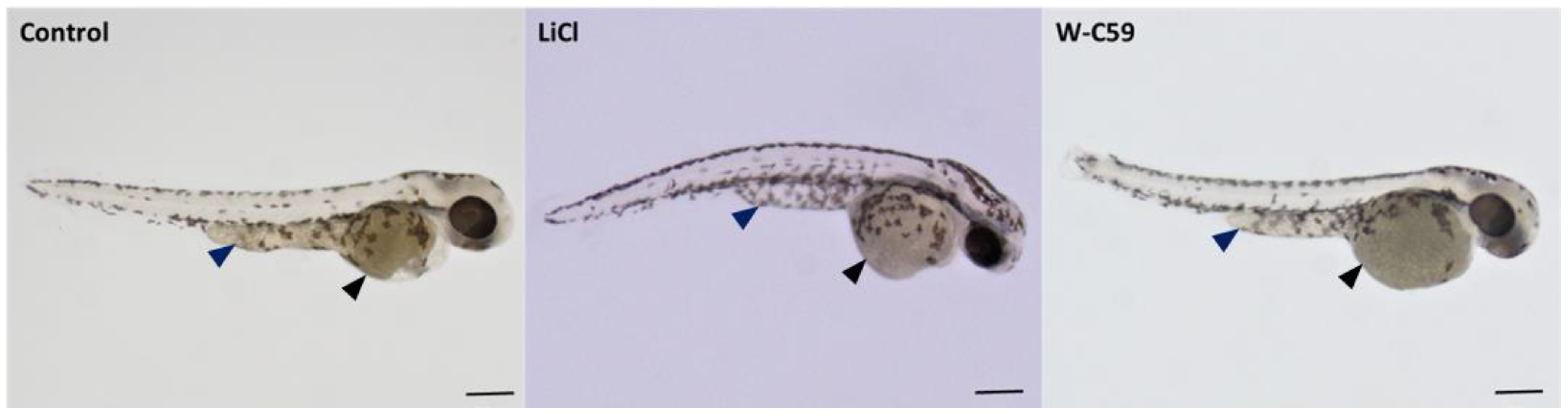
2.1. Microscopic Examination of Melanocyte Formation of the Control and Wnt Chemical-Treated Zebrafish Larvae
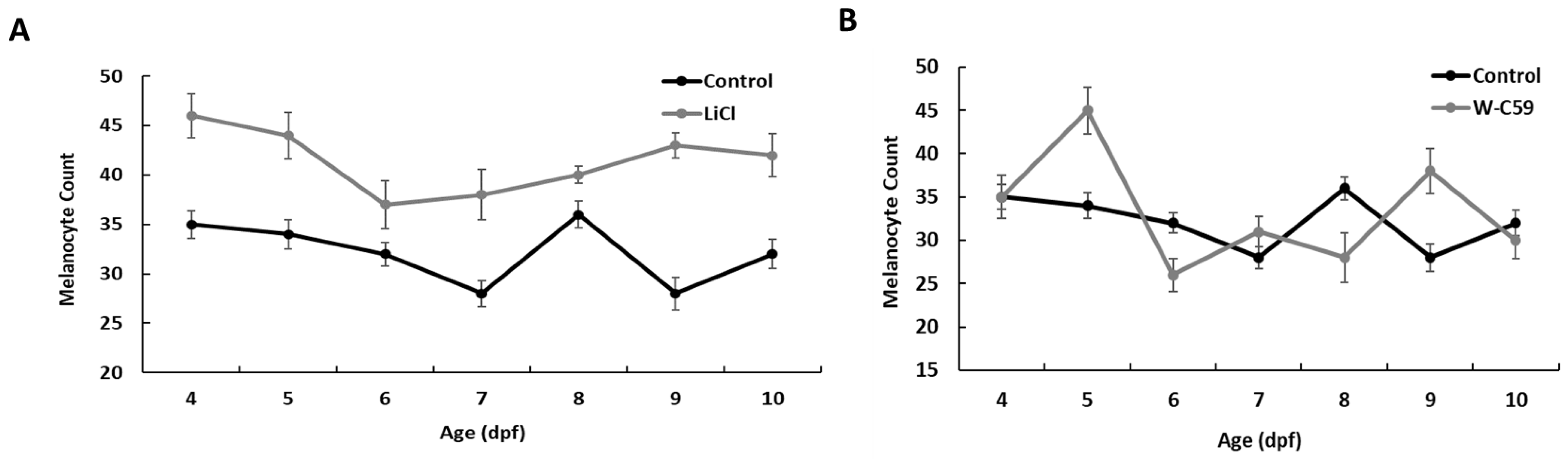
2.2. Comparison of the Variation in Melanocyte Count of the Control and Wnt Chemical-Treated Zebrafish Larvae
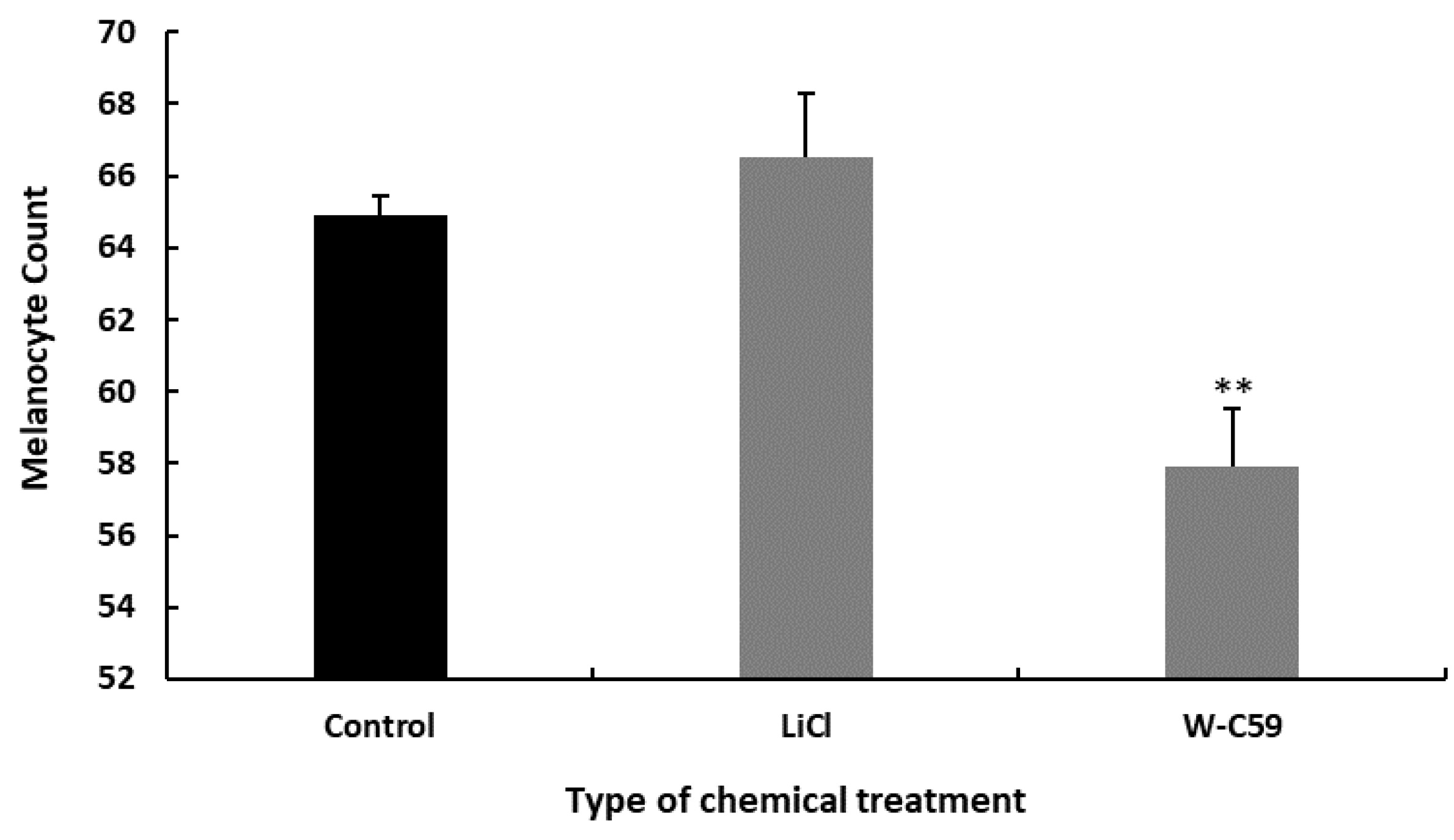
2.3. Comparison of the Variation of Melanocyte Count at 8 dpf against Wnt Chemical Treatments
2.4. Comparison of the Variation of Melanocyte Count at 15 dpf against Wnt Chemical Treatments
2.5. Comparison of the Differences in Melanocyte Morphology and Morphometry against Wnt Chemical Treatments
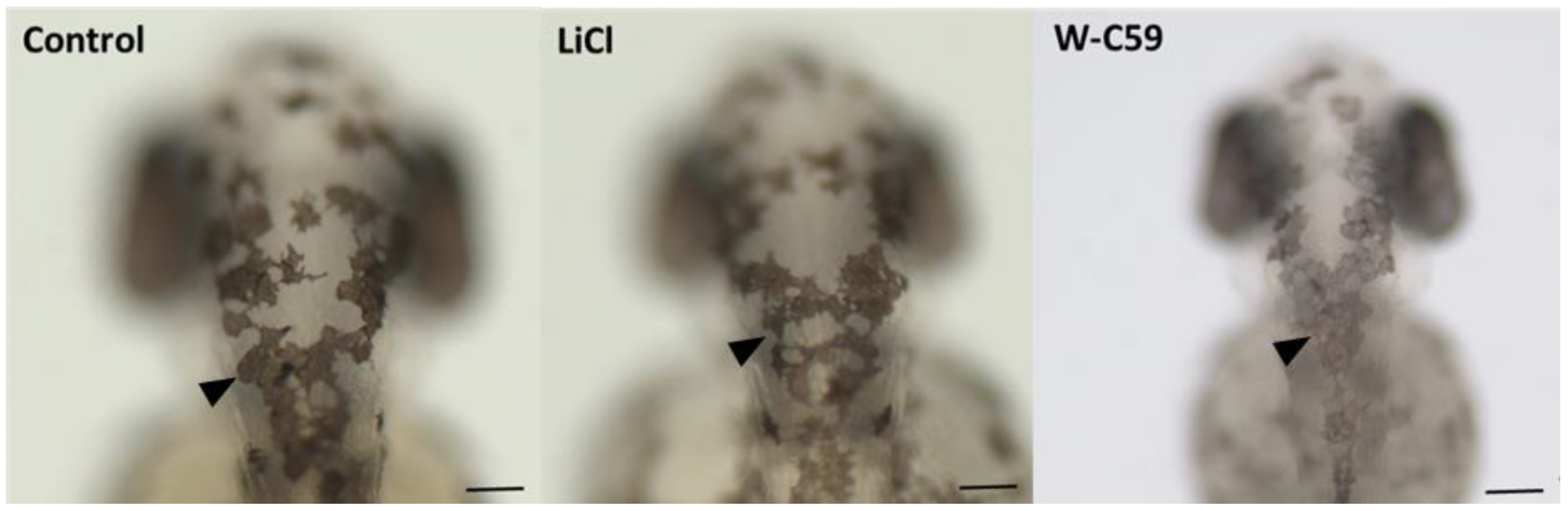
2.5.1. Melanocyte Phenotypic Differences at 2 dpf Embryonic Stage of Zebrafish
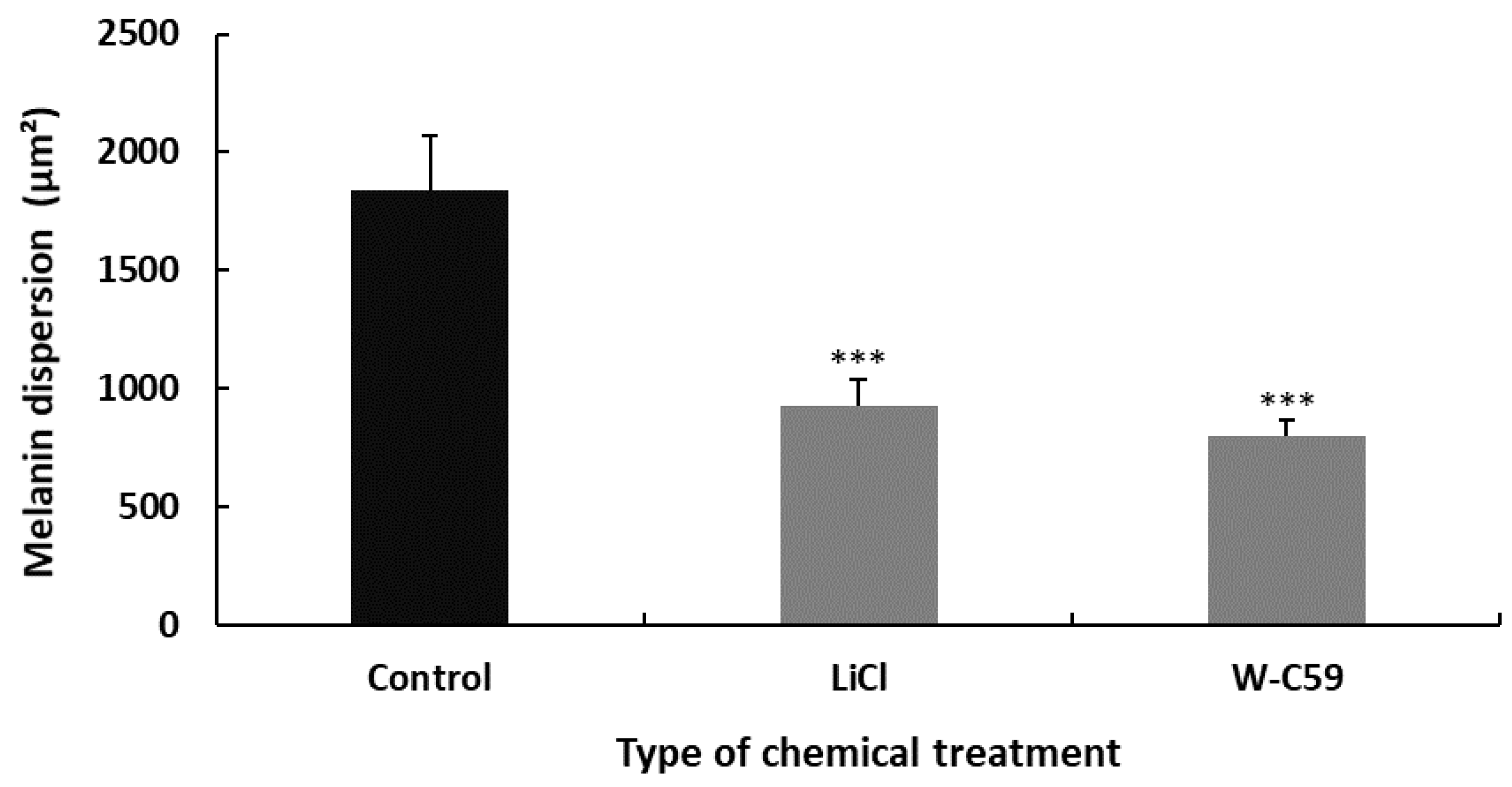
Differences in Melanin Dispersion at 2 dpf Embryonic Stage of Zebrafish
2.5.2. Melanocyte Phenotypic Differences at 6 dpf Larval Stage of Zebrafish
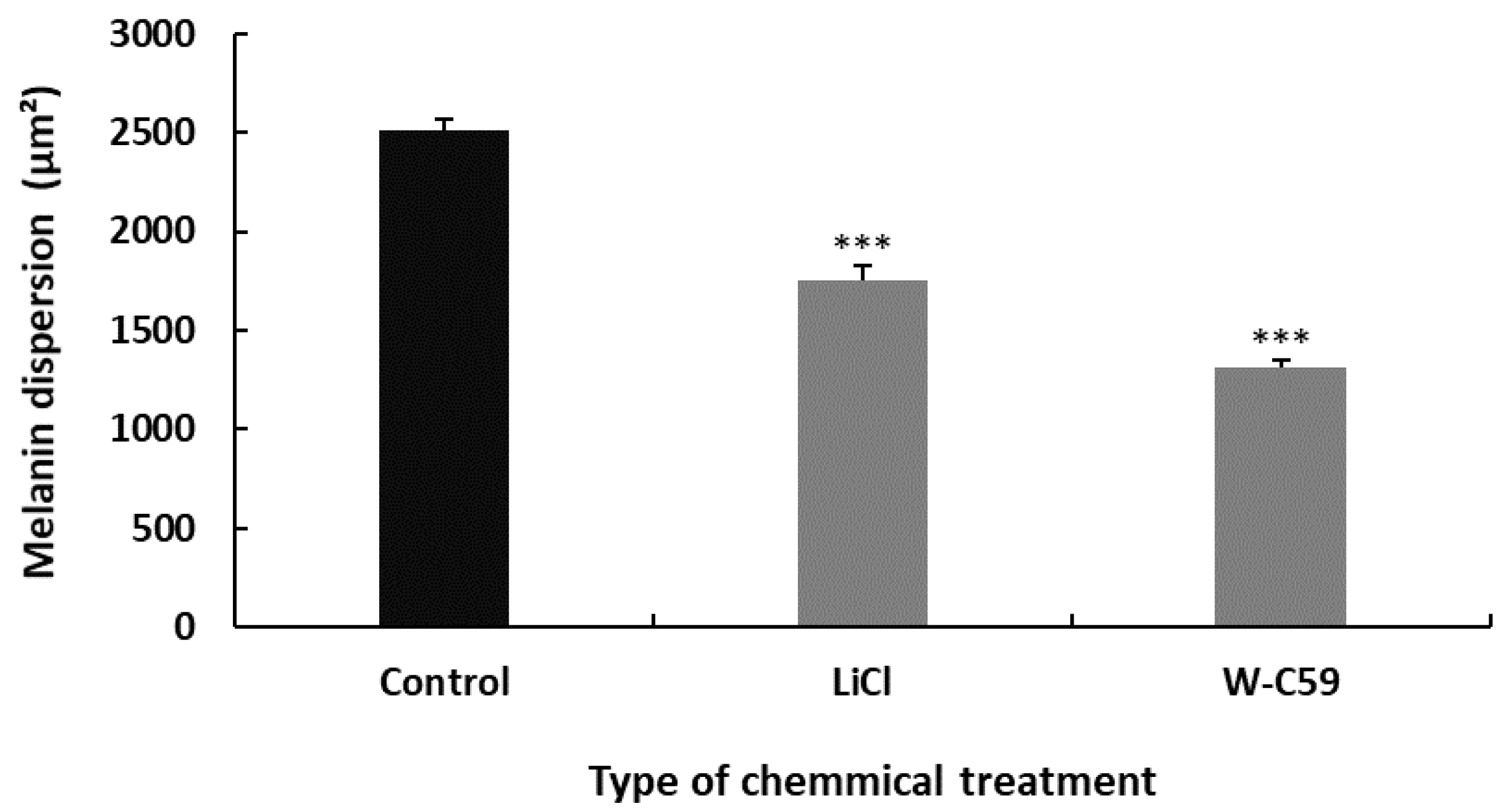
Differences in Melanin Dispersion at 6 dpf Larval Stage of Zebrafish
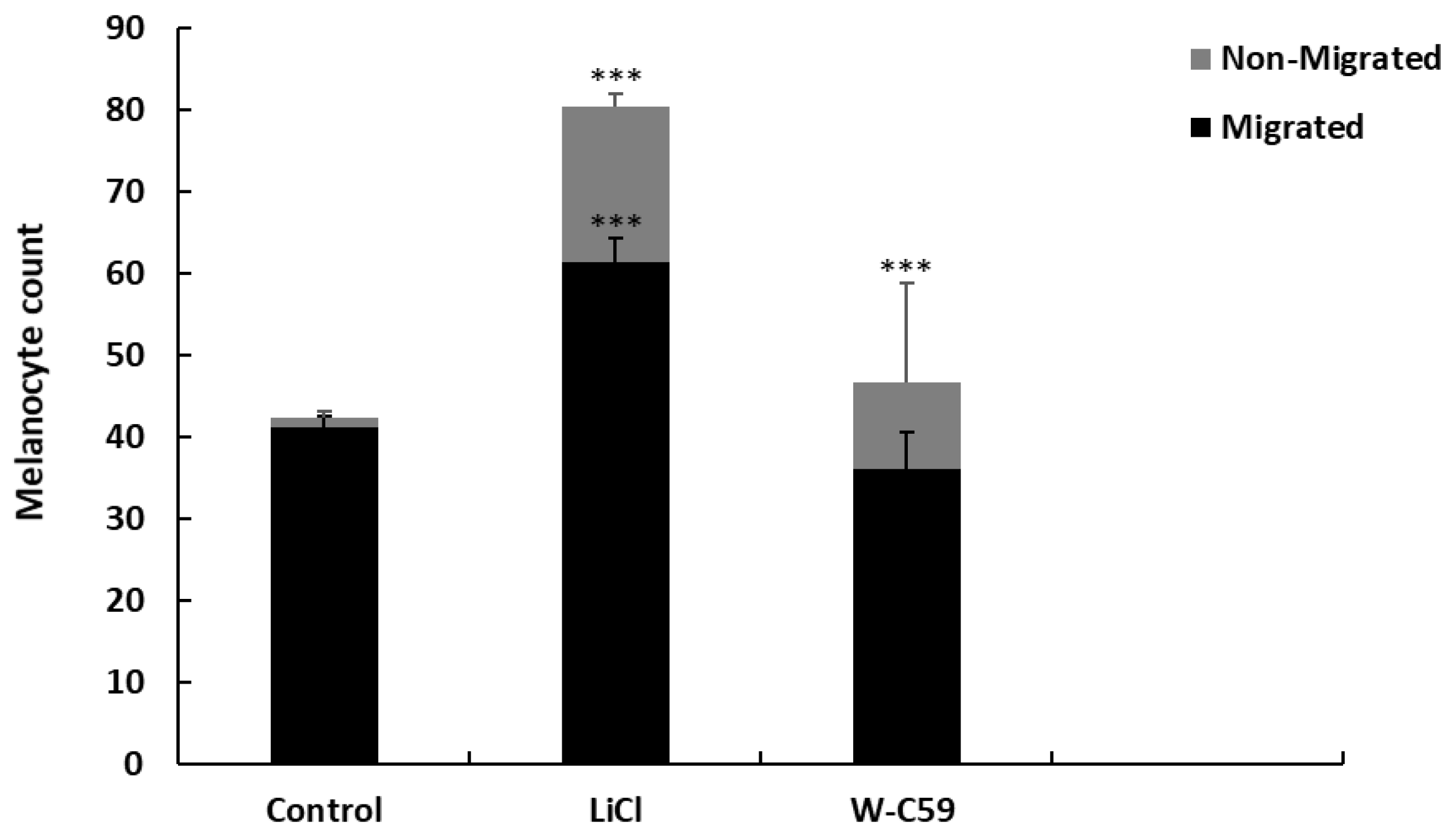
2.6. Examination of Melanocyte Migratory Differences against Wnt Chemical Treatments
2.7. Analysis of Melanocyte Migratory Differences against Wnt Chemical Treatments
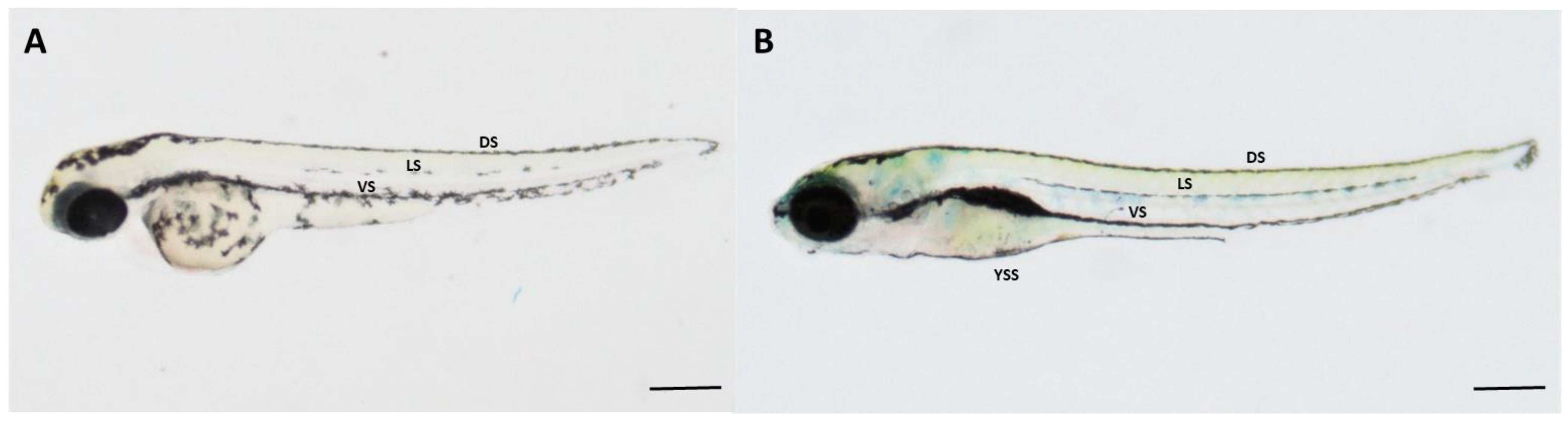
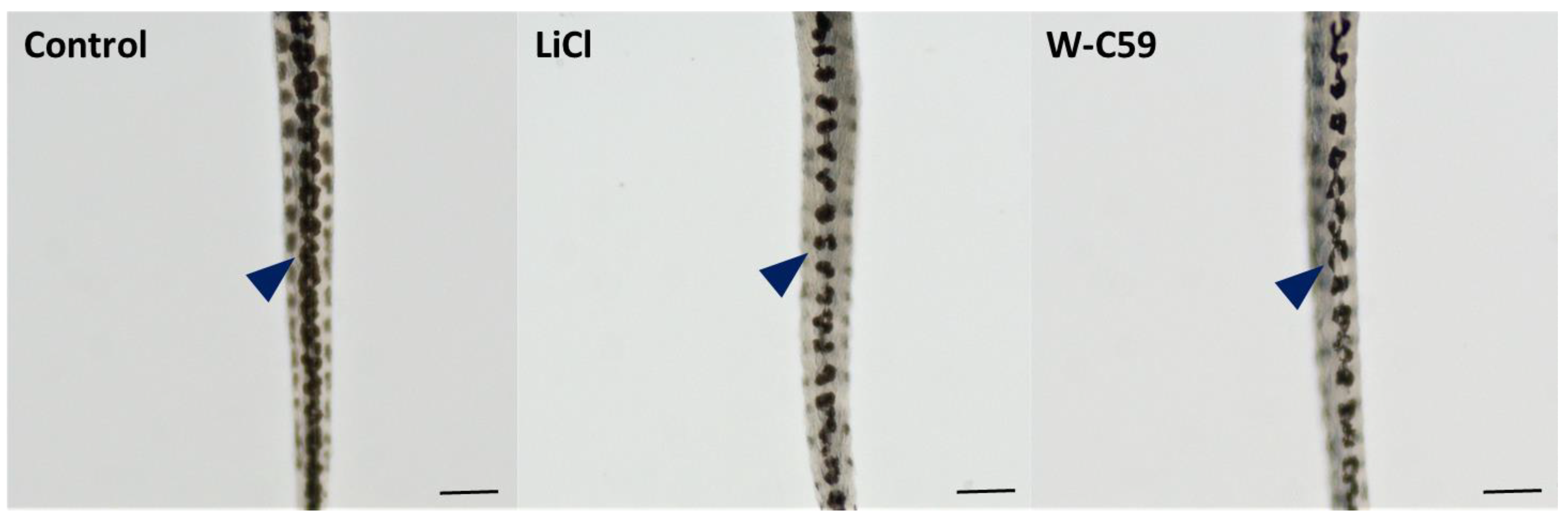
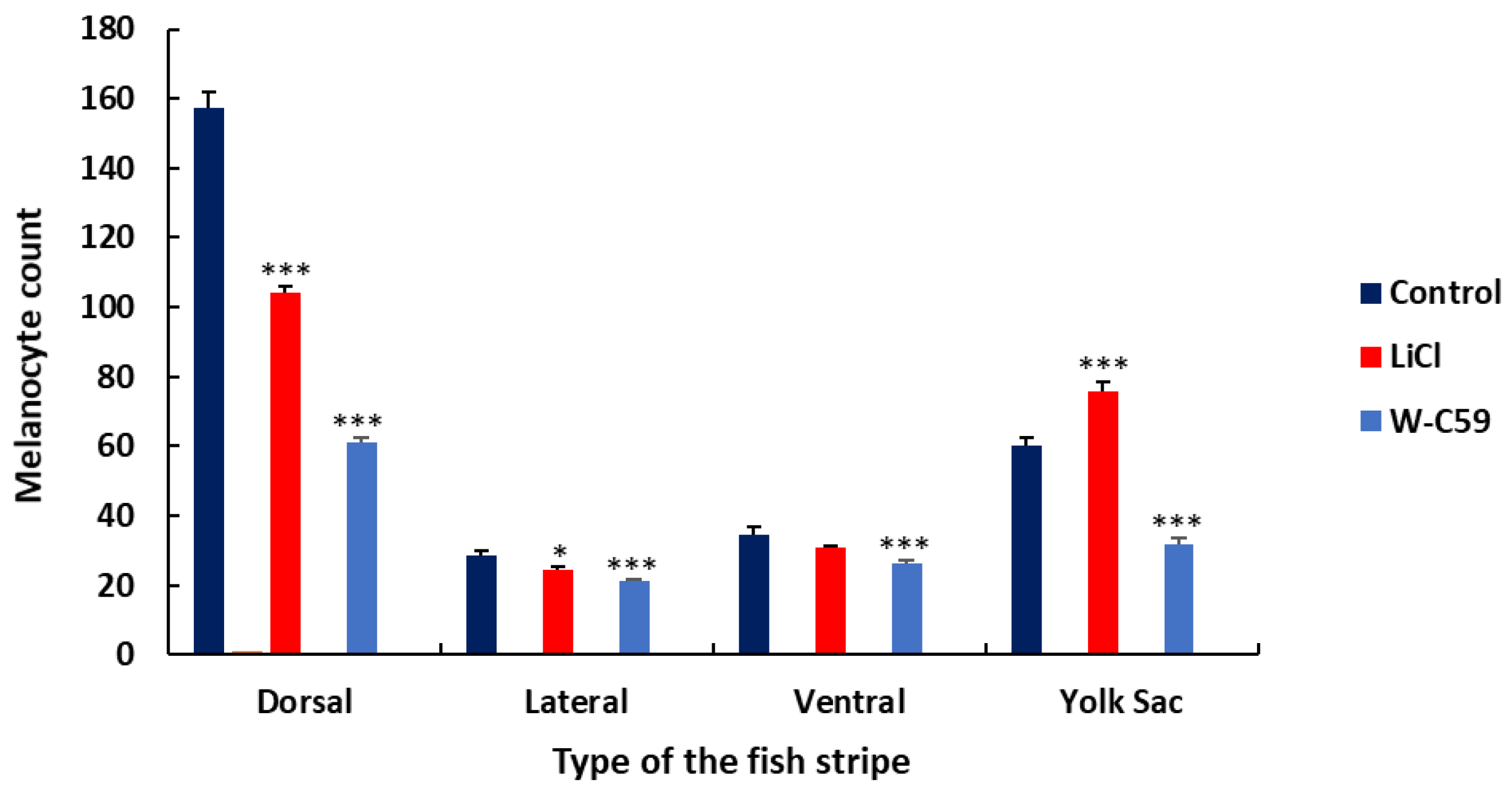
2.8. Variations of Melanocyte Arrangement in Stripe Formation against Wnt Chemical Treatments
2.8.1. Variations of Dorsal Stripe Melanocyte Arrangement
2.8.2. Variations of Yolk Sac Stripe Melanocyte Arrangement
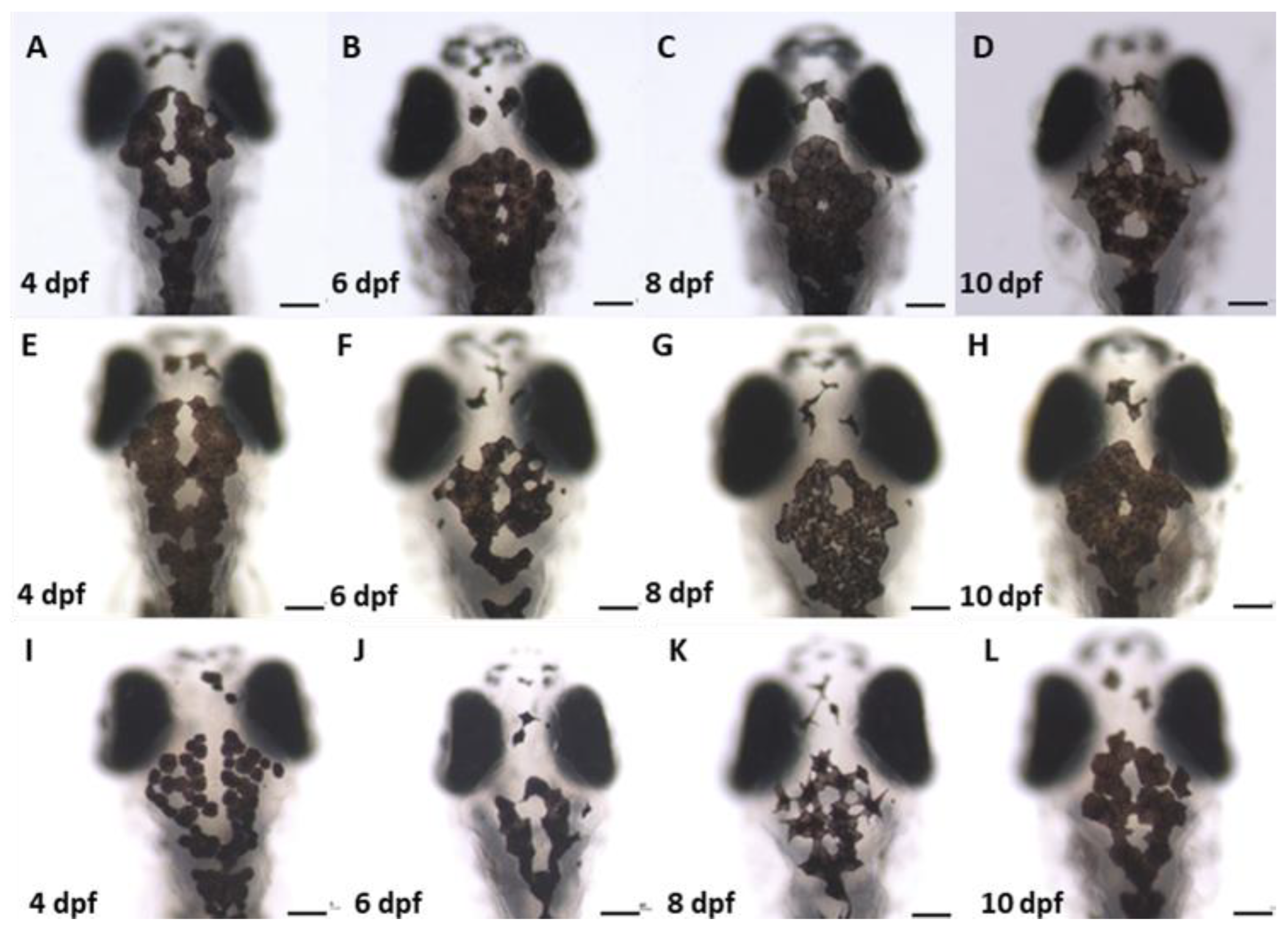
2.8.3. Analysis of Melanocyte Arrangement at Early Larval Stage upon Wnt Chemical Treatments
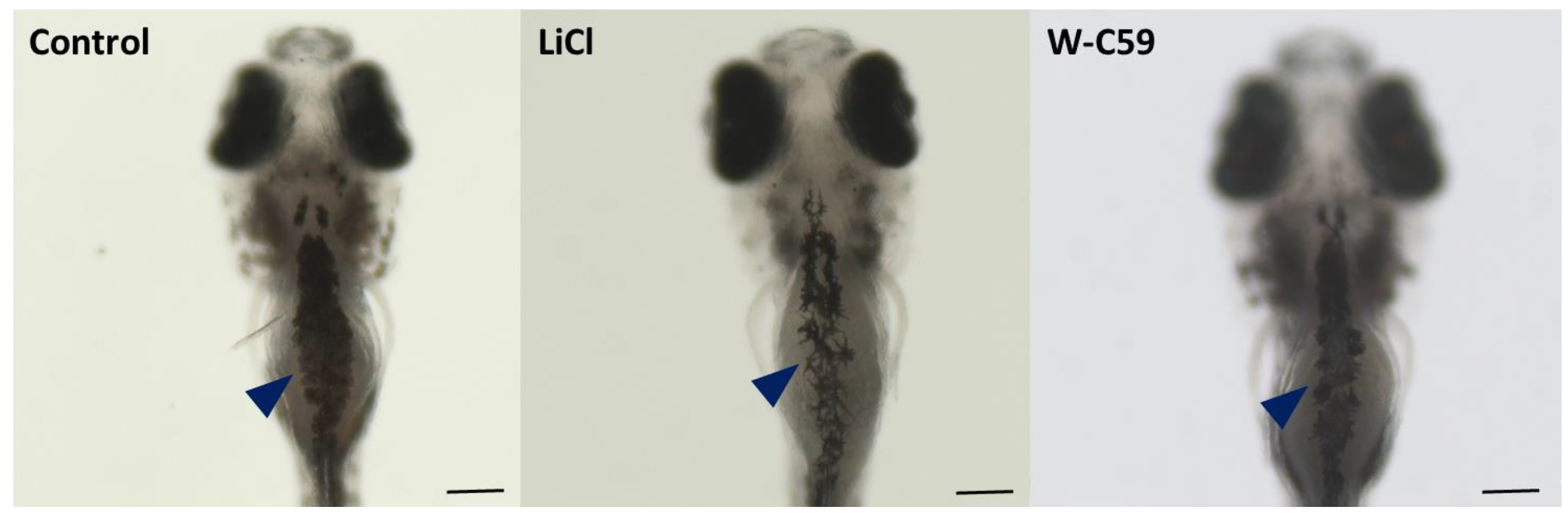
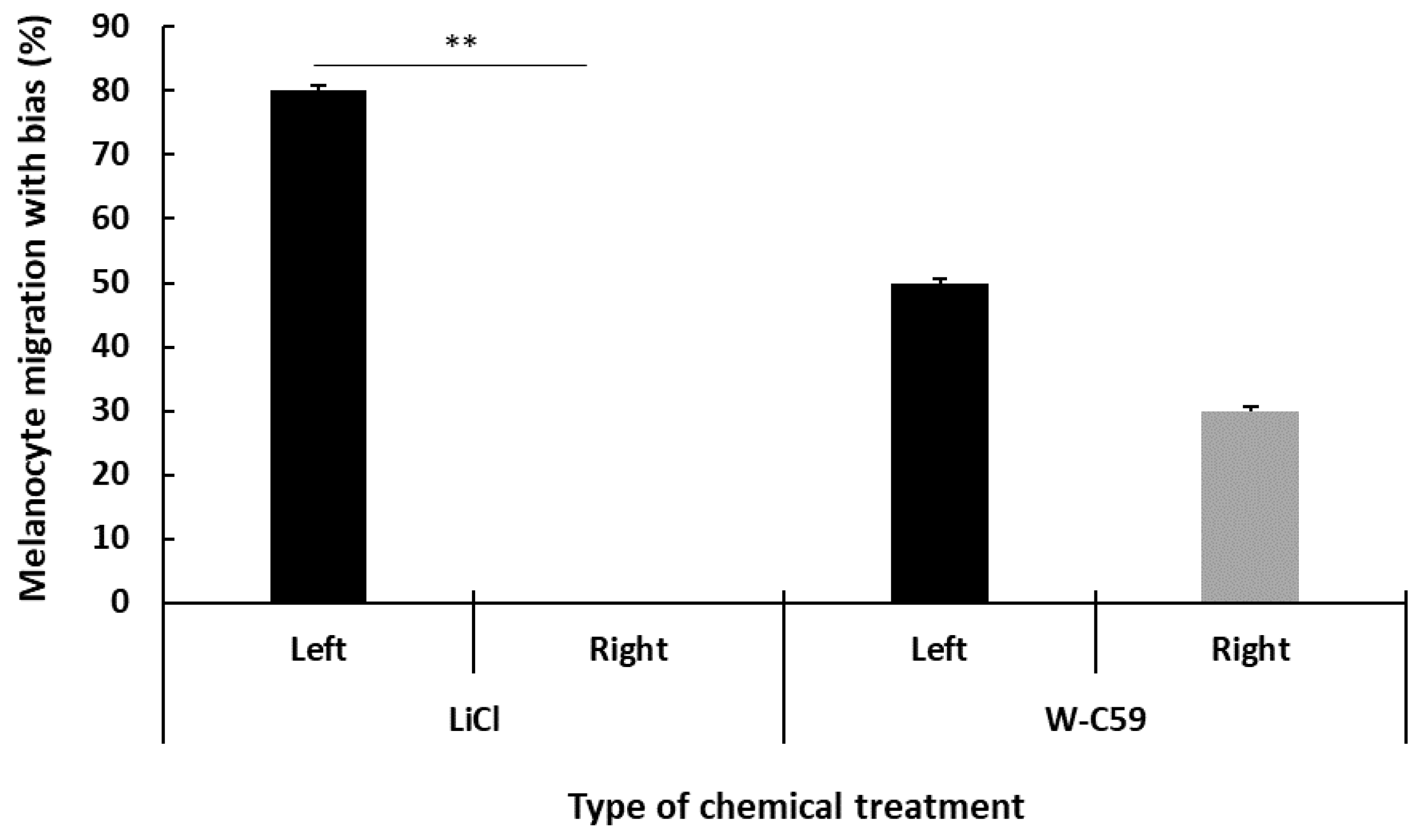
2.9. Analysis of Biased Migration of Melanocytes along the L–R Axis of Zebrafish Larvae upon Wnt Chemical Treatments
3. Discussion
4. Materials and Methods
4.1. Zebrafish Strains and Maintenance
4.2. Chemical Treatment of Wnt Modulators
4.3. Analysing the Melanocyte Count
4.4. Examining Morphology and Measuring Melanin Dispersion of Melanocytes
4.5. Analysing the Melanocyte Migration
4.6. Analysing the Melanocyte Arrangement
Analysis of Biased Migration of Melanocytes along the L–R Axis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- van Amerongen, R.; Nusse, R. Towards an integrated view of Wnt signaling in development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Angers, S.; Moon, R.T. Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 2009, 10, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, L.; Howe, D.G.; Ramachandran, S.; Toro, S.; E Van Slyke, C.; Bradford, Y.M.; Eagle, A.; Fashena, D.; Frazer, K.; Kalita, P.; et al. The Zebrafish Information Network: New support for non-coding genes, richer Gene Ontology annotations and the Alliance of Genome Resources. Nucleic Acids Res. 2019, 47, D867–D873. [Google Scholar] [CrossRef]
- Dorsky, R.I.; Moon, R.T.; Raible, D.W. Control of neural crest cell fate by the Wnt signalling pathway. Nature 1998, 396, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Dorsky, R.I.; Raible, D.W.; Moon, R.T. Direct regulation of nacre, a zebrafish MITF homolog required for pigment cell formation, by the Wnt pathway. Genes Dev. 2000, 14, 158–162. [Google Scholar] [CrossRef]
- Dunn, K.J.; Brady, M.; Snyder, S.; Incao, A.; Pavan, W.J.; Ochsenbauer-Jambor, C. WNT1 and WNT3a promote expansion of melanocytes through distinct modes of action. Pigment Cell Res. 2005, 18, 167–180. [Google Scholar] [CrossRef]
- Jin, E.-J.; Erickson, C.A.; Takada, S.; Burrus, L.W. Wnt and BMP signaling govern lineage segregation of melanocytes in the avian embryo. Dev. Biol. 2001, 233, 22–37. [Google Scholar] [CrossRef]
- Fujii, R. The regulation of motile activity in fish chromatophores. Pigment Cell Res. 2000, 13, 300–319. [Google Scholar] [CrossRef]
- Fujii, R. Cytophysiology of fish chromatophores. Int. Rev. Cytol. 1993, 143, 191–255. [Google Scholar] [CrossRef]
- Cooper, C.D.; Raible, D.W. Mechanisms for reaching the differentiated state: Insights from neural crest-derived melanocytes. Semin. Cell Dev. Biol. 2009, 20, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Stuart-Fox, D.; Ballen, C. Genetics and evolution of colour patterns in reptiles. Semin. Cell Dev. Biol. 2013, 24, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Grether, G.F.; Kolluru, G.R.; Nersissian, K. Individual colour patches as multicomponent signals. Biol. Rev. 1999, 79, 583–610. [Google Scholar] [CrossRef] [PubMed]
- Schartl, M.; Larue, L.; Goda, M.; Bosenberg, M.W.; Hashimoto, H.; Kelsh, R.N. What is a vertebrate pigment cell? Pigment Cell Melanoma Res. 2016, 29, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Kim, Y.E.; Jang, Y. Colour and pattern change against visually heterogeneous backgrounds in the tree frog Hyla japonica. Sci. Rep. 2016, 6, 22601. [Google Scholar] [CrossRef]
- Caro, T. The Colours of Extant Mammals. In Seminars in Cell & Developmental Biology; Elsevier: Coventry, UK, 2013. [Google Scholar]
- Jablonski, D.; Alena, A.; Vlček, P.; Jandzik, D. Axanthism in amphibians: A review and the first record in the widespread toad of the Bufotes viridis complex (Anura: Bufonidae). Belg. J. Zool. 2014, 144, 93–101. [Google Scholar] [CrossRef]
- Haffter, P.; Mullins, M.C.; Lin, S.; Farrell, M.J.; Vogelsang, E.; Haas, F.; Brand, M.; van Eeden, F.J.M.; Furutani-Seiki, M.; Granato, M.; et al. Mutations affecting pigmentation and shape of the adult zebrafish. Dev. Genes Evolut. 1996, 206, 260–276. [Google Scholar] [CrossRef]
- Kelsh, R.N.; Brand, M.; Jiang, Y.-J.; Heisenberg, C.-P.; Lin, S.; Haffter, P.; Odenthal, J.; Mullins, M.C.; van Eeden, F.J.M.; Furutani-Seiki, M.; et al. Zebrafish pigmentation mutations and the processes of neural crest development. Development 1996, 123, 369–389. [Google Scholar] [CrossRef]
- Silva, G.T.; Mahaulpatha, W.D. Distribution of colour and pattern polymorphism in montane frogs of Sri Lanka. J. Entomol. Zool. Studies 2019, 7, 486–491. [Google Scholar]
- Aspengren, S.; Hedberg, D.; Wallin, M. Studies of pigment transfer between Xenopus laevis melanophores and fibroblasts in vitro and in vivo 1. Pigment Cell Res. 2006, 19, 136–145. [Google Scholar] [CrossRef]
- Hultman, K.A.; Johnson, S.L. Differential contribution of direct-developing and stem cell-derived melanocytes to the zebrafish larval pigment pattern. Dev. Biol. 2010, 337, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Dooley, C.M.; Mongera, A.; Walderich, B.; Nüsslein-Volhard, C. On the embryonic origin of adult melanophores: The role of ErbB and Kit signalling in establishing melanophore stem cells in zebrafish. Development 2013, 140, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Le Guellec, D.; Morvan-Dubois, G.; Sire, J.-Y. Sire, Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio). Int. J. Dev. Biol. 2003, 48, 217–231. [Google Scholar] [CrossRef]
- Budi, E.H.; Patterson, L.B.; Parichy, D.M. Embryonic requirements for ErbB signaling in neural crest development and adult pigment pattern formation. Development 2008, 135, 2603–2614. [Google Scholar] [CrossRef]
- Quigley, I.K.; Parichy, D.M. Pigment pattern formation in zebrafish: A model for developmental genetics and the evolution of form. Microsc. Res. Tech. 2002, 58, 442–455. [Google Scholar] [CrossRef]
- Kelsh, R.N.; Harris, M.L.; Colanesi, S.; Erickson, C.A. Stripes and belly-spots—A review of pigment cell morphogenesis in vertebrates. Semin. Cell Dev. Biol. 2009, 20, 90–104. [Google Scholar] [CrossRef]
- Cooper, C.D. Insights from zebrafish on human pigment cell disease and treatment. Dev. Dyn. 2017, 246, 889–896. [Google Scholar] [CrossRef]
- Rouzaud, F.; Kadekaro, A.L.; Abdel-Malek, Z.A.; Hearing, V.J. MC1R and the response of melanocytes to ultraviolet radiation. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 571, 133–152. [Google Scholar] [CrossRef]
- Leal, L.F.; Bueno, A.C.; Gomes, D.C.; Abduch, R.; de Castro, M.; Antonini, S.R. Inhibition of the Tcf/beta-catenin complex increases apoptosis and impairs adrenocortical tumor cell proliferation and adrenal steroidogenesis. Oncotarget 2015, 6, 43016. [Google Scholar] [CrossRef]
- Henderson, W.R., Jr.; Chi, E.Y.; Ye, X.; Nguyen, C.; Tien, Y.T.; Zhou, B.; Borok, Z.; Knight, D.A.; Kahn, M. Inhibition of Wnt/β-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 2010, 107, 14309–14314. [Google Scholar] [CrossRef] [PubMed]
- Henion, P.D.; Weston, J.A. Timing and pattern of cell fate restrictions in the neural crest lineage. Development 1997, 124, 4351–4359. [Google Scholar] [CrossRef] [PubMed]
- Reedy, M.V.; Faraco, C.D.; Erickson, C.A. The delayed entry of thoracic neural crest cells into the dorsolateral path is a consequence of the late emigration of melanogenic neural crest cells from the neural tube. Dev. Biol. 1998, 200, 234–246. [Google Scholar] [CrossRef]
- Erickson, C.A.; Goins, T.L. Avian neural crest cells can migrate in the dorsolateral path only if they are specified as melanocytes. Development 1995, 121, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Saint-Germain, N.; Gyda, M.; Magner-Fink, E.; Lee, Y.-H.; Credidio, C.; Saint-Jeannet, J.-P. Sox10 regulates the development of neural crest-derived melanocytes in Xenopus. Dev. Biol. 2003, 259, 19–33. [Google Scholar] [CrossRef]
- Galli, C.; Piemontese, M.; Lumetti, S.; Manfredi, E.; Macaluso, G.M.; Passeri, G. GSK 3b-inhibitor lithium chloride enhances activation of Wnt canonical signaling and osteoblast differentiation on hydrophilic titanium surfaces. Clin. Oral Implant. Res. 2013, 24, 921–927. [Google Scholar] [CrossRef]
- Madan, B.; Patel, M.B.; Zhang, J.; Bunte, R.M.; Rudemiller, N.P.; Griffiths, R.; Virshup, D.M.; Crowley, S.D. Experimental inhibition of porcupine-mediated Wnt O-acylation attenuates kidney fibrosis. Kidney Int. 2016, 89, 1062–1074. [Google Scholar] [CrossRef]
- Smith, S.M.; Garic, A.; Flentke, G.R.; Berres, M.E. Neural crest development in fetal alcohol syndrome. Birth Defects Res. Part C Embryo Today Rev. 2014, 102, 210–220. [Google Scholar] [CrossRef]
- Silva, P.; Azimian Zavareh, P.; Atukorallaya, D. Teleost Fish as Model Animals to Understand Alcohol Teratology. In Fetal Alcohol Spectrum Disorder: Advances in Research and Practice; Springer: Cham, Switzerland, 2022; pp. 31–48. [Google Scholar]
- Zavareh, P.A.; Silva, P.; Gimhani, N.; Atukorallaya, D. Effect of Embryonic Alcohol Exposure on Craniofacial and Skin Melanocyte Development: Insights from Zebrafish (Danio rerio). Toxics 2022, 10, 544. [Google Scholar] [CrossRef]
- Rao, A.S.; Kremenevskaja, N.; Resch, J.; Brabant, G. Lithium stimulates proliferation in cultured thyrocytes by activating Wnt/β-catenin signalling. Eur. J. Endocrinol. 2005, 153, 929–938. [Google Scholar] [CrossRef]
- Lister, J.A.; Robertson, C.P.; Lepage, T.; Johnson, S.L.; Raible, D.W. Nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 1999, 126, 3757–3767. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.-I.; Takeda, K.; Yasumoto, K.-I.; Udono, T.; Saito, H.; Ikeda, K.; Takasaka, T.; Takahashi, K.; Kobayashi, T.; Tachibana, M.; et al. Identification of a distal enhancer for the melanocyte-specific promoter of the MITF gene. Pigment Cell Res. 2002, 15, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Cheli, Y.; Ohanna, M.; Ballotti, R.; Bertolotto, C. Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment Cell Melanoma Res. 2010, 23, 27–40. [Google Scholar] [CrossRef]
- Levy, C.; Khaled, M.; Fisher, D.E. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Hari, L.; Miescher, I.; Shakhova, O.; Suter, U.; Chin, L.; Taketo, M.; Richardson, W.D.; Kessaris, N.; Sommer, L. Temporal control of neural crest lineage generation by Wnt/β-catenin signaling. Development 2012, 139, 2107–2117. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Kléber, M.; Hari, L.; Brault, V.; Suter, U.; Taketo, M.M.; Kemler, R.; Sommer, L. Instructive role of Wnt/ß-catenin in sensory fate specification in neural crest stem cells. Science 2004, 303, 1020–1023. [Google Scholar] [CrossRef]
- Bellei, B.; Flori, E.; Izzo, E.; Maresca, V.; Picardo, M. GSK3β inhibition promotes melanogenesis in mouse B16 melanoma cells and normal human melanocytes. Cell. Signal. 2008, 20, 1750–1761. [Google Scholar] [CrossRef]
- Alexander, C.; Piloto, S.; Le Pabic, P.; Schilling, T.F. Wnt signaling interacts with bmp and edn1 to regulate dorsal-ventral patterning and growth of the craniofacial skeleton. PLoS Genet. 2014, 10, e1004479. [Google Scholar] [CrossRef]
- Moro, E.; Ozhan-Kizil, G.; Mongera, A.; Beis, D.; Wierzbicki, C.; Young, R.M.; Bournele, D.; Domenichini, A.; Valdivia, L.E.; Lum, L.; et al. In vivo Wnt signaling tracing through a transgenic biosensor fish reveals novel activity domains. Dev. Biol. 2012, 366, 327–340. [Google Scholar] [CrossRef]
- Vibert, L.; Aquino, G.; Gehring, I.; Subkankulova, T.; Schilling, T.F.; Rocco, A.; Kelsh, R.N. An ongoing role for Wnt signaling in differentiating melanocytes in vivo. Pigment Cell Melanoma Res. 2017, 30, 219–232. [Google Scholar] [CrossRef]
- Funck-Brentano, T.; Nilsson, K.H.; Brommage, R.; Henning, P.; Lerner, U.; Koskela, A.; Tuukkanen, J.; Cohen-Solal, M.; Movérare-Skrtic, S.; Ohlsson, C. Porcupine inhibitors impair trabecular and cortical bone mass and strength in mice. J. Endocrinol. 2018, 238, 13. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Phoon, Y.P.; Jin, X.; Chong, S.Y.S.; Ip, J.C.Y.; Wong, B.W.Y.; Lung, M.L. Wnt-C59 arrests stemness and suppresses growth of nasopharyngeal carcinoma in mice by inhibiting the Wnt pathway in the tumor microenvironment. Oncotarget 2015, 6, 14428. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.; Barwick, B.G.; Moreno, C.S.; Ordanic-Kodani, M.; Chen, Z.; Oprea-Ilies, G.; Tang, W.; Catzavelos, C.; Kerstann, K.F.; Sledge, G.W.; et al. Wnt signaling in triple negative breast cancer is associated with metastasis. BMC Cancer 2013, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- Milos, N.; Dingle, A.D. Dynamics of pigment pattern formation in the zebrafish, Brachydanio rerio. I. Establishment and regulation of the lateral line melanophore stripe during the first eight days of development. J. Exp. Zool. 1978, 205, 205–216. [Google Scholar] [CrossRef]
- McMenamin, S.K.; Bain, E.J.; McCann, A.E.; Patterson, L.B.; Eom, D.S.; Waller, Z.P.; Hamill, J.C.; Kuhlman, J.A.; Eisen, J.S.; Parichy, D.M. Thyroid hormone–dependent adult pigment cell lineage and pattern in zebrafish. Science 2014, 345, 1358–1361. [Google Scholar] [CrossRef]
- Johnson, S.L.; Africa, D.; Walker, C.; Weston, J.A. Genetic control of adult pigment stripe development in zebrafish. Dev. Biol. 1995, 167, 27–33. [Google Scholar] [CrossRef]
- Richardson, J.; Lundegaard, P.R.; Reynolds, N.L.; Dorin, J.R.; Porteous, D.J.; Jackson, I.J.; Patton, E.E. mc1r Pathway regulation of zebrafish melanosome dispersion. Zebrafish 2008, 5, 289–295. [Google Scholar] [CrossRef]
- Hammer, J.A.; Wu, X.S. Organelle motility: Running on unleadened. Curr. Biol. 2007, 17, R1017–R1019. [Google Scholar] [CrossRef]
- Logan, D.; Burn, S.; Jackson, I.J. Regulation of pigmentation in zebrafish melanophores. Pigment Cell Res. 2006, 19, 206–213. [Google Scholar] [CrossRef]
- Sheets, L.; Ransom, D.G.; Mellgren, E.M.; Johnson, S.L.; Schnapp, B.J. Zebrafish melanophilin facilitates melanosome dispersion by regulating dynein. Curr. Biol. 2007, 17, 1721–1734. [Google Scholar] [CrossRef]
- Choi, T.-Y.; Kim, J.-H.; Ko, D.H.; Kim, C.-H.; Hwang, J.-S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.-H.; Yoon, T.-J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Theveneau, E.; Mayor, R. Neural crest delamination and migration: From epithelium-to-mesenchyme transition to collective cell migration. Dev. Biol. 2012, 366, 34–54. [Google Scholar] [CrossRef] [PubMed]
- Ikeya, M.; Lee, S.M.K.; Johnson, J.E.; McMahon, A.P.; Takada, S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature 1997, 389, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Serbedzija, G.N.; McMahon, A.P. Analysis of neural crest cell migration in Splotch mice using a neural crest-specific LacZ reporter. Dev. Biol. 1997, 185, 139–147. [Google Scholar] [CrossRef]
- Borchers, A.; David, R.; Wedlich, D. Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development 2001, 128, 3049–3060. [Google Scholar] [CrossRef]
- Dickinson, M.E.; Selleck, M.A.J.; McMahon, A.P.; Bronner-Fraser, M. Dorsalization of the neural tube by the non-neural ectoderm. Development 1995, 121, 2099–2106. [Google Scholar] [CrossRef]
- Roussigné, M.; Blader, P.; Wilson, S.W. Breaking symmetry: The zebrafish as a model for understanding left-right asymmetry in the developing brain. Dev. Neurobiol. 2012, 72, 269–281. [Google Scholar] [CrossRef]
- Smith, K.A.; Uribe, V. Getting to the Heart of Left–Right Asymmetry: Contributions from the Zebrafish Model. J. Cardiovasc. Dev. Dis. 2021, 8, 64. [Google Scholar] [CrossRef]
- Grimes, D.T.; Patterson, V.L.; Luna-Arvizu, G.; Schottenfeld-Roames, J.; Irons, Z.H.; Burdine, R.D. Left-right asymmetric heart jogging increases the robustness of dextral heart looping in zebrafish. Dev. Biol. 2020, 459, 79–86. [Google Scholar] [CrossRef]
- Grimes, D.T.; Burdine, R.D. Left–right patterning: Breaking symmetry to asymmetric morphogenesis. Trends Genet. 2017, 33, 616–628. [Google Scholar] [CrossRef]
- Matsui, T.; Bessho, Y. Left–right asymmetry in zebrafish. Cell. Mol. Life Sci. 2012, 69, 3069–3077. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.P.; Vandenberg, L.N.; Blackiston, D.; Levin, M. Neurally derived tissues in Xenopus laevis embryos exhibit a consistent bioelectrical left-right asymmetry. Stem Cells Int. 2012, 2012, 353491. [Google Scholar] [CrossRef] [PubMed]
- A Hultman, K.; Bahary, N.; Zon, L.I.; Johnson, S.L. Gene duplication of the zebrafish kit ligand and partitioning of melanocyte development functions to kit ligand a. PLoS Genet. 2007, 3, e17. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, P.; Atukorallaya, D. Characterising the Effect of Wnt/β-Catenin Signalling on Melanocyte Development and Patterning: Insights from Zebrafish (Danio rerio). Int. J. Mol. Sci. 2023, 24, 10692. https://doi.org/10.3390/ijms241310692
Silva P, Atukorallaya D. Characterising the Effect of Wnt/β-Catenin Signalling on Melanocyte Development and Patterning: Insights from Zebrafish (Danio rerio). International Journal of Molecular Sciences. 2023; 24(13):10692. https://doi.org/10.3390/ijms241310692
Chicago/Turabian StyleSilva, Praneeth, and Devi Atukorallaya. 2023. "Characterising the Effect of Wnt/β-Catenin Signalling on Melanocyte Development and Patterning: Insights from Zebrafish (Danio rerio)" International Journal of Molecular Sciences 24, no. 13: 10692. https://doi.org/10.3390/ijms241310692
APA StyleSilva, P., & Atukorallaya, D. (2023). Characterising the Effect of Wnt/β-Catenin Signalling on Melanocyte Development and Patterning: Insights from Zebrafish (Danio rerio). International Journal of Molecular Sciences, 24(13), 10692. https://doi.org/10.3390/ijms241310692





