RNA Sequencing Reveals Unique Transcriptomic Signatures of the Thyroid in a Murine Lung Cancer Model Treated with PD-1 and PD-L1 Antibodies
Abstract
1. Introduction
2. Results
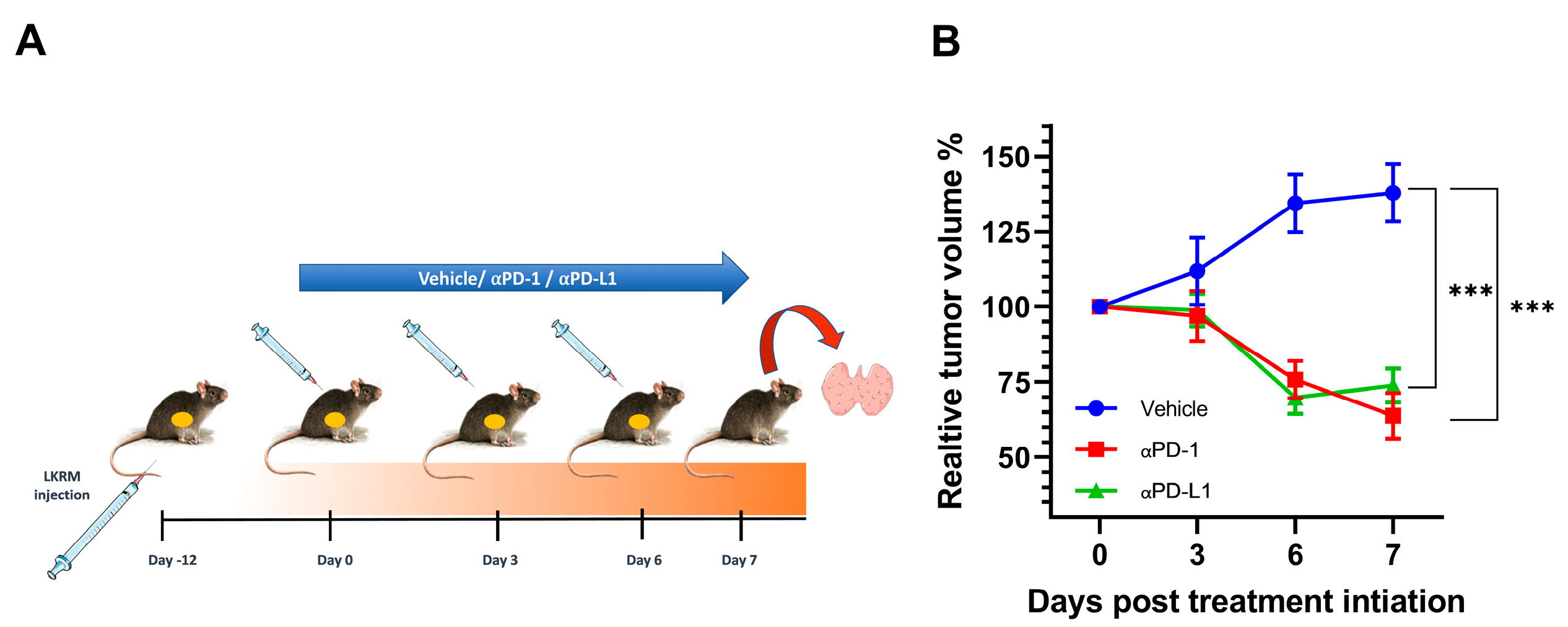
2.1. αPD-1 and αPD-L1 Treatment Induced an Anti-Tumor Response in Treated Mice
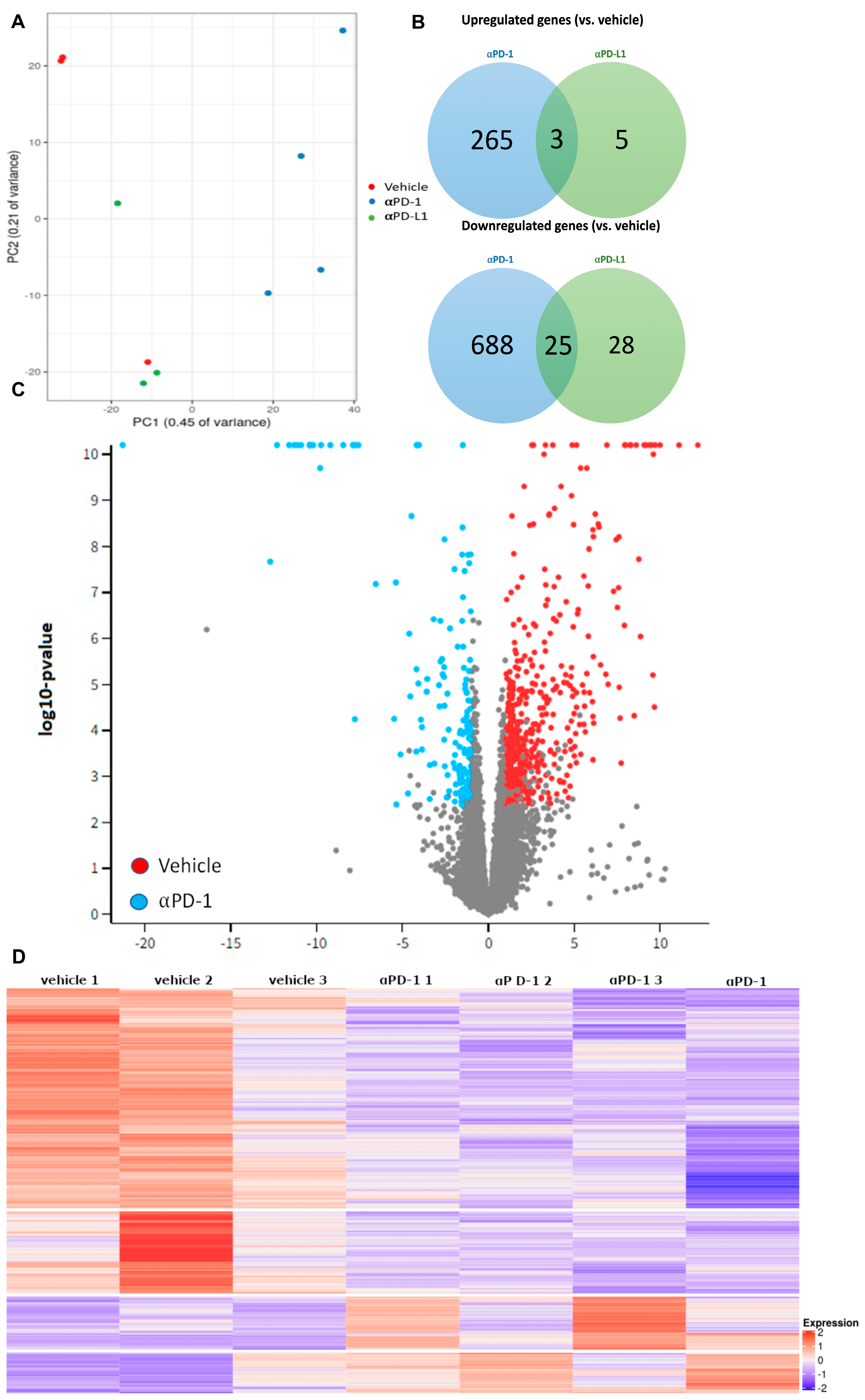
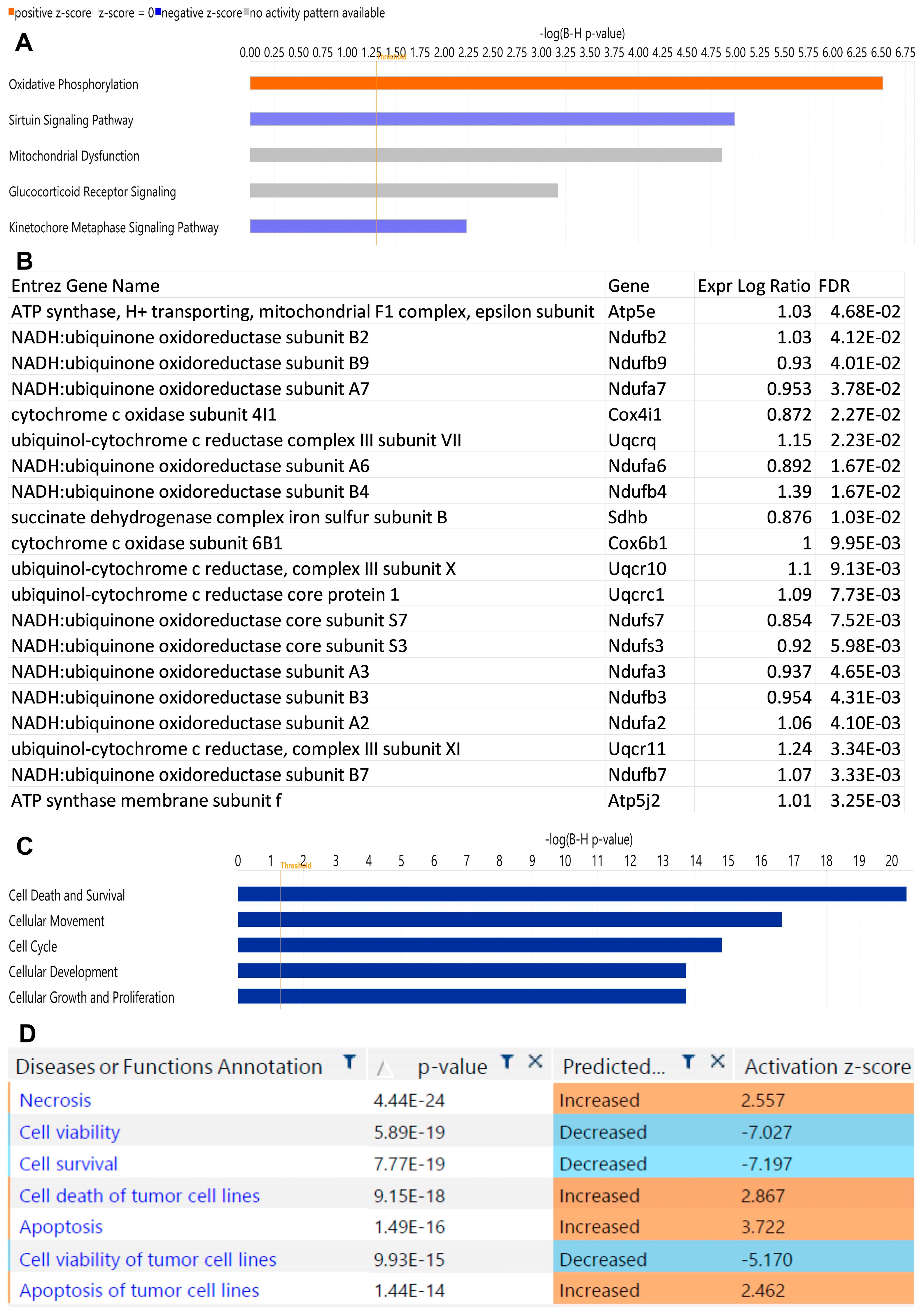
2.2. αPD-1 Treatment Induced Unique Transcriptional Changes in the Thyroid
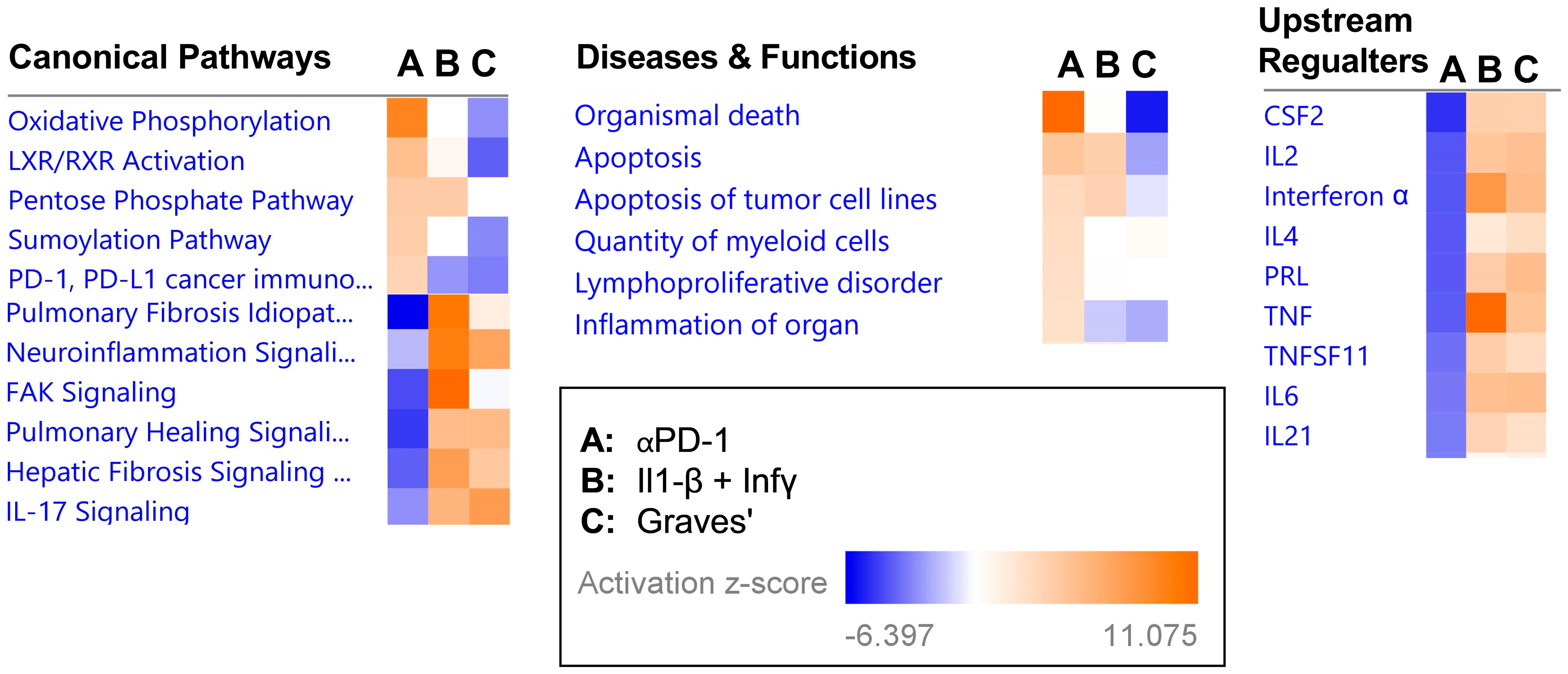
2.3. Transcriptional Signature following αPD-1 Treatment Is Distinct when Compared with Other Forms of Immune Thyroid Dysfunction
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Cell Line and Tumor Injection
4.3. RNA Extraction, Library Preparation, and RNA Sequencing
4.4. Pathway Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beatty, G.L.; Gladney, W.L. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 2015, 21, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Pollack, R.M.; Kagan, M.; Lotem, M.; Dresner-Pollak, R. Baseline Tsh Level Is Associated with Risk of Anti-Pd-1-Induced Thyroid Dysfunction. Endocr. Pract. 2019, 25, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Pollack, R.; Ashash, A.; Cahn, A.; Rottenberg, Y.; Stern, H.; Dresner-Pollak, R. Immune Checkpoint Inhibitor-Induced Thyroid Dysfunction is Associated with Higher Body Mass Index. J. Clin. Endocrinol. Metab. 2020, 105, e3620–e3627. [Google Scholar] [CrossRef]
- Brody, H.M.; Macherla, S.; Bulumulle, A.; Namireddy, P.; Cherry, C.R. The real-world incidence of immunotherapy-related thyroid dysfunction: A retrospective analysis of a single center’s experience over five years. J. Clin. Oncol. 2020, 38, 98. [Google Scholar] [CrossRef]
- Morganstein, D.L.; Lai, Z.; Spain, L.; Diem, S.; Levine, D.; Mace, C.; Gore, M.; Larkin, J. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin. Endocrinol. 2017, 86, 614–620. [Google Scholar] [CrossRef]
- Muir, C.A.; Clifton-Bligh, R.J.; Long, G.V.; Scolyer, R.A.; Lo, S.N.; Carlino, M.S.; Tsang, V.H.M.; Menzies, A.M. Thyroid Immune-related Adverse Events Following Immune Checkpoint Inhibitor Treatment. J. Clin. Endocrinol. Metab. 2021, 106, e3704–e3713. [Google Scholar] [CrossRef]
- Rogado, J.; Sanchez-Torres, J.M.; Romero-Laorden, N.; Ballesteros, A.I.; Pacheco-Barcia, V.; Ramos-Levi, A.; Arranz, R.; Lorenzo, A.; Gullon, P.; Donnay, O.; et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur. J. Cancer 2019, 109, 21–27. [Google Scholar] [CrossRef]
- Lima Ferreira, J.; Costa, C.; Marques, B.; Castro, S.; Victor, M.; Oliveira, J.; Santos, A.P.; Sampaio, I.L.; Duarte, H.; Marques, A.P.; et al. Improved survival in patients with thyroid function test abnormalities secondary to immune-checkpoint inhibitors. Cancer Immunol. Immunother. 2021, 70, 299–309. [Google Scholar] [CrossRef]
- Kobayashi, T.; Iwama, S.; Yasuda, Y.; Okada, N.; Tsunekawa, T.; Onoue, T.; Takagi, H.; Hagiwara, D.; Ito, Y.; Morishita, Y.; et al. Patients With Antithyroid Antibodies Are Prone To Develop Destructive Thyroiditis by Nivolumab: A Prospective Study. J. Endocr. Soc. 2018, 2, 241–251. [Google Scholar] [CrossRef]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef]
- Hollowell, J.G.; Staehling, N.W.; Flanders, W.D.; Hannon, W.H.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef]
- Wang, S.H.; Van Antwerp, M.; Kuick, R.; Gauger, P.G.; Doherty, G.M.; Fan, Y.Y.; Baker, J.R., Jr. Microarray analysis of cytokine activation of apoptosis pathways in the thyroid. Endocrinology 2007, 148, 4844–4852. [Google Scholar] [CrossRef]
- Huber, A.K.; Finkelman, F.D.; Li, C.W.; Concepcion, E.; Smith, E.; Jacobson, E.; Latif, R.; Keddache, M.; Zhang, W.; Tomer, Y. Genetically driven target tissue overexpression of CD40: A novel mechanism in autoimmune disease. J. Immunol. 2012, 189, 3043–3053. [Google Scholar] [CrossRef]
- Bai, X.; Chen, X.; Wu, X.; Huang, Y.; Zhuang, Y.; Lin, X. Immune checkpoint inhibitor-associated thyroid dysfunction: A disproportionality analysis using the WHO Adverse Drug Reaction Database, VigiBase. Eur. J. Endocrinol. 2020, 182, 1–9. [Google Scholar] [CrossRef]
- Yoon, J.H.; Hong, A.R.; Kim, H.K.; Kang, H.C. Characteristics of Immune-Related Thyroid Adverse Events in Patients Treated with PD-1/PD-L1 Inhibitors. Endocrinol. Metab. 2021, 36, 413–423. [Google Scholar] [CrossRef]
- Kurimoto, C.; Inaba, H.; Ariyasu, H.; Iwakura, H.; Ueda, Y.; Uraki, S.; Takeshima, K.; Furukawa, Y.; Morita, S.; Yamamoto, Y.; et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci. 2020, 111, 1468–1477. [Google Scholar] [CrossRef]
- Yasuda, Y.; Iwama, S.; Sugiyama, D.; Okuji, T.; Kobayashi, T.; Ito, M.; Okada, N.; Enomoto, A.; Ito, S.; Yan, Y.; et al. CD4+ T cells are essential for the development of destructive thyroiditis induced by anti–PD-1 antibody in thyroglobulin-immunized mice. Sci. Transl. Med. 2021, 13, eabb7495. [Google Scholar] [CrossRef]
- Lechner, M.G.; Cheng, M.I.; Patel, A.Y.; Hoang, A.T.; Yakobian, N.; Astourian, M.; Pioso, M.S.; Rodriguez, E.D.; McCarthy, E.C.; Hugo, W.; et al. Inhibition of IL-17A Protects against Thyroid Immune-Related Adverse Events while Preserving Checkpoint Inhibitor Antitumor Efficacy. J. Immunol. 2022, 209, 696–709. [Google Scholar] [CrossRef]
- Kimbara, S.; Fujiwara, Y.; Iwama, S.; Ohashi, K.; Kuchiba, A.; Arima, H.; Yamazaki, N.; Kitano, S.; Yamamoto, N.; Ohe, Y. Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci. 2018, 109, 3583–3590. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Iwama, S.; Okuji, T.; Kobayashi, T.; Yasuda, Y.; Wada, E.; Onoue, T.; Goto, M.; Sugiyama, M.; Tsunekawa, T.; et al. Anti-thyroid antibodies and thyroid echo pattern at baseline as risk factors for thyroid dysfunction induced by anti-programmed cell death-1 antibodies: A prospective study. Br. J. Cancer 2020, 122, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Phoon, Y.P.; Karlinsey, K.; Tian, Y.F.; Thapaliya, S.; Thongkum, A.; Qu, L.; Matz, A.J.; Cameron, M.; Cameron, C.; et al. A high OXPHOS CD8 T cell subset is predictive of immunotherapy resistance in melanoma patients. J. Exp. Med. 2022, 219, e20202084. [Google Scholar] [CrossRef] [PubMed]
- Kaisar-Iluz, N.; Arpinati, L.; Shaul, M.E.; Mahroum, S.; Qaisi, M.; Tidhar, E.; Fridlender, Z.G. The Bilateral Interplay between Cancer Immunotherapies and Neutrophils’ Phenotypes and Sub-Populations. Cells 2022, 11, 783. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Kenigsberg, E.; Jaitin, D.A.; David, E.; Paul, F.; Tanay, A.; Amit, I. MARS-seq2.0: An experimental and analytical pipeline for indexed sorting combined with single-cell RNA sequencing. Nat. Protoc. 2019, 14, 1841–1862. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 2011, 17, 3. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Koster, J.; Rahmann, S. Snakemake—A scalable bioinformatics workflow engine. Bioinformatics 2012, 28, 2520–2522. [Google Scholar] [CrossRef]
- Fan, Y.; Xie, W.; Huang, H.; Wang, Y.; Li, G.; Geng, Y.; Hao, Y.; Zhang, Z. Association of Immune Related Adverse Events With Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Cancers: A Systemic Review and Meta-analysis. Front. Oncol. 2021, 11, 633032. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollack, R.; Stokar, J.; Lishinsky, N.; Gurt, I.; Kaisar-Iluz, N.; Shaul, M.E.; Fridlender, Z.G.; Dresner-Pollak, R. RNA Sequencing Reveals Unique Transcriptomic Signatures of the Thyroid in a Murine Lung Cancer Model Treated with PD-1 and PD-L1 Antibodies. Int. J. Mol. Sci. 2023, 24, 10526. https://doi.org/10.3390/ijms241310526
Pollack R, Stokar J, Lishinsky N, Gurt I, Kaisar-Iluz N, Shaul ME, Fridlender ZG, Dresner-Pollak R. RNA Sequencing Reveals Unique Transcriptomic Signatures of the Thyroid in a Murine Lung Cancer Model Treated with PD-1 and PD-L1 Antibodies. International Journal of Molecular Sciences. 2023; 24(13):10526. https://doi.org/10.3390/ijms241310526
Chicago/Turabian StylePollack, Rena, Joshua Stokar, Natan Lishinsky, Irina Gurt, Naomi Kaisar-Iluz, Merav E. Shaul, Zvi G. Fridlender, and Rivka Dresner-Pollak. 2023. "RNA Sequencing Reveals Unique Transcriptomic Signatures of the Thyroid in a Murine Lung Cancer Model Treated with PD-1 and PD-L1 Antibodies" International Journal of Molecular Sciences 24, no. 13: 10526. https://doi.org/10.3390/ijms241310526
APA StylePollack, R., Stokar, J., Lishinsky, N., Gurt, I., Kaisar-Iluz, N., Shaul, M. E., Fridlender, Z. G., & Dresner-Pollak, R. (2023). RNA Sequencing Reveals Unique Transcriptomic Signatures of the Thyroid in a Murine Lung Cancer Model Treated with PD-1 and PD-L1 Antibodies. International Journal of Molecular Sciences, 24(13), 10526. https://doi.org/10.3390/ijms241310526






