Systemic Review of Clot Retraction Modulators
Abstract
1. Introduction
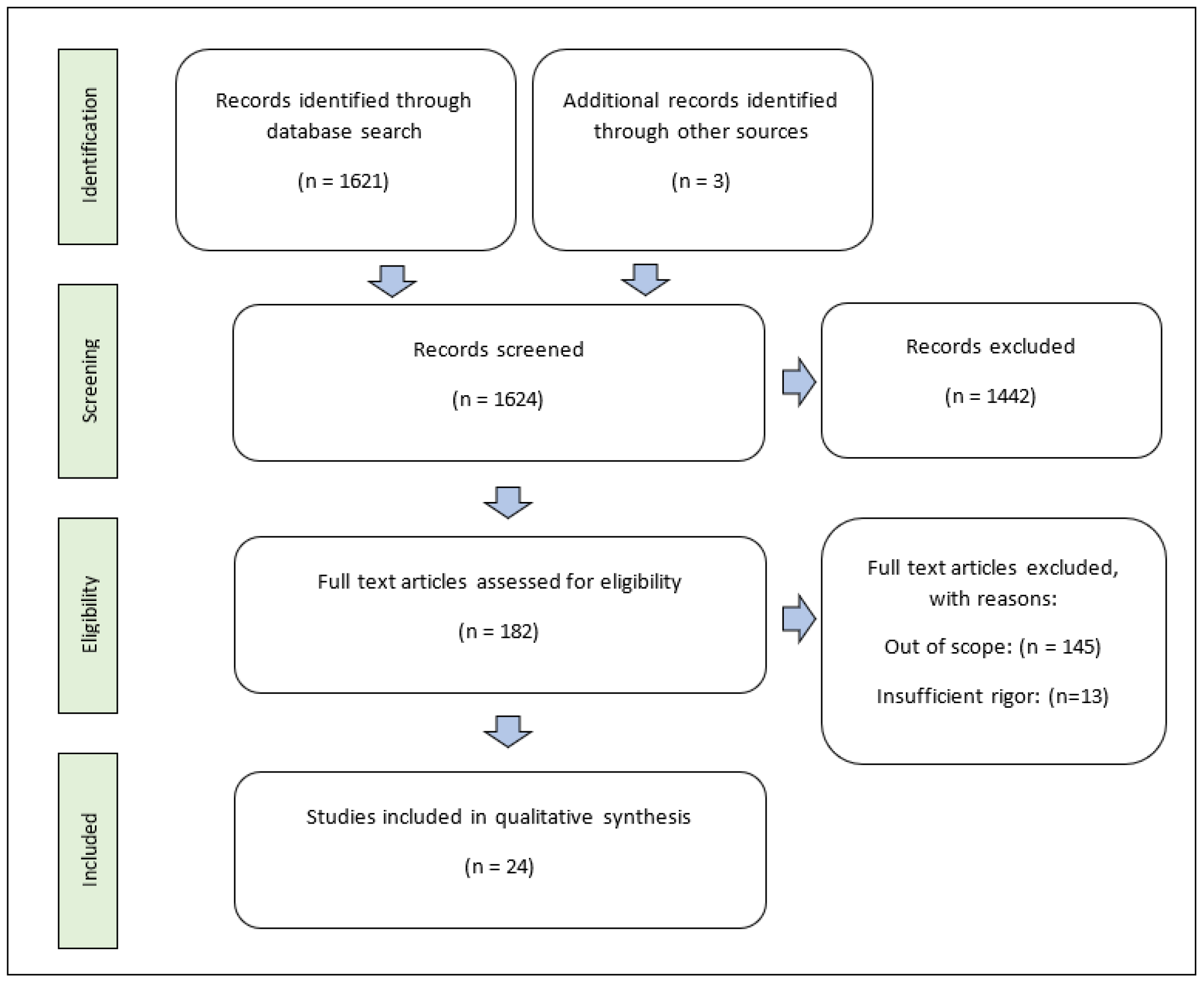
2. Methods
3. Results and Discussion
3.1. Exogenous Clot Retraction Modulators
3.2. Endogenous Modulators
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nurden, A.T. Molecular basis of clot retraction and its role in wound healing. Thromb. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Akkaya, S.; Preuss, M.; Rademacher, F.; Tohidnezhad, M.; Kubo, Y.; Behrendt, P.; Weitkamp, J.T.; Wedel, T.; Lucius, R.; et al. Platelet-Released Growth Factors Influence Wound Healing-Associated Genes in Human Keratinocytes and Ex Vivo Skin Explants. Int. J. Mol. Sci. 2022, 23, 2827. [Google Scholar] [CrossRef]
- Samson, A.L.; Alwis, I.; Maclean, J.A.A.; Priyananda, P.; Hawkett, B.; Schoenwaelder, S.M.; Jackson, S.P. Endogenous fibrinolysis facilitates clot retraction in vivo. Blood 2017, 130, 2453–2462. [Google Scholar] [CrossRef]
- Jansen, E.E.; Hartmann, M. Clot Retraction: Cellular Mechanisms and Inhibitors, Measuring Methods, and Clinical Implications. Biomedicines 2021, 9, 1064. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet integrin alphaIIbbeta3: Signal transduction, regulation, and its therapeutic targeting. J. Hematol. Oncol. 2019, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.; Kim, N.; Lee, H.; Woo, E.R.; Kim, Y.C.; Oh, H.; Lee, D.S. Anti-Inflammatory Effects of Compounds from Cudrania tricuspidata in HaCaT Human Keratinocytes. Int. J. Mol. Sci. 2021, 22, 7472. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Yang, J.H.; Seo, K.H.; Shin, S.M.; Park, E.Y.; Cho, S.S.; Jo, G.U.; Eo, J.H.; Park, J.S.; Oh, D.S.; et al. Cudrania Tricuspidata Extract and Its Major Constituents Inhibit Oxidative Stress-Induced Liver Injury. J. Med. Food 2019, 22, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, Z.; Jiang, X.; Sun, J.; Ran, G.; Yang, X.; Zhao, Y.; Yan, Y.; Chen, Z.; Tian, L.; et al. Bioactive compounds from Cudrania tricuspidata: A natural anticancer source. Crit. Rev. Food Sci. Nutr. 2020, 60, 494–514. [Google Scholar] [CrossRef]
- Oh, N.S.; Lee, J.Y.; Kim, Y.T.; Kim, S.H.; Lee, J.H. Cancer-protective effect of a synbiotic combination between Lactobacillus gasseri 505 and a Cudrania tricuspidata leaf extract on colitis-associated colorectal cancer. Gut Microbes 2020, 12, 1785803. [Google Scholar] [CrossRef]
- Oh, J.K.; Amoranto MB, C.; Oh, N.S.; Kim, S.; Lee, J.Y.; Oh, Y.N.; Shin, Y.K.; Yoon, Y.; Kang, D.K. Synergistic effect of Lactobacillus gasseri and Cudrania tricuspidata on the modulation of body weight and gut microbiota structure in diet-induced obese mice. Appl. Microbiol. Biotechnol. 2020, 104, 6273–6285. [Google Scholar] [CrossRef]
- Jo, Y.H.; Kim, S.B.; Ahn, J.H.; Turk, A.; Kwon, E.B.; Kim, M.O.; Hwang, B.Y.; Lee, M.K. Xanthones from the stems of Cudrania tricuspidata and their inhibitory effects on pancreatic lipase and fat accumulation. Bioorg. Chem. 2019, 92, 103234. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, M.K.; Yeo, S.H.; Kim, S. Short-term Cudrania tricuspidata fruit vinegar administration attenuates obesity in high-fat diet-fed mice by improving fat accumulation and metabolic parameters. Sci. Rep. 2020, 10, 21102. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Irfan, M.; Rhee, M.H.; Kwon, H.W. Derrone Inhibits Platelet Aggregation, Granule Secretion, Thromboxane A(2) Generation, and Clot Retraction: An In Vitro Study. Evid. Based Complement. Altern. Med. 2021, 2021, 8855980. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Irfan, M.; Rhee, M.H.; Kwon, H.W. Antiplatelet efect of cudraxanthone B is related to inhibition of calcium mobilization, αIIbβ3 activation, and clot retraction. Appl. Biol. Chem. 2021, 64, 4. [Google Scholar] [CrossRef]
- Kwon, H.-W.; Irfan, M.; Lee, Y.Y.; Rhee, M.H.; Shin, J.-H. Artocarpesin acts on human platelet aggregation through inhibition of cyclic nucleotides and MAPKs. Appl. Biol. Chem. 2022, 65, 22. [Google Scholar] [CrossRef]
- Nam, G.S.; Lee, K.S.; Nam, K.S. Morin hydrate inhibits platelet activation and clot retraction by regulating integrin alpha(IIb)beta(3), TXA(2), and cAMP levels. Eur. J. Pharmacol. 2019, 865, 172734. [Google Scholar] [CrossRef]
- Nam, G.S.; Nam, K.S. Arctigenin attenuates platelet activation and clot retraction by regulation of thromboxane A(2) synthesis and cAMP pathway. Biomed. Pharm. 2020, 130, 110535. [Google Scholar] [CrossRef]
- He, Y.; Fan, Q.; Cai, T.; Huang, W.; Xie, X.; Wen, Y.; Shi, Z. Molecular mechanisms of the action of Arctigenin in cancer. Biomed. Pharm. 2018, 108, 403–407. [Google Scholar] [CrossRef]
- Shabgah, A.G.; Suksatan, W.; Achmad, M.H.; Bokov, D.O.; Abdelbasset, W.K.; Ezzatifar, F.; Hemmati, S.; Mohammadi, H.; Soleimani, D.; Jadidi-Niaragh, F.; et al. Arctigenin, an anti-tumor agent; a cutting-edge topic and up-to-the-minute approach in cancer treatment. Eur. J. Pharmacol. 2021, 909, 174419. [Google Scholar] [CrossRef]
- Guo, S.; Chen, Y.; Shi, S.; Wang, X.; Zhang, H.; Zhan, Y.; An, H. Arctigenin, a novel TMEM16A inhibitor for lung adenocarcinoma therapy. Pharmacol. Res. 2020, 155, 104721. [Google Scholar] [CrossRef]
- Zhong, Y.; Lee, K.; Deng, Y.; Ma, Y.; Chen, Y.; Li, X.; Wei, C.; Yang, S.; Wang, T.; Wong, N.J.; et al. Arctigenin attenuates diabetic kidney disease through the activation of PP2A in podocytes. Nat. Commun. 2019, 10, 4523. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.A.; Mohamadin, A.M.; Abdel-Bakky, M.S. Arctigenin alleviates cadmium-induced nephrotoxicity: Targeting endoplasmic reticulum stress, Nrf2 signaling, and the associated inflammatory response. Life Sci. 2021, 287, 120121. [Google Scholar] [CrossRef]
- Xu, X.; Piao, H.N.; Aosai, F.; Zeng, X.Y.; Cheng, J.H.; Cui, Y.X.; Li, J.; Ma, J.; Piao, H.R.; Jin, X.; et al. Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-kappaB and TNF-alpha/TNFR1/NF-kappaB pathways. Br. J. Pharmacol. 2020, 177, 5224–5245. [Google Scholar] [CrossRef]
- Nam, G.S.; Park, H.J.; Nam, K.S. The antithrombotic effect of caffeic acid is associated with a cAMP-dependent pathway and clot retraction in human platelets. Thromb. Res. 2020, 195, 87–94. [Google Scholar] [CrossRef]
- Busbee, P.B.; Menzel, L.; Alrafas, H.R.; Dopkins, N.; Becker, W.; Miranda, K.; Tang, C.; Chatterjee, S.; Singh, U.; Nagarkatti, M.; et al. Indole-3-carbinol prevents colitis and associated microbial dysbiosis in an IL-22-dependent manner. JCI Insight 2020, 5, e127551. [Google Scholar] [CrossRef]
- Munakarmi, S.; Chand, L.; Shin, H.B.; Jang, K.Y.; Jeong, Y.J. Indole-3-Carbinol Derivative DIM Mitigates Carbon Tetrachloride-Induced Acute Liver Injury in Mice by Inhibiting Inflammatory Response, Apoptosis and Regulating Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 2048. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Nisani, S.; Chamovitz, D.A. Indole-3-carbinol: A plant hormone combatting cancer. F1000Research 2018, 7, 689. [Google Scholar] [CrossRef]
- Wu, Y.; Li, R.W.; Huang, H.; Fletcher, A.; Yu, L.; Pham, Q.; Yu, L.; He, Q.; Wang, T.T.Y. Inhibition of Tumor Growth by Dietary Indole-3-Carbinol in a Prostate Cancer Xenograft Model May Be Associated with Disrupted Gut Microbial Interactions. Nutrients 2019, 11, 467. [Google Scholar] [CrossRef]
- Lim, H.M.; Park, S.H.; Nam, M.J. Induction of apoptosis in indole-3-carbinol-treated lung cancer H1299 cells via ROS level elevation. Hum. Exp. Toxicol. 2021, 40, 812–825. [Google Scholar] [CrossRef]
- Tamer, F.; Tullemans, B.M.E.; Kuijpers, M.J.E.; Claushuis, T.A.M.; Heemskerk, J.W.M. Nutrition Phytochemicals Affecting Platelet Signaling and Responsiveness: Implications for Thrombosis and Hemostasis. Thromb. Haemost. 2022, 122, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, P.; Chauhan, G.; Gautam, D.; Dash, D.; Patne, S.C.U.; Krishnamurthy, S. Indole-3-carbinol improves neurobehavioral symptoms in a cerebral ischemic stroke model. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 613–625. [Google Scholar] [CrossRef]
- Ramakrishna, K.; Singh, N.; Krishnamurthy, S. Diindolylmethane ameliorates platelet aggregation and thrombosis: In silico, in vitro, and in vivo studies. Eur. J. Pharmacol. 2022, 919, 174812. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.W. Inhibitory Effects of Ginsenoside Ro on Clot Retraction through Suppressing PI3K/Akt Signaling Pathway in Human Platelets. Prev. Nutr. Food Sci. 2019, 24, 56–63. [Google Scholar] [CrossRef]
- Zhang, N.; Prasad, S.; Huyghues Despointes, C.E.; Young, J.; Kima, P.E. Leishmania parasitophorous vacuole membranes display phosphoinositides that create conditions for continuous Akt activation and a target for miltefosine in Leishmania infections. Cell Microbiol. 2018, 20, e12889. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.M.; Pinto, T.S.; da Costa Fernandes, C.J.; da Silva, R.A.; Zambuzzi, W.F. Wortmannin targeting phosphatidylinositol 3-kinase suppresses angiogenic factors in shear-stressed endothelial cells. J. Cell Physiol. 2020, 235, 5256–5269. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wei, G.; Wang, X.; Xu, X.; Ju, W.; Li, Z.; Gardiner, E.E.; Andrews, R.K.; Zeng, L.; Xu, K.; et al. All-Trans Retinoic Acid Impairs Platelet Function and Thrombus Formation and Inhibits Protein Kinase CssI/delta Phosphorylation. Thromb. Haemost. 2019, 119, 1655–1664. [Google Scholar] [CrossRef]
- Vilahur, G.; Gutierrez, M.; Arzanauskaite, M.; Mendieta, G.; Ben-Aicha, S.; Badimon, L. Intracellular platelet signalling as a target for drug development. Vasc. Pharmacol. 2018, 111, 22–25. [Google Scholar] [CrossRef]
- Beke Debreceni, I.; Mezei, G.; Batar, P.; Illes, A.; Kappelmayer, J. Dasatinib Inhibits Procoagulant and Clot Retracting Activities of Human Platelets. Int. J. Mol. Sci. 2019, 20, 5430. [Google Scholar] [CrossRef]
- Cheng, F.; Xu, Q.; Li, Q.; Cui, Z.; Li, W.; Zeng, F. Adverse reactions after treatment with dasatinib in chronic myeloid leukemia: Characteristics, potential mechanisms, and clinical management strategies. Front. Oncol. 2023, 13, 1113462. [Google Scholar] [CrossRef]
- Nam, G.S.; Kim, S.; Kwon, Y.S.; Kim, M.K.; Nam, K.S. A new function for MAP4K4 inhibitors during platelet aggregation and platelet-mediated clot retraction. Biochem. Pharmacol. 2021, 188, 114519. [Google Scholar] [CrossRef]
- Okoroiwu, I.L.; Obeagu, E.I.; Obeagu, G.U. Determination of Clot Retraction in Pregnant Women Attending Antenatal Clinc in Federal Medical Centre. Madonna Univ. J. Med. Health Sci. 2022, 2, 91–97. [Google Scholar]
- Tutwiler, V.; Peshkova, A.D.; Le Minh, G.; Zaitsev, S.; Litvinov, R.I.; Cines, D.B.; Weisel, J.W. Blood clot contraction differentially modulates internal and external fibrinolysis. J. Thromb. Haemost. 2019, 17, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Misztal, T.; Golaszewska, A.; Tomasiak-Lozowska, M.M.; Iwanicka, M.; Marcinczyk, N.; Leszczynska, A.; Chabielska, E.; Rusak, T. The myeloperoxidase product, hypochlorous acid, reduces thrombus formation under flow and attenuates clot retraction and fibrinolysis in human blood. Free Radic. Biol. Med. 2019, 141, 426–437. [Google Scholar] [CrossRef]
- Le Minh, G.; Peshkova, A.D.; Andrianova, I.A.; Sibgatullin, T.B.; Maksudova, A.N.; Weisel, J.W.; Litvinov, R.I. Impaired contraction of blood clots as a novel prothrombotic mechanism in systemic lupus erythematosus. Clin. Sci. 2018, 132, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Delgado Lagos, F.; Elgheznawy, A.; Kyselova, A.; Meyer Zu Heringdorf, D.; Ratiu, C.; Randriamboavonjy, V.; Mann, A.W.; Fisslthaler, B.; Siragusa, M.; Fleming, I. Secreted modular calcium-binding protein 1 binds and activates thrombin to account for platelet hyperreactivity in diabetes. Blood 2021, 137, 1641–1651. [Google Scholar] [CrossRef]
- Rodriguez, F.; Blum, M.R.; Falasinnu, T.; Hastings, K.G.; Hu, J.; Cullen, M.R.; Palaniappan, L.P. Diabetes-attributable mortality in the United States from 2003 to 2016 using a multiple-cause-of-death approach. Diabetes Res. Clin. Pr. 2019, 148, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Calzavarini, S.; Prince-Eladnani, R.; Saller, F.; Bologna, L.; Burnier, L.; Brisset, A.C.; Quarroz, C.; Reina Caro, M.D.; Ermolayev, V.; Matsumura, Y.; et al. Platelet protein S limits venous but not arterial thrombosis propensity by controlling coagulation in the thrombus. Blood 2020, 135, 1969–1982. [Google Scholar] [CrossRef]
- Brouns, S.L.N.; Tullemans, B.M.E.; Bulato, C.; Perrella, G.; Campello, E.; Spiezia, L.; van Geffen, J.P.; Kuijpers, M.J.E.; van Oerle, R.; Spronk, H.M.H.; et al. Protein C or Protein S deficiency associates with paradoxically impaired platelet-dependent thrombus and fibrin formation under flow. Res. Pr. Thromb. Haemost. 2022, 6, e12678. [Google Scholar] [CrossRef] [PubMed]
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality From Ischemic Heart Disease. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005375. [Google Scholar] [CrossRef]

| Origin | Compound | Increased | Decreased |
|---|---|---|---|
| Cudrania tricuspidata | derrone | p-VASP, p-IP3RI, cAMP, cGMP | p-cPLA2, p-p38, p-JNK, p-PI3K, Ca++, PltAgg, αIIbβIIIaff, TXA2, 5-HT |
| Cudraxanthone B | |||
| artocarpesin | |||
| morin hydrate | p-IP3RI, cAMP, cGMP | P-selectinex, ATP, p-PLCγ2, p-ERK, p-cPLA2, p-p38, p-JNK, p-I3K, Ca++, PltAgg, αIIbβIIIaff, TXA2, 5-HT | |
| Arctium lappa | arctigenin | p-VASP, p-IP3RI, cAMP | PltAgg, ATP, 5-HT, Ca++, αIIbβIIIaff, P-selectinex, TXA2, COX-1act |
| Many plants | caffeic acid | cAMP, p-VASP, p-IP3RI | |
| Cruciferous vegetables | diindolylmethane | GPVImod, PRY12mod, cAMP | ROS, TXB2, COX-1, PGE2 |
| Panax ginseng | Ginsenoside Ro | PltAgg, αIIbβIIIaff, | p-Akt, p-PI3K, |
| Synthetic | all-trans retinoic acid | P- PLCγ2, p-syk | |
| Synthetic | Dasatinib | p-Src, p-Fyn, p-Lyn, PSex, thrombin, αIIbβIIIex | |
| Synthetic | GNE 495 and PF06260933 | p-VASP, p-IP3RI, cAMP, p-PKAc | TXB2, ATP, 5-HT, αIIbβIIIaff, |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guilbeau, A.; Majumder, R. Systemic Review of Clot Retraction Modulators. Int. J. Mol. Sci. 2023, 24, 10602. https://doi.org/10.3390/ijms241310602
Guilbeau A, Majumder R. Systemic Review of Clot Retraction Modulators. International Journal of Molecular Sciences. 2023; 24(13):10602. https://doi.org/10.3390/ijms241310602
Chicago/Turabian StyleGuilbeau, Alaina, and Rinku Majumder. 2023. "Systemic Review of Clot Retraction Modulators" International Journal of Molecular Sciences 24, no. 13: 10602. https://doi.org/10.3390/ijms241310602
APA StyleGuilbeau, A., & Majumder, R. (2023). Systemic Review of Clot Retraction Modulators. International Journal of Molecular Sciences, 24(13), 10602. https://doi.org/10.3390/ijms241310602






