Abstract
The risk of losing a transplanted organ is high, and non-invasive markers to warn of this phenomenon are still being sought. We investigated the impact of post-transplant microchimerism on the function of the transplanted kidney. The study included 100 kidney transplant recipients, mostly women. All transplanted organs were from opposite-sex deceased donors. Microchimerism was assessed using multiplex PCR. Male DNA was detected in all urine samples from female recipients and in 13/56 blood samples from female kidney recipients. Female DNA was found in 31/44 urine samples from male recipients, but in none of the blood samples. Microchimerism in the urine of female recipients correlated positively with blood urea (Rs = 0.45; p = 5.84 × 10−4) and K+ ions (Rs = 0.29; p = 0.03), while microchimerism in the blood of female recipients also correlated positively with blood urea (Rs = 0. 28; p = 0.04), cystatin C (Rs = 0.31; p = 0.02) and the number of incompatible HLA alleles (Rs = 0.42; p = 0.01). A history of DGF was associated with higher urinary donor DNA concentrations in female recipients.: Post-transplant microchimerism may serve as a potential marker of chronic kidney rejection.
1. Introduction
Kidney transplantation is widely recognized as the most effective treatment for chronic kidney failure, offering not only the highest survival rate compared with other treatments, but also the highest level of patient comfort [1,2]. In Poland, the number of kidney transplants performed in 2021 was 753 [3], with the annual survival rate of patients after kidney transplantation fluctuating around 95%
Many years of experience of specialists and continuous intensive development of knowledge of possible immunosuppressive treatment methods result in a yearly decrease in the number of acute rejection episodes and an increase in the number of functioning organs in the first post-transplantation period. Unfortunately, modern transplantation medicine is still struggling with the problem of chronic rejection, which leads to dysfunction and ultimately organ loss [4]. According to available data, 90–95% of kidneys function properly after the first year following transplantation, and after 10 years, only one in two living donor kidneys and one in three deceased donor kidneys are still functioning [5]. As a result, researchers are constantly looking for ways to slow or stop chronic graft rejection. At the same time, the search is on for non-invasive markers that would warn of this process and possibly illustrate its dynamics. One such marker could be quantified post-transplant microchimerism.
Microchimerism refers to the coexistence of two genetically distinct populations in an organism, one of which is present in very small numbers. The simplest mechanism for this phenomenon in the human body is the mixing of blood from two organisms, the best example being maternal–fetal microchimerism. A woman has been shown to have cells from previous pregnancies in addition to her own genetic material [6,7,8]. Men, on the other hand, retain traces of maternal blood in their bodies [6,7,8]. Another source of microchimerism may be hematopoietic cell and organ transplants and transfusions of blood products [9].
Microchimerism appears to be particularly important in the context of transplantation. Donor DNA in the recipient’s organism originates from damaged cells of the transplanted organ. The source of damage can be the cytotoxic effects of immunosuppressive drugs, acute infection and the process of acute or chronic rejection. DNA from dying cells is broken down into nucleosomes and oligomers and then mostly recycled. However, some DNA does not undergo phagocytosis and ends up in the blood. Importantly, the amount of DNA in circulation correlates with the number of dying cells. The renal barrier partially transmits polymeric DNA from apoptotic cells in sufficient quantities to be detected by genetic analysis techniques such as PCR and hybridization [10]. The natural source of kidney donor cells is the sediment from the recipient’s urine. Damaged cells or cells that die in the process of apoptosis are ‘swept up’ by the urine that forms and is excreted from the body.
The role of microchimerism in the recipient’s organism remains controversial, as scientists are still unable to agree whether it contributes to immunological tolerance or activates the process of chronic rejection. However, a clear answer to this question could make the quantification of microchimerism a useful marker for assessing the success and risks of transplantation. This could lead to the replacement of an invasive procedure such as organ biopsy with a non-invasive and patient-friendly test. The present study is an attempt to determine the impact of microchimerism on the function of the transplanted kidney based on biochemical analytes measured at the time of microchimerism detection. In addition, these indices were evaluated in relation to the state of the recipient’s immune system by determining selected immunocompetent cells in the blood: T lymphocytes—CD8+ and CD4+, NK cells, B lymphocytes, monocytes and CD4+ CD25+ regulatory cells. To exclude the possible influence of the presence of maternal–fetal microchimerism and the patient’s previous blood transfusion on the results obtained, a comparison was made of the microchimerism levels in recipients with and without PRBC and in women with and without male offspring.
2. Results
2.1. Characteristics of the Study Group
The study group included 100 patients aged 20–78 years with a mean age of 49.91 years, 56% of whom were women (n = 56). The mean follow-up was 1817 days after surgery. Body mass index ranged from 18 to 44 kg/m2 (mean 26.41). Detailed characteristics of the study group are shown in Table 1.

Table 1.
Clinical characteristics of studied patients.
The main causes of renal failure in the study group were glomerulonephritis (36.63%), ADPKD (12.87%), reflux (11.88%), arterial hypertension (10.89%), diabetes (7.92%), IGA nephropathy (1.98%) and urolithiasis (1.98%). Other causes were found in 15.84% of patients.
2.2. Microchimerism in Blood and Urine of Female Kidney Recipients from Male Donors
2.2.1. Presence of Male DNA in Female Urine
The presence of male DNA was detected in all urine samples from female kidney recipients. A sample plot is shown in Figure 1. The highest concentration was 6.061994 ng/μL, and the lowest was 0.001752 ng/μL.
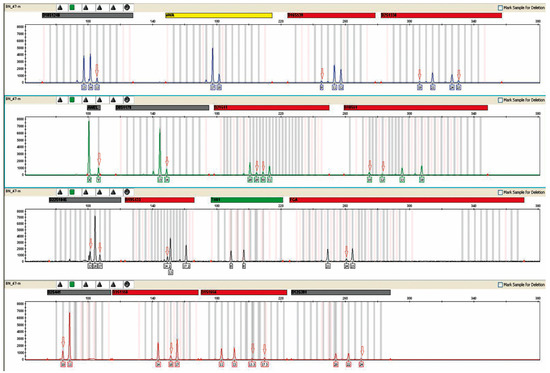
Figure 1.
Presence of male DNA in the urine of patient 16. Result obtained after a multiplex PCR reaction performed with an AmpFlSTR® NGM™ PCR Amplification Kit from Applied Biosystems (Waltham, MA, USA). A mixed genetic profile obtained from the urine of the patient under study is shown. A mixture with male DNA is visible, as evidenced by the appearance of the male allele in the amelogenin marker (AMEL). Red arrows indicate alleles derived from donor DNA. The concentration of male DNA in this case was 0.153803 ng/μL. The markers of the systems shown above were labeled with different fluorescent dyes during the course of the multiplex PCR reaction, which translates into different colored peaks corresponding to the alleles.
2.2.2. Presence of Male DNA in Women’s Blood
The presence of male DNA was detected in 13/56 blood samples from female kidney recipients. The highest concentration was 0.037444 ng/μL, and the lowest was 0.00173 ng/μL. The sample result is shown in Figure 2.
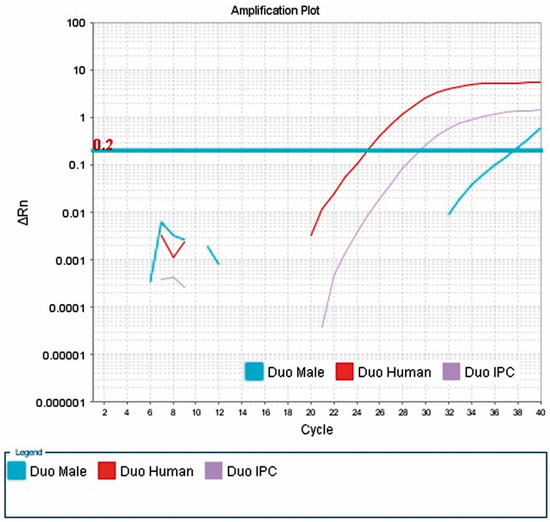
Figure 2.
Presence of male DNA in the blood of patient 79. Result of a real-time PCR reaction performed with a Quantifiler® Duo DNA Quantification Kit from Applied Biosystems. The presence of male DNA in the patient’s blood is shown. The Y-chromosome-specific marker used in this kit, multiplying in real time, allows the concentration of male DNA to be determined (Duo Male curve). The X-axis shows successive cycles of the PCR reaction and the Y-axis shows ΔRn, which determines the magnitude of the signal generated by a given set of PCR reaction components. Three curves can be seen: (1) Duo Male (amplification curve of the marker for the SRY gene, located on chromosome Y at position 11.3, for which the probe was labeled with FAM™ dye); (2) Duo Human (amplification curve of the marker for human DNA, i.e., (2) Duo Human (amplification curve of a marker for human DNA, i.e., the 140 bp RPPH1 gene located on the long arm of chromosome 14 at position 11.2, for which the probe was labeled with VIC dye); (3) Duo IPC (amplification curve for the internal IPC standard of 130 bp, for which the probe was labeled with NED™ dye). The threshold line is shown in blue. It is placed above the non-specific deviation ΔRn, within the exponential increase. The copy threshold (CT) number, which is the cycle number at which the amplification curve crosses the threshold line (detectable increase in fluorescence), was determined for the samples.
2.3. Microchimerism in Blood and Urine of Male Kidney Recipients from Female Donors
The presence of female DNA was found in 31/44 urine samples from male recipients. The highest concentration was 80–90%, and the lowest was 20–30% (Figure 3).
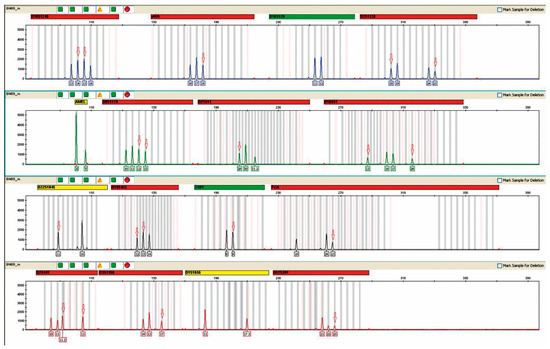
Figure 3.
Microchimerism in the urine of patient #74, a recipient of a female donor kidney. The result was obtained after the multiplex PCR reaction performed in the above figure using an AmpFlSTR® NGM™ PCR Amplification Kit from Applied Biosystems. The mixed genetic profile obtained from the urine of the study patient is shown. Red arrows indicate alleles derived from donor DNA. The percentage of female DNA was 80–90%.
In men’s blood, the presence of female DNA was not detected in any case by the available test methodology. A representative example of the analysis is shown in Figure 4.
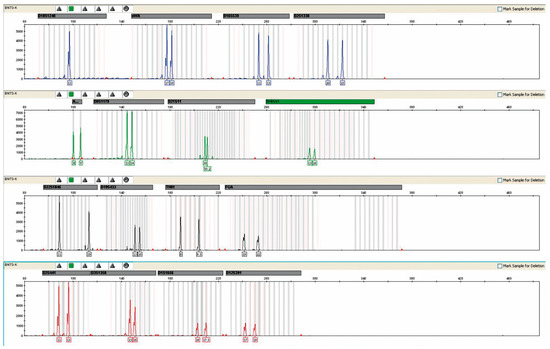
Figure 4.
The result of the genetic profile obtained from the blood of patient No. 42, without the presence of foreign DNA. Result obtained after a multiplex PCR reaction.
2.4. Associations between Microchimerism Present in Blood and Urine of Kidney Recipients and Selected Parameters
Microchimerism in the urine of female recipients correlated positively with urea (Rs = 0.45; p = 5.84 × 10−4) and K+ ions (Rs = 0.29; p = 0.03), while microchimerism in the blood of female recipients also correlated positively with urea (Rs = 0. 28; p = 0.04) and cystatin C (Rs = 0.31; p = 0.02), the number of CD34+CD133+ cells (Rs = 0.26; p = 0.06; a statistical tendency) and the number of incompatible HLA alleles (Rs = 0.42; p = 0.01). Detailed data are shown in Table 2.

Table 2.
Correlations of microchimerism with some parameters.
If there was a history of DGF, there were higher concentrations of donor DNA in the urine of both female recipients and male recipients (p = 0.041 and p = 0.099) than that in recipients with IGF. Data are presented in Table 3.

Table 3.
Comparison of the presence of microchimerism depending on immediate (IGF) or delayed graft function (DGF).
2.5. Comparison of Microchimerism Levels in Women with Male Offspring and Microchimerism in Women without Male Offspring
The study found that having a male offspring did not affect the level of microchimerism in female recipients. In addition, to exclude the possible influence of the presence of maternal–fetal microchimerism on the results obtained, 651 women whose blood was used for paternity testing were analyzed as a control group. The study followed an identical methodology (PCR-STR) (Figure 5). No maternal–fetal microchimerism was detected in any of the samples tested. The data are presented in Table 4.
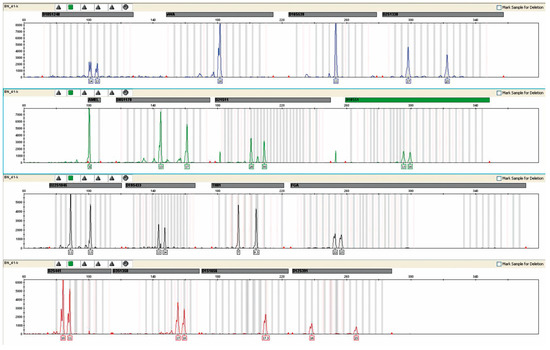
Figure 5.
Assessment of maternal–fetal chimerism exemplified by the female profile in one case of disputed paternity showing the maternal profile without any contribution of fetal (male) DNA.

Table 4.
Comparison of microchimerism depending on having a male offspring.
2.6. Comparison of Microchimerism Levels in Recipients after a History of Red Cell Concentrate Transfusions and Recipients without Transfusions
A history of previous blood transfusions had no effect on microchimerism levels (Table 5, Figure 5).

Table 5.
Comparison of microchimerism depending on past PRBC transfusions.
3. Discussion
A transplanted kidney functioning in the recipient’s body is undoubtedly a phenomenon of modern transplant medicine. Unfortunately, the process of chronic rejection, which often leads to organ loss, remains a problem. The search is still on for markers that herald this process and give a picture of its dynamic changes. In our study involving a group of 100 patients who underwent kidney transplantation at different time points (from 19 days to more than 10 years), quantitative levels of donor DNA were measured in the blood and urine of the recipients. The function of the transplanted kidney was assessed using cystatin C. In addition, the study analyzed the immune status of the recipients by assessing selected immunocompetent cells, such as CD4+ CD25+ regulatory lymphocytes, CD8+ and CD4+ T cells, NK cells, B lymphocytes and monocytes, and their association with the presence of microchimerism. Cystatin C levels in the study group were measured during routine outpatient follow-up, during simultaneous blood and urine sampling for microchimerism and immunocompetent cell analysis. The key parameter analyzed in the study was the level of post-transplant microchimerism in the blood and urine of the recipients. The study required material from patients who had received an organ from a donor of the opposite sex. The selected methods of microchimerism analysis, which are standard in forensic medicine, allowed the differentiation of the study material of different sexes. Using the same tools for single-sex transplants would not have been as conclusive.
The presence of male DNA was detected in all urine samples from female recipients and in 13/56 blood samples from these patients. The positive results for urine were predictable because the transplanted kidney is an organ that is also genetically foreign to the recipient. The urine must therefore contain fragments of the shed epithelium and, therefore, the donor’s cells and DNA. The presence of male DNA in the blood of female patients is a phenomenon similar to that in urine, but much less intense and less detectable. The presence of donor DNA in the recipient’s bloodstream is associated with the ‘scavenging’ of cells from the transplanted organ as the blood flows through the capillaries of the kidney. In women in whom no microchimerism was detected in the blood, its presence cannot be ruled out, but due to its very low level it could not be detected with the techniques used. The presence of female DNA was detected in 31/34 male urine samples. The presence of female DNA was not detected in any male blood sample using the methodology used. In the case of urine, the results obtained can be explained via analogy with those obtained for female recipients. On the other hand, the presence of female DNA in male blood using the molecular techniques used did not give the desired results due to their imperfection. This does not mean that the phenomenon of microchimerism does not exist; it is due to the limitations of the research method and its inability to only be applied to female genetic material.
Many researchers believe that a weakness in the use of microchimerism as a marker of the immune response to a transplanted organ is the described phenomenon of maternal–fetal or embryonic microchimerism. Fetal cells pass through the placenta into the mother’s bloodstream during pregnancy and can eventually take up residence in many tissues [11]. Each pregnancy is a source of fetal cells that migrate into the mother’s body, with multiple pregnancies being the source of a many times larger pool of fetal cells due to the greater placental flow compared with single pregnancies [12,13,14]. On the other hand, there is a bidirectional transfer of fetal and maternal cells during pregnancy, so maternal cells can also be found in the adult offspring [15,16]. Fetal microchimerism is detected in the mother’s body even decades after pregnancy [17,18,19]. Studies suggest that the occurrence of this phenomenon in both mother and child can trigger the development of many autoimmune diseases [20,21]. The presence of maternal–fetal microchimerism and its impact on the development of autoimmune disease remains unclear. It may contribute to the hyperreactivity of the immune system, but it is not known whether it is itself a consequence of autoimmune disease [11].
The donor DNA in the recipient’s blood and urine, determined in this study using PCR-STR methodology, was free of a ‘background’ of maternal–fetal microchimerism. To confirm this, the results of 651 women whose blood was used for paternity testing and who were also mothers of a male offspring were analyzed as a control group. The presence of maternal–fetal microchimerism was not detected in any of the samples tested. In addition, microchimerism levels were compared between women with a male offspring (21/56) and those without a male offspring (35/56). The study clearly showed that the presence of a male offspring had no effect on the microchimerism levels measured in the blood and urine of the recipient women.
Microchimerism also occurs in the body as a result of transfusions of blood products [22]. The sensitive STR method allows detection of 1% of donor cells after transfusion. This raises the question of how long donor cells remain in the recipient’s body after transfusion. A study by Lee et al. found that up to 99.9% of leukocytes are cleared within two days of transfusion, with the cell pool increasing over the course of a week before being completely cleared [23]. According to Busch’s study, no donor lymphocytes were found in the recipient 14 days after transfusion. It is generally believed that donor leukocytes have the ability to expand in the recipient’s body, but their survival is usually short and does not exceed one week [24]. In the study group, 19/100 patients had received a blood transfusion more than three months before the study. Patients with a history of PRBC transfusion were compared with a group of patients without transfusion, taking into account microchimerism levels in blood and urine. No statistically significant differences were found between the two groups. By excluding the presence of maternal microchimerism in female recipients and post-transfusion microchimerism in recipients with a history of transfusions, post-transplant microchimerism was objectively determined in the blood and urine of recipient patients.
Statistical analysis of the results obtained in our study showed a clear positive correlation between cystatin C, urea concentrations and the level of microchimerism in the blood of female recipients, but not males. Such correlations were, however, not found in urine. On the other hand, microchimerism in urine correlated positively with urea and potassium concentrations. Among these variables, cystatin is the strongest one linked to graft function [25]. However, others, like urea [26] and potassium [27], might be indirectly linked to renal failure as well [28]. These results might indicate that deterioration of transplanted kidney function is associated with an increase in post-transplant microchimerism levels. Notably, one should remember that the correlations were only found in a small subgroup of samples analyzed (13/56 female recipients) and need further research to prove their validity.
In our study, a positive correlation was also observed between the degree of microchimerism in female blood and the degree of HLA incompatibility between donor and recipient. This phenomenon is theoretically consistent with existing immunological knowledge stating that HLA matching matters in organ transplantation procedures [29,30,31]. The degree of HLA incompatibility between donor and recipient is one of the main factors determining the immunogenicity of an allograft, especially in the context of direct presentation. A higher number of incompatible antigens in the donor results in a more intense response from the recipient’s immune system. This should be associated with a greater recruitment of CD4+ cells with a helper lymphocyte phenotype, followed by induction of CD8+ cytotoxic lymphocyte function. In this context, microchimerism could result from the release of DNA from transplanted cells following cytotoxic reactions.
In our study, we also compared urine microchimerism in patients with IGF and patients with DGF. A history of DGF was associated with higher levels of donor DNA in the urine of female recipients only. In case of men, a statistical tendency was found. We assume that a higher number of tested patients would definitely explain whether such linkage is present. Gender differences impact aspects of kidney care [32]; however, it does not seem possible that it also applies to the detection of DGF, thus its link with microchimerism. Further studies on this aspect should be taken. Nevertheless, the most common cause of DGF is acute renal tubular necrosis. The most common factors for its occurrence are prolonged cold ischemia time, older age and recipient hypotension [33,34,35,36,37,38,39]. In their study, Bai et al. showed a positive correlation between microchimerism levels and prolonged cold ischemia time, the most common cause of DGF [40]. The cold ischemia phenomenon is associated with the reperfusion process, during which a variety of substances are rapidly washed out of the transplanted organ, including DNA and large amounts of proteins, including antigens recognized by Toll-like receptors (TLRs) present on both transplanted kidney cells and donor and recipient APCs. Reperfusion itself, together with the subsequent local induction of the inflammatory response and the subsequent release of APC-mediated acquired response mechanisms, is likely to account for the association of cold ischemia time with increased microchimerism in the context of DGF. These data suggest that a higher degree of HLA incompatibility between donor and recipient or a history of DGF leads to an increase in microchimerism in the recipient. However, a full explanation of the relationship between the degree of HLA incompatibility and the degree of microchimerism requires further studies in a more homogeneous group of recipients.
The current literature is conflicting on the relationship between the renal rejection process and microchimerism. Some studies suggest a positive role for microchimerism in the recipient, suggesting that it contributes to immune tolerance. Pujal et al. conducted a study in a group of 84 kidney transplant patients at 2, 6, 12 and 18 months after surgery. They found its presence in 56.2% of patients at 2 months and 30.1% at 12 months after surgery. The authors found a positive correlation between donor DNA and a lower incidence of acute rejection [41]. A study by El-Ansary of 20 patients who underwent living donor kidney transplantation showed that higher levels of donor cell microchimerism were associated with better kidney function up to 1 month after surgery and predicted improvement in kidney function at 1 and 3 years of follow-up. The authors concluded that donor cell microchimerism may contribute to a lower immune response and transplant acceptance, although this requires further research and a larger group of patients [42]. Similar results were found by Villari et al. in a 2020 study of 12 female kidney transplant recipients [43]. In the literature, there are also papers describing the occurrence of microchimerism after transplantation, in which it was not found to influence the rejection process of the transplanted organ, as in the studies by Fourtounas et al. and Suberbielle et al. [44,45].
In contrast, Lo et al. investigated the presence of donor DNA in the serum of recipients of 8 livers and 28 kidneys and demonstrated the presence of microchimerism in all liver recipients and in 82% of kidney recipients. They suggested that the source of microchimerism could be cells from the transplanted organ that had undergone apoptosis or ‘homing’ of donor hematopoietic cells, particularly in the liver. The researchers concluded that DNA quantification could be a marker of rejection, but it could also be triggered by infections (e.g., CMV), vascular or biliary complications or cancer developing in the transplanted organ [46]. The concept of a negative impact of donor microchimerism on the function of the transplanted kidney observed in the present study is also supported by reports from other researchers. In 2012, Curcio et al. published the results of a 2-year follow-up of 54 kidney transplant patients. The authors analyzed the recipients’ blood microchimerism levels using quantitative real-time PCR for HLA-DRB1. They correlated the results with the function of the transplanted organ. After 2 years, 38 patients had stable kidney function, while 16 patients showed clear signs of dysfunction. Donor DNA was detected in the serum of 75% of the patients with renal dysfunction. In patients with normal renal function, donor DNA was detected in only 11%. At the end of 2 years of follow-up, 18.1% of patients who had lost a kidney had a high level of microchimerism compared with 7.4% of patients who had this titer [47]. Zhang et al. hypothesized that donor DNA may be present in the recipient’s cell-free urine and that its levels may be associated with rejection of the transplanted kidney. They conducted a study of female recipients of a male donor kidney. The SRY gene on the Y chromosome and beta-globin were used as markers for male DNA. Real-time PCR was used for analysis. The SRY sequence was found in the urine of 82% of female recipients. Patients with acute rejection had a dramatic increase in β-globin DNA levels, which decreased rapidly after the immunosuppressive treatment was changed [48].
Schlitt et al. described the case of a liver transplant patient who developed acute rejection of the organ eight years after the procedure, and therefore underwent another liver transplant. Histopathology of the removed liver showed chronic rejection in addition to severe acute rejection. Before the second transplant, the patient received blood, a piece of skin and bone marrow and, during the procedure, lymph nodes from the small intestine. The presence of donor microchimerism from the first liver was then detected in the recipient’s liver, skin, mesenteric nodes and blood using oligonucleotide probes specific for donor and recipient DRB1 alleles. Recipient tissues were then collected 9 weeks after the second transplantation and the presence of the new microchimerism was detected. On this basis, Schlitt et al. concluded that microchimerism remains in constant equilibrium and its presence is not associated with normal function of the transplanted organ [49]. Gadi et al. analyzed the presence of microchimerism in 42 pancreas–kidney recipients and correlated it with the process of acute rejection confirmed via histopathological examination. They assessed microchimerism using real-time PCR. One week after transplantation, they detected donor DNA in 94% of patients. Patients were followed up for 5 years after transplantation. The amount of donor DNA was higher in patients with a history of organ rejection compared with a group of patients without rejection. After pharmacological intervention in the rejection process, the amount of donor DNA decreased significantly. Despite the results obtained, Gadi et al. concluded that the presence of donor DNA in the recipient’s serum did not correspond to the rejection process and cannot be used as a parameter of dysfunction. The presence of donor DNA can be part of an algorithm to determine the onset of rejection of the transplanted organ. In conclusion, the researchers found that assessing the level of donor DNA in the recipient’s body is effective in managing immunosuppression [50]. Summarizing the above research and as the highest proof in evidence-based medicine, a meta-analysis by Knight et al. [51] clearly proved that the presence of a donor-specific, cell-free DNA negatively correlated with a solid transplant function and might serve as marker of a graft injury. Our study has some limitations. The major one is that due to financial constraints, microchimerism testing was performed only once in each patient with different time post-surgery. This for sure influenced the results, as the time frame between time from transplantation (days) varied significantly. However, as elegantly demonstrated, a rapid fall in cfDNA content of a donor by approximately 10 days post-transplantation occurred and then tended to stabilize; of course, this had some variation for living vs. deceased donors and different solid organs transplanted [51]. Also, the study required material from patients who had received an organ from a donor of the opposite sex. The selected methods of microchimerism analysis, which are standard in forensic medicine, allowed the differentiation of opposite-sex study material. Applying the same tools to unisex transplants would not have been as conclusive. Also, the results of correlation analyses refer to a relatively small subgroup of patients (13/56 female recipients); thus, the data we obtained needs to be verified in further research. Indeed, a correlation (found in the present study) does not exactly mean causation.
Both positive and negative effects of post-transplant microchimerism on transplanted kidney function have been reported in the literature. The discrepancy in results is due to the lack of standardized research methods. Also, studies evaluating microchimerism used different fractions of blood to obtain DNA, for instance separated mononuclear cells or whole peripheral blood (similarly to our approach). This might result in different fractions of DNA isolated and, thus, analyzed of intact cell origin DNA vs. cell-free DNA. In summary, the results obtained by researchers, although so different, are not necessarily mutually exclusive. It is very likely that the presence of traces of donor DNA in the recipient of a transplanted organ promotes the phenomenon of immunological tolerance. Certain external conditions, such as a history of severe infections (especially CMV) and cytotoxic effects of drugs, disrupt immune homeostasis and the function of the transplanted kidney deteriorates. In this situation, an increase in microchimerism levels should be a signal to modify the current immunosuppressive treatment. Standardization of testing methods for donor DNA traces may contribute to the recognition of microchimerism testing as one of the markers of organ function risk.
4. Materials and Methods
4.1. Study Group
After approval by the Bioethics Committee of the Medical University of Pomerania in Szczecin (Resolution No. KB-0080/128/09), there were 100 kidney recipients aged 20–78 years (mean 49.91 years) from the Transplantation Outpatient Clinic of the Department of Nephrology, Transplantation and Internal Medicine, Independent Public Clinical Hospital no. 2 of the Pomeranian Medical University in Szczecin and the Outpatient Transplant Clinic of the Department of Nephrology and Kidney Transplantation with Dialysis Unit of the Independent Regional Public Hospital in Szczecin. All patients included in the study received detailed information about the study and gave written informed consent for the study to be conducted. All transplanted organs were from deceased donors. To be included in the study, patients had to have received a kidney from a donor of the opposite sex.
All recipients were further analyzed for body mass index (BMI), blood group, duration (in months) and type of dialysis, cause of renal failure, previous red blood cell (RBC) transfusions, occurrence of delayed graft function (DGF) after transplantation, occurrence of histopathologically confirmed acute rejection, postoperative immunosuppression (primary and current), average daily urine output (in liters), biochemical tests, baseline blood count and number of incompatible alleles in the HLA system between donor and recipient. All analyses were carried out according to the rules of the clinical laboratory.
HLA compatibility between recipients and their donors was calculated according to the current recommendations of the Polish allocation system. Transplants without mismatches were scored as follows: 2 points for each absence of mismatch at A loci, 5 points at B loci and 10 points at DR loci.
The study used the results of the genetic profiles of 651 women from paternity analyses to determine maternal–fetal microchimerism. The presence of donor DNA (intact) in the recipient’s blood and urine (post-transplant microchimerism) was analyzed by quantifying the ratio of donor to recipient DNA content.
4.2. Determination of Cystatin C
An immunonephelometric method was used for the determination of cystatin C. The analysis was performed on a Siemens BN ProSpec nephelometer. A Siemens N La-tex Cystatin C diagnostic test was used for the determination. The procedure was performed according to the manufacturer’s protocol.
4.3. Determination of Donor DNA in Blood and Urine of the Recipient
4.3.1. Cell-Free DNA Isolation
DNA was isolated from two types of biological material: whole peripheral blood and urine. A commercially available PrepFiler Forensic DNA Extraction Kit was used for isolation. The biological material was properly prepared prior to isolation. Urine was centrifuged and the precipitate collected on a sterile swab. Blood was applied to an FTA card, from which small sections (approximately 25 mm2) were cut after drying. Before starting the process, the magnetic beads provided were incubated at 37 °C for 10 min and then vortexed. Isolation was performed according to the manufacturer’s protocol [52].
4.3.2. DNA Amplification
The PCR reaction was performed on a GeneAmp® PCR System 9700 thermocycler [53], and an AmpFlSTR® NGM™ PCR Amplification Kit from Applied Biosystems [54] was used for the multiplex PCR reaction, amplifying 15 loci from 34 short tandem repeats (STRs): D10S1248, vWA, D16S539, D2S1338, D8S1179, D21S11, D18S51, D22S1045, D19S433, TH01, FGA, D2S441, D3S1358, D1S1656, D12S391 and a marker located on the sex chromosomes—amelogenin. The reaction mixture consisted of (per 25 µL sample)
- -
- 10 µL AmpFlSTR® NGM™ Master Mix;
- -
- 5 µL AmpFlSTR® NGM™ Primer Set;
- -
- 10 µL test DNA.
The positive control consisted of 10 µL AmpFlSTR® NGM™ Master Mix, 5 µL AmpFlSTR® NGM™ Primer Set and 10 µL control DNA, and the negative control consisted of 10 µL AmpFlSTR® NGM™ Master Mix, 5 µL AmpFlSTR® NGM™ Primer Set and 10 µL deionized water. The amplification program followed the manufacturer’s recommendations [55].
4.3.3. Capillary Electrophoresis
Samples were analyzed using a four capillary Applied Biosystems 3130 Genetic Analyzer [56]. GeneMapper® ID Software 3.1 and 3.2 was used to process the results [57]. After amplification, the DNA was separated electrophoretically in a 96-well plate. To create the reaction mixture, the following were used:
- 0.3 µL GS 500 LIZ® Size Standard;
- 8.7 µL Hi-Di™ Formamide;
- 1 µL of PCR product/allelic ladder.
The composition of a negative control included:
- 0.3 µL GS 500 LIZ® Size Standard;
- 8.7 µL Hi-Di™ Formamide [58].
4.3.4. Assessment of DNA Concentration
The microchimerism observed after the multiplex PCR reaction was further confirmed by assessing the concentration of male DNA in samples from recipient patients. This reaction, in addition to confirming the presence of foreign DNA, allowed a quantitative assessment of the microchimerism phenomenon.
The analysis was based on the real-time polymerase chain reaction method (real-time PCR). The reaction was performed using a Quantifiler® Duo DNA Quantification Kit from Applied Biosystems [59]. It allowed both the detection of total nuclear DNA concentration and the measurement of male DNA concentration. As an internal standard, a series of 8 dilutions of the concentration standards were prepared according to the manufacturer’s recommendations, with the following concentrations: 50, 16.7, 5.56, 1.85, 0.62, 0.21, 0.068, 0.023 ng/µL. The reaction mixture (for one sample) consisted of:
- 10.5 µL Quantifiler Duo Primer Mix;
- 12.5 µL Quantifiler Duo PCR Reaction Mix;
- 2 µL of test DNA.
A negative control was also performed. Samples were added to a 96-well plate according to the manufacturer’s recommended scheme and the reaction was performed on an Applied Biosystems 7500 RealTime PCR System machine [60]. Results were analyzed using HID RealTime PCR-Analysis Software v. 1.1 [61].
4.3.5. Quantitative Evaluation of Female DNA in The Urine of Male Recipients
Genetic profiles obtained after multiplex PCR reactions using an AmpFlSTR® NGM™ PCR Amplification Kit from Applied Biosystems were analyzed. Those with a mixed profile indicative of foreign DNA were evaluated. Evaluation was based on peak height measurements for the donor and recipient alleles using GeneMapper® software 3.1 and 3.2 [57] based on the ratio of donor to recipient peak heights read from electropherograms measured with relative fluorescence units (RFUs) in male patients who received a transplant from a female donor. Peak heights were used to estimate the percentage of donor DNA content in the recipient.
4.4. Immunocompetent and Stem Cell Research
Analysis of immunocompetent and stem cells was performed using flow cytometry according to the methodology described in the author’s previously published articles [62,63,64].
4.5. Statistical Analysis
Spearman’s rank correlation coefficient (Rs) was used to assess associations between tested variables of continuous type. Correlations with p < 0.05 were considered statistically significant. The t-test was used to assess the association between qualitative variables and the parameters tested. Calculations were carried out using Statistica 10.
5. Conclusions
- -
- Increased levels of post-transplant microchimerism in female graft recipients might correlate with deterioration of transplanted kidney function as expressed by cystatin C levels and HLA match and indirectly by potassium and urea.
- -
- A history of DGF leads to increased levels of microchimerism.
- -
- The degree of microchimerism in recipients might be considered as a new predictive/prognostic marker in renal transplantation.
Author Contributions
Data curation, J.S., M.K., K.T. and S.Z.; Formal analysis, J.S., M.K., W.C., A.P., K.T., K.K., S.Z., Z.C. and K.S.-Ż.; Funding acquisition, J.S.; Investigation, J.S., M.K., K.T., K.K. and Z.C.; Methodology, J.S.; Resources, J.S., M.K., W.C., A.P., K.T. and K.K.; Supervision, K.S.-Ż.; Validation, M.K., K.T. and K.K.; Writing—original draft, J.S.; Writing—review and editing, K.S.-Ż. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Ministry of Science and Higher Education No. N N403 143939.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Pomeranian Medical University in Szczecin (Resolution No. KB-0080/128/09).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are available upon request from the first author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hariharan, S.; Israni, A.K.; Danovitch, G. Long-Term Survival after Kidney Transplantation. N. Engl. J. Med. 2021, 385, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Podestà, M.A.; Sykes, M. Chimerism-Based Tolerance to Kidney Allografts in Humans: Novel Insights and Future Perspectives. Front. Immunol. 2022, 12, 791725. [Google Scholar] [CrossRef] [PubMed]
- Biuletyn Informacyjny Poltransplantu. Available online: https://www.poltransplant.org.pl/biuletyny.html (accessed on 18 June 2023).
- Krajewska, M.; Weyde, W.; Klinger, M. Tolerancja Immunologiczna Po Przeczepieniu Nerki. Post. Hig. 2006, 60, 163–169. [Google Scholar]
- Trzonkowski, P.; Basak, G.W.; Stokłosa, T.; Gaciong, Z. Immunologia Transplantacyjna; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2012. [Google Scholar]
- Gammill, H.S.; Nelson, J.L. Naturally Acquired Microchimerism. Int. J. Dev. Biol. 2010, 54, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Sunami, R.; Komuro, M.; Tagaya, H.; Hirata, S. Migration of Microchimeric Fetal Cells into Maternal Circulation before Placenta Formation. Chimerism 2010, 1, 66–68. [Google Scholar] [CrossRef]
- Yan, Z.; Lambert, N.C.; Guthrie, K.A.; Porter, A.J.; Loubiere, L.S.; Madeleine, M.M.; Stevens, A.M.; Hermes, H.M.; Nelson, J.L. Male Microchimerism in Women without Sons: Quantitative Assessment and Correlation with Pregnancy History. Am. J. Med. 2005, 118, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Reed, W.; Lee, T.-H.; Norris, P.J.; Utter, G.H.; Busch, M.P. Transfusion-Associated Microchimerism: A New Complication of Blood Transfusions in Severely Injured Patients. Semin. Hematol. 2007, 44, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Botezatu, I.; Serdyuk, O.; Potapova, G.; Shelepov, V.; Alechina, R.; Molyaka, Y.; Ananév, V.; Bazin, I.; Garin, A.; Narimanov, M.; et al. Genetic Analysis of DNA Excreted in Urine: A New Approach for Detecting Specific Genomic DNA Sequences from Cells Dying in an Organism. Clin. Chem. 2000, 46, 1078–1084. [Google Scholar] [CrossRef]
- Szarynska, M. Mikrochimeryzm plodowo-matczyny i jego znaczenie kliniczne. Postępy Biologii Komórki 2007, 34, 85–102. [Google Scholar]
- Ariga, H.; Ohto, H.; Busch, M.P.; Imamura, S.; Watson, R.; Reed, W.; Lee, T.H. Kinetics of Fetal Cellular and Cell-Free DNA in the Maternal Circulation during and after Pregnancy: Implications for Noninvasive Prenatal Diagnosis. Transfusion 2001, 41, 1524–1530. [Google Scholar] [CrossRef]
- Krabchi, K.; Gros-Louis, F.; Yan, J.; Bronsard, M.; Massé, J.; Forest, J.C.; Drouin, R. Quantification of All Fetal Nucleated Cells in Maternal Blood between the 18th and 22nd Weeks of Pregnancy Using Molecular Cytogenetic Techniques. Clin. Genet. 2001, 60, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Al-Mufti, R.; Hambley, H.; Farzaneh, F.; Nicolaides, K.H. Distribution of Fetal Erythroblasts Enriched from Maternal Blood in Multifetal Pregnancies. Hum. Reprod. 2003, 18, 1933–1936. [Google Scholar] [CrossRef] [PubMed]
- Lambert, N.C.; Erickson, T.D.; Yan, Z.; Pang, J.M.; Guthrie, K.A.; Furst, D.E.; Nelson, J.L. Quantification of Maternal Microchimerism by HLA-Specific Real-Time Polymerase Chain Reaction: Studies of Healthy Women and Women with Scleroderma. Arthritis Rheum. 2004, 50, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Boddy, A.M.; Fortunato, A.; Wilson Sayres, M.; Aktipis, A. Fetal Microchimerism and Maternal Health: A Review and Evolutionary Analysis of Cooperation and Conflict beyond the Womb. Bioessays 2015, 37, 1106–1118. [Google Scholar] [CrossRef]
- Guetta, E.; Gordon, D.; Simchen, M.J.; Goldman, B.; Barkai, G. Hematopoietic Progenitor Cells as Targets for Non-Invasive Prenatal Diagnosis: Detection of Fetal CD34+ Cells and Assessment of Post-Delivery Persistence in the Maternal Circulation. Blood Cells Mol. Dis. 2003, 30, 13–21. [Google Scholar] [CrossRef]
- O’Donoghue, K.; Chan, J.; de la Fuente, J.; Kennea, N.; Sandison, A.; Anderson, J.R.; Roberts, I.A.G.; Fisk, N.M. Microchimerism in Female Bone Marrow and Bone Decades after Fetal Mesenchymal Stem-Cell Trafficking in Pregnancy. Lancet 2004, 364, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.F.N.; Gurnot, C.; Montine, T.J.; Sonnen, J.A.; Guthrie, K.A.; Nelson, J.L. Male Microchimerism in the Human Female Brain. PLoS ONE 2012, 7, e45592. [Google Scholar] [CrossRef]
- Śliwa, L. Microchimerism a Potential cause of autoimmune diseases. Med. Rodz. 2009, 12, 25–28. [Google Scholar]
- Nelson, J.L. Microchimerism and Human Autoimmune Diseases. Lupus 2002, 11, 651–654. [Google Scholar] [CrossRef]
- Bojer, E. Transfusion-Associated Microchimerism. Acta Haematol. Pol. 2011, 42, 435–444. [Google Scholar]
- Lee, T.H.; Donegan, E.; Slichter, S.; Busch, M.P. Transient Increase in Circulating Donor Leukocytes after Allogeneic Transfusions in Immunocompetent Recipients Compatible with Donor Cell Proliferation. Blood 1995, 85, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Paglieroni, T.; Ohto, H.; Holland, P.V.; Busch, M.P. Survival of Donor Leukocyte Subpopulations in Immunocompetent Transfusion Recipients: Frequent Long-Term Microchimerism in Severe Trauma Patients. Blood 1999, 93, 3127–3139. [Google Scholar] [CrossRef]
- Pan, P.; Binjie, H.; Min, L.; Lipei, F.; Yanli, N.; Junwen, Z.; Xianghua, S. A Meta-Analysis on Diagnostic Value of Serum Cystatin C and Creatinine for the Evaluation of Glomerular Filtration Function in Renal Transplant Patients. Afr. Health Sci. 2014, 14, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Zheng, X.; Mathew, J.M.; Gallon, L.; Leventhal, J.R.; Zhang, Z.J. Tackling Chronic Kidney Transplant Rejection: Challenges and Promises. Front. Immunol. 2021, 12, 661643. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Matsushita, K.; Sang, Y.; Brunskill, N.J.; Carrero, J.J.; Chodick, G.; Hasegawa, T.; Heerspink, H.L.; Hirayama, A.; Landman, G.W.D.; et al. Serum Potassium and Adverse Outcomes across the Range of Kidney Function: A CKD Prognosis Consortium Meta-Analysis. Eur. Heart J. 2018, 39, 1535–1542. [Google Scholar] [CrossRef]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.K.; Vernekar, S.N. Markers of Renal Function Tests. N. Am. J. Med. Sci. 2010, 2, 170–173. [Google Scholar]
- Kim, J.J.; Fuggle, S.V.; Marks, S.D. Does HLA Matching Matter in the Modern Era of Renal Transplantation? Pediatr. Nephrol. 2021, 36, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.C.; Opelz, G.; Weil, E.J.; McGarvey, C.J.; Chakkera, H.A. The Risk of Transplant Failure With HLA Mismatch in First Adult Kidney Allografts 2: Living Donors, Summary, Guide. Transplant. Direct 2017, 3, e152. [Google Scholar] [CrossRef]
- Shi, X.; Lv, J.; Han, W.; Zhong, X.; Xie, X.; Su, B.; Ding, J. What Is the Impact of Human Leukocyte Antigen Mismatching on Graft Survival and Mortality in Renal Transplantation? A Meta-Analysis of 23 Cohort Studies Involving 486,608 Recipients. BMC Nephrol. 2018, 19, 116. [Google Scholar] [CrossRef]
- Ashuntantang, G.E.; Garovic, V.D.; Heilberg, I.P.; Lightstone, L. Kidneys and Women’s Health: Key Challenges and Considerations. Nat. Rev. Nephrol. 2018, 14, 203–210. [Google Scholar] [CrossRef]
- Asderakis, A.; Dyer, P.; Augustine, T.; Worthington, J.; Campbell, B.; Johnson, R.W. Effect of Cold Ischemic Time and HLA Matching in Kidneys Coming from “Young” and “Old” Donors: Do Not Leave for Tomorrow What You Can Do Tonight. Transplantation 2001, 72, 674–678. [Google Scholar] [CrossRef]
- Cecka, J.M. The UNOS Renal Transplant Registry. Clin. Transpl. 2001, 1–18. [Google Scholar]
- Ivanovski, N.; Popov, Z.; Kolevski, P.; Cakalaroski, K.; Stojkovski, L.; Spasovski, G.; Zafirovska, K. Living Related Renal Transplantation--the Use of Advanced Age Donors. Clin. Nephrol. 2001, 55, 309–312. [Google Scholar] [PubMed]
- Peters, T.G.; Shaver, T.R.; Ames, J.E.; Santiago-Delpin, E.A.; Jones, K.W.; Blanton, J.W. Cold Ischemia and Outcome in 17,937 Cadaveric Kidney Transplants. Transplantation 1995, 59, 191–196. [Google Scholar] [CrossRef]
- Kyllönen, L.; Salmela, K. Transplantation of Both Kidneys from 408 Donors; Comparison of Results. Transpl. Int. 2000, 13 (Suppl. S1), S95–S98. [Google Scholar] [CrossRef]
- Hetzel, G.R.; Klein, B.; Brause, M.; Westhoff, A.; Willers, R.; Sandmann, W.; Grabensee, B. Risk Factors for Delayed Graft Function after Renal Transplantation and Their Significance for Long-Term Clinical Outcome. Transpl. Int. 2002, 15, 10–16. [Google Scholar] [CrossRef]
- Pacholczyk, M.; Łągiewska, B.; Wałaszewski, J.; Mokrzycka-Michalik, M.; Rowiński, M.; Lao, M. Przyczyny Pierwotnego Braku Czynności Przeszczepu Allogennego Nerki Pobranej Ze Zwłok. Pol. Prz. Chir. 1994, 66, 1267–1271. [Google Scholar]
- Bai, H.; Shi, B.; Qian, Y.; Na, Y.; Zeng, X.; Zhong, D.; Lu, M.; Zou, W.; Wu, S. Endothelial Cell Chimerism by Fluorescence in Situ Hybridization in Gender Mismatched Renal Allograft Biopsies. Chin. Med. J. 2007, 120, 859–862. [Google Scholar] [CrossRef]
- Pujal, J.-M.; Grinyó, J.M.; Gil-Vernet, S.; Caldes, A.; Hernández, P.; Mestre, M.; Encuentra, M.; Perez-Garcia, A.; Gallardo, D. Early Hematopoietic Microchimerism Predicts Clinical Outcome after Kidney Transplantation. Transplantation 2007, 84, 1103–1111. [Google Scholar] [CrossRef]
- El-Ansary, M.; Saadi, G.; Hassaballa, M.; Zidan, M.; Abdel Fattah, W.; Kelany, A.K.; Hanna, M.O.F. Donor Cell Microchimerism in Kidney Transplantation: Implications for Graft Function. Int. J. Immunogenet. 2020, 47, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Villari, D.; Salvianti, F.; Zanazzi, M.; Martini, A.; Spatafora, P.; Grisanti, S.C.; Sebastianelli, A.; Nicita, G.; Serni, S.; Pinzani, P. Quantitative Polymerase Chain Reaction Detection of Microchimerism in Female Transplant Renal Recipients. UIN 2020, 104, 865–870. [Google Scholar] [CrossRef]
- Fourtounas, C.; Spyridonidis, A.; Dousdampanis, P.; Savidaki, E.; Kalliakmani, P.; Papachristou, E.; Goumenos, D.; Vlachojannis, J.G. Microchimerism in Peripheral Blood and Urine in Renal Transplant Recipients: Preliminary Results. Transplant. Proc. 2008, 40, 3434–3436. [Google Scholar] [CrossRef] [PubMed]
- Suberbielle, C.; Caillat-Zucman, S.; Legendre, C.; Bodemer, C.; Noël, L.H.; Kreis, H.; Bach, J.F. Peripheral Microchimerism in Long-Term Cadaveric-Kidney Allograft Recipients. Lancet 1994, 343, 1468–1469. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.; Tein, M.S.; Pang, C.C.; Yeung, C.K.; Tong, K.L.; Hjelm, N.M. Presence of Donor-Specific DNA in Plasma of Kidney and Liver-Transplant Recipients. Lancet 1998, 351, 1329–1330. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Cantarovich, D.; Barbuti, S.; Boggi, U.; Chelazzi, S.; Focosi, D.; Lapi, S.; Paleologo, G.; Rizzo, G.; Scatena, F.; et al. Association of Donor-Specific Microchimerism with Graft Dysfunction in Kidney Transplant Patients. Transpl. Immunol. 2012, 26, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tong, K.L.; Li, P.K.; Chan, A.Y.; Yeung, C.K.; Pang, C.C.; Wong, T.Y.; Lee, K.C.; Lo, Y.M. Presence of Donor- and Recipient-Derived DNA in Cell-Free Urine Samples of Renal Transplantation Recipients: Urinary DNA Chimerism. Clin. Chem. 1999, 45, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Schlitt, H.J.; Hundrieser, J.; Ringe, B.; Pichlmayr, R. Donor-Type Microchimerism Associated with Graft Rejection Eight Years after Liver Transplantation. N. Engl. J. Med. 1994, 330, 646–647. [Google Scholar] [CrossRef]
- Gadi, V.K.; Nelson, J.L.; Boespflug, N.D.; Guthrie, K.A.; Kuhr, C.S. Soluble Donor DNA Concentrations in Recipient Serum Correlate with Pancreas-Kidney Rejection. Clin. Chem. 2006, 52, 379–382. [Google Scholar] [CrossRef]
- Knight, S.R.; Thorne, A.; Lo Faro, M.L. Donor-Specific Cell-Free DNA as a Biomarker in Solid Organ Transplantation. A Systematic Review. Transplantation 2019, 103, 273–283. [Google Scholar] [CrossRef]
- PrepFiler® and PrepFiler® BTA Forensic DNA Extraction Kits. Publication Part Number 4463348 Rev. C. 2012. Available online: https://www.thermofisher.cn/order/catalog/product/4463348 (accessed on 18 June 2023).
- LaboShop|Products|GeneAmp PCR System 9700 with 96-Well Gold-Plated Silver Sample Block. Available online: https://laboshop.ae/product/geneamp-pcr-system-9700-with-96-well-gold-plated-silver-sample-block-293 (accessed on 18 June 2023).
- AmpFLSTR|Thermo Fisher Scientific. Available online: https://www.thermofisher.com/search/results?query=AmpFLSTR&focusarea=Search%20All (accessed on 18 June 2023).
- AmpFLSTRTM NGM SElectTM PCR Amplification Kit. Available online: https://www.thermofisher.com/order/catalog/product/4457889 (accessed on 18 June 2023).
- 3130XLR. Available online: https://www.thermofisher.com/order/catalog/product/3130XLR (accessed on 18 June 2023).
- GeneMapper ID Software—PL. Available online: https://www.thermofisher.com/uk/en/home/technical-resources/software-downloads/genemapper-id-software.html (accessed on 18 June 2023).
- Hi-DiTM Formamide. Available online: https://www.thermofisher.com/order/catalog/product/4311320 (accessed on 18 June 2023).
- Quantifiler® Duo DNA Quantification Kit|Thermo Fisher Scientific. Available online: https://www.thermofisher.com/search/results?query=Quantifiler%C2%AE%20Duo%20DNA%20Quantification%20Kit&focusarea=Search%20All (accessed on 18 June 2023).
- Applied Biosystems® 7500 Fast and 7500 Real-Time PCR System—PL. Available online: https://www.thermofisher.com/uk/en/home/life-science/pcr/real-time-pcr/real-time-pcr-instruments/7500-fast-real-time-pcr-system.html (accessed on 18 June 2023).
- HID RealTime PCR Analysis Software|Thermo Fisher Scientific. Available online: https://www.thermofisher.com/search/results?query=HID%20RealTime%20PCR%20Analysis%20Software%20&focusarea=Search%20All (accessed on 18 June 2023).
- Sieńko, J.; Kotowski, M.; Safranow, K.; Paczkowska, E.; Wojciuk, B.; Piątek, J.; Ossowski, A.; Wiśniewska, M.; Ostrowski, M.; Jasiczek, A.; et al. Role of Platelets in the Modulation of Kidney Allograft Recipients’ Immune Systems. Ann. Transplant. 2013, 18, 76–81. [Google Scholar] [CrossRef]
- Sieńko, J.; Kotowski, M.; Dziewanowski, K.; Wojciuk, B.; Paczkowska, E.; Kędzierska, K.; Tejchman, K.; Stasiuk, R.; Zair, L.; Ostrowski, M.; et al. Correlation Between Percentage of Immuncompetent CD4+ and CD4+CD25+ Cells and Compatibility in the HLA System and Selected Parameters Assessing Transplanted Kidney Function. Transplant. Proc. 2018, 50, 1738–1743. [Google Scholar] [CrossRef] [PubMed]
- Sieńko, J.; Kotowski, M.; Paczkowska, E.; Sobuś, A.; Tejchman, K.; Piątek, J.; Pilichowska, E.; Kędzierska-Kapuza, K.; Ostrowski, M. Correlation Between Stem and Progenitor Cells Number and Immune Response in Patients After Allogeneic Kidney Transplant. Ann. Transplant. 2018, 23, 874–878. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).