Antioxidant Supplementation Alleviates Mercury-Induced Cytotoxicity and Restores the Implantation-Related Functions of Primary Human Endometrial Cells
Abstract
1. Introduction
2. Results
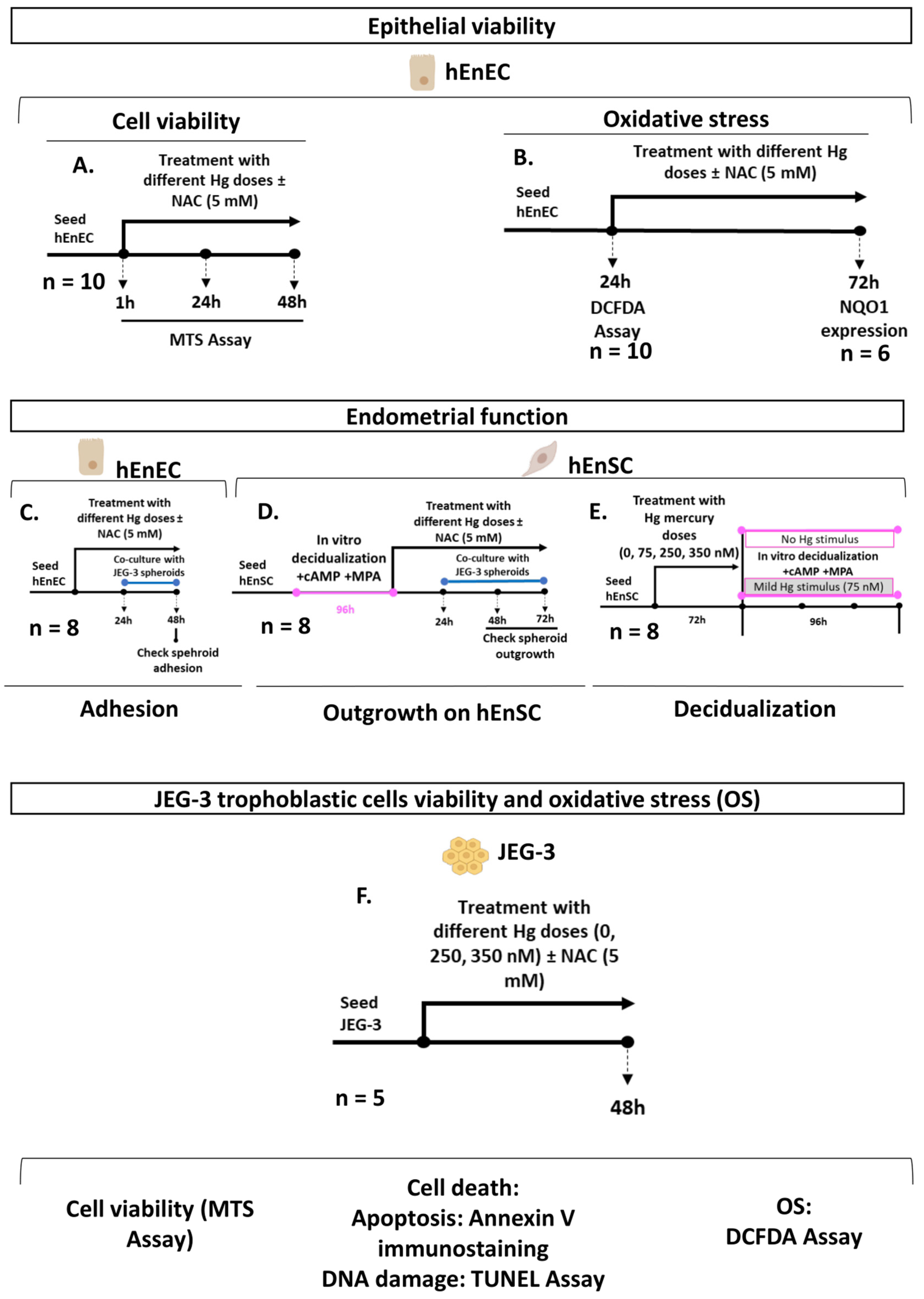
2.1. Hg Treatment Compromises the Viability of Human Primary Epithelial Cells (hEnEC) by Inducing Oxidative Stress (OS)
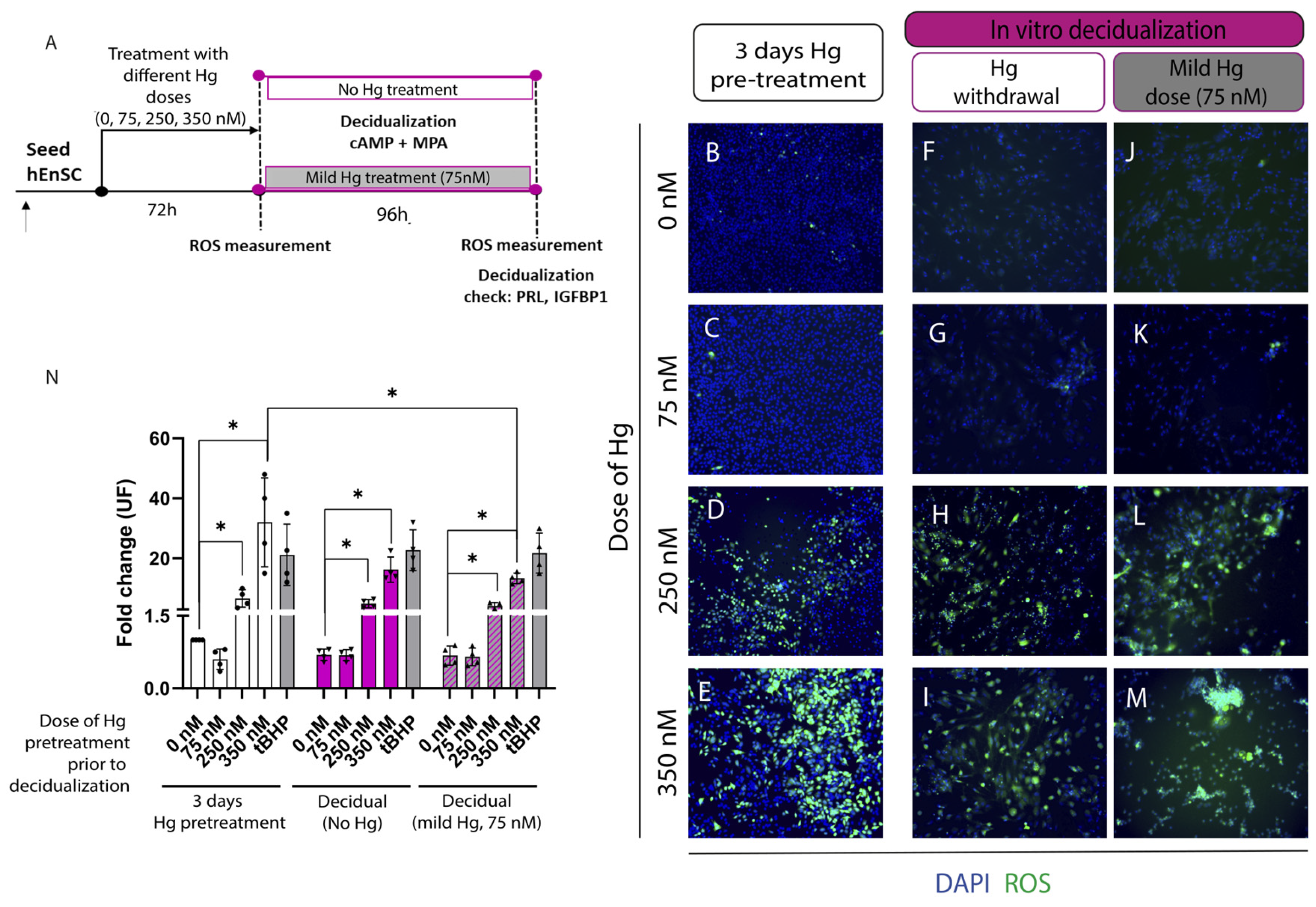
2.2. Decidual Function Is Unaffected by Withdrawing or Maintaining a Mild Mercury (Hg) Treatment during Decidualization, Regardless of Prior Metal Treatment
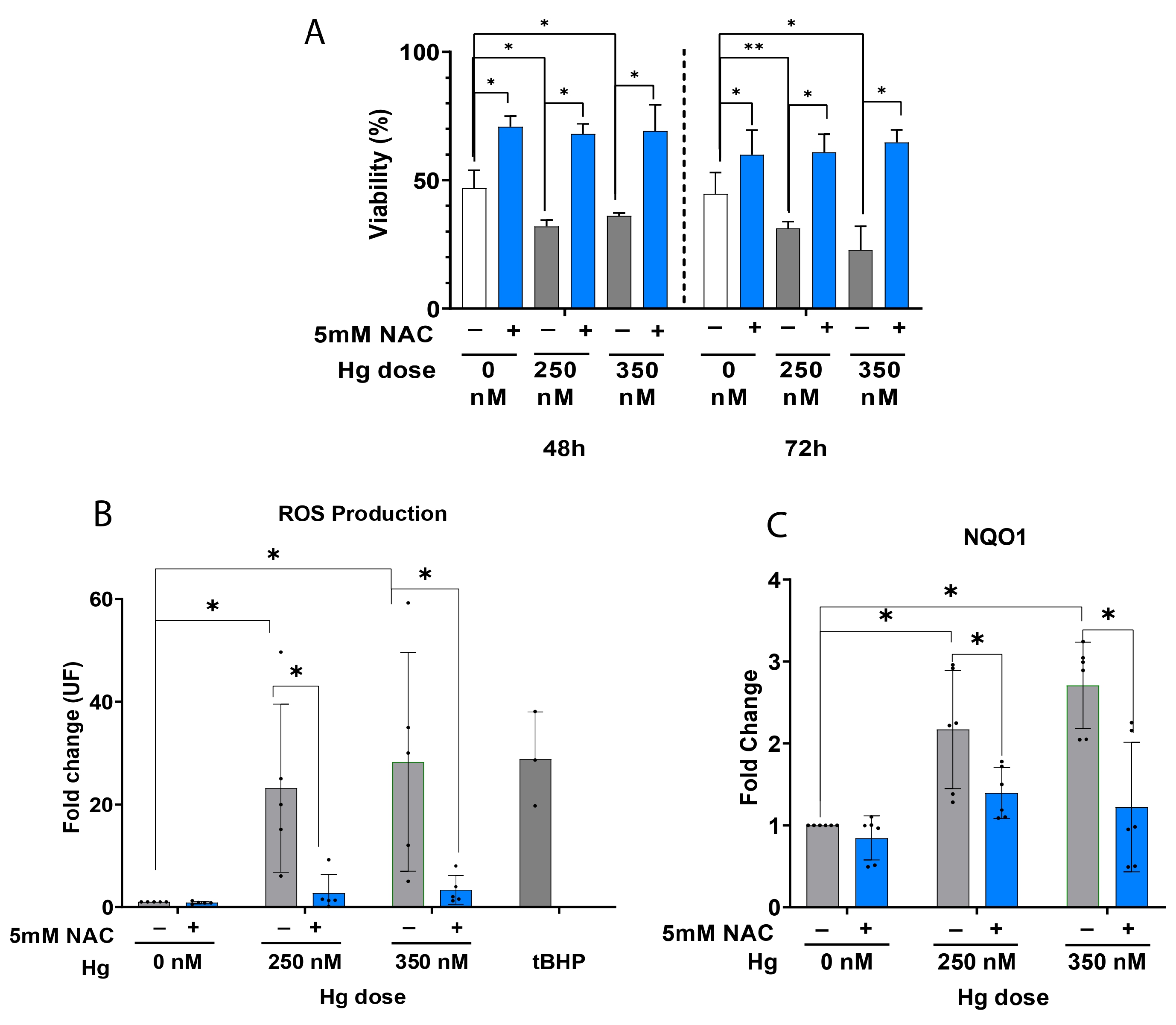
2.3. Antioxidant Supplementation Enhances Cell Viability by Alleviating OS in Primary hEnEC Exposed to Hg
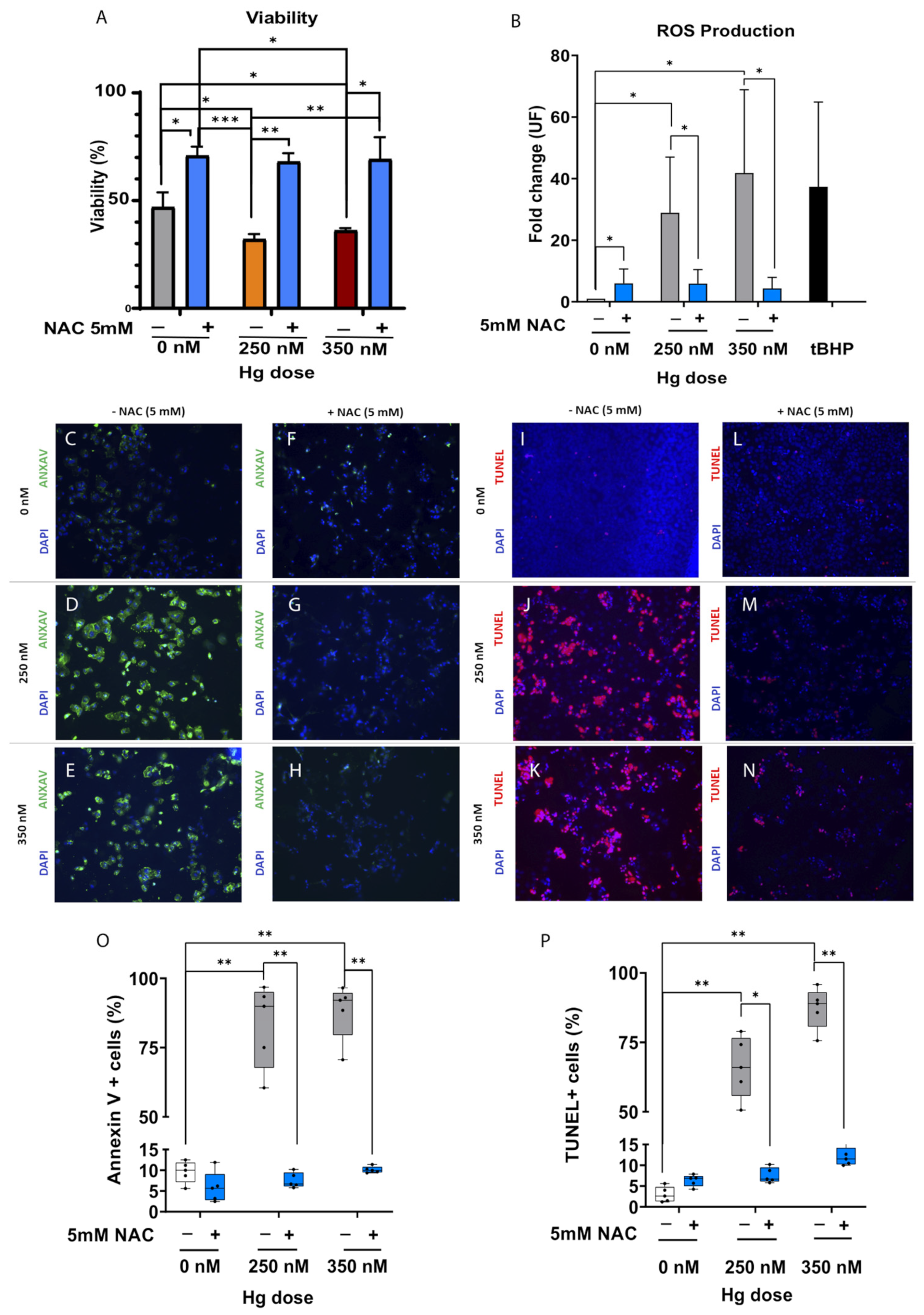
2.4. Antioxidant Supplementation Enhances Cell Survival by Alleviating Oxidative Stress (OS), Reducing Apoptosis and Protecting DNA Integrity in JEG-3 Trophoblast Cells Exposed to Mercury (Hg)
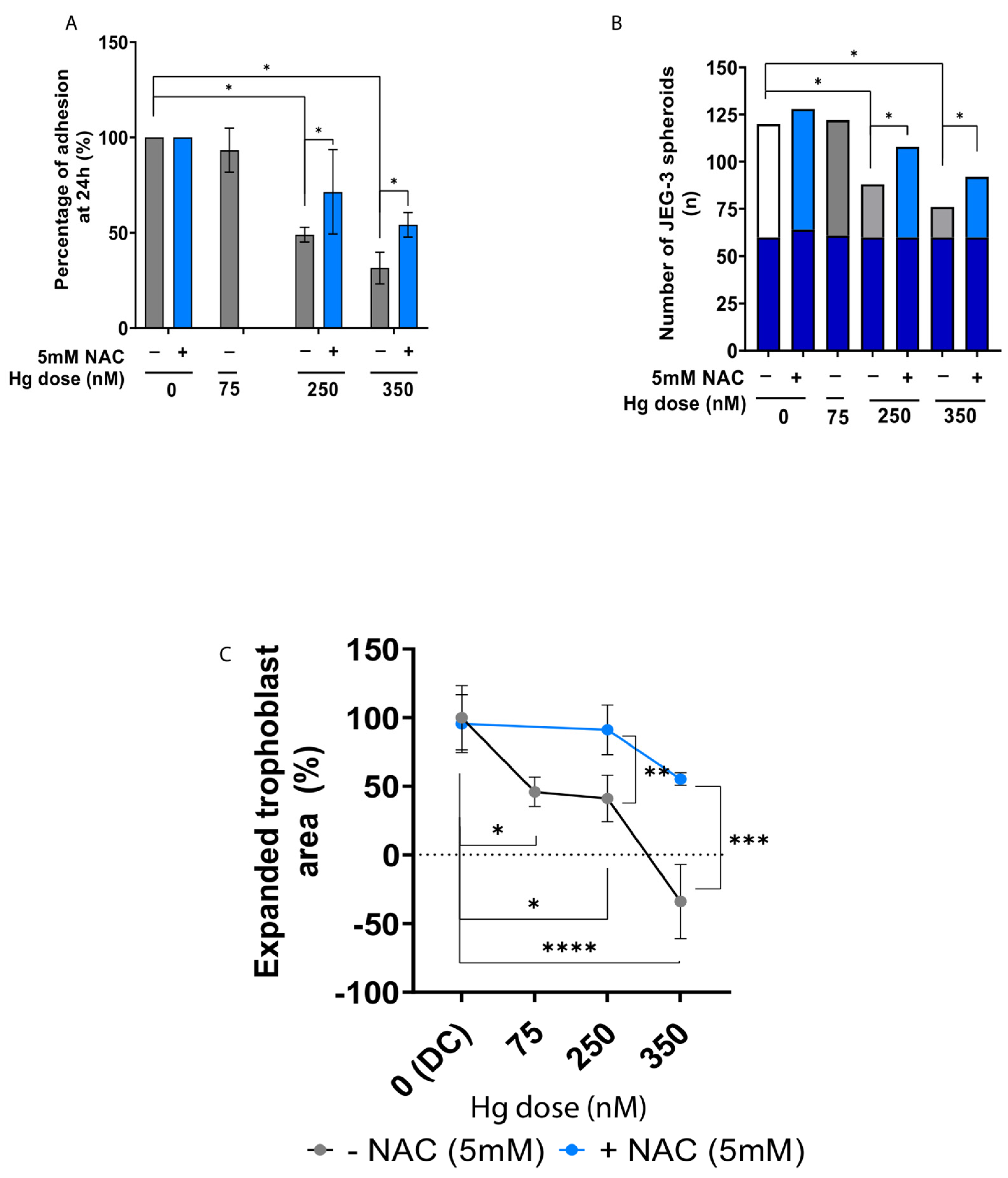
2.5. Antioxidant Supplementation Rescues Trophoblast Adhesion and Outgrowth following Mercury-Induced Damage
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Participants and Biological Samples
4.3. Isolation and Culture of hEnSC and hEnEC
4.4. In Vitro Hg Exposure and Antioxidant Supplementation
4.5. Cell Viability Assays
4.6. Cell Death Assessments: Annexin V Immunostaining and TUNEL
4.7. Quantification of ROS
4.8. Quantification of NQO1 Gene Expression
4.9. In Vitro Decidualization of Primary hEnSC
4.10. Culture of the JEG-3 Trophoblast Cells and Generation of Trophoblastic Spheroids
4.11. Adhesion Assays
4.12. Outgrowth Assays
4.13. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ojosnegros, S.; Seriola, A.; Godeau, A.L.; Veiga, A. Embryo Implantation in the Laboratory: An Update on Current Techniques. Hum. Reprod. Update 2021, 27, 501–530. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Vargas, P.; Muñoz, M.; Domínguez, F. Identifying Biomarkers for Predicting Successful Embryo Implantation: Applying Single to Multi-OMICs to Improve Reproductive Outcomes. Hum. Reprod. Update 2020, 26, 264–301. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.; Zhou, C.; Chiang, C.; Mahalingam, S. Exposure to Endocrine Disruptors during Adulthood: Consequences for Female Fertility. J. Endocrinol. 2017, 233, R109–R129. [Google Scholar] [CrossRef] [PubMed]
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R.H. Low Dose Mercury Toxicity and Human Health. Environ. Toxicol. Pharmacol. 2005, 20, 351–360. [Google Scholar] [CrossRef]
- Fujimoto, V.Y.; Bloom, M.S. Role of Environmental Factors and Gonadotoxin Exposure in Unexplained Female Infertility. In Unexplained Infertility; Schattman, G., Esteves, S., Agarwal, A., Eds.; Springer: New York, NY, USA, 2015; pp. 161–173. ISBN 9781493921409. [Google Scholar]
- Ye, B.J.; Kim, B.G.; Jeon, M.J.; Kim, S.Y.; Kim, H.C.; Jang, T.W.; Chae, H.J.; Choi, W.J.; Ha, M.N.; Hong, Y.S. Evaluation of Mercury Exposure Level, Clinical Diagnosis and Treatment for Mercury Intoxication. Ann. Occup. Environ. Med. 2016, 28, 5. [Google Scholar] [CrossRef]
- Tan, S.W.; Meiller, J.C.; Mahaffey, K.R. The Endocrine Effects of Mercury in Humans and Wildlife. Crit. Rev. Toxicol. 2009, 39, 228–269. [Google Scholar] [CrossRef]
- Bjørklund, G.; Aaseth, J.; Dadar, M.; Butnariu, M.; Chirumbolo, S. Exposure to Environmental Organic Mercury and Impairments in Human Fertility. J. Reprod. Infertil. 2019, 20, 195–197. [Google Scholar]
- Bjørklund, G.; Chirumbolo, S.; Dadar, M.; Pivina, L.; Lindh, U.; Butnariu, M.; Aaseth, J. Mercury Exposure and Its Effects on Fertility and Pregnancy Outcome. Basic Clin. Pharmacol. Toxicol. 2019, 125, 317–327. [Google Scholar] [CrossRef]
- Wang, G.; Tang, W.-Y.; Ji, H.; Wang, X. Prenatal Exposure to Mercury and Precocious Puberty: A Prospective Birth Cohort Study. Hum. Reprod. 2020, 36, 712–720. [Google Scholar] [CrossRef]
- Henriques, M.C.; Loureiro, S.; Fardilha, M.; Herdeiro, M.T. Exposure to Mercury and Human Reproductive Health: A Systematic Review. Reprod. Toxicol. 2019, 85, 93–103. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, C.; Zhang, Y.; Chen, S.; Huang, X.; Li, J.; Wang, Y.; Liu, X.; Deng, G.; Gao, J. Elevated Blood Mercury Level Has a Non-Linear Association with Infertility in U.S. Women: Data from the NHANES 2013–2016. Reprod. Toxicol. 2020, 91, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.B.; Reiter, R.; Pham, T.; Avellanet, Y.R.; Camara, J.; Lahm, M.; Pentecost, E.; Pratap, K.; Gilmore, B.A.; Divekar, S.; et al. Estrogen-Like Activity of Metals in Mcf-7 Breast Cancer Cells. Endocrinology 2003, 144, 2425–2436. [Google Scholar] [CrossRef] [PubMed]
- Guyot, E.; Solovyova, Y.; Tomkiewicz, C.; Leblanc, A.; Pierre, S.; El Balkhi, S.; Le Frère-Belda, M.A.; Lecuru, F.; Poupon, J.; Barouki, R.; et al. Determination of Heavy Metal Concentrations in Normal and Pathological Human Endometrial Biopsies and in Vitro Regulation of Gene Expression by Metals in the Ishikawa and Hec-1b Endometrial Cell Line. PLoS ONE 2015, 10, e0142590. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhu, M.; Miao, L.; Zhang, X.; Dong, X.; Zou, X. Mercuric Chloride Induced Ovarian Oxidative Stress by Suppressing Nrf2-Keap1 Signal Pathway and Its Downstream Genes in Laying Hens. Biol. Trace Elem. Res. 2018, 185, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Unoki, T.; Akiyama, M.; Kumagai, Y.; Gonçalves, F.M.; Farina, M.; da Rocha, J.B.T.; Aschner, M. Molecular Pathways Associated with Methylmercury-Induced Nrf2 Modulation. Front. Genet. 2018, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yin, C.; Zhou, Y.; Wang, Q.; Jiang, Y.; Bai, Y.; Qian, H.; Xing, G.; Wang, S.; Li, F.; et al. Curcumin Protects against Methylmercury-Induced Cytotoxicity in Primary Rat Astrocytes by Activating the Nrf2/ARE Pathway Independently of PKCδ. Toxicology 2019, 425, 152248. [Google Scholar] [CrossRef] [PubMed]
- Palomar, A.; González-Martín, R.; Pérez-Debén, S.; Medina-Laver, Y.; Quiñonero, A.; Domínguez, F. Mercury Impairs Human Primary Endometrial Stromal Cell Function. Biol. Reprod. 2022, 106, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Aplin, J.D.; Ruane, P.T. Embryo–Epithelium Interactions during Implantation at a Glance. J. Cell Sci. 2017, 130, 15–22. [Google Scholar] [CrossRef]
- Martinez, C.S.; Peçanha, F.M.; Brum, D.S.; Santos, F.W.; Franco, J.L.; Zemolin, A.P.P.; Anselmo-Franci, J.A.; Junior, F.B.; Alonso, M.J.; Salaices, M.; et al. Reproductive Dysfunction after Mercury Exposure at Low Levels: Evidence for a Role of Glutathione Peroxidase (GPx) 1 and GPx4 in Male Rats. Reprod. Fertil. Dev. 2016, 29, 1803–1812. [Google Scholar] [CrossRef]
- Mojica-Vázquez, L.H.; Madrigal-Zarraga, D.; García-Martínez, R.; Boube, M.; Calderón-Segura, M.E.; Oyallon, J. Mercury Chloride Exposure Induces DNA Damage, Reduces Fertility, and Alters Somatic and Germline Cells in Drosophila Melanogaster Ovaries. Environ. Sci. Pollut. Res. 2019, 26, 32322–32332. [Google Scholar] [CrossRef]
- Hossain, K.F.B.; Hosokawa, T.; Saito, T.; Kurasaki, M. Zinc-Pretreatment Triggers Glutathione and Nrf2-Mediated Protection against Inorganic Mercury-Induced Cytotoxicity and Intrinsic Apoptosis in PC12 Cells. Ecotoxicol. Environ. Saf. 2021, 207, 111320. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.F.B.; Rahman, M.; Sikder, T.; Hosokawa, T.; Saito, T.; Kurasaki, M. Selenium Modulates Inorganic Mercury Induced Cytotoxicity and Intrinsic Apoptosis in PC12 Cells. Ecotoxicol. Environ. Saf. 2021, 207, 111262. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.F.B.; Hosokawa, T.; Saito, T.; Kurasaki, M. Amelioration of Butylated Hydroxytoluene against Inorganic Mercury Induced Cytotoxicity and Mitochondrial Apoptosis in PC12 Cells via Antioxidant Effects. Food Chem. Toxicol. 2020, 146, 111819. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.F.B.; Rahman, M.; Sikder, T.; Hosokawa, T.; Saito, T.; Kurasaki, M. Regulatory Effects of Dihydrolipoic Acid against Inorganic Mercury-Mediated Cytotoxicity and Intrinsic Apoptosis in PC12 Cells. Ecotoxicol. Environ. Saf. 2020, 192, 110238. [Google Scholar] [CrossRef]
- Fatema, K.; Shoily, S.S.; Ahsan, T.; Haidar, Z.; Sumit, A.F.; Sajib, A.A. Effects of Arsenic and Heavy Metals on Metabolic Pathways in Cells of Human Origin: Similarities and Differences | Elsevier Enhanced Reader. Toxicol. Rep. 2021, 8, 1109–1120. [Google Scholar] [CrossRef]
- Han, B.; Lv, Z.; Han, X.; Li, S.; Han, B.; Yang, Q.; Wang, X.; Wu, P.; Li, J.; Deng, N.; et al. Harmful Effects of Inorganic Mercury Exposure on Kidney Cells: Mitochondrial Dynamics Disorder and Excessive Oxidative Stress. Biol. Trace Elem. Res. 2021, 200, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Shi, X.; Tang, B.Z.; Wang, W.-X. Real-Time In Vitro Monitoring of the Subcellular Toxicity of Inorganic Hg and Methylmercury in Zebrafish Cells. Aquat. Toxicol. 2021, 236, 105859. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.S.; Vasconcelos, C.P.; Mitre, G.P.; Kataoka, M.S.D.S.; Bittencourt, L.O.; Lima, M.O.; de Oliveira, E.H.C.; Crespo-Lopez, M.E.; Lima, R.R. Metabolic and Oxidative Impairments in Human Salivary Gland Cells Line Exposed to MeHg. J. Trace Elem. Med. Biol. 2021, 66, 126747. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, J.; Kim, J.; Kim, J.-Y.; An, M.-J.; Shin, G.-S.; Lee, H.-M.; Kim, C.-H.; Kim, J.-W. Transcriptome Analysis Reveals HgCl2 Induces Apoptotic Cell Death in Human Lung Carcinoma H1299 Cells through Caspase-3-Independent Pathway. Int. J. Mol. Sci. 2021, 22, 2006. [Google Scholar] [CrossRef]
- Granitzer, S.; Widhalm, R.; Forsthuber, M.; Ellinger, I.; Desoye, G.; Hengstschläger, D.; Zeisler, H.; Salzer, H.; Gundacker, C. Amino Acid Transporter LAT1 (SLC7A5) Mediates MeHg-Induced Oxidative Stress Defense in the Human Placental Cell Line HTR-8/SVneo. Int. J. Mol. Sci. 2021, 22, 1707. [Google Scholar] [CrossRef]
- Maggisano, V.; Bulotta, S.; Celano, M.; Maiuolo, J.; Lepore, S.M.; Abballe, L.; Iannone, M.; Russo, D. Low Doses of Methylmercury Induce the Proliferation of Thyroid Cells in Vitro through Modulation of ERK Pathway. Int. J. Mol. Sci. 2020, 21, 1556. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Xu, S.; Nakano, R.; Hasegawa, T.; Endo, N.; Takahashi, T.; Lee, J.Y.; Naganuma, A.; Hwang, G.W. The Nuclear Protein HOXB13 Enhances Methylmercury Toxicity by Inducing Oncostatin M and Promoting Its Binding to TNFR3 in Cultured Cells. Cells 2019, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Takanezawa, Y.; Nakamura, R.; Matsuda, H.; Yagi, T.; Egawa, Z.; Sone, Y.; Uraguchi, S.; Adachi, T.; Kiyono, M. Intracellular Demethylation of Methylmercury to Inorganic Mercury by Organomercurial Lyase (MerB) Strengthens Cytotoxicity. Toxicol. Sci. 2019, 170, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Sudo, K.; van Dao, C.; Miyamoto, A.; Shiraishi, M. Comparative Analysis of in Vitro Neurotoxicity of Methylmercury, Mercury, Cadmium, and Hydrogen Peroxide on SH-SY5Y Cells. J. Vet. Med. Sci. 2019, 81, 828–837. [Google Scholar] [CrossRef]
- Orr, S.E.; Barnes, M.C.; Joshee, L.; Uchakina, O.; McKallip, R.J.; Bridges, C.C. Potential Mechanisms of Cellular Injury Following Exposure to a Physiologically Relevant Species of Inorganic Mercury. Toxicol. Lett. 2019, 304, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, B.; Gao, J.; Liu, Q.S.; Liu, R.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Methylmercury Exposure Alters RNA Splicing in Human Neuroblastoma SK-N-SH Cells: Implications from Proteomic and Post-Transcriptional Responses. Environ. Pollut. 2018, 238, 213–221. [Google Scholar] [CrossRef]
- Miranda, R.R.; Bezerra, A.G.; Oliveira Ribeiro, C.A.; Randi, M.A.F.; Voigt, C.L.; Skytte, L.; Rasmussen, K.L.; Kjeldsen, F.; Filipak Neto, F. Toxicological Interactions of Silver Nanoparticles and Non-Essential Metals in Human Hepatocarcinoma Cell Line. Toxicol. Vitr. 2017, 40, 134–143. [Google Scholar] [CrossRef]
- Lu, X.; Xiang, Y.; Yang, G.; Zhang, L.; Wang, H.; Zhong, S. Transcriptomic Characterization of Zebrafish Larvae in Response to Mercury Exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 192, 40–49. [Google Scholar] [CrossRef]
- Morcillo, P.; Esteban, M.; Cuesta, A. Heavy Metals Produce Toxicity, Oxidative Stress and Apoptosis in the Marine Teleost Fish SAF-1 Cell Line. Chemosphere 2016, 144, 225–233. [Google Scholar] [CrossRef]
- Pérez-Debén, S.; Gonzalez-Martin, R.; Palomar, A.; Quiñonero, A.; Salsano, S.; Dominguez, F. Copper and Lead Exposures Disturb Reproductive Features of Primary Endometrial Stromal and Epithelial Cells. Reprod. Toxicol. 2020, 93, 106–117. [Google Scholar] [CrossRef]
- Philbert, M.A.; Billingsley, M.L.; Reuhl, K.R. Mechanisms of Injury in the Central Nervous System. Toxicol. Pathol. 2000, 28, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Bhaumik, S.; Purkayastha, M.; Basu, S.; Nag Chaudhuri, A.; das Gupta, S. Apoptosis and Necrosis in Developing Brain Cells Due to Arsenic Toxicity and Protection with Antioxidants. Toxicol. Lett. 2002, 136, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Tucker, E.K.; Nowak, R.A. Methylmercury Alters Proliferation, Migration, and Antioxidant Capacity in Human HTR8/SV-Neo Trophoblast Cells. Reprod. Toxicol. 2018, 78, 60. [Google Scholar] [CrossRef]
- Kucki, M.; Aengenheister, L.; Diener, L.; Rippl, A.V.; Vranic, S.; Newman, L.; Vazquez, E.; Kostarelos, K.; Wick, P.; Buerki-Thurnherr, T. Impact of Graphene Oxide on Human Placental Trophoblast Viability, Functionality and Barrier Integrity. 2D Mater. 2018, 5, 035014. [Google Scholar] [CrossRef]
- Pirković, A.; Vilotić, A.; Borozan, S.; Nacka-Aleksić, M.; Bojić-Trbojević, Ž.; Krivokuća, M.J.; Battino, M.; Giampieri, F.; Dekanski, D. Oleuropein Attenuates Oxidative Stress in Human Trophoblast Cells. Antioxidants 2023, 12, 197. [Google Scholar] [CrossRef]
- Bruić, M.; Pirković, A.; Vilotić, A.; Jovanović-Krivokuća, M.; Spremo-Potparević, B. Cytoprotective and Genoprotective Effects of Taxifolin against Oxidative Damage in HTR-8/SVneo Human Trophoblast Cells. Mutagenesis 2023, 38, 64–70. [Google Scholar] [CrossRef]
- World Health Organization. Air Quality Guidelines for Europe. In WHO Regional Publication; European Series; WHO: Geneva, Italy, 2000; pp. 157–161. [Google Scholar]
- Dominguez, F.; Gadea, B.; Mercader, A.; Esteban, F.J.; Pellicer, A.; Simón, C. Embryologic Outcome and Secretome Profile of Implanted Blastocysts Obtained after Coculture in Human Endometrial Epithelial Cells versus the Sequential System. Fertil. Steril. 2010, 93, 774–782.e1. [Google Scholar] [CrossRef]
- Salsano, S.; Pérez-Debén, S.; Quiñonero, A.; González-Martín, R.; Domínguez, F. Phytoestrogen Exposure Alters Endometrial Stromal Cells and Interferes with Decidualization Signaling. Fertil. Steril. 2019, 112, 947–958.e3. [Google Scholar] [CrossRef]
- Salsano, S.; González-Martín, R.; Quiñonero, A.; López-Martín, S.; Gómez-Escribano, A.P.; Pérez-Debén, S.; Yañez-Mo, M.; Domínguez, F. Novel Nonclassic Progesterone Receptor PGRMC1 Pulldown-Precipitated Proteins Reveal a Key Role during Human Decidualization. Fertil. Steril. 2020, 113, 1050–1066.e7. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ Ecosystem: An Open Platform for Biomedical Image Analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Salsano, S.; Quinonero, A.; Perez, S.; Garrido Gomez, T.; Simon, C.; Dominguez, F. Dynamic Expression of PGRMC1 and SERBP1 in Human Endometrium: An Implication in the Human Decidualization Process. Fertil. Steril. 2017, 108, 832–842.e1. [Google Scholar] [CrossRef] [PubMed]
- Gellersen, B.; Brosens, I.A.; Brosens, J.J. Decidualization of the Human Endometrium: Mechanisms, Functions, and Clinical Perspectives. Semin. Reprod. Med. 2007, 25, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Turco, M.Y.; Gardner, L.; Hughes, J.; Cindrova-Davies, T.; Gomez, M.J.; Farrell, L.; Hollinshead, M.; Marsh, S.G.E.; Brosens, J.J.; Critchley, H.O.; et al. Long-Term, Hormone-Responsive Organoid Cultures of Human Endometrium in a Chemically Defined Medium. Nat. Cell. Biol. 2017, 19, 568–577. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomar, A.; Quiñonero, A.; Medina-Laver, Y.; Gonzalez-Martin, R.; Pérez-Debén, S.; Alama, P.; Domínguez, F. Antioxidant Supplementation Alleviates Mercury-Induced Cytotoxicity and Restores the Implantation-Related Functions of Primary Human Endometrial Cells. Int. J. Mol. Sci. 2023, 24, 8799. https://doi.org/10.3390/ijms24108799
Palomar A, Quiñonero A, Medina-Laver Y, Gonzalez-Martin R, Pérez-Debén S, Alama P, Domínguez F. Antioxidant Supplementation Alleviates Mercury-Induced Cytotoxicity and Restores the Implantation-Related Functions of Primary Human Endometrial Cells. International Journal of Molecular Sciences. 2023; 24(10):8799. https://doi.org/10.3390/ijms24108799
Chicago/Turabian StylePalomar, Andrea, Alicia Quiñonero, Yassmin Medina-Laver, Roberto Gonzalez-Martin, Silvia Pérez-Debén, Pilar Alama, and Francisco Domínguez. 2023. "Antioxidant Supplementation Alleviates Mercury-Induced Cytotoxicity and Restores the Implantation-Related Functions of Primary Human Endometrial Cells" International Journal of Molecular Sciences 24, no. 10: 8799. https://doi.org/10.3390/ijms24108799
APA StylePalomar, A., Quiñonero, A., Medina-Laver, Y., Gonzalez-Martin, R., Pérez-Debén, S., Alama, P., & Domínguez, F. (2023). Antioxidant Supplementation Alleviates Mercury-Induced Cytotoxicity and Restores the Implantation-Related Functions of Primary Human Endometrial Cells. International Journal of Molecular Sciences, 24(10), 8799. https://doi.org/10.3390/ijms24108799





