Degradation of a New Herbicide Florpyrauxifen-Benzyl in Water: Kinetics, Various Influencing Factors and Its Reaction Mechanisms
Abstract
1. Introduction
2. Results and Discussion
2.1. Hydrolytic Characteristics of Florpyrauxifen-Benzyl
2.2. Effect of Environmental Factors on Hydrolysis of Florpyrauxifen-Benzyl
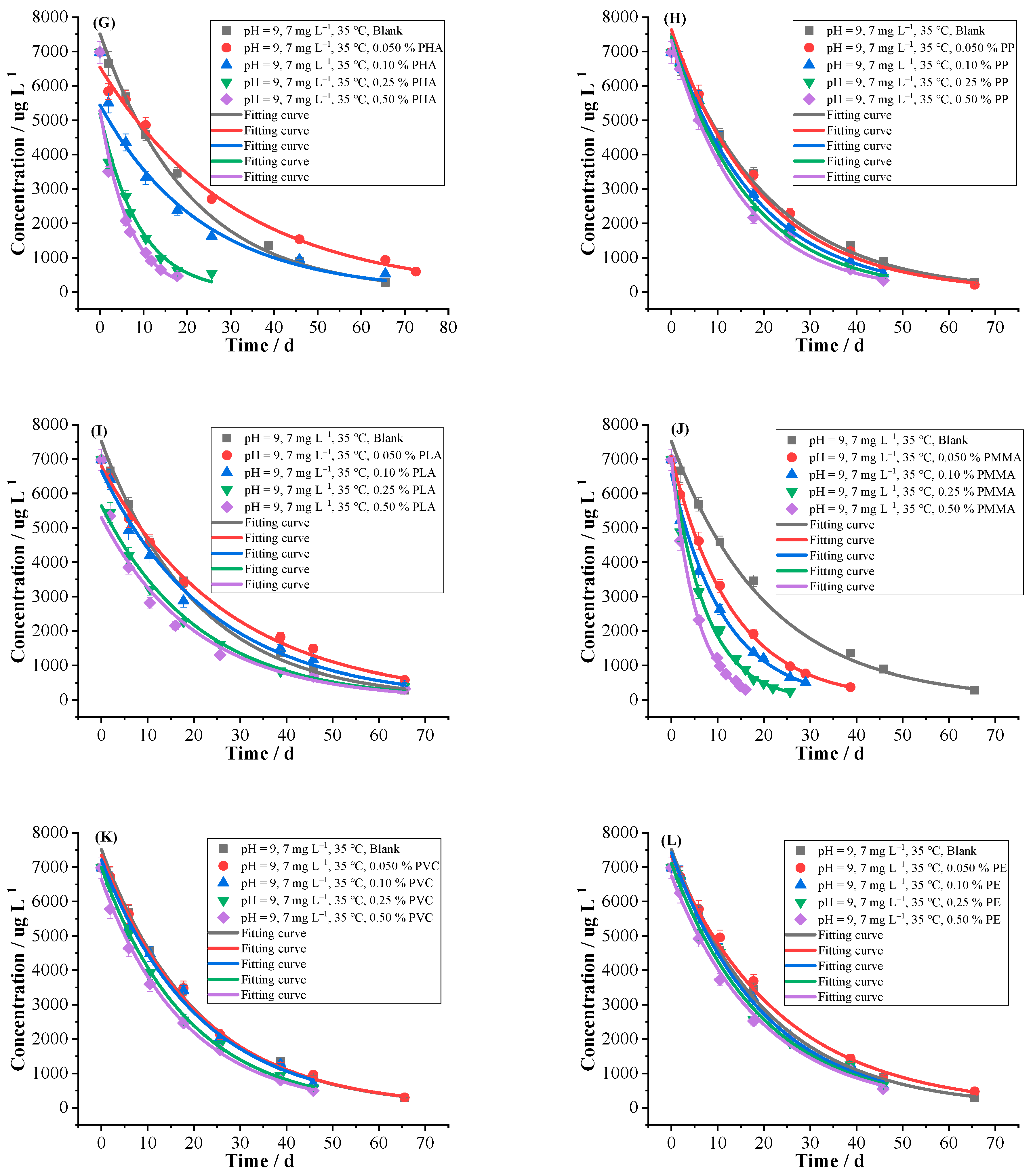
2.2.1. MPs
2.2.2. DFMs
2.2.3. Fertilizers
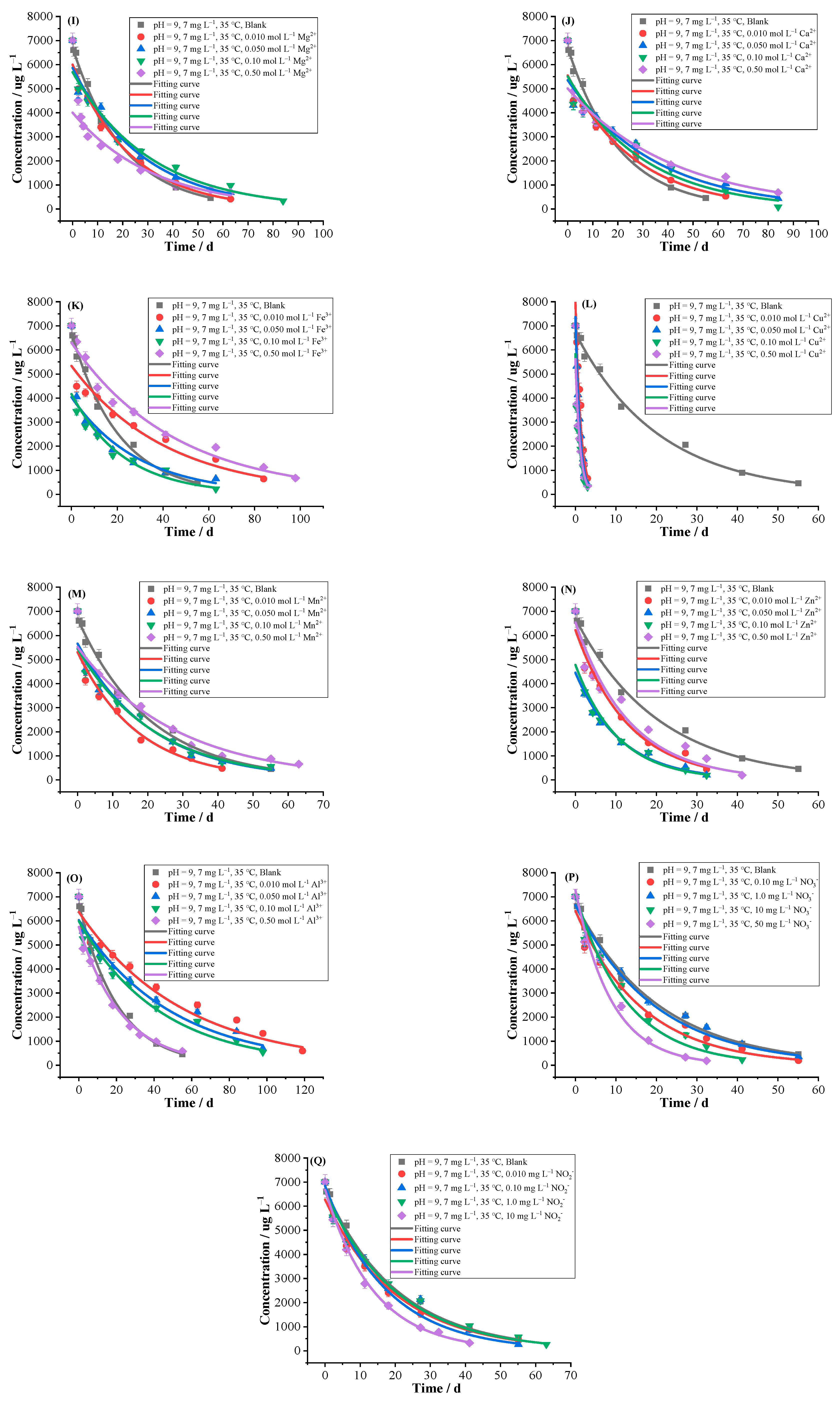
2.2.4. Cations
2.2.5. Anions
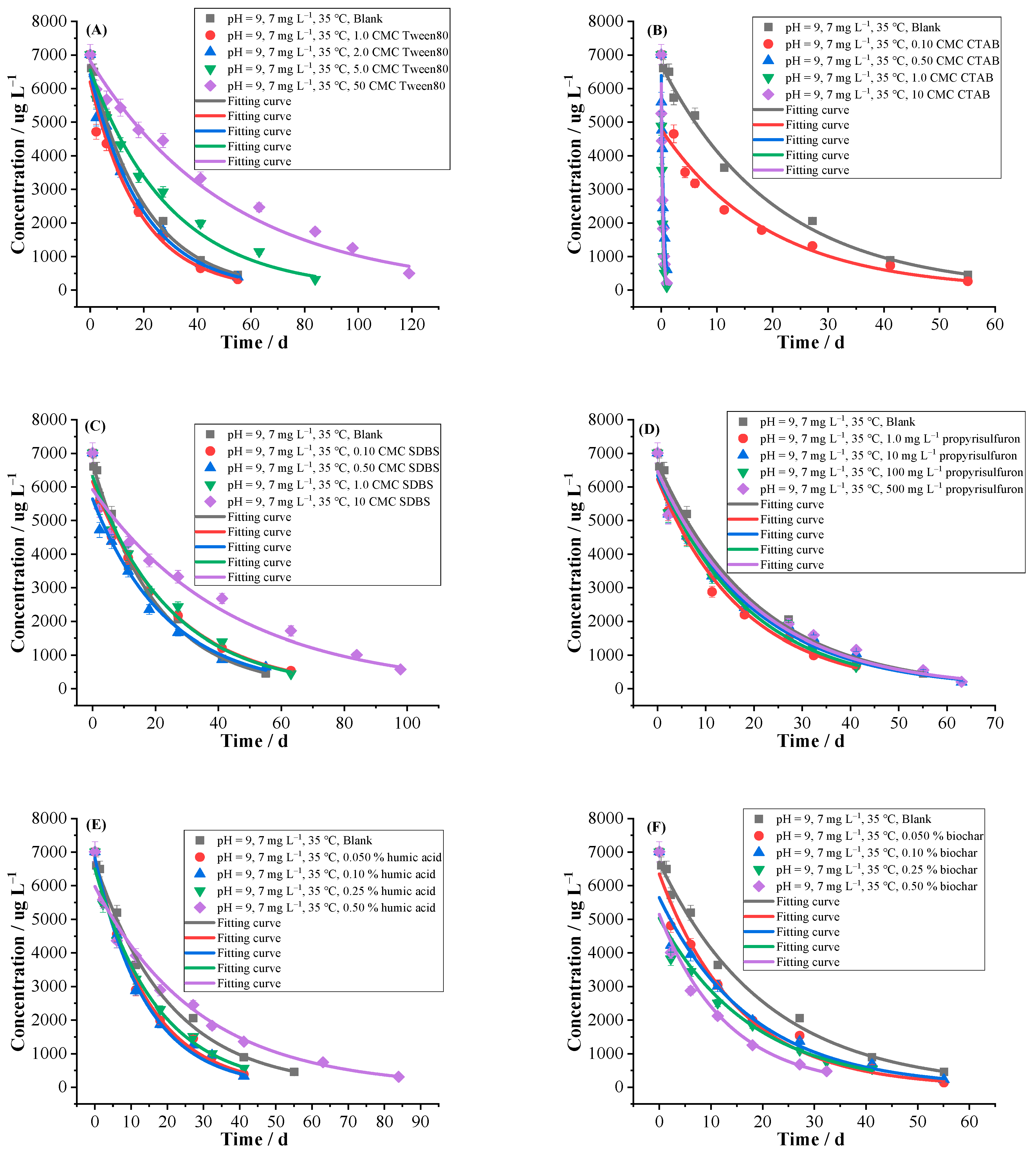
2.2.6. Surfactants
2.2.7. Coexisting Herbicide of Propyrisulfuron
2.2.8. Humic Acid and Biochar
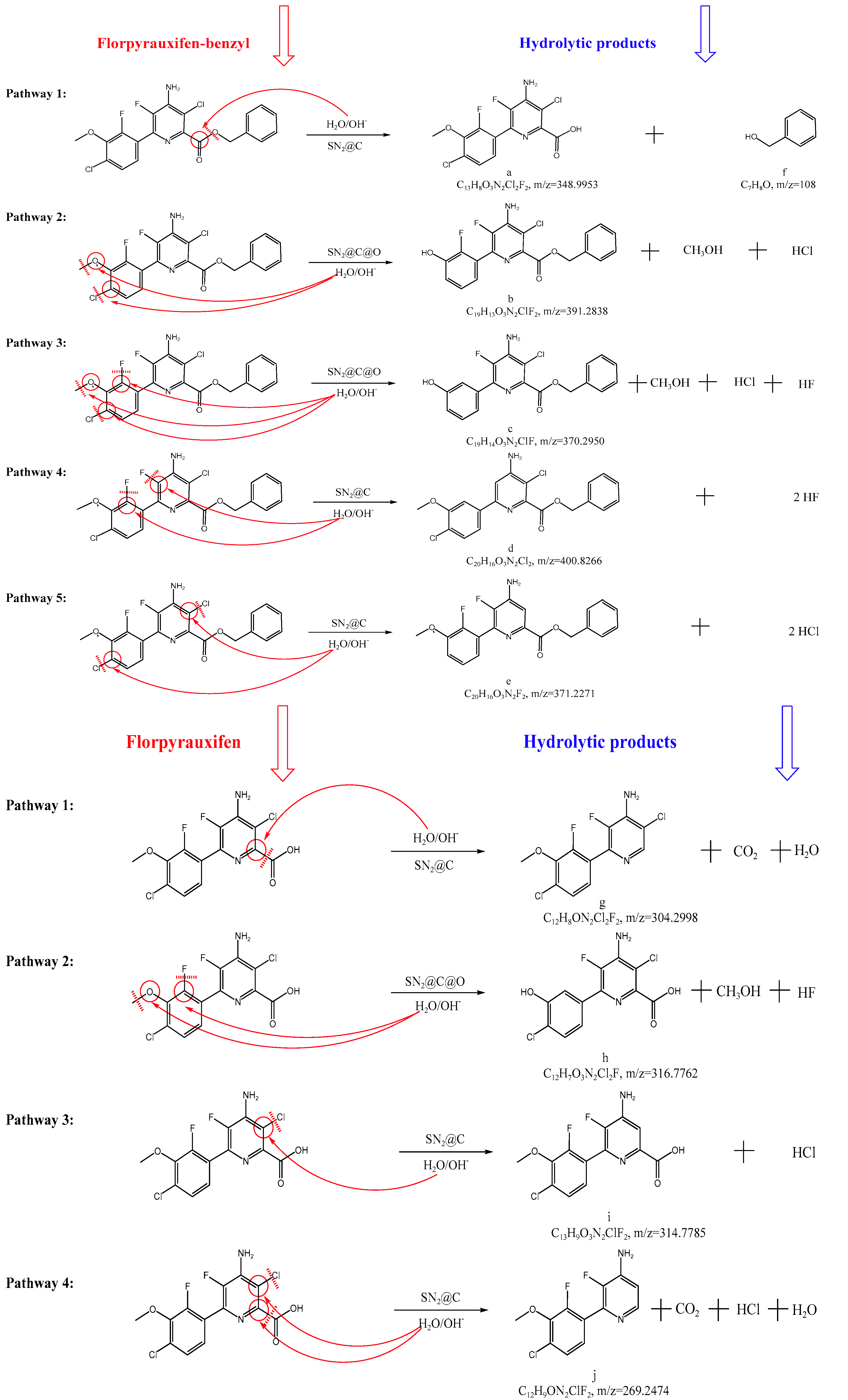
2.3. Hydrolytic Products and Mechanisms
3. Materials and Methods
3.1. Instruments and Reagents
3.2. Preparation of Buffer Solutions
3.3. Hydrolysis Test
- (1)
- An amount of 1 mg L−1 of florpyrauxifen-benzyl aqueous solutions was prepared, and its hydrolysis was conducted at different temperatures (15, 25, 35, 50 °C) and pH values (4, 7, 9), respectively.
- (2)
- The initial mass concentrations of 1, 2 and 5 mg L−1 of florpyrauxifen-benzyl were prepared with the pH = 7 buffer solutions, and its hydrolysis was conducted at 25 °C, respectively.
- (3)
- An amount of 1 mg L−1 of florpyrauxifen-benzyl aqueous solutions was prepared with ultrapure water, tap water, lake water, paddy water and artificial seawater, and its hydrolysis was conducted at 25 °C, respectively.
- (4)
- An amount of 7 mg L−1 of florpyrauxifen-benzyl was prepared with the pH = 9 buffer solutions, and the effects of different environmental factors on hydrolysis were conducted at 35 °C, respectively, including 12 kinds of common MPs (PA, PHB, PS, PBS, PBAT, LDPE, PHA, PP, PLA, PMMA, PVC, PE) with different contents (0.050, 0.10, 0.25, 0.50%), different contents (0.050, 0.10, 0.25, 0.50%) of DFMs which were divided into the whole mask, outer layer, middle layer, inner layer and ear band and they were respectively cut into tiny pieces, 6 kinds of common fertilizers (CMPF, urea, organic fertilizer, potash fertilizer, compound fertilizer, OICF) with different contents (0.050, 0.10, 0.25, 0.50%), 9 kinds of cations (Na+, K+, Mg2+, Ca2+, Fe3+, Cu2+, Mn2+, Zn2+, Al3+) with different concentrations (0.010, 0.050, 0.10, 0.50 mol L−1), 2 kinds of anions with different concentrations (0.10, 1.0, 10, 50 mg L−1 of NO3− and 0.010, 0.10, 1.0, 10 mg L−1 of NO2−), 3 kinds of surfactants with different critical micelle concentrations (1.0, 2.0, 5.0, 50 CMC of Tween80, 0.10, 0.50, 1.0, 10 CMC of CTAB and 0.10, 0.50, 1.0, 10 CMC of SDBS), coexisting herbicide propyrisulfuron (1.0, 10, 100, 500 mg L−1), humic acid and biochar with different contents (0.050, 0.10, 0.25, 0.50%), as well as the blank control group.
3.4. Sample Analysis Method
3.4.1. Preparation of Standard and Matrix-Matched Standard Working Solutions
3.4.2. Sample Pretreatment
3.4.3. Liquid Chromatography and Mass Spectrometry Conditions
3.5. Data Analysis
3.6. Hydrolytic Mechanism Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, F.W.; Li, Y.X.; Ren, F.Z.; Wang, R.; Pang, G.F. Toxicity, residue, degradation and detection methods of the insecticide triazophos. Environ. Chem. Lett. 2019, 17, 1769–1785. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, S.K. A treatise on organophosphate pesticide pollution: Current strategies and advancements in their environmental degradation and elimination. Ecotoxicol. Environ. Saf. 2021, 207, 111483. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, P.S.; Vo, D.N.; Rajamohan, N.; Saravanan, R. Microbial degradation of recalcitrant pesticides: A review. Environ. Chem. Lett. 2021, 19, 3209–3228. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2016, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.S.; Sharma, R.; Singh, S.K.; Singh, D.K. A comprehensive review of environmental fate and degradation of fipronil and its toxic metabolites. Environ. Res. 2021, 199, 111316. [Google Scholar] [CrossRef]
- Xu, X.; Li, C.M.; Sun, J.; Wang, H.L.; Wang, D.H.; Song, H.W.; Wang, Z.J. Residue characteristics and ecological risk assessment of twenty-nine pesticides in surface water of major river-basin in China. Asian J. Ecotoxicol. 2016, 11, 347–354. [Google Scholar] [CrossRef]
- Peng, Y.; Fang, W.D.; Krauss, M.; Brack, W.; Wang, Z.H.; Li, F.L.; Zhang, X.W. Screening hundreds of emerging organic pollutants (EOPs) in surface water from the Yangtze River Delta (YRD): Occurrence, distribution, ecological risk. Environ. Pollut. 2018, 241, 484–493. [Google Scholar] [CrossRef]
- Stone, W.W.; Gilliom, R.J.; Ryberg, K.R. Pesticides in U.S. streams and rivers: Occurrence and trends during 1992–2011. Environ. Sci. Technol. 2014, 48, 11025–11030. [Google Scholar] [CrossRef]
- Souza, R.M.D.; Seibert, D.; Quesada, H.B.; Bassetti, F.D.J.; Fagundes-Klen, M.R.; Bergamasco, R. Occurrence, impacts and general aspects of pesticides in surface water: A review. Process Saf. Environ. Prot. 2020, 135, 22–37. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, X.Z.; Lin, Z.Q.; Pang, S.M.; Mishra, S.; Chen, S.H. Biodegradation of fpronil: Current state of mechanisms of biodegradation and future perspectives. Appl. Microbiol. Biotechnol. 2021, 105, 7695–7708. [Google Scholar] [CrossRef]
- Li, M.M.; Wang, R.; Kong, Z.Q.; Gao, T.F.; Wang, F.Z.; Fan, B. Cyflumetofen degradation in different aquatic environments and identification of hydrolytic products. J. Environ. Chem. Eng. 2020, 8, 104512. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Y.B.; Lu, Z.Y.; Pan, J.; Li, M. A novel biosensor-based method for the detection of p-nitrophenol in agricultural soil. Chemosphere 2023, 313, 137306. [Google Scholar] [CrossRef]
- Gan, Q.; Singh, R.M.; Wu, T.; Jans, U. Kinetics and mechanism of degradation of dichlorvos in aqueous solutions containing reduced sulfur species. Environ. Sci. Technol. 2006, 40, 5717–5723. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.K.; He, W.Q.; Yan, C.R. “White revolution” to “white pollution”—Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 9, 091001. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.H.; Li, Y.; Powell, T.; Wang, X.; Wang, G.Y.; Zhang, P.P. Microplastics as contaminants in the soil environment: A mini-review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Akanyange, S.N.; Zhang, Y.; Zhao, X.H.; Adom-Asamoah, G.; Ature, A.R.A.; Anning, C.; Chen, T.P.; Zhao, H.Q.; Lyu, X.J.; Crittenden, J.C. A holistic assessment of microplastic ubiquitousness: Pathway for source identification in the environment. Sustain. Prod. Consump. 2022, 33, 113–145. [Google Scholar] [CrossRef]
- Leslie, H.A.; Velzen, M.J.M.V.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Tesfaldet, Y.T.; Ndeh, N.T. Assessing face masks in the environment by means of the DPSIR framework. Sci. Total Environ. 2022, 814, 152859. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, H.; Noh, T.K.; Lim, J.S.; Yook, M.J.; Kim, J.W.; Yi, J.H.; Kim, D.S. Baseline sensitivity of Echinochloa crus-gall and E. oryzicola to florpyrauxifen-benzyl, a new synthetic auxin herbicide, in korea. Front. Plant. Sci. 2021, 12, 656642. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Norsworthy, J.K. Florpyrauxifen-benzyl weed control spectrum and tank-mix compatibility with other commonly applied herbicides in rice. Weed Technol. 2018, 32, 319–325. [Google Scholar] [CrossRef]
- Teló, G.M.; Webster, E.P.; Blouin, D.C.; Benjamin, M.M.; RustomJr, S.Y. Florpyrauxifen-benzyl activity on perennial grass weeds found in Louisiana rice production. Weed Technol. 2019, 33, 246–252. [Google Scholar] [CrossRef]
- Wright, H.E.; Norsworthy, J.K.; Roberts, T.L.; Scott, R.C.; Hardke, J.T.; Gbur, E.E. Use of florpyrauxifen-benzyl in non-flooded rice production systems. Crop Forage Turfgrass Manag. 2021, 7, e20081. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Yang, W.H.; Yin, X.E.; Zhang, D. Analysis of florpyrauxifen-benzyl TC by HPLC. Agrochemicals 2021, 60, 32–34. [Google Scholar] [CrossRef]
- Tu Wang, M.C.; Ma, Y.N.; Pan, J.Y.; Huang, S.Q.; Chen, M.X. Identification of hydrolysis products of florpyrauxifen-benzyl and establishment of detection method. Qual. Saf. Agro Prod. 2021, 3, 40–48. [Google Scholar] [CrossRef]
- Zhou, R.D.; Dong, Z.M.; Bian, C.F.; Wang, L.; Wu, T.Q.; Zhou, W.W.; Li, Y.Q.; Li, B.T. Residue analysis, dissipation behavior, storage stability and dietary risk assessment of florpyrauxifen-benzyl in natural paddy field environment using UPLC-QTOF-MS/MS. J. Food Compos. Anal. 2022, 114, 104781. [Google Scholar] [CrossRef]
- Bian, C.F.; Wang, L.; Cui, Z.Y.; Dong, Z.M.; Shi, X.L.; Li, Y.Q.; Li, B.T. Adsorption-desorption and transport behavior of pydiflumetofen in eight different types of soil. Ecotoxicol. Environ. Saf. 2022, 234, 113378. [Google Scholar] [CrossRef]
- Zheng, W.; Yates, S.R.; Papiernik, S.K. Transformation kinetics and mechanism of the sulfonylurea herbicides pyrazosulfuron ethyl and halosulfuron methyl in aqueous solutions. J. Agric. Food Chem. 2008, 56, 7367–7372. [Google Scholar] [CrossRef]
- Qi, Y.J.; Yang, Y.; Zheng, Z.Y.; Shi, H.Y.; Wang, M.H. Study on the photolysis and hydrolysis properties of thifluzamide. Chinese J. Pestic. Sci. 2016, 18, 540–544. [Google Scholar] [CrossRef]
- Han, D.W.; Yan, D.D.; Cao, A.C.; Fang, W.S.; Wang, X.L.; Song, Z.X.; Li, Y.; Ouyang, C.B.; Guo, M.X.; Wang, Q.X. Study on the hydrolysis kinetics of dimethyl disulfide. Water Air Soil Pollut. 2017, 228, 234. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.X.; Liao, X.P.; Luo, Y.W.; Wu, S.S.; Wang, J.W. Hydrolysis mechanism of methyl parathion evidenced by Q-Exactive mass spectrometry. Environ. Sci. Pollut. Res. 2015, 22, 19747–19755. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.B.; Xiong, Y.Q. Influencing factors of the hydrolysis of bispyribac-sodium. J. Agro-Environ. Sci. 2006, 25, 1299–1302. [Google Scholar] [CrossRef]
- Man, Y.L.; Stenrod, M.; Wu, C.; Almvik, M.; Holten, R.; Clarke, J.H.L.; Yuan, S.K.; Wu, X.H.; Xu, J.; Dong, F.S.; et al. Degradation of difenoconazole in water and soil: Kinetics, degradation pathways, transformation products identification and ecotoxicity assessment. J. Hazard. Mater. 2021, 418, 126303. [Google Scholar] [CrossRef]
- Wang, W.F.; Wang, J. Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplastics. Chemosphere 2017, 193, 567–573. [Google Scholar] [CrossRef]
- Zhang, S.W.; Han, B.; Sun, Y.H.; Wang, F.Y. Microplastics influence the adsorption and desorption characteristics of Cd in an agricultural soil. J. Hazard. Mater. 2020, 388, 121775. [Google Scholar] [CrossRef]
- Xu, B.; Liu, F.C.; Brookes, P.; Xu, J.M. Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter. Environ. Pollut. 2018, 240, 87–94. [Google Scholar] [CrossRef]
- Fresne, M.; Jordan, P.; Fenton, O.; Mellander, P.; Daly, K. Soil chemical and fertilizer influences on soluble and medium-sized colloidal phosphorus in agricultural soils. Sci. Total Environ. 2021, 754, 142112. [Google Scholar] [CrossRef]
- Meng, X.G.; Wang, N.; Long, X.F.; Hu, D.Y. Degradation of a novel pesticide antiviral agent vanisulfane in aqueous solution: Kinetics, identification of photolysis products, and pathway. ACS Omega 2020, 5, 24881–24889. [Google Scholar] [CrossRef]
- Song, S.M.; Zhang, C.F.; Chen, Z.J.; Wei, J.; Tan, H.H.; Li, X.S. Hydrolysis and photolysis of bentazone in aqueous abiotic solutions and identification of its degradation products using quadrupole time-of-flight mass spectrometry. Environ. Sci. Pollut. Res. 2019, 26, 10127–10135. [Google Scholar] [CrossRef]
- Chen, X.X.; Chen, Q.L.; Song, W.; Chen, M.; Yuan, D.X. Abiotic degradation and influencing factors of acetochlor, butachlor and metolachlor in different waters under natural conditions. Environ. Chem. 2014, 33, 2136–2143. [Google Scholar] [CrossRef]
- Bao, Z.P.; Wu, Y.L.; Song, R.D.; Gao, Y.X.; Zhang, S.H.; Zhao, K.F.; Wu, T.Y.; Zhang, C.H.; Du, F.P. The simple strategy to improve pesticide bioavailability and minimize environmental risk by effective and ecofriendly surfactants. Sci. Total Environ. 2022, 851, 158169. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.T.; Wei, Y.M.; Zhai, W.J.; Wang, P.; Liu, D.H.; Zhou, Z.Q. Effects of three surfactants on the degradation and environmental risk of metolachlor in aquatic environment. Chemosphere 2022, 300, 134295. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.G.; Cheng, X.K.; Liu, G.L.; Zhang, T. The influences of sodium dodecylbenzenesulfonate and humus on hydrolysis of aldicarb and its oxidation products. China Environ. Sci. 2002, 22, 193–197. [Google Scholar]
- Salamanez, K.C.; Baltazar, A.M.; Rodriguez, E.B.; Lacsamana, M.S.; Ismail, A.M.; Johnson, D.E. Acetolactate synthase activity and growth of rice (Oryza sativa L.) and weed species treated with the herbicide propyrisulfuron. Philipp. J. Crop Sci. 2015, 40, 23–32. [Google Scholar]
- Sarmah, A.K.; Sabadie, J. Hydrolysis of sulfonylurea herbicides in soils and aqueous solutions: A review. J. Agric. Food Chem. 2002, 50, 6253–6265. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.W.; Li, X.; Shan, Y.L.; Yu, S.T.; Yu, W.L.; Liu, Y.B.; Zhao, W.T.; Li, X.K.; Liu, M.X.; Ding, Y.Q. Super facile one-step synthesis of aromatic amine waste residue derived N-rich porous carbon for hyper efficient p-nitrophenol adsorption. J. Environ. Chem. Eng. 2021, 9, 105106. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, H.W.; Yu, L.; Sun, T.H. Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biochars. J. Hazard. Mater. 2013, 244–245, 217–224. [Google Scholar] [CrossRef]
- Glinski, D.A.; Purucker, S.T.; Meter, R.J.V.; Black, M.C.; Henderson, W.M. Analysis of pesticides in surface water, stemflow, and throughfall in an agricultural area in South Georgia, USA. Chemosphere 2018, 209, 496–507. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Zuskova, E.; Kubec, J.; Buric, M.; Kouba, A. Chronic toxicity of metolachlor OA on growth, ontogenetic development, antioxidant biomarkers and histopathology of early life stages of marbled crayfish. Sci. Total Environ. 2018, 643, 1456–1463. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Guha, S. Kinetics of biotransformation of chlorpyrifos in aqueous and soil slurry environments. Water Res. 2014, 51, 73–85. [Google Scholar] [CrossRef]
- Sevilla-Moran, B.; Alonso-Prados, J.L.; Garcia-Baudin, J.M.; Sandin-Espana, P. Indirect photodegradation of clethodim in aqueous media. Byproduct identification by quadrupole time-of-flight mass spectrometry. J. Agric. Food Chem. 2010, 58, 3068–3076. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Norsworthy, J.K. Assessment of Florpyrauxifen-benzyl Potential to Carryover to Subsequent Crops. Weed Technol. 2018, 32, 404–409. [Google Scholar] [CrossRef]
- Lin, L.J.; Yuan, B.; Hong, H.L.; Li, H.Y.; He, L.; Lu, H.L.; Liu, J.C.; Yan, C.L. Post COVID-19 pandemic: Disposable face masks as a potential vector of antibiotics in freshwater and seawater. Sci. Total Environ. 2022, 820, 153049. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Parambadath, S.; Kim, S.Y.; Ha, H.M.; Ha, C.S. Diffusion mediated selective adsorption of Zn2+ from artificial seawater by MCM-41. Microporous Mesoporous Mater. 2016, 229, 124–133. [Google Scholar] [CrossRef]
- GB/T 31270. 2-2014; Test Guidelines on Environmental Safety Assessment for Chemical Pesticides-Part 2: Hydrolysis. Standards Press of China: Beijing, China, 2015.
- Chen, K.Y.; Tian, F.J.; Wu, C.; Wu, X.H.; Xu, J.; Dong, F.S.; Liu, X.G.; Zheng, Y.Q. Degradation products and pathway of ethiprole in water and soil. Water Res. 2019, 161, 531–539. [Google Scholar] [CrossRef]
- Williams, K.L.; Tjeerdema, R.S. Hydrolytic activation kinetics of the herbicide benzobicyclon in simulated aquatic systems. J. Agric. Food Chem. 2016, 64, 4838–4844. [Google Scholar] [CrossRef]
- Li, M.L.; Zhuang, B.L.; Lu, Y.Y.; An, L.J.; Wang, Z.G. Salt-induced liquid-liquid phase separation: Combined experimental and theoretical investigation of water-acetonitrile-salt mixtures. J. Am. Chem. Soc. 2021, 143, 773–784. [Google Scholar] [CrossRef]
- Salahudeen, N.; Rasheed, A.A. Kinetics and thermodynamics of hydrolysis of crystal violet at ambient and below ambient temperatures. Sci. Rep. 2020, 10, 21929. [Google Scholar] [CrossRef]
- Xie, G.H.; Lv, J.X.; Liu, G.G.; Sun, D.Z.; Zheng, L.Q. Effects of surfactants on the kinetics of acetamiprid photolysis. React. Kinet. Catal. Lett. 2008, 95, 289–299. [Google Scholar] [CrossRef]




| Water | Mass Concentration/mg L−1 | pH | Temperature/°C | Kinetic Equation | R2 | Rate Constant (k)/d−1 | Half-Life (T0.5)/d |
|---|---|---|---|---|---|---|---|
| Ultrapure water | 1 | 4 | 15 | Ct = 1021.11e−0.0042t | 0.9772 | 0.0042 | 163.48 |
| 25 | Ct = 960.23e−0.0031t | 0.9537 | 0.0031 | 220.75 | |||
| 35 | Ct = 972.30e−0.0034t | 0.9630 | 0.0034 | 202.67 | |||
| 50 | Ct = 994.75e−0.0034t | 0.8538 | 0.0034 | 205.68 | |||
| 7 | 15 | Ct = 1011.04e−0.0052t | 0.9429 | 0.0052 | 134.59 | ||
| 25 | Ct = 1154.46e−0.0409t | 0.9708 | 0.0409 | 16.96 | |||
| 35 | Ct = 1194.53e−0.0506t | 0.9250 | 0.0506 | 13.70 | |||
| 50 | Ct = 1199.24e−0.0702t | 0.9154 | 0.0702 | 9.87 | |||
| 9 | 15 | Ct = 970.39e−0.0086t | 0.9403 | 0.0086 | 80.88 | ||
| 25 | Ct = 1128.19e−0.0529t | 0.9706 | 0.0529 | 13.10 | |||
| 35 | Ct = 1111.91e−0.3573t | 0.9875 | 0.3573 | 1.94 | |||
| 50 | Ct = 989.59e−4.6981t | 0.9850 | 4.6981 | 0.15 | |||
| Ultrapure water | 1 | 7 | 25 | Ct = 1154.46e−0.0409t | 0.9708 | 0.0409 | 16.96 |
| 2 | Ct = 2286.14e−0.0238t | 0.9507 | 0.0238 | 29.10 | |||
| 5 | Ct = 5016.71e−0.0039t | 0.9717 | 0.0039 | 176.82 | |||
| Ultrapure water | 1 | 7.12 | 25 | Ct = 1154.46e−0.0409t | 0.9708 | 0.0409 | 16.96 |
| Tap water | 7.34 | Ct = 1056.08e−0.0144t | 0.9278 | 0.0144 | 48.04 | ||
| Lake water | 6.54 | Ct = 1150.18e−0.0287t | 0.9541 | 0.0287 | 24.13 | ||
| Paddy water | 7.41 | Ct = 1159.70e−0.0238t | 0.9551 | 0.0238 | 29.11 | ||
| Seawater | 8.18 | Ct = 969.31e−0.0270t | 0.8850 | 0.0270 | 25.66 |
| Influencing Factors | Kinetic Equation | R2 | Rate Constant (k)/d−1 | Half-Life (T0.5)/d | Promoting or Inhibiting Ratio (PR/IR)/% | |
|---|---|---|---|---|---|---|
| Blank control group 1 | Ct = 7510.60e−0.0480t | 0.9933 | 0.0480 | 14.43 | / | |
| 1 PA/% | 0.050 | Ct = 7177.88e−0.0589t | 0.9893 | 0.0589 | 11.78 | 22.57 |
| 0.10 | Ct = 7285.07e−0.0681t | 0.9984 | 0.0681 | 10.18 | 41.84 | |
| 0.25 | Ct = 6158.84e−0.0700t | 0.9780 | 0.0700 | 9.91 | 45.67 | |
| 0.50 | Ct = 6229.83e−0.0914t | 0.9669 | 0.0914 | 7.59 | 90.25 | |
| 1 PHB/% | 0.050 | Ct = 6602.58e−0.0321t | 0.9718 | 0.0321 | 21.57 | −33.07 |
| 0.10 | Ct = 5067.25e−0.0409t | 0.9280 | 0.0409 | 16.95 | −14.85 | |
| 0.25 | Ct = 5100.17e−0.0888t | 0.8597 | 0.0888 | 7.81 | 84.90 | |
| 0.50 | Ct = 5014.89e−0.1317t | 0.9503 | 0.1317 | 5.26 | 174.22 | |
| 1 PS/% | 0.050 | Ct = 7179.06e−0.0532t | 0.9800 | 0.0532 | 13.03 | 10.77 |
| 0.10 | Ct = 7283.69e−0.0654t | 0.9958 | 0.0654 | 10.59 | 36.28 | |
| 0.25 | Ct = 6760.08e−0.0755t | 0.9967 | 0.0755 | 9.18 | 57.27 | |
| 0.50 | Ct = 6670.89e−0.1036t | 0.9966 | 0.1036 | 6.69 | 115.76 | |
| 1 PBS/% | 0.050 | Ct = 5585.40e−0.0420t | 0.9602 | 0.0420 | 16.50 | −12.49 |
| 0.10 | Ct = 5248.99e−0.0553t | 0.9187 | 0.0553 | 12.54 | 15.10 | |
| 0.25 | Ct = 5468.54e−0.1190t | 0.9550 | 0.1190 | 5.83 | 147.75 | |
| 0.50 | Ct = 5528.74e−0.2173t | 0.9484 | 0.2173 | 3.19 | 352.42 | |
| 1 PBAT/% | 0.050 | Ct = 5148.41e−0.0403t | 0.9436 | 0.0403 | 17.20 | −16.06 |
| 0.10 | Ct = 5029.84e−0.0676t | 0.9391 | 0.0676 | 10.25 | 40.84 | |
| 0.25 | Ct = 5413.17e−0.1281t | 0.9543 | 0.1281 | 5.41 | 166.83 | |
| 0.50 | Ct = 5804.29e−0.2334t | 0.9642 | 0.2334 | 2.97 | 385.99 | |
| 1 LDPE/% | 0.050 | Ct = 7504.82e−0.0481t | 0.9853 | 0.0481 | 14.41 | 0.19 |
| 0.10 | Ct = 7561.63e−0.0508t | 0.9897 | 0.0508 | 13.65 | 5.77 | |
| 0.25 | Ct = 7118.21e−0.0526t | 0.9850 | 0.0526 | 13.18 | 9.50 | |
| 0.50 | Ct = 6797.73e−0.0538t | 0.9948 | 0.0538 | 12.89 | 11.97 | |
| 1 PHA/% | 0.050 | Ct = 6541.85e−0.0320t | 0.9924 | 0.0320 | 21.69 | −33.47 |
| 0.10 | Ct = 5442.54e−0.0424t | 0.9355 | 0.0424 | 16.34 | −11.66 | |
| 0.25 | Ct = 5193.77e−0.1115t | 0.9167 | 0.1115 | 6.22 | 132.15 | |
| 0.50 | Ct = 5280.78e−0.1490t | 0.9519 | 0.1490 | 4.65 | 210.37 | |
| 1 PP/% | 0.050 | Ct = 7633.40e−0.0512t | 0.9893 | 0.0512 | 13.54 | 6.58 |
| 0.10 | Ct = 7420.42e−0.0551t | 0.9958 | 0.0551 | 12.58 | 14.72 | |
| 0.25 | Ct = 7422.28e−0.0598t | 0.9943 | 0.0598 | 11.59 | 24.51 | |
| 0.50 | Ct = 7318.79e−0.0649t | 0.9911 | 0.0649 | 10.68 | 35.15 | |
| 1 PLA/% | 0.050 | Ct = 6795.70e−0.0364t | 0.9952 | 0.0364 | 19.06 | −24.26 |
| 0.10 | Ct = 6657.12e−0.0412t | 0.9929 | 0.0412 | 16.80 | −14.10 | |
| 0.25 | Ct = 5643.14e−0.0476t | 0.9694 | 0.0476 | 14.56 | −0.85 | |
| 0.50 | Ct = 5300.35e−0.0485t | 0.9528 | 0.0485 | 14.29 | 1.04 | |
| 1 PMMA/% | 0.050 | Ct = 7072.02e−0.0762t | 0.9986 | 0.0762 | 9.10 | 58.66 |
| 0.10 | Ct = 6569.37e−0.0883t | 0.9955 | 0.0883 | 7.85 | 83.92 | |
| 0.25 | Ct = 6930.55e−0.1323t | 0.9867 | 0.1323 | 5.24 | 175.57 | |
| 0.50 | Ct = 7023.22e−0.1895t | 0.9902 | 0.1895 | 3.66 | 294.65 | |
| 1 PVC/% | 0.050 | Ct = 7348.99e−0.0473t | 0.9946 | 0.0473 | 14.66 | −1.52 |
| 0.10 | Ct = 7212.53e−0.0478t | 0.9916 | 0.0478 | 14.49 | −0.37 | |
| 0.25 | Ct = 6991.86e−0.0537t | 0.9982 | 0.0537 | 12.91 | 11.81 | |
| 0.50 | Ct = 6633.69e−0.0553t | 0.9971 | 0.0553 | 12.53 | 15.22 | |
| 1 PE/% | 0.050 | Ct = 7319.65e−0.0423t | 0.9914 | 0.0423 | 16.37 | −11.83 |
| 0.10 | Ct = 7418.69e−0.0500t | 0.9907 | 0.0500 | 13.86 | 4.16 | |
| 0.25 | Ct = 7104.14e−0.0508t | 0.9898 | 0.0508 | 13.64 | 5.83 | |
| 0.50 | Ct = 6745.45e−0.0512t | 0.9872 | 0.0512 | 13.52 | 6.73 | |
| 1 DFMs/% | 0.050 | Ct = 7126.03e−0.0451t | 0.9984 | 0.0451 | 15.38 | −6.16 |
| 0.10 | Ct = 6795.57e−0.0547t | 0.9808 | 0.0547 | 12.67 | 13.95 | |
| 0.25 | Ct = 5208.87e−0.0576t | 0.9160 | 0.0576 | 12.03 | 19.97 | |
| 0.50 | Ct = 5184.28e−0.0631t | 0.9166 | 0.0631 | 10.98 | 31.42 | |
| 1 Outer layer of DFMs/% | 0.050 | Ct = 6702.83e−0.0365t | 0.9904 | 0.0365 | 18.97 | −23.91 |
| 0.10 | Ct = 5953.48e−0.0384t | 0.9635 | 0.0384 | 18.04 | −19.97 | |
| 0.25 | Ct = 5906.58e−0.0399t | 0.9627 | 0.0399 | 17.38 | −16.95 | |
| 0.50 | Ct = 5248.56e−0.0424t | 0.9756 | 0.0424 | 16.36 | −11.79 | |
| 1 Middle layer of DFMs/% | 0.050 | Ct = 7123.12e−0.0521t | 0.9860 | 0.0521 | 13.31 | 8.43 |
| 0.10 | Ct = 6481.00e−0.0544t | 0.9899 | 0.0544 | 12.75 | 13.20 | |
| 0.25 | Ct = 6387.03e−0.0622t | 0.9649 | 0.0622 | 11.15 | 29.47 | |
| 0.50 | Ct = 5060.24e−0.0600t | 0.9532 | 0.0600 | 11.55 | 24.97 | |
| 1 Inner layer of DFMs/% | 0.050 | Ct = 7189.70e−0.0453t | 0.9983 | 0.0453 | 15.29 | −5.62 |
| 0.10 | Ct = 6981.13e−0.0504t | 0.9932 | 0.0504 | 13.75 | 4.96 | |
| 0.25 | Ct = 6198.20e−0.0542t | 0.9909 | 0.0542 | 12.79 | 12.85 | |
| 0.50 | Ct = 5230.34e−0.0648t | 0.9538 | 0.0648 | 10.70 | 34.84 | |
| 1 Ear band of DFMs/% | 0.050 | Ct = 6874.53e−0.0405t | 0.9624 | 0.0405 | 17.12 | −15.70 |
| 0.10 | Ct = 6913.16e−0.0574t | 0.9973 | 0.0574 | 12.08 | 19.49 | |
| 0.25 | Ct = 7009.87e−0.0763t | 0.9987 | 0.0763 | 9.09 | 58.83 | |
| 0.50 | Ct = 7189.25e−0.1168t | 0.9894 | 0.1168 | 5.93 | 143.21 | |
| Blank control group 2 | Ct = 6703.39e−0.0483t | 0.9948 | 0.0483 | 14.34 | / | |
| 2 CMPF/% | 0.050 | Ct = 6005.36e−0.0409t | 0.9885 | 0.0409 | 16.95 | −15.36 |
| 0.10 | Ct = 5816.78e−0.0424t | 0.9853 | 0.0424 | 16.34 | −12.19 | |
| 0.25 | Ct = 5036.41e−0.0398t | 0.9658 | 0.0398 | 17.40 | −17.57 | |
| 0.50 | Ct = 4961.20e−0.0435t | 0.9604 | 0.0435 | 15.92 | −9.89 | |
| 2 Urea/% | 0.050 | Ct = 6725.00e−0.0483t | 0.9898 | 0.0483 | 14.36 | −0.08 |
| 0.10 | Ct = 6597.26e−0.0453t | 0.9949 | 0.0453 | 15.30 | −6.25 | |
| 0.25 | Ct = 6387.59e−0.0453t | 0.9900 | 0.0453 | 15.29 | −6.21 | |
| 0.50 | Ct = 6717.64e−0.0535t | 0.9912 | 0.0535 | 12.97 | 10.64 | |
| 2 Organic fertilizer/% | 0.050 | Ct = 6616.29e−0.0552t | 0.9698 | 0.0552 | 12.57 | 14.16 |
| 0.10 | Ct = 6539.83e−0.0576t | 0.9775 | 0.0576 | 12.04 | 19.18 | |
| 0.25 | Ct = 6051.00e−0.0582t | 0.9829 | 0.0582 | 11.91 | 20.49 | |
| 0.50 | Ct = 5739.93e−0.0773t | 0.9852 | 0.0773 | 8.96 | 60.06 | |
| 2 Potash fertilizer/% | 0.050 | Ct = 6115.86e−0.0435t | 0.9909 | 0.0435 | 15.92 | −9.89 |
| 0.10 | Ct = 6516.86e−0.0471t | 0.9936 | 0.0471 | 14.72 | −2.52 | |
| 0.25 | Ct = 6475.41e−0.0421t | 0.9972 | 0.0421 | 16.46 | −12.83 | |
| 0.50 | Ct = 6454.15e−0.0421t | 0.9943 | 0.0421 | 16.46 | −12.85 | |
| 2 Compound fertilizer/% | 0.050 | Ct = 6513.16e−0.0399t | 0.9920 | 0.0399 | 17.36 | −17.38 |
| 0.10 | Ct = 6551.27e−0.0386t | 0.9559 | 0.0386 | 17.95 | −20.07 | |
| 0.25 | Ct = 6528.45e−0.0322t | 0.9918 | 0.0322 | 21.56 | −33.46 | |
| 0.50 | Ct = 6306.80e−0.0254t | 0.9826 | 0.0254 | 27.32 | −47.50 | |
| 2 OICF/% | 0.050 | Ct = 5650.74e−0.0343t | 0.9836 | 0.0343 | 20.23 | −29.10 |
| 0.10 | Ct = 6418.24e−0.0413t | 0.9185 | 0.0413 | 16.80 | −14.61 | |
| 0.25 | Ct = 5815.25e−0.0409t | 0.9500 | 0.0409 | 16.96 | −15.40 | |
| 0.50 | Ct = 5575.26e−0.0420t | 0.9557 | 0.0420 | 16.50 | −13.06 | |
| 2 Na+/mol L−1 | 0.010 | Ct = 6603.34e−0.0452t | 0.9870 | 0.0452 | 15.32 | −6.37 |
| 0.050 | Ct = 6237.02e−0.0380t | 0.9905 | 0.0380 | 18.23 | −21.32 | |
| 0.10 | Ct = 6392.31e−0.0370t | 0.9796 | 0.0370 | 18.73 | −23.43 | |
| 0.50 | Ct = 6215.34e−0.0218t | 0.9798 | 0.0218 | 31.87 | −54.99 | |
| 2 K+/mol L−1 | 0.010 | Ct = 5848.30e−0.0480t | 0.9741 | 0.0480 | 14.45 | −0.75 |
| 0.050 | Ct = 6020.21e−0.0400t | 0.9877 | 0.0400 | 17.32 | −17.20 | |
| 0.10 | Ct = 5786.43e−0.0334t | 0.9790 | 0.0334 | 20.72 | −30.77 | |
| 0.50 | Ct = 6154.19e−0.0250t | 0.9795 | 0.0250 | 27.76 | −48.32 | |
| 2 Mg2+/mol L−1 | 0.010 | Ct = 6003.34e−0.0425t | 0.9888 | 0.0425 | 16.32 | −12.11 |
| 0.050 | Ct = 5865.90e−0.0354t | 0.9821 | 0.0354 | 19.56 | −26.68 | |
| 0.10 | Ct = 5690.02e−0.0321t | 0.9728 | 0.0321 | 21.62 | −33.65 | |
| 0.50 | Ct = 4007.15e−0.0311t | 0.9425 | 0.0311 | 22.32 | −35.72 | |
| 2 Ca2+/mol L−1 | 0.010 | Ct = 5552.82e−0.0371t | 0.9783 | 0.0371 | 18.70 | −23.30 |
| 0.050 | Ct = 5358.55e−0.0287t | 0.9684 | 0.0287 | 24.17 | −40.65 | |
| 0.10 | Ct = 5508.63e−0.0327t | 0.9518 | 0.0327 | 21.18 | −32.28 | |
| 0.50 | Ct = 5008.52e−0.0236t | 0.9615 | 0.0236 | 29.36 | −51.14 | |
| 2 Fe3+/mol L−1 | 0.010 | Ct = 5334.64e−0.0240t | 0.9660 | 0.0240 | 28.86 | −50.29 |
| 0.050 | Ct = 4038.75e−0.0343t | 0.8630 | 0.0343 | 20.18 | −28.93 | |
| 0.10 | Ct = 4166.93e−0.0434t | 0.9291 | 0.0434 | 15.99 | −10.29 | |
| 0.50 | Ct = 6300.30e−0.0220t | 0.9820 | 0.0220 | 31.46 | −54.41 | |
| 2 Cu2+/mol L−1 | 0.010 | Ct = 8057.62e−0.8071t | 0.9662 | 0.8071 | 0.86 | 1570.24 |
| 0.050 | Ct = 7394.43e−0.9257t | 0.9870 | 0.9257 | 0.75 | 1815.79 | |
| 0.10 | Ct = 5852.22e−1.0024t | 0.9558 | 1.0024 | 0.69 | 1974.57 | |
| 0.50 | Ct = 5642.79e−0.9059t | 0.9655 | 0.9059 | 0.77 | 1774.79 | |
| 2 Mn2+/mol L−1 | 0.010 | Ct = 5309.73e−0.0574t | 0.9643 | 0.0574 | 12.08 | 18.75 |
| 0.050 | Ct = 5664.72e−0.0474t | 0.9635 | 0.0474 | 14.62 | −1.90 | |
| 0.10 | Ct = 5456.66e−0.0455t | 0.9555 | 0.0455 | 15.23 | −5.79 | |
| 0.50 | Ct = 5522.17e−0.0355t | 0.9584 | 0.0355 | 19.54 | −26.57 | |
| 2 Zn2+/mol L−1 | 0.010 | Ct = 6230.32e−0.0762t | 0.9593 | 0.0762 | 9.09 | 57.80 |
| 0.050 | Ct = 4460.53e−0.0886t | 0.9478 | 0.0886 | 7.82 | 83.44 | |
| 0.10 | Ct = 4785.48e−0.0947t | 0.9599 | 0.0947 | 7.32 | 95.99 | |
| 0.50 | Ct = 6544.89e−0.0748t | 0.9168 | 0.0748 | 9.27 | 54.70 | |
| 2 Al3+/mol L−1 | 0.010 | Ct = 6369.25e−0.0180t | 0.9578 | 0.0180 | 38.51 | −62.75 |
| 0.050 | Ct = 6034.06e−0.0203t | 0.9696 | 0.0203 | 34.13 | −57.97 | |
| 0.10 | Ct = 5991.31e−0.0230t | 0.9759 | 0.0230 | 30.19 | −52.48 | |
| 0.50 | Ct = 5749.62e−0.0437t | 0.9803 | 0.0437 | 15.85 | −9.52 | |
| 2 NO3−/mg L−1 | 0.10 | Ct = 6421.40e−0.0603t | 0.9730 | 0.0603 | 11.49 | 24.81 |
| 1.0 | Ct = 6661.17e−0.0508t | 0.9810 | 0.0508 | 13.66 | 5.03 | |
| 10 | Ct = 7093.17e−0.0783t | 0.9686 | 0.0783 | 8.86 | 61.96 | |
| 50 | Ct = 7284.60e−0.1124t | 0.9857 | 0.1124 | 6.17 | 132.60 | |
| 2 NO2−/mg L−1 | 0.010 | Ct = 6270.93e−0.0489t | 0.9911 | 0.0489 | 14.18 | 1.16 |
| 0.10 | Ct = 6840.22e−0.0567t | 0.9817 | 0.0567 | 12.22 | 17.43 | |
| 1.0 | Ct = 6602.46e−0.0490t | 0.9864 | 0.0490 | 14.15 | 1.41 | |
| 10 | Ct = 6681.25e−0.0717t | 0.9933 | 0.0717 | 9.67 | 48.39 | |
| 2 Tween80/CMC | 1.0 | Ct = 6199.92e−0.0537t | 0.9865 | 0.0537 | 12.90 | 11.18 |
| 2.0 | Ct = 6420.75e−0.0507t | 0.9932 | 0.0507 | 13.67 | 4.90 | |
| 5.0 | Ct = 6549.50e−0.0331t | 0.9804 | 0.0331 | 20.95 | −31.52 | |
| 50 | Ct = 6823.51e−0.0190t | 0.9695 | 0.0190 | 36.44 | −60.64 | |
| 2 CTAB/CMC | 0.10 | Ct = 4786.30e−0.0517t | 0.9597 | 0.0517 | 13.40 | 7.08 |
| 0.50 | Ct = 6448.72e−2.3920t | 0.9843 | 2.3920 | 0.29 | 4850.43 | |
| 1.0 | Ct = 5752.74e−4.2599t | 0.9584 | 4.2599 | 0.16 | 8716.06 | |
| 10 | Ct = 6234.73e−3.5119t | 0.9867 | 3.5119 | 0.20 | 7168.03 | |
| 2 SDBS/CMC | 0.10 | Ct = 6162.35e−0.0392t | 0.9928 | 0.0392 | 17.66 | −18.77 |
| 0.50 | Ct = 5639.97e−0.0421t | 0.9697 | 0.0421 | 16.45 | −12.79 | |
| 1.0 | Ct = 6309.99e−0.0403t | 0.9874 | 0.0403 | 17.21 | −16.66 | |
| 10 | Ct = 5923.69e−0.0227t | 0.9798 | 0.0227 | 30.54 | −53.02 | |
| 2 Propyrisulfuron/mg L−1 | 1.0 | Ct = 6234.50e−0.0557t | 0.9837 | 0.0557 | 12.44 | 15.27 |
| 10 | Ct = 6336.75e−0.0498t | 0.9654 | 0.0498 | 13.92 | 3.02 | |
| 100 | Ct = 6450.54e−0.0537t | 0.9838 | 0.0537 | 12.90 | 11.22 | |
| 500 | Ct = 6461.64e−0.0486t | 0.9736 | 0.0486 | 14.27 | 0.52 | |
| 2 Humic acid/% | 0.050 | Ct = 6778.75e−0.0665t | 0.9829 | 0.0665 | 10.43 | 37.56 |
| 0.10 | Ct = 6821.76e−0.0705t | 0.9816 | 0.0705 | 9.83 | 45.92 | |
| 0.25 | Ct = 6502.14e−0.0582t | 0.9934 | 0.0582 | 11.90 | 20.53 | |
| 0.50 | Ct = 5976.80e−0.0349t | 0.9879 | 0.0349 | 19.88 | −27.84 | |
| 2 Biochar/% | 0.050 | Ct = 6350.65e−0.0648t | 0.9719 | 0.0648 | 10.69 | 34.15 |
| 0.10 | Ct = 5646.26e−0.0562t | 0.9759 | 0.0562 | 12.34 | 16.20 | |
| 0.25 | Ct = 5040.18e−0.0561t | 0.9565 | 0.0561 | 12.36 | 16.08 | |
| 0.50 | Ct = 5147.28e−0.0761t | 0.9591 | 0.0761 | 9.11 | 57.41 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, R.; Dong, Z.; Wang, L.; Zhou, W.; Zhao, W.; Wu, T.; Chang, H.; Lin, W.; Li, B. Degradation of a New Herbicide Florpyrauxifen-Benzyl in Water: Kinetics, Various Influencing Factors and Its Reaction Mechanisms. Int. J. Mol. Sci. 2023, 24, 10521. https://doi.org/10.3390/ijms241310521
Zhou R, Dong Z, Wang L, Zhou W, Zhao W, Wu T, Chang H, Lin W, Li B. Degradation of a New Herbicide Florpyrauxifen-Benzyl in Water: Kinetics, Various Influencing Factors and Its Reaction Mechanisms. International Journal of Molecular Sciences. 2023; 24(13):10521. https://doi.org/10.3390/ijms241310521
Chicago/Turabian StyleZhou, Rendan, Zemin Dong, Long Wang, Wenwen Zhou, Weina Zhao, Tianqi Wu, Hailong Chang, Wei Lin, and Baotong Li. 2023. "Degradation of a New Herbicide Florpyrauxifen-Benzyl in Water: Kinetics, Various Influencing Factors and Its Reaction Mechanisms" International Journal of Molecular Sciences 24, no. 13: 10521. https://doi.org/10.3390/ijms241310521
APA StyleZhou, R., Dong, Z., Wang, L., Zhou, W., Zhao, W., Wu, T., Chang, H., Lin, W., & Li, B. (2023). Degradation of a New Herbicide Florpyrauxifen-Benzyl in Water: Kinetics, Various Influencing Factors and Its Reaction Mechanisms. International Journal of Molecular Sciences, 24(13), 10521. https://doi.org/10.3390/ijms241310521