Transcriptome Analysis of the Developmental Effects of Bisphenol F Exposure in Chinese Medaka (Oryzias sinensis)
Abstract
1. Introduction
2. Results
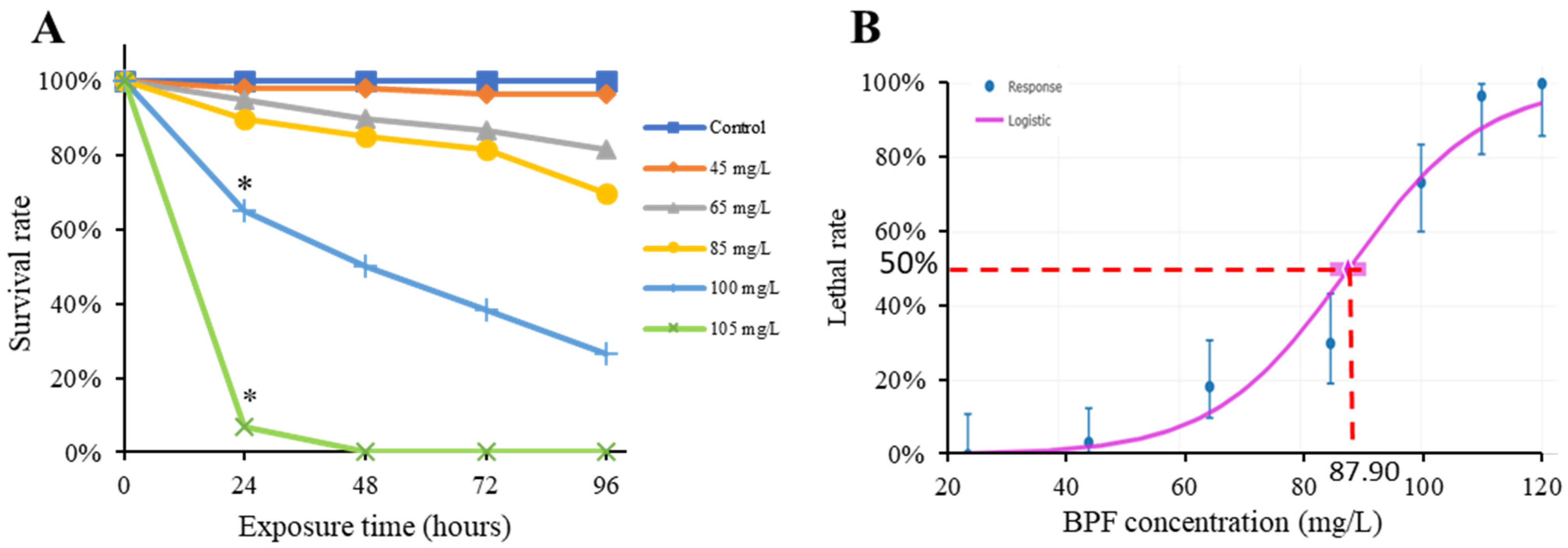
2.1. Acute Toxicity of BPF to Chinese Medaka Embryo
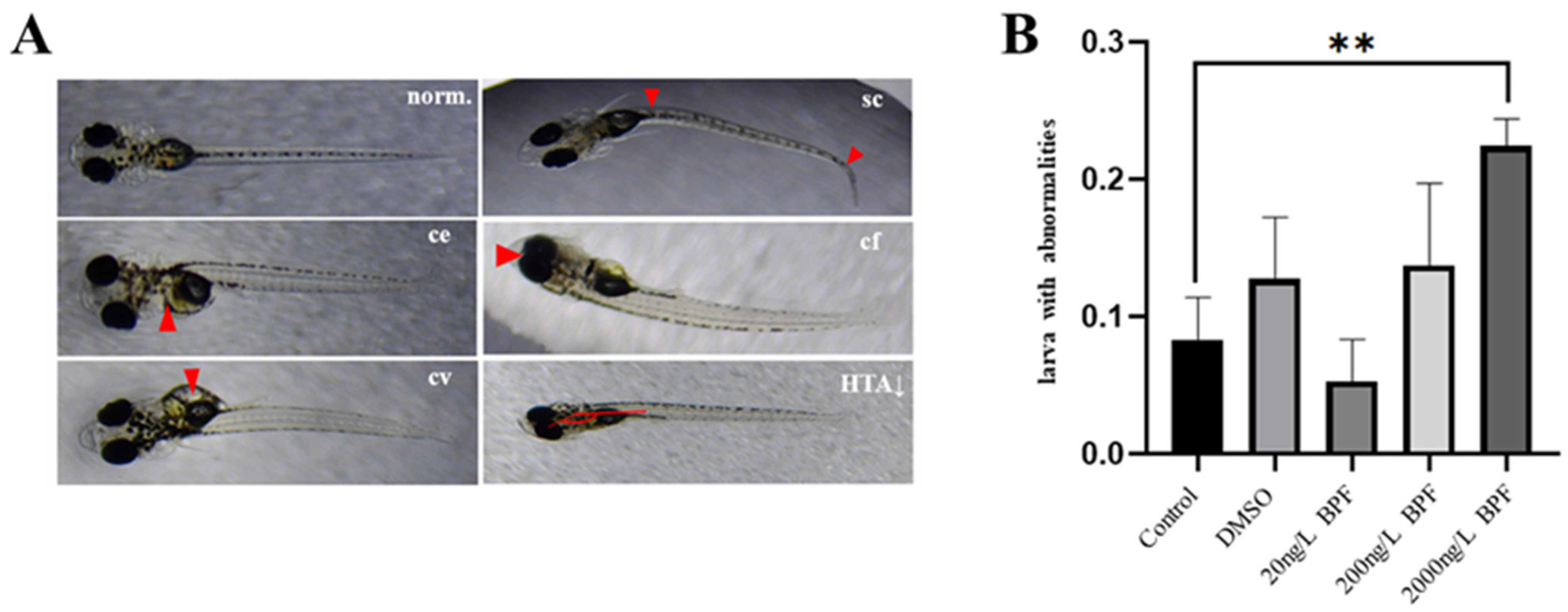
2.2. Developmental Abnormalities Induced by BPF Exposure
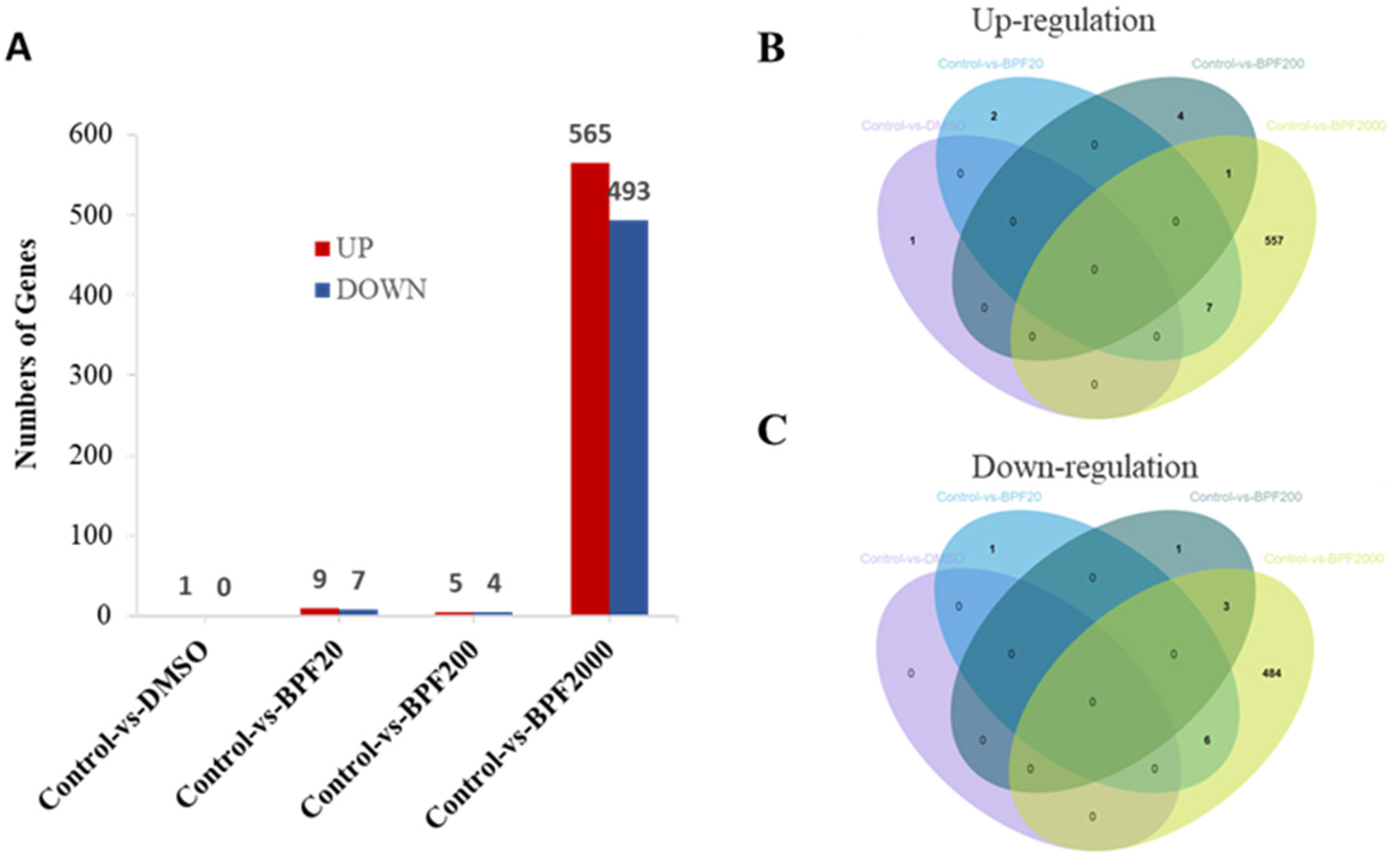
2.3. Differential Expression of Genes (DEGs) in the Larvae
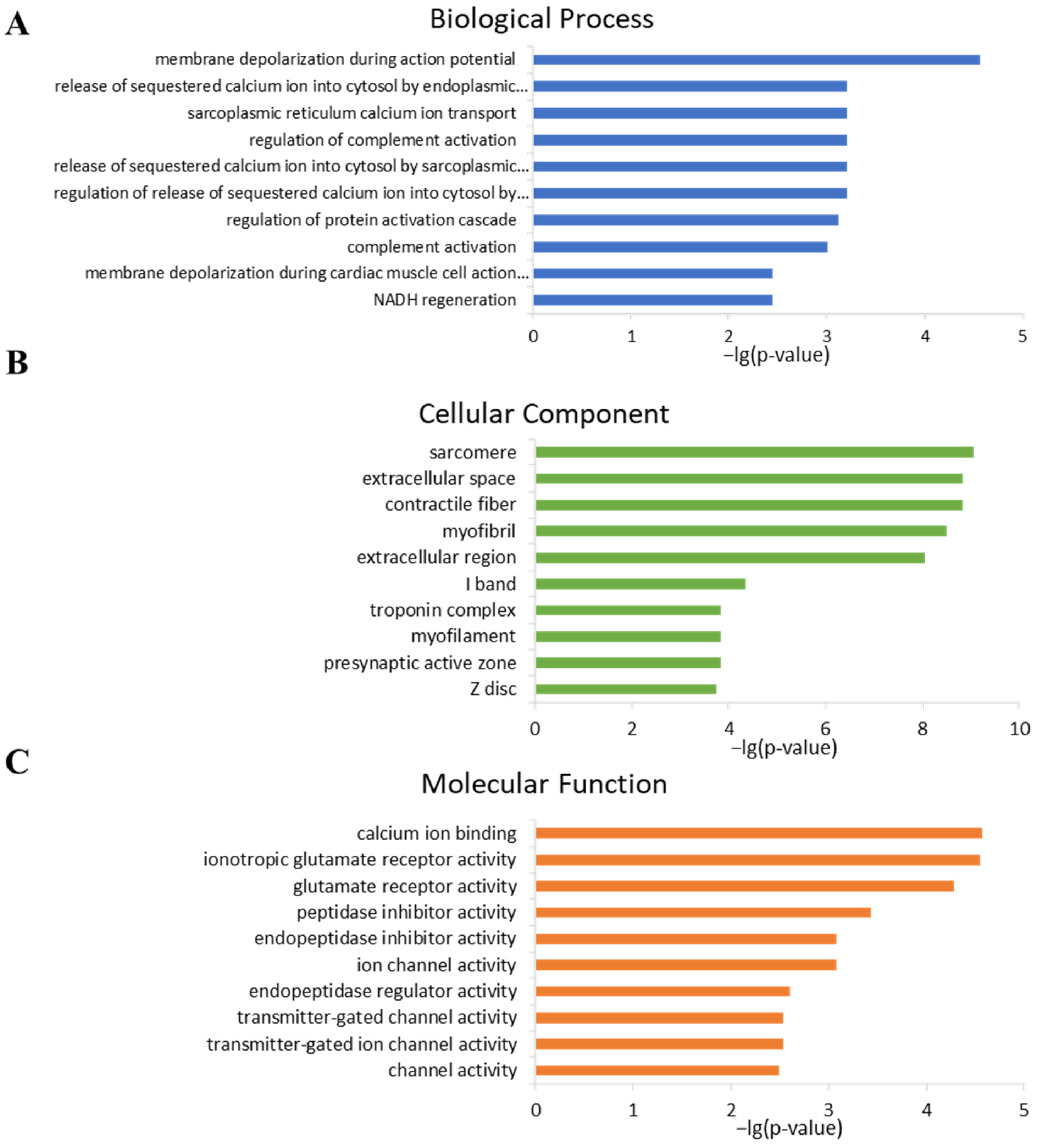
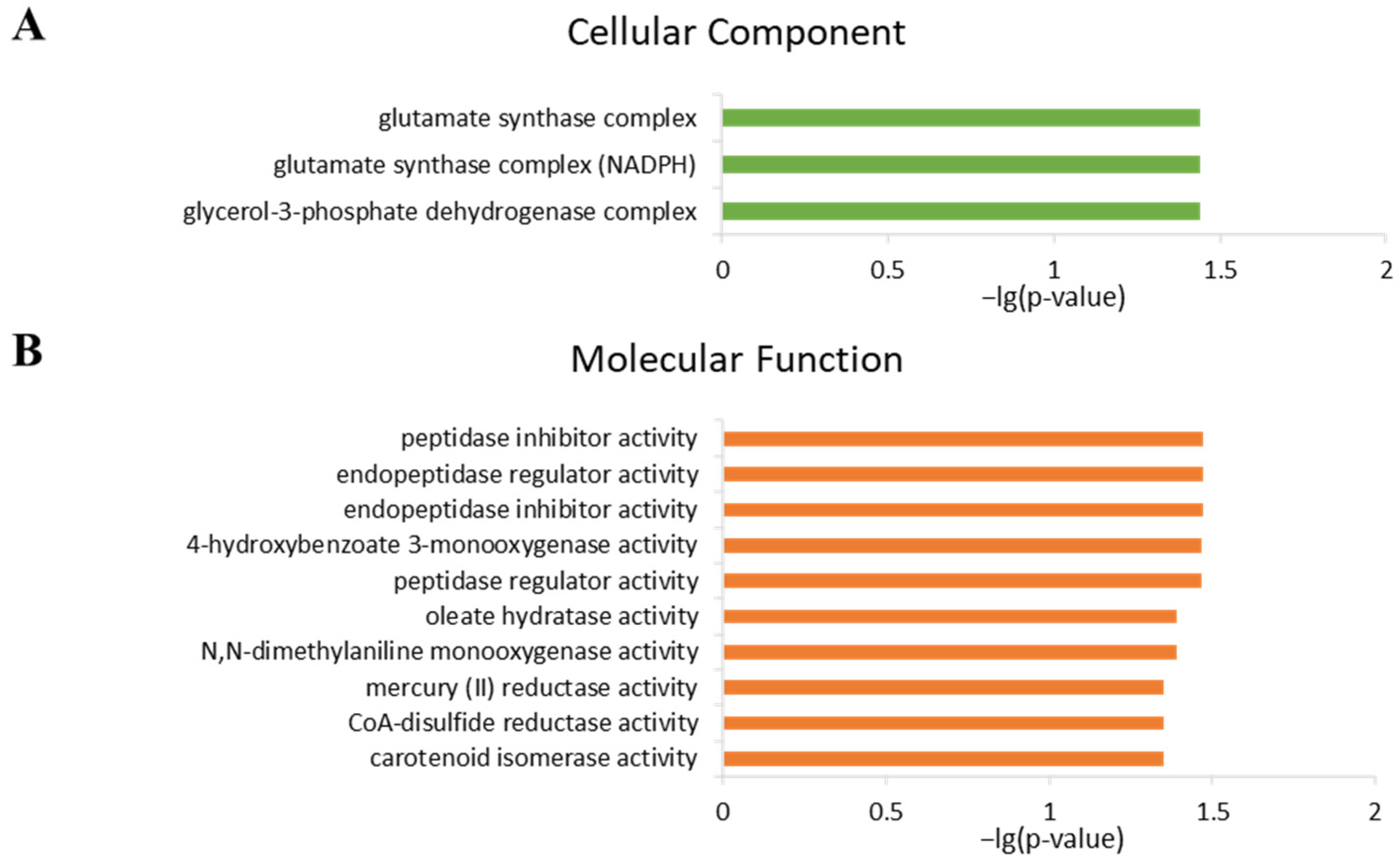
2.4. Analysis of Gene Ontology
2.5. Analysis of Key KEGG Pathways
3. Discussion
4. Materials and Methods
4.1. Fish Husbandry
4.2. Chemicals and Reagents
4.3. Exposure Methods
4.4. Acute Toxic Exposure
4.5. Developmental Toxic Exposure
4.6. RNA-Seq and Bioinformatics Analysis
4.6.1. Library Preparation and Sequencing
4.6.2. Data Processing
4.6.3. GO Enrichment Analysis
4.6.4. Pathway Enrichment Analysis
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Nguyen, T.V.; Cuprys, A.K.; Ratnaweera, H. Bisphenols in water: Occurrence, effects, and mitigation strategies. Chemosphere 2023, 328, 138560. [Google Scholar] [CrossRef] [PubMed]
- Thayil, A.J.; Wang, X.; Bhandari, P.; vom Saal, F.S.; Tillitt, D.E.; Bhandari, R.K. Bisphenol A and 17α-ethinylestradiol-induced transgenerational gene expression differences in the brain–pituitary–testis axis of medaka, Oryzias latipes. Biol. Reprod. 2020, 103, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.K.; Wang, X.; Saal, F.S.V.; Tillitt, D.E. Transcriptome analysis of testis reveals the effects of developmental exposure to bisphenol a or 17α-ethinylestradiol in medaka (Oryzias latipes). Aquat. Toxicol. 2020, 225, 105553. [Google Scholar] [CrossRef] [PubMed]
- Timms, B.G.; Howdeshell, K.L.; Barton, L.; Bradley, S.; Richter, C.A.; vom Saal, F.S. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc. Natl. Acad. Sci. USA 2005, 102, 7014–7019. [Google Scholar] [CrossRef]
- vom Saal, F.S.; Nagel, S.C.; Coe, B.L.; Angle, B.M.; Taylor, J.A. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol. Cell. Endocrinol. 2012, 354, 74–84. [Google Scholar] [CrossRef]
- Vrooman, L.A.; Oatley, J.M.; Griswold, J.E.; Hassold, T.J.; Hunt, P.A. Estrogenic exposure alters the spermatogonial stem cells in the developing testis, permanently reducing crossover levels in the adult. PLoS Genet. 2015, 11, e1004949. [Google Scholar] [CrossRef]
- Mandrah, K.; Satyanarayana, G.N.V.; Roy, S.K. A dispersive liquid-liquid microextraction based on solidification of floating organic droplet followed by injector port silylation coupled with gas chromatography–tandem mass spectrometry for the determination of nine bisphenols in bottled carbonated beverages. J. Chromatogr. A Incl. Electrophor. Other Sep. Methods 2017, 1528, 10–17. [Google Scholar]
- Huang, C.; Wu, L.H.; Liu, G.Q.; Shi, L.; Guo, Y. Occurrence and Ecological Risk Assessment of Eight Endocrine-Disrupting Chemicals in Urban River Water and Sediments of South China. Arch. Environ. Contam. Toxicol. 2018, 75, 224–235. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, L. Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China. Water Res. 2016, 103, 343–351. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Song, N.; Guo, R.; Chen, M.; Mai, D.; Yan, Z.; Han, Z.; Chen, J. Occurrence, distribution and sources of bisphenol analogues in a shallow Chinese freshwater lake (Taihu Lake): Implications for ecological and human health risk. Sci. Total Environ. 2017, 599–600, 1090–1098. [Google Scholar] [CrossRef]
- Yamazaki, E.; Yamashita, N.; Taniyasu, S.; Lam, J.; Lam, P.K.; Moon, H.B.; Jeong, Y.; Kannan, P.; Achyuthan, H.; Munuswamy, N.; et al. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf. 2015, 122, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Küchler, T.; Otto, T.; Pilz, K.; Müller, J.; Wenzel, A. Occurrence of phthalates and bisphenol A and F in the environment. Water Res. 2002, 36, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, Y.; Li, Y.; Ashfaq, M.; Dai, L.; Xie, X.; Yu, C.P. Fate and mass balance of bisphenol analogues in wastewater treatment plants in Xiamen City, China. Environ. Pollut. 2017, 225, 542–549. [Google Scholar] [CrossRef]
- Sun, X.; Peng, J.; Wang, M.; Wang, J.; Chen, J. Determination of nine bisphenols in sewage and sludge using dummy molecularly imprinted solid-phase extraction coupled with liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2018, 1552, 10–16. [Google Scholar] [CrossRef]
- Česen, M.; Lenarčič, K.; Mislej, V.; Levstek, M.; Kovačič, A.; Cimrmančič, B.; Uranjek, N.; Kosjek, T.; Heath, D.; Dolenc, M.S.; et al. The occurrence and source identification of bisphenol compounds in wastewaters. Sci. Total Environ. 2017, 616–617, 744–752. [Google Scholar] [CrossRef]
- Esen, M.; Ahel, M.; Terzi, S.; Heath, D.J.; Heath, E. The occurrence of contaminants of emerging concern in Slovenian and Croatian wastewaters and receiving Sava river. Sci. Total Environ. 2018, 650 Pt 2, 2446–2453. [Google Scholar]
- Zhang, H.; Zhang, Y.; Li, J.; Yang, M. Occurrence and exposure assessment of bisphenol analogues in source water and drinking water in China. Sci. Total Environ. 2019, 655, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-E.; Liao, C.; Moon, H.-B. Occurrence and exposure assessment of bisphenol analogues through different types of drinking water in Korea. Expo. Health 2023, 15, 185–197. [Google Scholar] [CrossRef]
- Liao, C.; Kannan, K. A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Arch. Environ. Contam. Toxicol. 2014, 67, 50–59. [Google Scholar] [CrossRef]
- Yamasaki, K.; Noda, S.; Imatanaka, N.; Yakabe, Y. Comparative study of the uterotrophic potency of 14 chemicals in a uterotrophic assay and their receptor-binding affinity. Toxicol. Lett. 2004, 146, 111–120. [Google Scholar] [CrossRef]
- Moreman, J.; Lee, O.; Trznadel, M.; David, A.; Kudoh, T.; Tyler, C.R. Acute Toxicity, Teratogenic, and Estrogenic Effects of Bisphenol A and Its Alternative Replacements Bisphenol S, Bisphenol F, and Bisphenol AF in Zebrafish Embryo-Larvae. Environ. Sci. Technol. 2017, 51, 12796–12805. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wu, J.; Xu, S.; Zhang, L.; Fan, D.; Shi, L.; Wang, J.; Ji, G. Bisphenol F exposure impairs neurodevelopment in zebrafish larvae (Danio rerio). Ecotoxicol. Environ. Saf. 2020, 188, 109870. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Qian, L.; Qian, Y.; Liu, J.; Yang, K.; Huang, Y.; Wang, C.; Li, Y.; Mu, X. Bisphenol F-Induced Neurotoxicity toward Zebrafish Embryos. Environ. Sci. Technol. 2019, 53, 14638–14648. [Google Scholar] [CrossRef] [PubMed]
- Cabaton, N.; Dumont, C.; Severin, I.; Perdu, E.; Zalko, D.; Cherkaoui-Malki, M.; Chagnon, M.C. Genotoxic and endocrine activities of bis(hydroxyphenyl)methane (bisphenol F) and its derivatives in the HepG2 cell line. Toxicology 2009, 255, 15–24. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Ren, X.-M.; Li, Y.-Y.; Yao, X.-F.; Li, C.-H.; Qin, Z.-F.; Guo, L.H. Bisphenol A alternatives bisphenol S and bisphenol F interfere with thyroid hormone signaling pathway in vitro and in vivo. Environ. Pollut. 2018, 237, 1072–1079. [Google Scholar] [CrossRef]
- Huang, G.-M.; Tian, X.-F.; Fang, X.-D.; Ji, F.-J. Waterborne exposure to bisphenol F causes thyroid endocrine disruption in zebrafish larvae. Chemosphere 2016, 147, 188–194. [Google Scholar] [CrossRef]
- Gu, J.; Li, L.; Yin, X.; Liang, M.; Zhu, Y.; Guo, M.; Zhou, L.; Fan, D.; Shi, L.; Ji, G. Long-term exposure of zebrafish to bisphenol F: Adverse effects on parental reproduction and offspring neurodevelopment. Aquat. Toxicol. 2022, 248, 106190. [Google Scholar] [CrossRef]
- Wang, H.; Qi, S.; Mu, X.; Yuan, L.; Li, Y.; Qiu, J. Bisphenol F induces liver-gut alteration in zebrafish. Sci. Total Environ. 2022, 851, 157974. [Google Scholar] [CrossRef]
- Qiu, W.; Fang, M.; Liu, J.; Fu, C.; Zheng, C.; Chen, B.; Wang, K.J. In vivo actions of Bisphenol F on the reproductive neuroendocrine system after long-term exposure in zebrafish. Sci. Total Environ. 2019, 665, 995–1002. [Google Scholar] [CrossRef]
- Lee, S.-J.; Baek, S.-K.; Kim, W.; Quah, Y.; Kim, S.-Y.; Jeong, J.-S.; Lee, J.; Yu, W.J. Reproductive and developmental toxicity screening of bisphenol F by oral gavage in rats. Regul. Toxicol. Pharmacol. 2022, 136, 105286. [Google Scholar] [CrossRef]
- Wang, X.; Bhandari, R.K. DNA methylation reprogramming in medaka fish, a promising animal model for environmental epigenetics research. Environ. Epigenet. 2020, 6, dvaa008. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhu, P.; Yan, L.; Li, R.; Hu, B.; Lian, Y.; Yan, J.; Ren, X.; Lin, S.; Li, J.; et al. The DNA methylation landscape of human early embryos. Nature 2014, 511, 606. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bhandari, R.K. DNA methylation dynamics during epigenetic reprogramming of medaka embryo. Epigenetics 2019, 14, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bhandari, R.K. The dynamics of DNA methylation during epigenetic reprogramming of primordial germ cells in medaka (Oryzias latipes). Epigenetics 2020, 15, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Nagahama, Y.; Shinomiya, A.; Sato, T.; Matsuda, C.; Kobayashi, T.; Morrey, C.E.; Shibata, N.; Asakawa, S.; Shimizu, N.; et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 2002, 417, 559. [Google Scholar] [CrossRef]
- Kasahara, M.; Naruse, K.; Sasaki, S.; Nakatani, Y.; Qu, W.; Ahsan, B.; Yamada, T.; Nagayasu, Y.; Doi, K.; Kasai, Y.; et al. The medaka draft genome and insights into vertebrate genome evolution. Nature 2007, 447, 714–719. [Google Scholar] [CrossRef]
- Cui, L.; Fan, M.; Belanger, S.; Li, J.; Wang, X.N.; Fan, B.; Li, W.; Gao, X.; Chen, J.; Liu, Z. Oryzias sinensis, a new model organism in the application of eco-toxicity and water quality criteria (WQC). Chemosphere 2020, 261, 127813. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.L.; Jia, X.J.; Luo, X.Z.; Zhou, Y.; Mao, X.T.; Fan, X.; Hu, H.; Zhu, H.; Jia, C.; et al. Genome and transcriptome of Chinese medaka (Oryzias sinensis) and its uses as a model fish for evaluating estrogenicity of surface water. Environ. Pollut. 2023, 317, 120724. [Google Scholar] [CrossRef]
- Fan, X.; Guo, J.; Jia, X.; Mao, X.; Zhou, Y.; Wang, Y.; Guo, X.; Shen, J.; Huai, N.; Zhang, K.; et al. Reproductive Toxicity and Teratogenicity of Fluorene-9-bisphenol on Chinese Medaka (Oryzias sinensis): A Study from Laboratory to Field. Environ. Sci. Technol. 2023, 57, 561–569. [Google Scholar] [CrossRef]
- Li, Y.; Fu, X.; Zhao, Y.; Su, H.; Qin, Z. Comparison on Acute Toxicity of Bisphenol A with Its Substitutes to Pelophylax nigromaculatus. Asian J. Ecotoxicol. 2015, 10, 251–257. [Google Scholar]
- Gao, Y.; Li, A.; Zhang, W.; Pang, S.; Liang, Y.; Song, M. Assessing the toxicity of bisphenol A and its six alternatives on zebrafish embryo/larvae. Aquat. Toxicol. 2022, 246, 106154. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, Y.; Yan, K.; Wu, S.; Han, Z.; Guo, R.; Chen, M.; Yang, Q.; Zhang, S.; Chen, J. Bisphenol analogues in surface water and sediment from the shallow Chinese freshwater lakes: Occurrence, distribution, source apportionment, and ecological and human health risk. Chemosphere 2017, 184, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, J.; Yao, J.; Wu, L.; Xiao, H.; Wang, J.; Gao, R. Simultaneous identification and quantification of bisphenol A and 12 bisphenol analogues in environmental samples using precolumn derivatization and ultra high performance liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2018, 41, 2269–2278. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.-P.; Zhou, W.; Guo, C.-H. The Role of Plant Peroxisomes in ROS Signalling Network. Zhongguo Shengwu Huaxue Yu Fenzi Shengwu Xuebao 2017, 33, 220–226. [Google Scholar]
- Ullah, A.; Pirzada, M.; Jahan, S.; Ullah, H.; Shaheen, G.; Rehman, H.; Siddiqui, M.F.; Butt, M.A. Bisphenol A and its analogs bisphenol B, bisphenol F, and bisphenol S: Comparative in vitro and in vivo studies on the sperms and testicular tissues of rats. Chemosphere 2018, 209, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.H.; Shao, H.Y.; Lei, P.H.; Zheng, C.M.; Qiu, C.X.; Yang, M.; Zheng, Y. Immunotoxicity of bisphenol S and F are similar to that of bisphenol A during zebrafish early development. Chemosphere 2018, 194, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.P.; Hu, X.M.; Rao, X.C. Apoptosis induced by Staphylococcus aureus toxins. Microbiol. Res. 2017, 205, 19–24. [Google Scholar] [CrossRef]
- Qiu, W.H.; Liu, S.; Yang, F.; Dong, P.Y.; Yang, M.; Wong, M.H.; Zheng, C. Metabolism disruption analysis of zebrafish larvae in response to BPA and BPA analogs based on RNA-Seq technique. Ecotoxicol. Environ. Saf. 2019, 174, 181–188. [Google Scholar] [CrossRef]
- Ando, H.; Urano, A. Molecular regulation of gonadotropin secretion by gonadotropin-releasing hormone in salmonid fishes. Zool. Sci. 2005, 22, 379–389. [Google Scholar] [CrossRef]
- OECD. Test No. 212: Fish, Short-Term Toxicity Test on Embryo and Sac-Fry Stages; OECD Publishing: Paris, France, 1998. [Google Scholar]
- OECD. Test No. 210: Fish, Early-Life Stage Toxicity Test; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. Erecipes Res. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.N.; Bo, L. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Guo, Y.; Pi, D.; Li, X.; Li, B.; Huang, Y.; Song, X.; Bhandari, R.K.; Wang, X. Transcriptome Analysis of the Developmental Effects of Bisphenol F Exposure in Chinese Medaka (Oryzias sinensis). Int. J. Mol. Sci. 2023, 24, 10898. https://doi.org/10.3390/ijms241310898
Liang Z, Guo Y, Pi D, Li X, Li B, Huang Y, Song X, Bhandari RK, Wang X. Transcriptome Analysis of the Developmental Effects of Bisphenol F Exposure in Chinese Medaka (Oryzias sinensis). International Journal of Molecular Sciences. 2023; 24(13):10898. https://doi.org/10.3390/ijms241310898
Chicago/Turabian StyleLiang, Zhiying, Yafen Guo, Duan Pi, Xiang Li, Bingying Li, Yongsi Huang, Xiaohong Song, Ramji Kumar Bhandari, and Xuegeng Wang. 2023. "Transcriptome Analysis of the Developmental Effects of Bisphenol F Exposure in Chinese Medaka (Oryzias sinensis)" International Journal of Molecular Sciences 24, no. 13: 10898. https://doi.org/10.3390/ijms241310898
APA StyleLiang, Z., Guo, Y., Pi, D., Li, X., Li, B., Huang, Y., Song, X., Bhandari, R. K., & Wang, X. (2023). Transcriptome Analysis of the Developmental Effects of Bisphenol F Exposure in Chinese Medaka (Oryzias sinensis). International Journal of Molecular Sciences, 24(13), 10898. https://doi.org/10.3390/ijms241310898





