Integrating Microbiome Analysis, Metabolomics, Bioinformatics, and Histopathology to Elucidate the Protective Effects of Pomegranate Juice against Benzo-alpha-pyrene-Induced Colon Pathologies
Abstract
1. Introduction
2. Results
2.1. Negligible Changes in Animal Weights along the Experiment
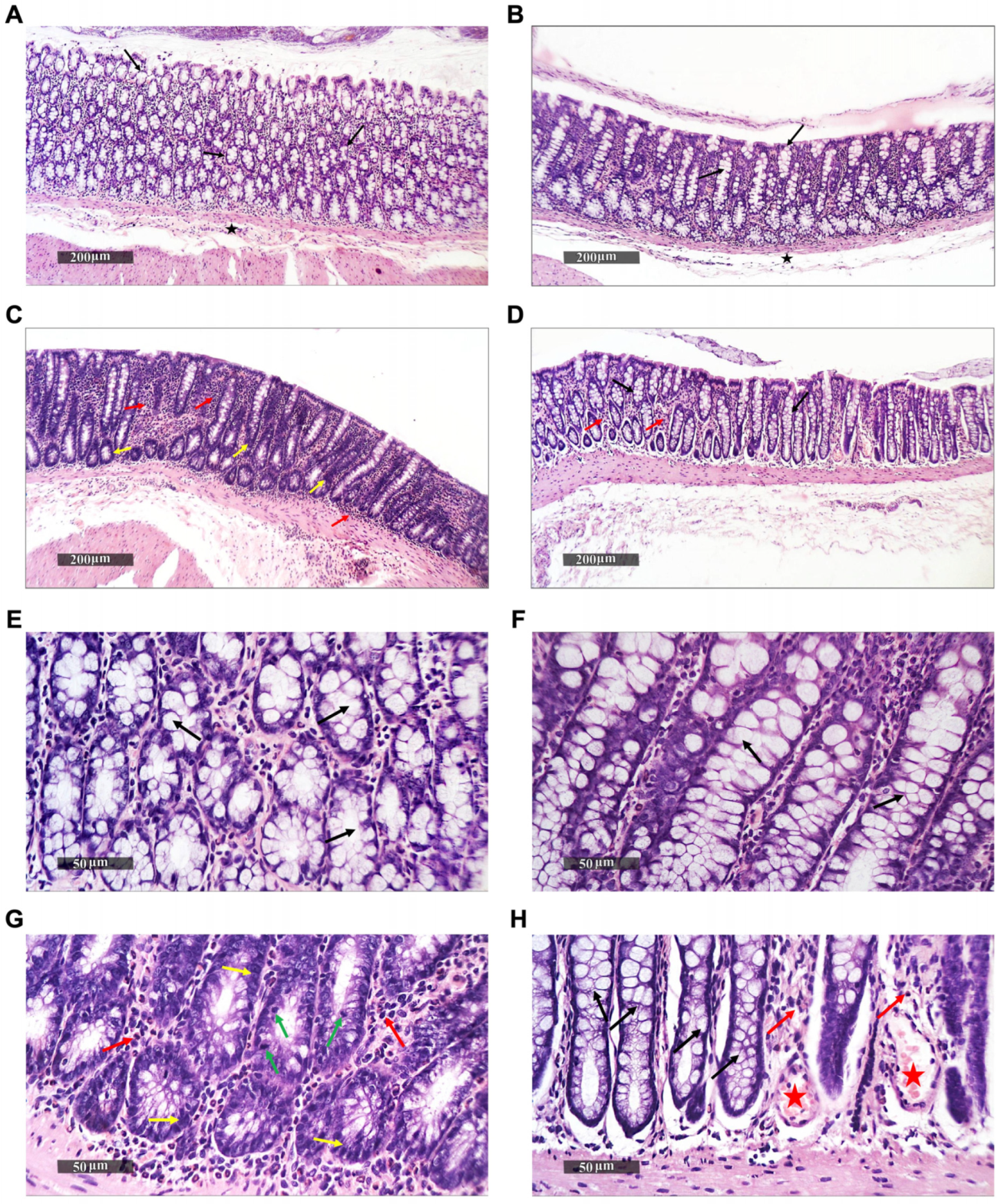
2.2. Histopathological Alterations in Rat Colons upon Treatment
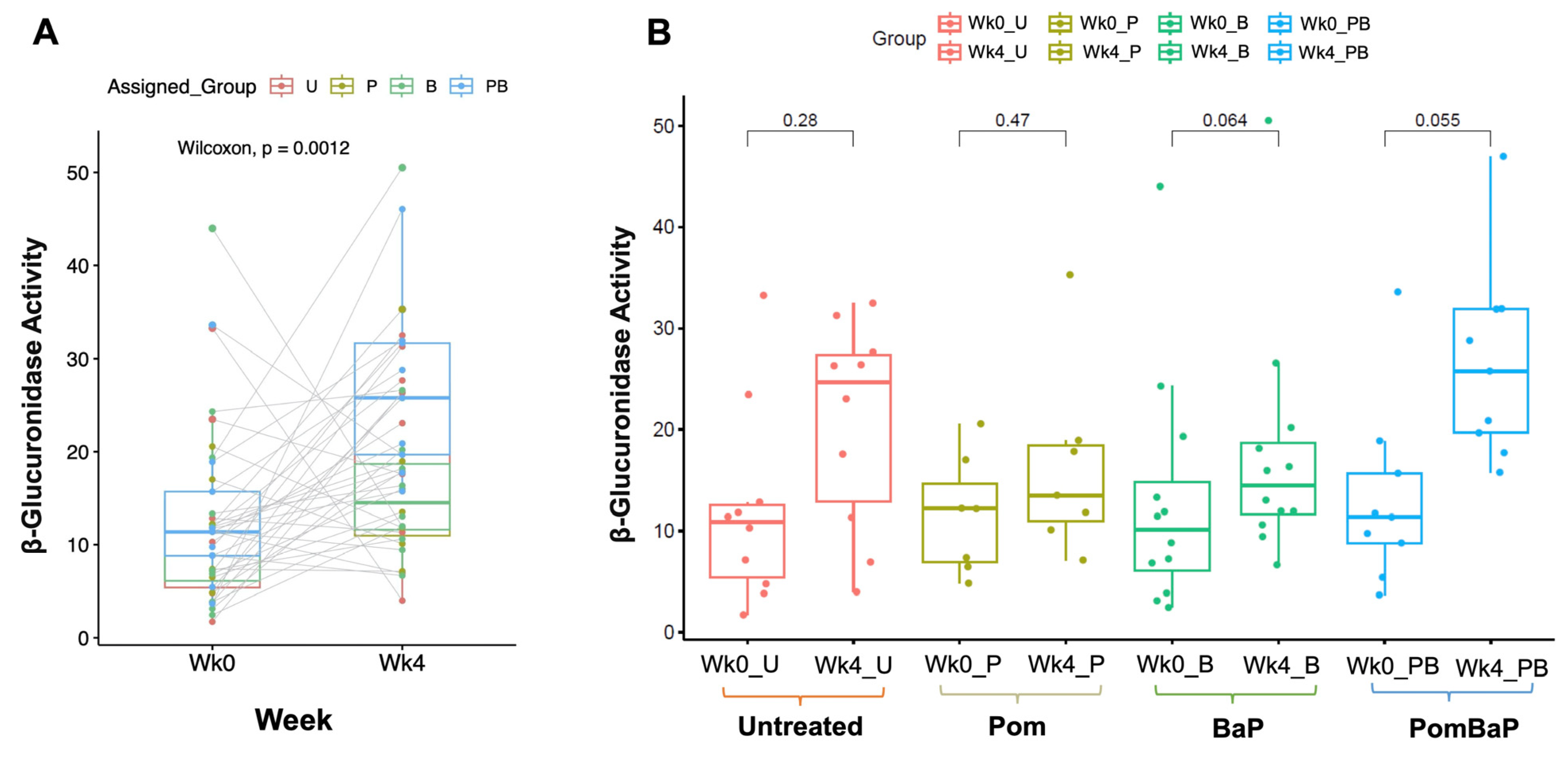
2.3. Uneven Increase in β-Glucuronidase Activity after Four Weeks
2.4. Microbiome Profiling by 16S rRNA Amplicon Sequencing
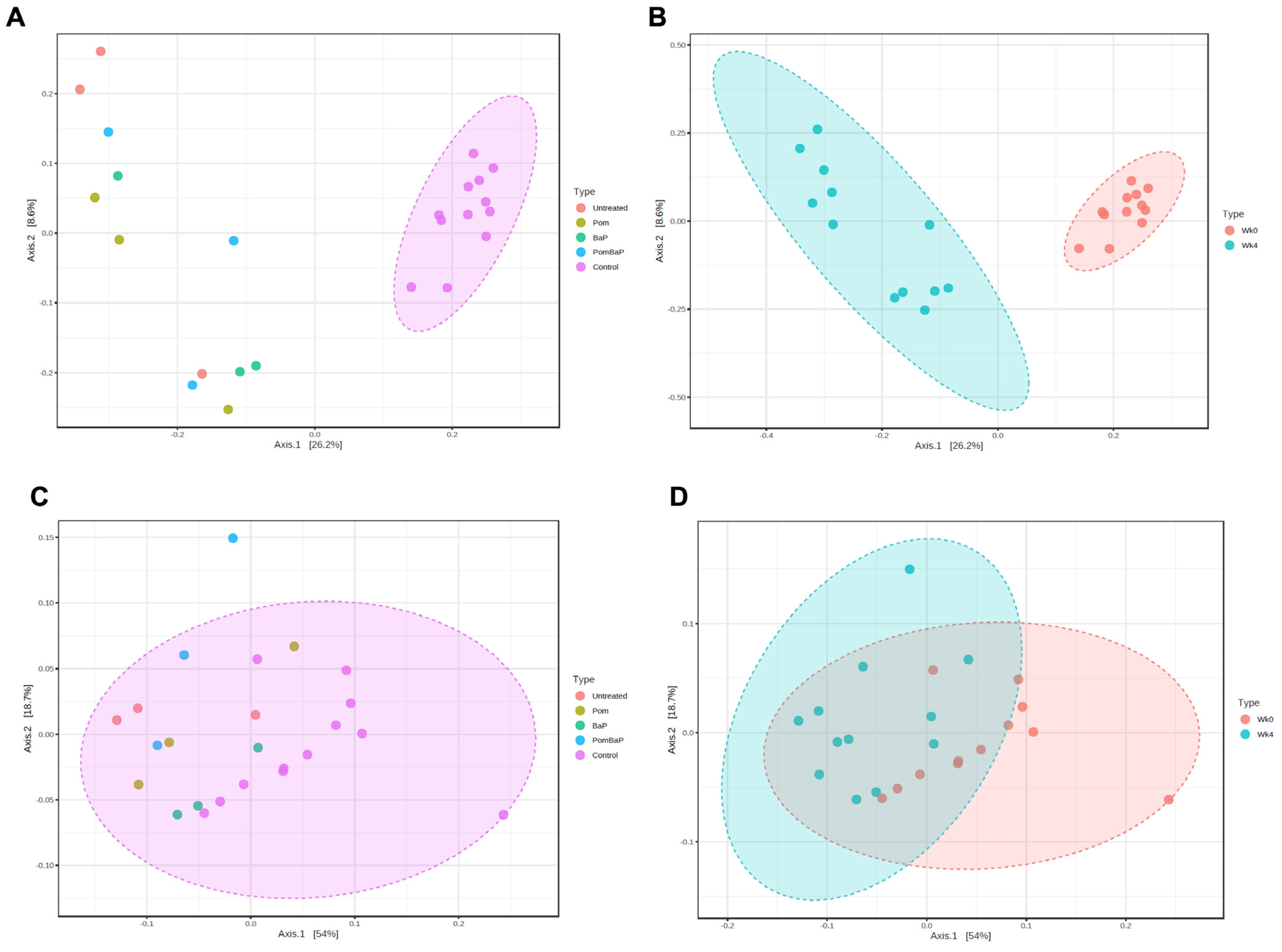
2.5. Alterations in Fecal Microbiota Diversity across Sample Groups
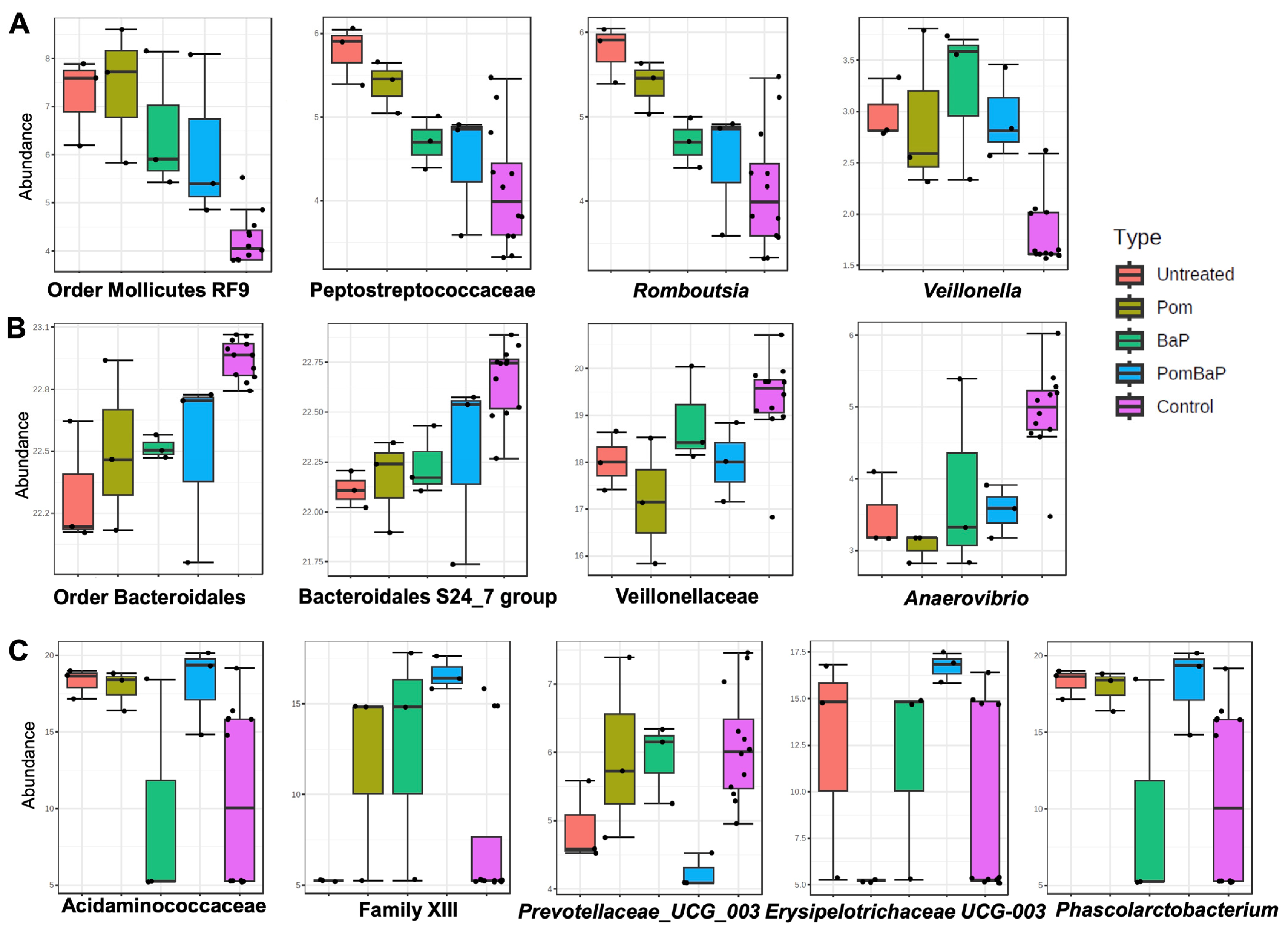
2.6. Alterations in Fecal Microbiome Profiles across Sample Groups
2.6.1. Influence of Temporal, Age-Related Factors on Fecal Microbiome Composition
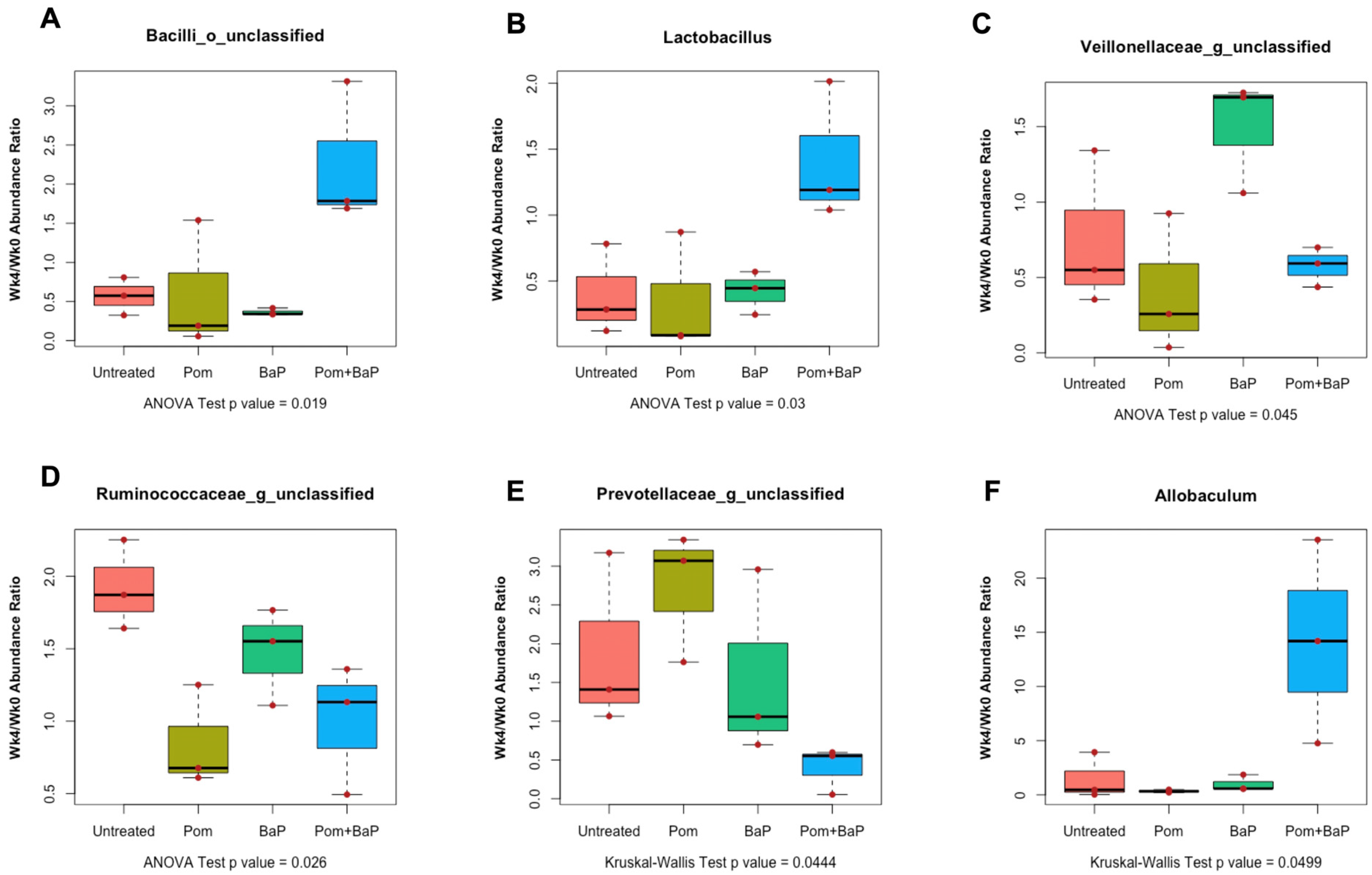
2.6.2. Alterations in Fecal Microbiome Profiles in Response to Daily Ingestion of Pom, BaP, and PomBaP
2.7. Alterations in Fecal Metabolome across Sample Groups
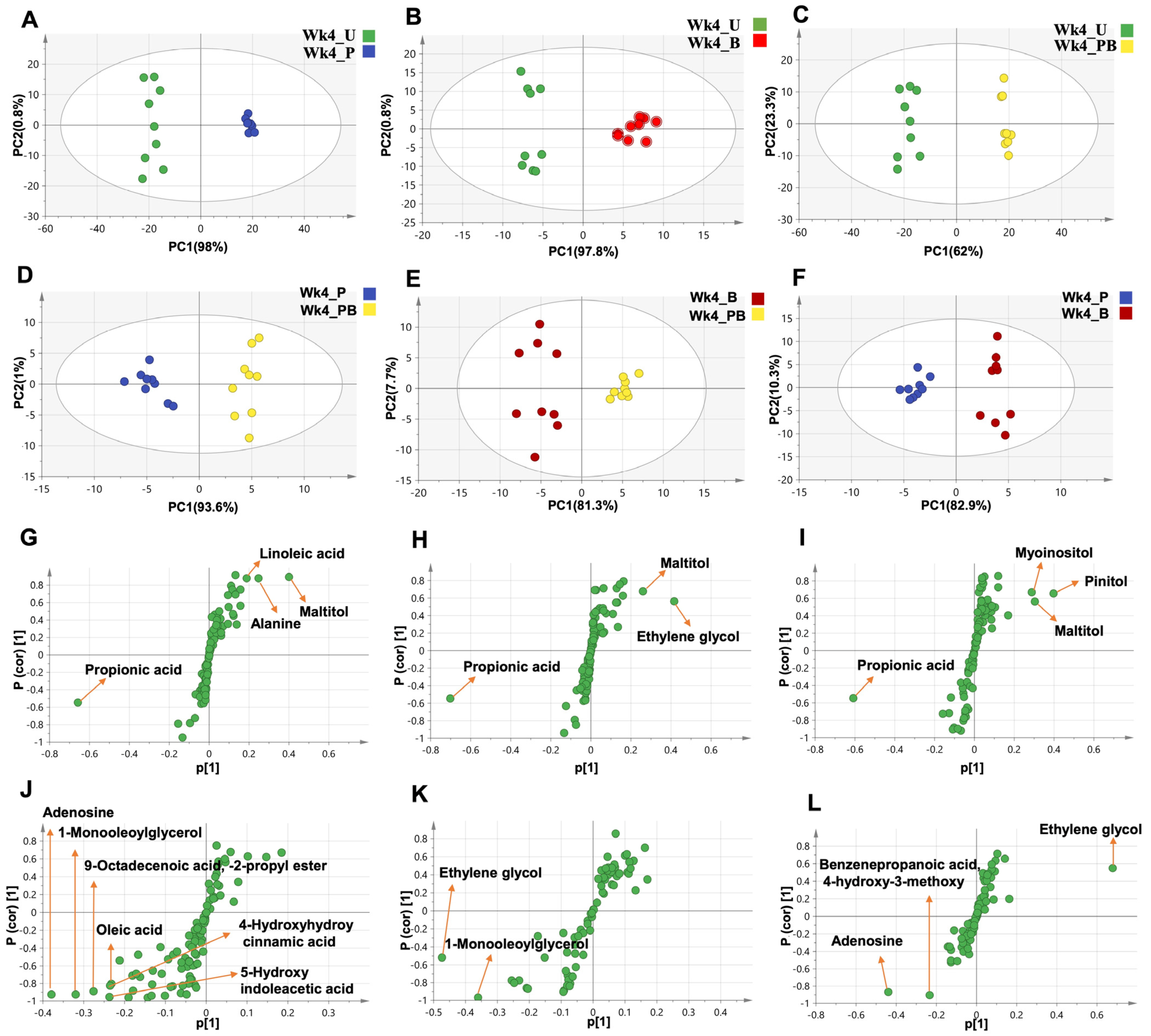
2.8. Unsupervised and Supervised Multivariate Data Analysis of GC-MS Datasets
2.8.1. Clustering of the Entire Dataset into Two Separate Clusters by PCA and OPLS-DA
2.8.2. Lack of Segregation between Week 0 Sample Groups
2.9. Alterations in Fecal Metabolomes in Response to Daily Ingestion of Pom, BaP, and PomBaP
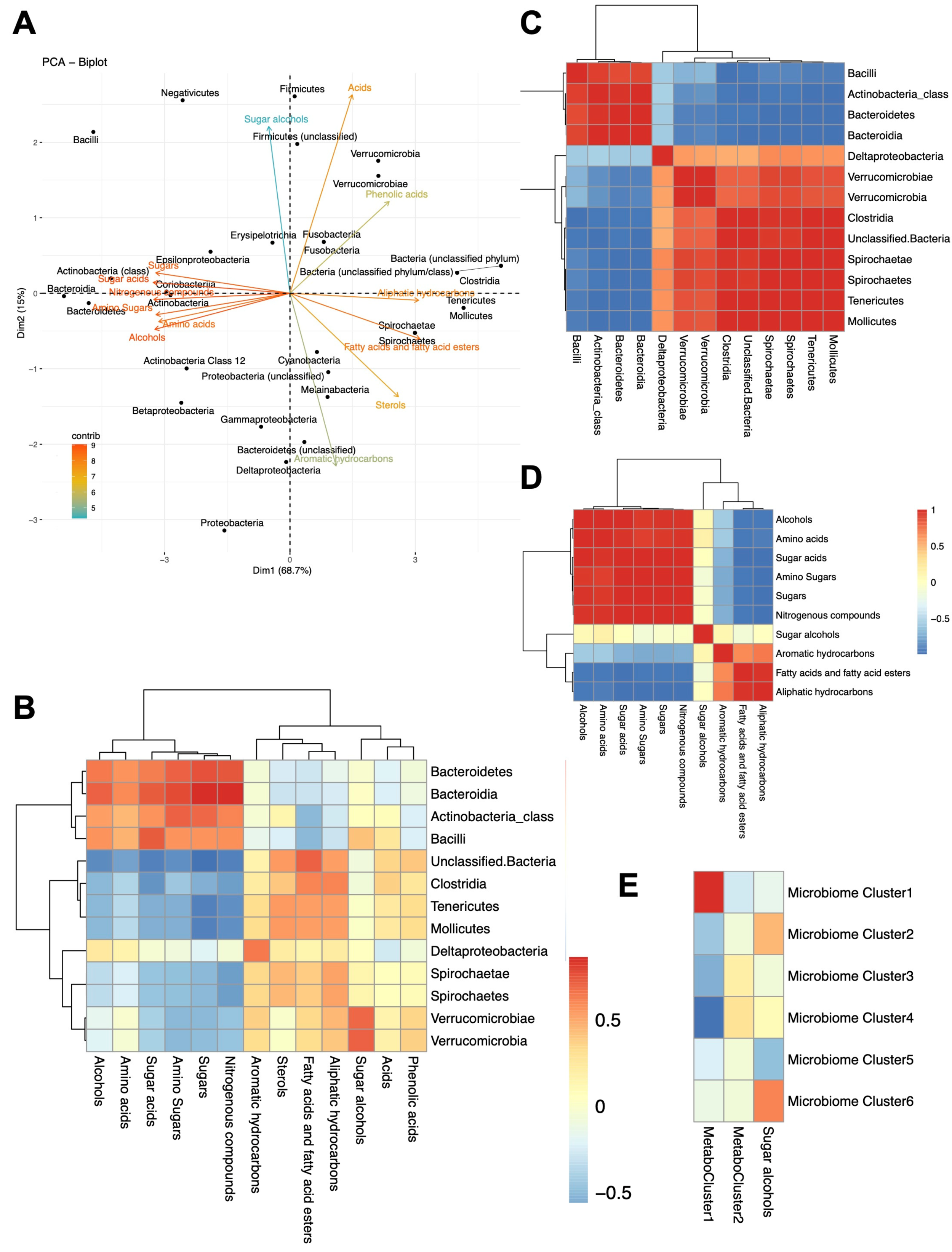
2.10. Microbiome–Metabolome Correlations
3. Discussion
3.1. Histopathological Alterations in Rat Colons
3.2. Fecal Microbiome and Metabolome Alterations
3.2.1. Temporal/Age Effects
3.2.2. Treatment Effects
3.2.3. Microbiome–Metabolome Correlations
3.3. Other Phenotypic Alterations
3.4. Limitations of This Study
3.5. Future Prospects
4. Materials and Methods
4.1. Study Design
4.2. Animals
4.3. Chemicals
4.4. Preparation and Standardization of Pomegranate Juice
4.5. Sample Collection
4.6. Beta-Glucuronidase Assay
4.7. Histopathological and Histochemical Examination
4.8. DNA Extraction and Quantification
4.9. 16S rRNA Amplicon Sequencing
4.10. Microbiome Analysis
4.11. Fecal Metabolite Profiling by Gas Chromatography Coupled with Mass Spectrometry Detection (GC-MS)
4.12. GC-MS Data Analysis
4.13. Data Visualization and Statistical Analysis
4.14. Sequence Deposition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Short, M.W.; Layton, M.C.; Teer, B.N.; Domagalski, J.E. Colorectal cancer screening and surveillance. Am. Fam. Physician 2015, 91, 93–100. [Google Scholar]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Wang, S.; Chanock, S.; Tang, D.; Li, Z.; Edwards, S.; Jedrychowski, W.; Perera, F.P. Effect of gene-environment Interactions on mental development in African American, Dominican, and Caucasian mothers and newborns. Ann. Hum. Genet. 2010, 74, 46–56. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ugai, T.; Sasamoto, N.; Lee, H.Y.; Ando, M.; Song, M.; Tamimi, R.M.; Kawachi, I.; Campbell, P.T.; Giovannucci, E.L.; Weiderpass, E.; et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 2022, 19, 656–673. [Google Scholar] [CrossRef]
- Bray, F.; Jemal, A.; Grey, N.; Ferlay, J.; Forman, D. Global cancer transitions according to the human development index (2008–2030): A population-based study. Lancet Oncol. 2012, 13, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Gado, A.; Ebeid, B.; Abdelmohsen, A.; Axon, A. Colorectal cancer in Egypt is commoner in young people: Is this cause for alarm? Alex. J. Med. 2014, 50, 197–201. [Google Scholar] [CrossRef]
- Abou-Zeid, A.A.; Khafagy, W.; Marzouk, D.M.; Alaa, A.; Mostafa, I.; Ela, M.A. Colorectal cancer in Egypt. Dis. Colon Rectum 2002, 45, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, N.A.; Abdel-Gawad, M.; Mahros, A.M.; Lashen, S.A.; Zaghloul, M.; Eliwa, A.; Elshemy, E.E.; Ali-Eldin, Z.; Abdeltawab, D.; El-Raey, F.; et al. Colorectal cancer in Arab world: A systematic review. World J. Gastrointest. Oncol. 2021, 13, 1791–1798. [Google Scholar] [CrossRef]
- Kerber, R.A.; Neklason, D.W.; Samowitz, W.S.; Burt, R.W. Frequency of familial colon cancer and hereditary nonpolyposis colorectal cancer (lynch syndrome) in a large population database. Fam. Cancer 2005, 4, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and familial colon cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef] [PubMed]
- Hadjipetrou, A.; Anyfantakis, D.; Galanakis, C.G.; Kastanakis, M.; Kastanakis, S. Colorectal cancer, screening and primary care: A mini literature review. World J. Gastroenterol. 2017, 23, 6049–6058. [Google Scholar] [CrossRef] [PubMed]
- Glade, M.J. Food, nutrition, and the prevention of cancer: A global perspective. American Institute for Cancer Research/World Cancer Research Fund, American Institute for Cancer Research, 1997. Nutrition 1999, 15, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Bishehsari, F.; Mahdavinia, M.; Vacca, M.; Malekzadeh, R.; Mariani-Costantini, R. Epidemiological transition of colorectal cancer in developing countries: Environmental factors, molecular pathways, and opportunities for prevention. World J. Gastroenterol. 2014, 20, 6055–6072. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Harrad, S. Spatiotemporal analysis and human exposure assessment on polycyclic aromatic hydrocarbons in indoor air, settled house dust, and diet: A review. Environ. Int. 2015, 84, 7–16. [Google Scholar] [CrossRef]
- Sinha, R.; Cross, A.; Curtin, J.; Zimmerman, T.; McNutt, S.; Risch, A.; Holden, J. Development of a food frequency questionnaire module and databases for compounds in cooked and processed meats. Mol. Nutr. Food Res. 2005, 49, 648–655. [Google Scholar] [CrossRef]
- Key, T.J.; Bradbury, K.E.; Perez-Cornago, A.; Sinha, R.; Tsilidis, K.K.; Tsugane, S. Diet, nutrition, and cancer risk: What do we know and what is the way forward? BMJ 2020, 368, m511. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 92, 853p. [Google Scholar]
- Renner, R. EPA to strengthen persistent, bioaccumulative, and toxic pollutant controls-Mercury first to be targeted. Environ. Sci. Technol. 1999, 33, 62A. [Google Scholar] [CrossRef]
- Conney, A.H.; Chang, R.L.; Jerina, D.M.; Wei, S.J. Studies on the metabolism of benzo[a]pyrene and dose-dependent differences in the mutagenic profile of its ultimate carcinogenic metabolite. Drug Metab. Rev. 1994, 26, 125–163. [Google Scholar] [CrossRef]
- Phillips, D.H. Polycyclic aromatic hydrocarbons in the diet. Mutat. Res. 1999, 443, 139–147. [Google Scholar] [CrossRef]
- Phillips, D.H. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis 2002, 23, 1979–2004. [Google Scholar] [CrossRef] [PubMed]
- Kazerouni, N.; Sinha, R.; Hsu, C.H.; Greenberg, A.; Rothman, N. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem. Toxicol. 2001, 39, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Walker, S.A.; Hood, D.B.; Guillen, M.D.; Schneider, K.; Weyand, E.H. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int. J. Toxicol. 2004, 23, 301–333. [Google Scholar] [CrossRef] [PubMed]
- Alomirah, H.; Al-Zenki, S.; Al-Hooti, S.; Zaghloul, S.; Sawaya, W.; Ahmed, N.; Kannan, K. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control 2011, 22, 2028–2035. [Google Scholar] [CrossRef]
- Collins, J.F.; Brown, J.P.; Dawson, S.V.; Marty, M.A. Risk assessment for benzo[a]pyrene. Regul. Toxicol. Pharmacol. 1991, 13, 170–184. [Google Scholar] [CrossRef]
- Ellard, S.; Mohammed, Y.; Dogra, S.; Wolfel, C.; Doehmer, J.; Parry, J.M. The use of genetically engineered V79 Chinese hamster cultures expressing rat liver CYP1A1, 1A2 and 2B1 cDNAs in micronucleus assays. Mutagenesis 1991, 6, 461–470. [Google Scholar] [CrossRef]
- Wester, P.W.; Muller, J.J.; Slob, W.; Mohn, G.R.; Dortant, P.M.; Kroese, E.D. Carcinogenic activity of benzo[a]pyrene in a 2 year oral study in Wistar rats. Food Chem. Toxicol. 2012, 50, 927–935. [Google Scholar] [CrossRef]
- Uno, S.; Makishima, M. Benzo [a] pyrene toxicity and inflammatory disease. Curr. Rheumatol. Rev. 2009, 5, 266–271. [Google Scholar] [CrossRef]
- Genies, C.; Maitre, A.; Lefebvre, E.; Jullien, A.; Chopard-Lallier, M.; Douki, T. The extreme variety of genotoxic response to benzo[a]pyrene in three different human cell lines from three different organs. PLoS ONE 2013, 8, e78356. [Google Scholar] [CrossRef]
- Miller, K.P.; Ramos, K.S. Impact of cellular metabolism on the biological effects of benzo[a]pyrene and related hydrocarbons. Drug Metab. Rev. 2001, 33, 1–35. [Google Scholar] [CrossRef]
- Kleiner, H.E.; Vulimiri, S.V.; Hatten, W.B.; Reed, M.J.; Nebert, D.W.; Jefcoate, C.R.; DiGiovanni, J. Role of cytochrome p4501 family members in the metabolic activation of polycyclic aromatic hydrocarbons in mouse epidermis. Chem. Res. Toxicol. 2004, 17, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.L.; Washington, M.K.; Hood, D.B.; Roberts, L.J., II; Ramesh, A. Dietary fat-influenced development of colon neoplasia in ApcMin mice exposed to benzo(a)pyrene. Toxicol. Pathol. 2009, 37, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Diggs, D.L.; Huderson, A.C.; Harris, K.L.; Myers, J.N.; Banks, L.D.; Rekhadevi, P.V.; Niaz, M.S.; Ramesh, A. Polycyclic aromatic hydrocarbons and digestive tract cancers: A perspective. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2011, 29, 324–357. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, J.; Seki, Y.; Hakura, A.; Hosokawa, S. Time course of the incidence/multiplicity and histopathological features of murine colonic dysplasia, adenoma and adenocarcinoma induced by benzo[a]pyrene and dextran sulfate sodium. J. Toxicol. Pathol. 2015, 28, 109–120. [Google Scholar] [CrossRef]
- Fu, Z.; Shrubsole, M.J.; Smalley, W.E.; Wu, H.; Chen, Z.; Shyr, Y.; Ness, R.M.; Zheng, W. Association of meat intake and meat-derived mutagen exposure with the risk of colorectal polyps by histologic type. Cancer Prev. Res. 2011, 4, 1686–1697. [Google Scholar] [CrossRef]
- Gunter, M.J.; Probst-Hensch, N.M.; Cortessis, V.K.; Kulldorff, M.; Haile, R.W.; Sinha, R. Meat intake, cooking-related mutagens and risk of colorectal adenoma in a sigmoidoscopy-based case-control study. Carcinogenesis 2005, 26, 637–642. [Google Scholar] [CrossRef]
- Sachse, C.; Smith, G.; Wilkie, M.J.; Barrett, J.H.; Waxman, R.; Sullivan, F.; Forman, D.; Bishop, D.T.; Wolf, C.R.; Colorectal Cancer Study, G. A pharmacogenetic study to investigate the role of dietary carcinogens in the etiology of colorectal cancer. Carcinogenesis 2002, 23, 1839–1849. [Google Scholar] [CrossRef]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef]
- Thathsarani, N.; Nayanajeehwi, G.; Jayawardena, U.; Nilakarawasam, N.; Jayasinghe, C.D. Cancer chemoprevention through functional food of plant origin. Asian J. Pharm. Pharmacol. 2020, 6, 132–143. [Google Scholar] [CrossRef]
- Teibo, J.O.; Ayinde, K.S.; Olaoba, O.T.; Adelusi, T.I.; Teibo, T.K.A.; Bamikunle, M.V.; Jimoh, Y.A.; Alghamdi, S.; Abdulaziz, O.; Rauf, A. Functional foods’ bioactive components and their chemoprevention mechanism in cervical, breast, and liver cancers: A systematic review. Funct. Food Health Dis. 2021, 11, 559–585. [Google Scholar] [CrossRef]
- AlAli, M.; Alqubaisy, M.; Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.S.; Lim, S.E. Nutraceuticals: Transformation of conventional foods into health promoters/disease preventers and safety considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Sallam, I.E.; Abdelwareth, A.; Attia, H.; Aziz, R.K.; Homsi, M.N.; von Bergen, M.; Farag, M.A. Effect of gut microbiota biotransformation on dietary tannins and human health implications. Microorganisms 2021, 9, 965. [Google Scholar] [CrossRef]
- Lansky, E.P.; Jiang, W.; Mo, H.; Bravo, L.; Froom, P.; Yu, W.; Harris, N.M.; Neeman, I.; Campbell, M.J. Possible synergistic prostate cancer suppression by anatomically discrete pomegranate fractions. Investig. New Drugs 2005, 23, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Viladomiu, M.; Hontecillas, R.; Lu, P.; Bassaganya-Riera, J. Preventive and prophylactic mechanisms of action of pomegranate bioactive constituents. Evid. Based Complement Alternat. Med. 2013, 2013, 789764. [Google Scholar] [CrossRef] [PubMed]
- Espin, J.C.; Gonzalez-Barrio, R.; Cerda, B.; Lopez-Bote, C.; Rey, A.I.; Tomas-Barberan, F.A. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J. Agric. Food Chem. 2007, 55, 10476–10485. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Gonzalez-Sarrias, A.; Garcia-Villalba, R.; Nunez-Sanchez, M.A.; Selma, M.V.; Garcia-Conesa, M.T.; Espin, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef]
- Espin, J.C.; Larrosa, M.; Garcia-Conesa, M.T.; Tomas-Barberan, F. Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: The evidence so far. Evid. Based Complement. Alternat. Med. 2013, 2013, 270418. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Syed, D.N.; Afaq, F.; Mukhtar, H. Pomegranate derived products for cancer chemoprevention. Semin. Cancer Biol. 2007, 17, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Kawaii, S.; Lansky, E.P. Differentiation-promoting activity of pomegranate (Punica granatum) fruit extracts in HL-60 human promyelocytic leukemia cells. J. Med. Food 2004, 7, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Lansky, E.P. Breast cancer chemopreventive properties of pomegranate (Punica granatum) fruit extracts in a mouse mammary organ culture. Eur. J. Cancer Prev. 2004, 13, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Mukhtar, H. Prostate cancer prevention through pomegranate fruit. Cell Cycle 2006, 5, 371–373. [Google Scholar] [CrossRef]
- Modaeinama, S.; Abasi, M.; Abbasi, M.M.; Jahanban-Esfahlan, R. Anti tumoral properties of Punica granatum (Pomegranate) peel extract on different human cancer cells. Asian Pac. J. Cancer Prev. 2015, 16, 5697–5701. [Google Scholar] [CrossRef]
- Afaq, F.; Saleem, M.; Krueger, C.G.; Reed, J.D.; Mukhtar, H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int. J. Cancer 2005, 113, 423–433. [Google Scholar] [CrossRef]
- Larrosa, M.; Tomas-Barberan, F.A.; Espin, J.C. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J. Nutr. Biochem. 2006, 17, 611–625. [Google Scholar] [CrossRef]
- Kasimsetty, S.G.; Bialonska, D.; Reddy, M.K.; Ma, G.; Khan, S.I.; Ferreira, D. Colon cancer chemopreventive activities of pomegranate ellagitannins and urolithins. J. Agric. Food Chem. 2010, 58, 2180–2187. [Google Scholar] [CrossRef]
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.; Sand, D.; Heber, D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J. Agric. Food Chem. 2006, 54, 980–985. [Google Scholar] [CrossRef]
- Moreira, H.; Slezak, A.; Szyjka, A.; Oszmianski, J.; Gasiorowski, K. Antioxidant and cancer chemopreventive activities of cistus and pomegranate polyphenols. Acta. Pol. Pharm. 2017, 74, 688–698. [Google Scholar]
- Boateng, J.; Verghese, M.; Shackelford, L.; Walker, L.T.; Khatiwada, J.; Ogutu, S.; Williams, D.S.; Jones, J.; Guyton, M.; Asiamah, D.; et al. Selected fruits reduce azoxymethane (AOM)-induced aberrant crypt foci (ACF) in Fisher 344 male rats. Food Chem. Toxicol. 2007, 45, 725–732. [Google Scholar] [CrossRef]
- Kohno, H.; Suzuki, R.; Yasui, Y.; Hosokawa, M.; Miyashita, K.; Tanaka, T. Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci. 2004, 95, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Abdelwareth, A.; Sallam, I.E.; El Shorbagi, M.; Jehmlich, N.; Fritz-Wallace, K.; Serena Schäpe, S.; Rolle-Kampczyk, U.; Ehrlich, A.; Wessjohann, L.A.; et al. Metabolomics reveals impact of seven functional foods on metabolic pathways in a gut microbiota model. J. Adv. Res. 2020, 23, 47–59. [Google Scholar] [CrossRef]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef] [PubMed]
- Huderson, A.C.; Myers, J.N.; Niaz, M.S.; Washington, M.K.; Ramesh, A. Chemoprevention of benzo(a)pyrene-induced colon polyps in ApcMin mice by resveratrol. J. Nutr. Biochem. 2013, 24, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Banks, L.D.; Amoah, P.; Niaz, M.S.; Washington, M.K.; Adunyah, S.E.; Ramesh, A. Olive oil prevents benzo(a)pyrene [B(a)P]-induced colon carcinogenesis through altered B(a)P metabolism and decreased oxidative damage in ApcMin mouse model. J. Nutr. Biochem. 2016, 28, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Aboulthana, W.M.; Shousha, W.G.; Essawy, E.A.; Saleh, M.H.; Salama, A.H. Assessment of the anti-cancer efficiency of silver Moringa oleifera Leaves nano-extract against colon cancer induced chemically in rats. Asian Pac. J. Cancer Prev. 2021, 22, 3267–3286. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Kim, N.Y.; Son, J.Y.; Park, J.H.; Lee, S.H.; Kim, H.R.; Kim, B.; Kim, Y.G.; Jeong, H.G.; Lee, B.M.; et al. Curcumin ameliorates benzo[a]pyrene-induced DNA damages in stomach tissues of Sprague-Dawley rats. Int. J. Mol. Sci. 2019, 20, 5533. [Google Scholar] [CrossRef]
- Aziz, R.K.; Hegazy, S.M.; Yasser, R.; Rizkallah, M.R.; ElRakaiby, M.T. Drug pharmacomicrobiomics and toxicomicrobiomics: From scattered reports to systematic studies of drug-microbiome interactions. Expert Opin. Drug Metab. Toxicol. 2018, 14, 1043–1055. [Google Scholar] [CrossRef]
- Abdelsalam, N.A.; Ramadan, A.T.; ElRakaiby, M.T.; Aziz, R.K. Toxicomicrobiomics: The human microbiome vs. pharmaceutical, dietary, and environmental xenobiotics. Front. Pharmacol. 2020, 11, 390. [Google Scholar] [CrossRef]
- Jass, J.R.; Roberton, A.M. Colorectal mucin histochemistry in health and disease: A critical review. Pathol. Int. 1994, 44, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; Mabrok, H.B. Protective effect of pomegranate peel powder against gastric ulcer in rats. Biointerface Res. Appl. Chem. 2021, 12, 4888–4899. [Google Scholar] [CrossRef]
- Bastide, N.M.; Naud, N.; Nassy, G.; Vendeuvre, J.L.; Tache, S.; Gueraud, F.; Hobbs, D.A.; Kuhnle, G.G.; Corpet, D.E.; Pierre, F.H. Red wine and pomegranate extracts suppress cured meat promotion of colonic mucin-depleted foci in carcinogen-induced Rats. Nutr. Cancer 2017, 69, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Tortora, K.; Femia, A.P.; Romagnoli, A.; Sineo, I.; Khatib, M.; Mulinacci, N.; Giovannelli, L.; Caderni, G. Pomegranate by-products in colorectal cancer chemoprevention: Effects in Apc-mutated Pirc rats and mechanistic studies in vitro and ex vivo. Mol. Nutr. Food Res. 2018, 62, 1700401. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Cardeno, A.; Aparicio-Soto, M.; Sanchez-Fidalgo, S.; Villegas, I.; de la Lastra, C.A. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol. Res. 2012, 66, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Cardeno, A.; de la Lastra, C.A. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of crohn’s disease. Biochem. Pharmacol. 2011, 82, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Flemer, B.; Gaci, N.; Borrel, G.; Sanderson, I.R.; Chaudhary, P.P.; Tottey, W.; O’Toole, P.W.; Brugere, J.F. Fecal microbiota variation across the lifespan of the healthy laboratory rat. Gut Microbes 2017, 8, 428–439. [Google Scholar] [CrossRef]
- Andreollo, N.A.; Santos, E.F.; Araujo, M.R.; Lopes, L.R. Rat’s age versus human’s age: What is the relationship? Arq. Bras. Cir. Dig. 2012, 25, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Quinn, R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition 2005, 21, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Riley, M.G.; Johnson, D.E. Spontaneous neoplasms in aged Sprague-Dawley rats. Arch. Toxicol. 1992, 66, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Zapata, H.J.; Quagliarello, V.J. The microbiota and microbiome in aging: Potential implications in health and age-related diseases. J. Am. Geriatr. Soc. 2015, 63, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Bartley, J.M.; Zhou, X.; Kuchel, G.A.; Weinstock, G.M.; Haynes, L. Impact of age, caloric restriction, and influenza infection on mouse gut microbiome: An exploratory study of the role of age-related microbiome changes on influenza responses. Front. Immunol. 2017, 8, 1164. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Su, J.; Zheng, X.; Wang, C.; Chen, S.; Liu, J.; Lv, Y.; Fan, S.; Zhao, A.; et al. Age-related compositional changes and correlations of gut microbiome, serum metabolome, and immune factor in rats. Geroscience 2021, 43, 709–725. [Google Scholar] [CrossRef]
- Lees, H.; Swann, J.; Poucher, S.M.; Nicholson, J.K.; Holmes, E.; Wilson, I.D.; Marchesi, J.R. Age and microenvironment outweigh genetic influence on the Zucker rat microbiome. PLoS ONE 2014, 9, e100916. [Google Scholar] [CrossRef]
- Sgro, M.; Iacono, G.; Yamakawa, G.R.; Kodila, Z.N.; Marsland, B.J.; Mychasiuk, R. Age matters: Microbiome depletion prior to repeat mild traumatic brain injury differentially alters microbial composition and function in adolescent and adult rats. PLoS ONE 2022, 17, e0278259. [Google Scholar] [CrossRef] [PubMed]
- Song, E.M.; Byeon, J.S.; Lee, S.M.; Yoo, H.J.; Kim, S.J.; Lee, S.H.; Chang, K.; Hwang, S.W.; Yang, D.H.; Jeong, J.Y. Fecal fatty acid profiling as a potential new screening biomarker in patients with colorectal cancer. Dig. Dis. Sci. 2018, 63, 1229–1236. [Google Scholar] [CrossRef]
- Varma, V.S.; Shabtay, A.; Yishay, M.; Mizrahi, I.; Shterzer, N.; Freilich, S.; Medina, S.; Agmon, R.; Laor, Y. Diet supplementation with pomegranate peel extract altered odorants emission from fresh and incubated calves’ feces. Front. Sustain. Food Syst. 2018, 2, 33. [Google Scholar] [CrossRef]
- Marhuenda-Munoz, M.; Laveriano-Santos, E.P.; Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Martinez-Huelamo, M.; Vallverdu-Queralt, A. Microbial phenolic metabolites: Which molecules actually have an effect on human health? Nutrients 2019, 11, 2725. [Google Scholar] [CrossRef]
- Serra, A.; Macia, A.; Romero, M.P.; Angles, N.; Morello, J.R.; Motilva, M.J. Distribution of procyanidins and their metabolites in rat plasma and tissues after an acute intake of hazelnut extract. Food Funct. 2011, 2, 562–568. [Google Scholar] [CrossRef]
- Zhao, L.; Xiao, H.T.; Mu, H.X.; Huang, T.; Lin, Z.S.; Zhong, L.L.D.; Zeng, G.Z.; Fan, B.M.; Lin, C.Y.; Bian, Z.X. Magnolol, a natural polyphenol, attenuates dextran sulfate sodium-induced colitis in mice. Molecules 2017, 22, 1218. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulos, S.; Dokou, S.; Papadopoulos, G.A.; Savvidou, S.; Christaki, S.; Kyriakoudi, A.; Dotas, V.; Tsiouris, V.; Bonos, E.; Skoufos, I. Dietary supplementation with pomegranate and onion aqueous and cyclodextrin encapsulated extracts affects broiler performance parameters, welfare and meat characteristics. Poultry 2022, 1, 74–93. [Google Scholar] [CrossRef]
- Dinh, D.M.; Volpe, G.E.; Duffalo, C.; Bhalchandra, S.; Tai, A.K.; Kane, A.V.; Wanke, C.A.; Ward, H.D. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J. Infect. Dis. 2015, 211, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Schaubeck, M.; Clavel, T.; Calasan, J.; Lagkouvardos, I.; Haange, S.B.; Jehmlich, N.; Basic, M.; Dupont, A.; Hornef, M.; von Bergen, M.; et al. Dysbiotic gut microbiota causes transmissible crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut 2016, 65, 225–237. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Hu, J.; Wu, X.; Pan, J.A.; Jiao, N.; Li, Y.; Huang, Y.; Lin, X.; Zou, Y.; Chen, Y.; et al. Altered gut microbiome in FUT2 loss-of-function mutants in support of personalized medicine for inflammatory bowel diseases. J. Genet. Genom. 2021, 48, 771–780. [Google Scholar] [CrossRef]
- Zhu, Q.; Jin, Z.; Wu, W.; Gao, R.; Guo, B.; Gao, Z.; Yang, Y.; Qin, H. Analysis of the intestinal lumen microbiota in an animal model of colorectal cancer. PLoS ONE 2014, 9, e90849. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Y.; Lu, P.; Wang, X.; Li, W.; Dong, H.; Fan, S.; Li, D. Gut metabolite Urolithin A mitigates ionizing radiation-induced intestinal damage. J. Cell Mol. Med. 2021, 25, 10306–10312. [Google Scholar] [CrossRef]
- He, T.; Cheng, X.; Xing, C. The gut microbial diversity of colon cancer patients and the clinical significance. Bioengineered 2021, 12, 7046–7060. [Google Scholar] [CrossRef]
- Park, J.Y.; Seo, H.; Kang, C.S.; Shin, T.S.; Kim, J.W.; Park, J.M.; Kim, J.G.; Kim, Y.K. Dysbiotic change in gastric microbiome and its functional implication in gastric carcinogenesis. Sci. Rep. 2022, 12, 4285. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Li, Y.; Poroyko, V.; Yan, Z.; Pan, L.; Feng, Y.; Zhao, P.; Xie, Z.; Hong, L. Characterization of intestinal microbiomes of hirschsprung’s disease patients with or without enterocolitis using Illumina-MiSeq high-throughput sequencing. PLoS ONE 2016, 11, e0162079. [Google Scholar] [CrossRef] [PubMed]
- Bamola, V.D.; Kapardar, R.; Lal, B.; Sharma, A.; Chaudhry, R. A metagenomic assessment of gut microbiota in Indian colon cancer patients. J. Cancer Res. Ther. 2022, 18, 96–102. [Google Scholar] [CrossRef]
- Cortez, R.V.; Moreira, L.N.; Padilha, M.; Bibas, M.D.; Toma, R.K.; Porta, G.; Taddei, C.R. Gut microbiome of children and adolescents with primary sclerosing cholangitis in association with ulcerative colitis. Front. Immunol. 2020, 11, 598152. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Henning, S.M.; Lee, R.P.; Lu, Q.Y.; Summanen, P.H.; Thames, G.; Corbett, K.; Downes, J.; Tseng, C.H.; Finegold, S.M.; et al. Pomegranate extract induces ellagitannin metabolite formation and changes stool microbiota in healthy volunteers. Food Funct. 2015, 6, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Y.; Han, B.; Deng, X.; Deng, S.Y.; Zhang, Y.Y.; Shen, P.X.; Hui, T.; Chen, R.H.; Li, X.; Zhang, Y. Pomegranate peel extract ameliorates the severity of experimental autoimmune encephalomyelitis via modulation of gut microbiota. Gut Microbes 2020, 12, 1857515. [Google Scholar] [CrossRef]
- Kendall, A.I. Some observations on the study of the intestinal bacteria. J. Biol. Chem. 1909, 6, 499–507. [Google Scholar] [CrossRef]
- Behr, C.; Slopianka, M.; Haake, V.; Strauss, V.; Sperber, S.; Kamp, H.; Walk, T.; Beekmann, K.; Rietjens, I.; van Ravenzwaay, B. Analysis of metabolome changes in the bile acid pool in feces and plasma of antibiotic-treated rats. Toxicol. Appl. Pharmacol. 2019, 363, 79–87. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, R.; Wang, D.; Zhu, W. The diversity of the fecal bacterial community and its relationship with the concentration of volatile fatty acids in the feces during subacute rumen acidosis in dairy cows. BMC Vet. Res. 2012, 8, 237. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, S.; Zhao, X.; Li, H.; Lin, W.; Li, B.; Lu, J.; Sun, Y.; Yuan, J. Community-metabolome correlations of gut microbiota from child-turcotte-pugh of A and B patients. Front. Microbiol. 2016, 7, 1856. [Google Scholar] [CrossRef]
- Kang, H.G.; Jeong, S.H.; Cho, M.H.; Cho, J.H. Changes of biomarkers with oral exposure to benzo(a)pyrene, phenanthrene and pyrene in rats. J. Vet. Sci. 2007, 8, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Long, X.; Yang, J.; Du, L.; Zhang, X.; Li, J.; Hou, C. Pomegranate peel polyphenols reduce chronic low-grade inflammatory responses by modulating gut microbiota and decreasing colonic tissue damage in rats fed a high-fat diet. Food Funct. 2019, 10, 8273–8285. [Google Scholar] [CrossRef] [PubMed]
- Blaut, M.; Schoefer, L.; Braune, A. Transformation of flavonoids by intestinal microorganisms. Int. J. Vitam. Nutr. Res. 2003, 73, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Pellock, S.J.; Redinbo, M.R. Glucuronides in the gut: Sugar-driven symbioses between microbe and host. J. Biol. Chem. 2017, 292, 8569–8576. [Google Scholar] [CrossRef]
- Dashnyam, P.; Mudududdla, R.; Hsieh, T.J.; Lin, T.C.; Lin, H.Y.; Chen, P.Y.; Hsu, C.Y.; Lin, C.H. β-glucuronidases of opportunistic bacteria are the major contributors to xenobiotic-induced toxicity in the gut. Sci. Rep. 2018, 8, 16372. [Google Scholar] [CrossRef]
- Gloux, K.; Berteau, O.; El Oumami, H.; Beguet, F.; Leclerc, M.; Dore, J. A metagenomic β-glucuronidase uncovers a core adaptive function of the human intestinal microbiome. Proc. Natl Acad. Sci. USA 2011, 108 (Suppl. S1), 4539–4546. [Google Scholar] [CrossRef]
- Masamune, H. Biochemical Studies on Carbohydrates IV. On an enzyme which catalyses the hydrolysis of biosynthetic osides of glucuronic acid. J. Biochem. 1934, 19, 353–375. [Google Scholar] [CrossRef]
- Haiser, H.J.; Turnbaugh, P.J. Developing a metagenomic view of xenobiotic metabolism. Pharmacol. Res. 2013, 69, 21–31. [Google Scholar] [CrossRef]
- Nakamura, J.; Kubota, Y.; Miyaoka, M.; Saitoh, T.; Mizuno, F.; Benno, Y. Comparison of four microbial enzymes in Clostridia and Bacteroides isolated from human feces. Microbiol. Immunol. 2002, 46, 487–490. [Google Scholar] [CrossRef]
- Dabek, M.; McCrae, S.I.; Stevens, V.J.; Duncan, S.H.; Louis, P. Distribution of β-glucosidase and β-glucuronidase activity and of β-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol. Ecol. 2008, 66, 487–495. [Google Scholar] [CrossRef]
- Walsh, J.; Olavarria-Ramirez, L.; Lach, G.; Boehme, M.; Dinan, T.G.; Cryan, J.F.; Griffin, B.T.; Hyland, N.P.; Clarke, G. Impact of host and environmental factors on β-glucuronidase enzymatic activity: Implications for gastrointestinal serotonin. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G816–G826. [Google Scholar] [CrossRef]
- de Moreno de LeBlanc, A.; Perdigon, G. Reduction of beta-glucuronidase and nitroreductase activity by yoghurt in a murine colon cancer model. Biocell 2005, 29, 15–24. [Google Scholar] [PubMed]
- Chang, J.; Chadwick, R.W.; Allison, J.C.; Hayes, Y.O.; Talley, D.L.; Autry, C.E. Microbial succession and intestinal enzyme activities in the developing rat. J. Appl. Bacteriol. 1994, 77, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Goldin, B.; Dwyer, J.; Gorbach, S.L.; Gordon, W.; Swenson, L. Influence of diet and age on fecal bacterial enzymes. Am. J. Clin. Nutr. 1978, 31, S136–S140. [Google Scholar] [CrossRef] [PubMed]
- McMahon, T.F.; Beierschmitt, W.P.; Weiner, M. Changes in phase I and phase II biotransformation with age in male Fischer 344 rat colon: Relationship to colon carcinogenesis. Cancer Lett. 1987, 36, 273–282. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Srinivas, N.R. Is pomegranate juice a potential perpetrator of clinical drug-drug interactions? Review of the in vitro, preclinical and clinical evidence. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 223–229. [Google Scholar] [CrossRef]
- Andishmand, H.; Azadmard-Damirchi, S.; Hamishekar, H.; Torbati, M.; Kharazmi, M.S.; Savage, G.P.; Tan, C.; Jafari, S.M. Nano-delivery systems for encapsulation of phenolic compounds from pomegranate peel. Adv. Colloid Interface Sci. 2023, 311, 102833. [Google Scholar] [CrossRef]
- Zuccari, G.; Baldassari, S.; Ailuno, G.; Turrini, F.; Alfei, S.; Caviglioli, G.J.A.S. Formulation strategies to improve oral bioavailability of ellagic acid. Appl. Sci. 2020, 10, 3353. [Google Scholar] [CrossRef]
- Avramia, I.; Amariei, S. Formulation, characterization and optimization of beta-glucan and pomegranate juice based films for its potential in diabetes. Nutrients 2022, 14, 2142. [Google Scholar] [CrossRef]
- Huderson, A.C.; Rekha Devi, P.V.; Niaz, M.S.; Adunyah, S.E.; Ramesh, A. Alteration of benzo(a)pyrene biotransformation by resveratrol in ApcMin/+ mouse model of colon carcinogenesis. Investig. New Drugs 2019, 37, 238–251. [Google Scholar] [CrossRef]
- National Institutes of Health (US). NIH Guidelines for the Laboratory Use of Chemical Carcinogens; US Department of Health and Human Services: Bethesda, MD, USA, 1981; p. 15.
- Odabasoglu, F.; Aslan, A.; Cakir, A.; Suleyman, H.; Karagoz, Y.; Halici, M.; Bayir, Y. Comparison of antioxidant activity and phenolic content of three lichen species. Phytother. Res. 2004, 18, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Blainski, A.; Lopes, G.C.; de Mello, J.C. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Ammar, N.M.; Kholeif, T.E.; Metwally, N.S.; El-Sheikh, N.M.; Wessjohann, L.A.; Abdel-Hamid, A.Z. Rats’ urinary metabolomes reveal the potential roles of functional foods and exercise in obesity management. Food Funct. 2017, 8, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Ruehl-Fehlert, C.; Kittel, B.; Morawietz, G.; Deslex, P.; Keenan, C.; Mahrt, C.R.; Nolte, T.; Robinson, M.; Stuart, B.P.; Deschl, U.; et al. Revised guides for organ sampling and trimming in rats and mice–Part 1. Exp. Toxicol. Pathol. 2003, 55, 91–106. [Google Scholar] [CrossRef]
- Lee, D.S.; Kim, Y.S.; Ko, C.N.; Cho, K.H.; Bae, H.S.; Lee, K.S.; Kim, J.J.; Park, E.K.; Kim, D.H. Fecal metabolic activities of herbal components to bioactive compounds. Arch. Pharm. Res. 2002, 25, 165–169. [Google Scholar] [CrossRef]
- Yoo, D.H.; Kim, I.S.; Van Le, T.K.; Jung, I.H.; Yoo, H.H.; Kim, D.H. Gut microbiota-mediated drug interactions between lovastatin and antibiotics. Drug Metab. Dispos. 2014, 42, 1508–1513. [Google Scholar] [CrossRef]
- Culling, C.F.A. Handbook of Histopathological and Histochemical Techniques: Including Museum Techniques; Butterworth-Heinemann: London, UK, 1974. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Otify, A.M.; Ibrahim, R.M.; Abib, B.; Laub, A.; Wessjohann, L.A.; Jiang, Y.; Farag, M.A. Unveiling metabolome heterogeneity and new chemicals in 7 tomato varieties via multiplex approach of UHPLC-MS/MS, GC-MS, and UV-Vis in relation to antioxidant effects as analyzed using molecular networking and chemometrics. Food Chem. 2023, 417, 135866. [Google Scholar] [CrossRef]
- Wiklund, S.; Johansson, E.; Sjostrom, L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Racine, J.S. RStudio: A Platform-Independent IDE for R and Sweave. J. Appl. Econom. 2012, 27, 167–172. [Google Scholar] [CrossRef]




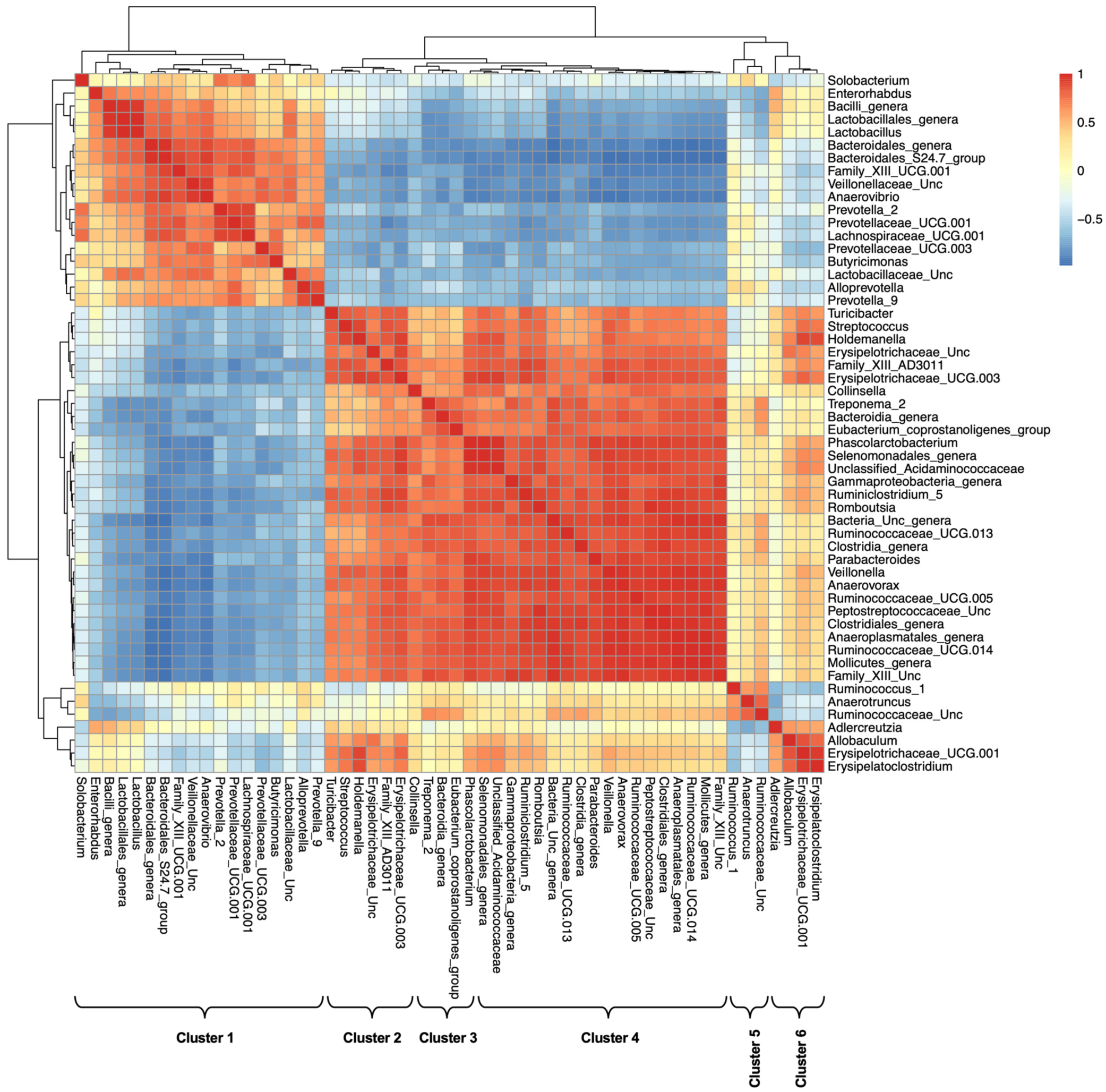
| Cluster | Major Members |
|---|---|
| Cluster 1: Lactobacillaceae and Prevoteallaceae | Enterorhabdus, Bacteroidales S24-7 group, Prevotellaceae, Lactobacillaceae, Family XIII UCG-001, Lachnospiraceae, Anaerovibrio |
| Cluster 2: Erysipelotrichaceae | Streptococcus, Family XIII AD3011 group, Erysipelotrichaceae UCG-003, Holdemanella, Turicibacter, unclassified Erysipelotrichaceae |
| Cluster 3: Collinsella_CAG | Collinsella, Unclassified Bacteroidia, [Eubacterium] coprostanoligenes group, Treponema 2 |
| Cluster 4: Ruminococcaceae 1 | Parabacteroides, Anaerovorax, Romboutsia, unclassified Peptostreptococcaceae, Ruminiclostridium 5, Ruminococcaceae UCG-005, Ruminococcaceae UCG-013, Ruminococcaceae UCG-014, unclassified Clostridiales family, Phascolarctobacterium, Veillonella, unclassified Selenomonadales, unclassified Anaeroplasmatales, unclassified Mollicutes |
| Cluster 5: Ruminococcaceae 2 | Anaerotruncus, Ruminococcus 1, unclassified Ruminococcaceae |
| Cluster 6: | Allobaculum, Erysipelatoclostridium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attia, H.; ElBanna, S.A.; Khattab, R.A.; Farag, M.A.; Yassin, A.S.; Aziz, R.K. Integrating Microbiome Analysis, Metabolomics, Bioinformatics, and Histopathology to Elucidate the Protective Effects of Pomegranate Juice against Benzo-alpha-pyrene-Induced Colon Pathologies. Int. J. Mol. Sci. 2023, 24, 10691. https://doi.org/10.3390/ijms241310691
Attia H, ElBanna SA, Khattab RA, Farag MA, Yassin AS, Aziz RK. Integrating Microbiome Analysis, Metabolomics, Bioinformatics, and Histopathology to Elucidate the Protective Effects of Pomegranate Juice against Benzo-alpha-pyrene-Induced Colon Pathologies. International Journal of Molecular Sciences. 2023; 24(13):10691. https://doi.org/10.3390/ijms241310691
Chicago/Turabian StyleAttia, Heba, Shahira A. ElBanna, Rania A. Khattab, Mohamed A. Farag, Aymen S. Yassin, and Ramy K. Aziz. 2023. "Integrating Microbiome Analysis, Metabolomics, Bioinformatics, and Histopathology to Elucidate the Protective Effects of Pomegranate Juice against Benzo-alpha-pyrene-Induced Colon Pathologies" International Journal of Molecular Sciences 24, no. 13: 10691. https://doi.org/10.3390/ijms241310691
APA StyleAttia, H., ElBanna, S. A., Khattab, R. A., Farag, M. A., Yassin, A. S., & Aziz, R. K. (2023). Integrating Microbiome Analysis, Metabolomics, Bioinformatics, and Histopathology to Elucidate the Protective Effects of Pomegranate Juice against Benzo-alpha-pyrene-Induced Colon Pathologies. International Journal of Molecular Sciences, 24(13), 10691. https://doi.org/10.3390/ijms241310691








