Effect of Paclobutrazol Application on Enhancing the Efficacy of Nitenpyram against the Brown Planthopper, Nilaparvata lugens
Abstract
1. Introduction
2. Results
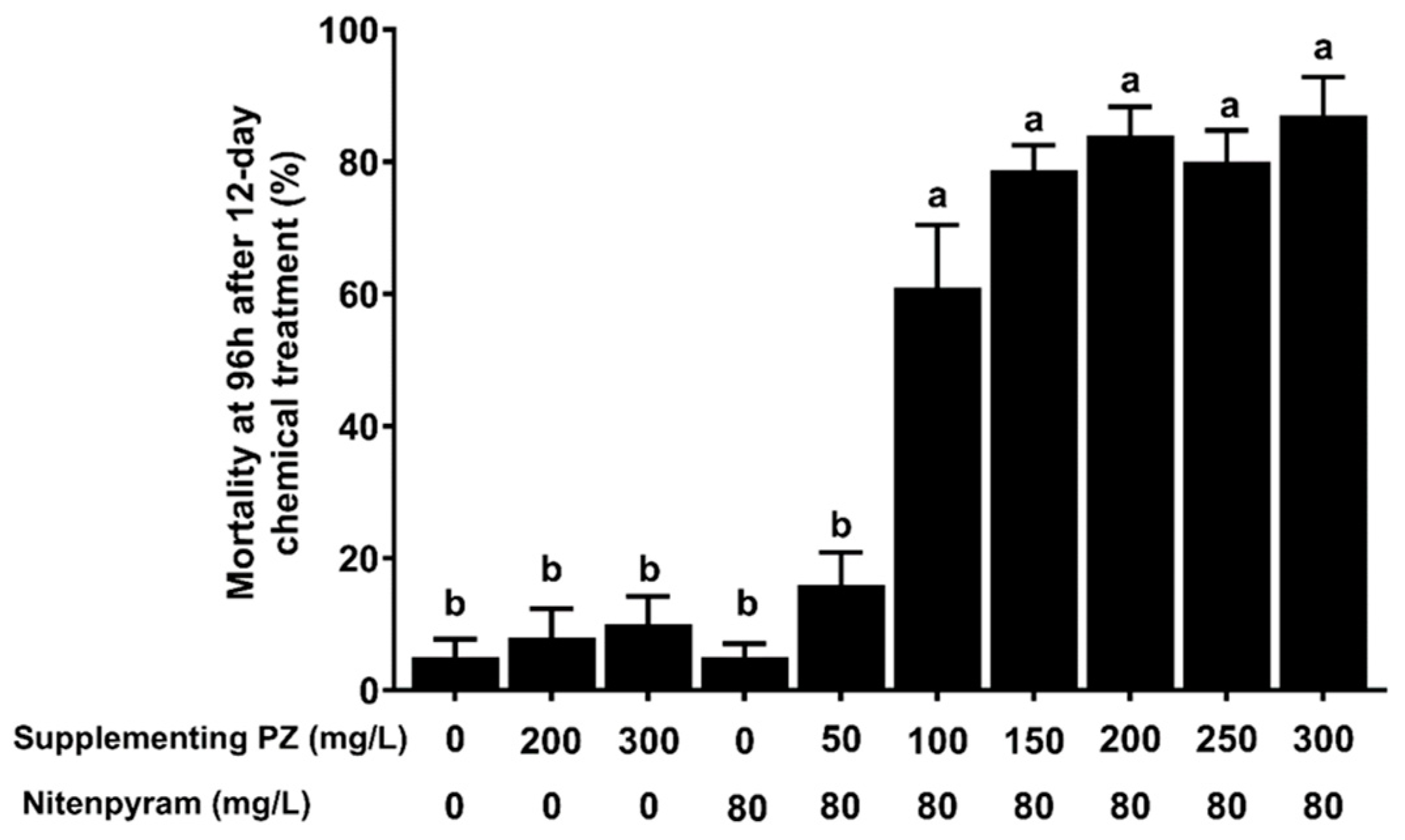
2.1. Synergistic Effect of Paclobutrazol on Nitenpyram against BPH
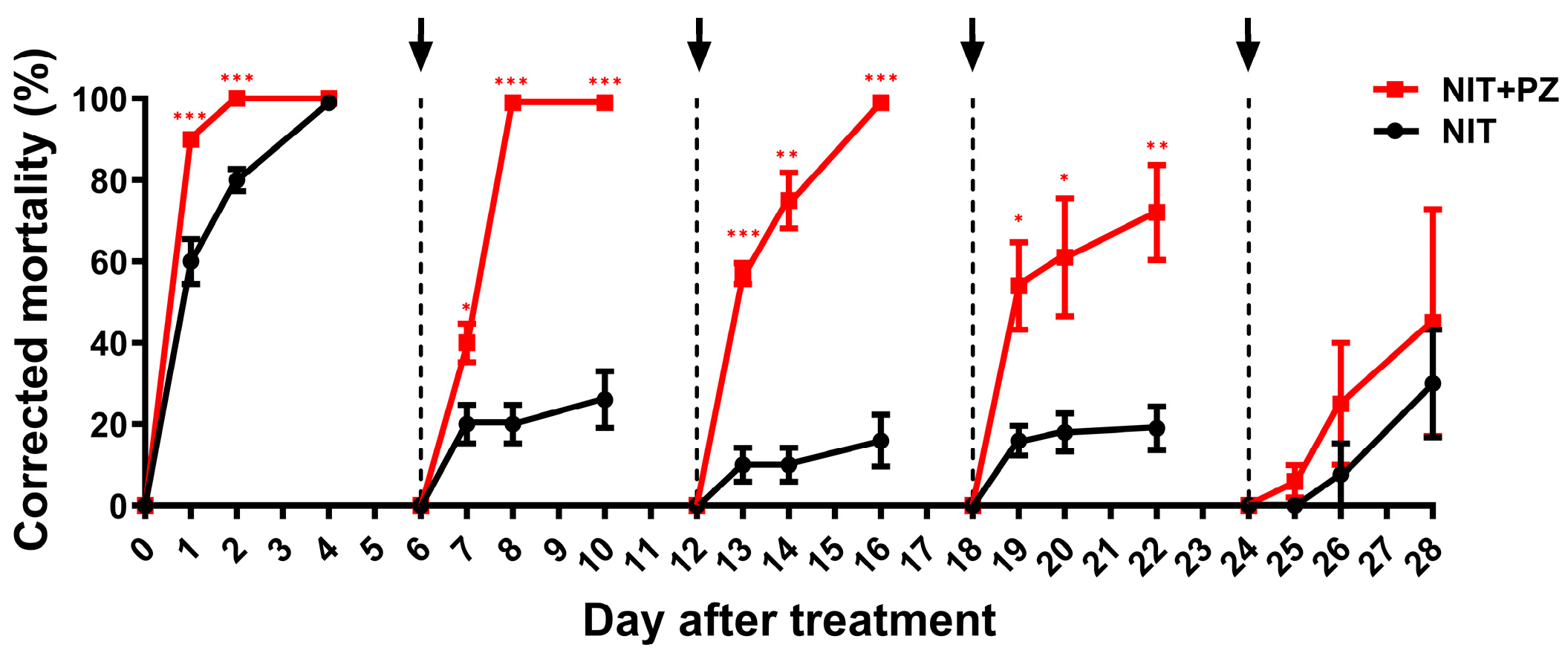
2.2. Paclobutrazol Prolongs the Persistence of Nitenpyram against BPH
2.3. Effect of Paclobutrazol on the Susceptibility of BPH to Nitenpyram
2.4. Paclobutrazol Induces the Biosynthesis of Constitutive and Elicited Secondary Metabolites
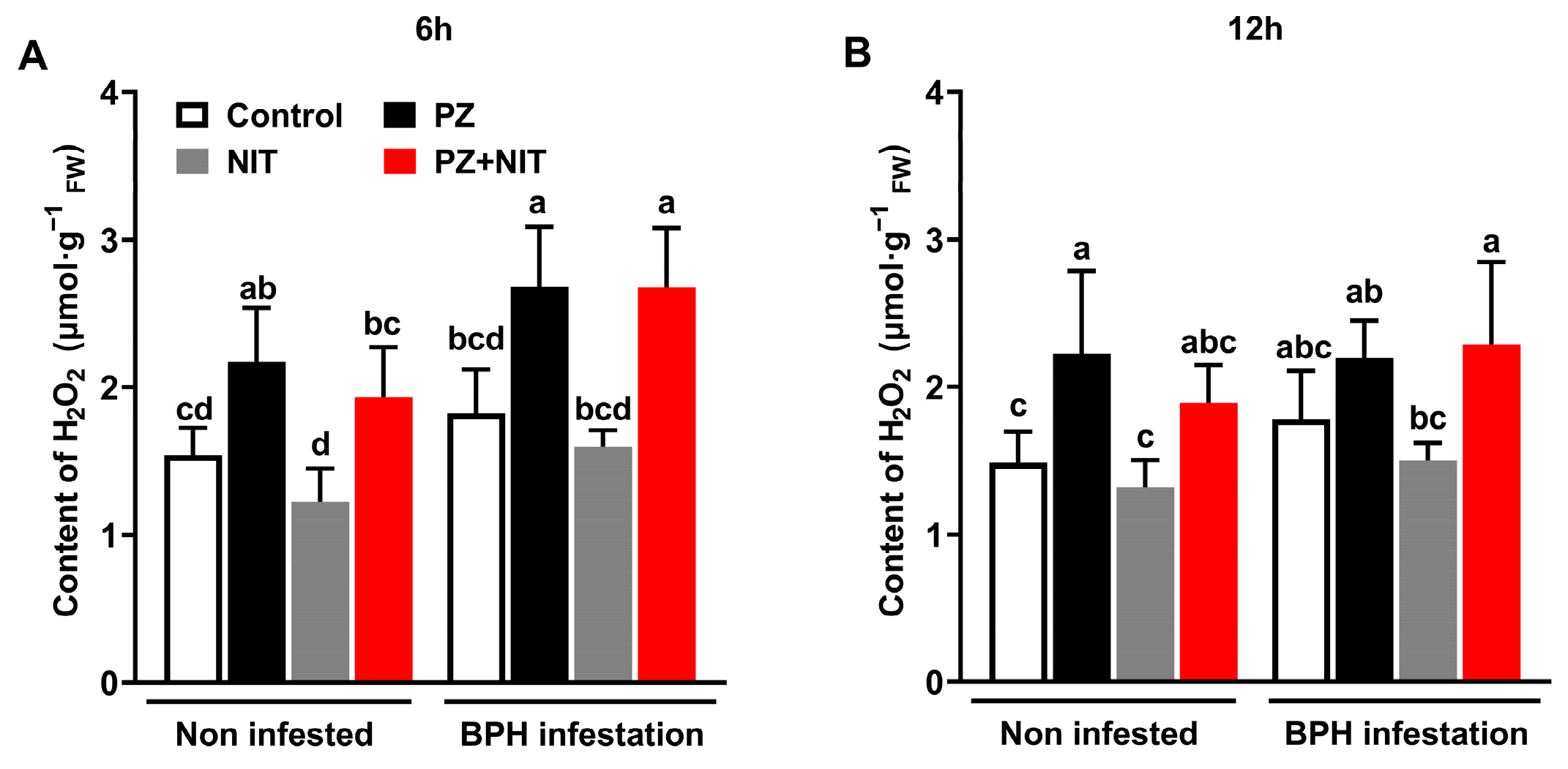
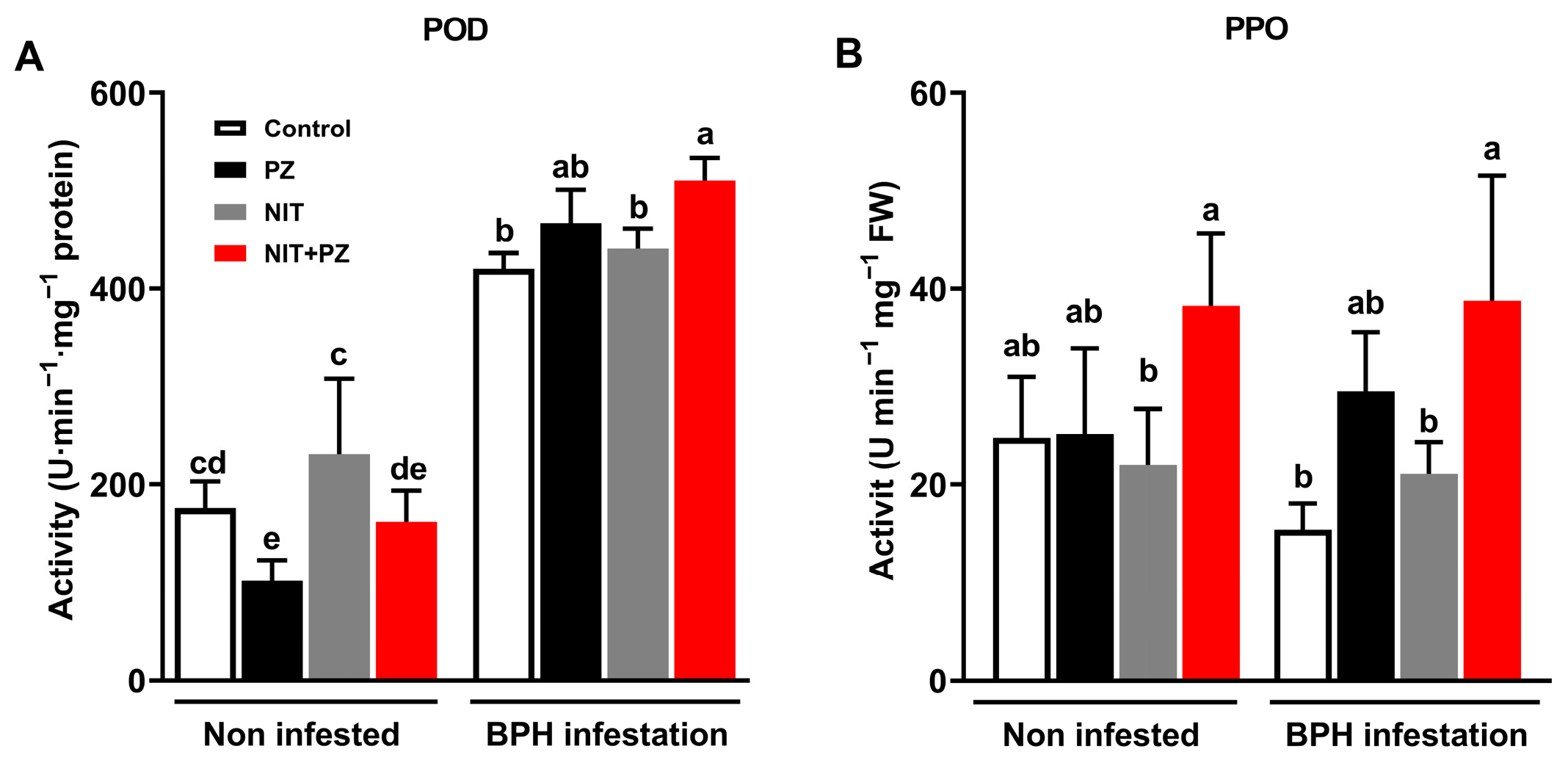
2.5. Paclobutrazol Alters the Biosynthesis of Constitutive and Elicited H2O2 and the Activities of Defense Enzymes
3. Discussion
4. Materials and Methods
4.1. Plants and Insects
4.2. Chemicals
4.3. Procedure of Chemical Solution and Plant Treatments
4.4. Bioassay
4.5. Determination of Total Secondary Metabolites, H2O2 and Defense Enzyme Activity
4.5.1. Secondary Metabolite Analysis
4.5.2. H2O2 Analysis
4.5.3. Defense Enzyme Activity
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPH | Brown planthopper |
| NIT | Nitenpyram |
| PZ | Paclobutrazol |
| PPO | Polyphenol oxidase |
| POD | Peroxidase |
| GA | Gibberellin |
| MDA | Malondialdehyde |
| ZIM | Zinc-finger protein |
| DELLA | Spartic acid (D), glutamic acid (E), leucine (L), leucine (L) and alanine (A) domain |
| WDG | Water-dispersible granules |
| FW | Fresh weight |
| S.E. | Standard error |
| HEPES | 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid |
| EDTA | Ethylene diamine tetraacetic acid |
| GSH | Glutathione |
| DTT | Dithiothreitol |
| PBS | Phosphate buffered saline |
| PVP | Polyvinyl pyrrolidone |
References
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [PubMed]
- Cheng, X.; Zhu, L.; He, G. Towards understanding of molecular interactions between rice and the brown planthopper. Mol. Plant 2013, 6, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Horgan, F.G.; Penalver Cruz, A.; Bernal, C.C.; Ramal, A.F.; Almazan, M.L.P.; Wilby, A. Resistance and tolerance to the brown planthopper, Nilaparvata lugens (Stål), in rice infested at different growth stages across a gradient of nitrogen applications. Field Crops Res. 2018, 217, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.T.; Li, Y.; Li, T.P.; Liang, Y.; Hu, L.; Zhang, D.; Zhou, C.Y.; Yang, C.; Zhang, X.; Zha, S.S.; et al. Stable Introduction of Plant-Virus-Inhibiting Wolbachia into Planthoppers for Rice Protection. Curr. Biol. 2020, 30, 4837–4845.e5. [Google Scholar] [CrossRef]
- Gorman, K.; Liu, Z.; Denholm, I.; Bruggen, K.U.; Nauen, R. Neonicotinoid resistance in rice brown planthopper, Nilaparvata lugens. Pestic. Manag. Sci. 2008, 64, 1122–1125. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Zhu, F.; Li, J.; You, H.; Lu, P. Field evolution of insecticide resistance in the brown planthopper (Nilaparvata lugens Stål) in China. Crop. Prot. 2014, 58, 61–66. [Google Scholar] [CrossRef]
- Minamida, I.; Iwanaga, K.; Tabuchi, T.; Uneme, H.; Dantsuji, H.; Tetsuo, O. Synthesis and insecticidal activity of acyclic nitroethene compounds containing a 3-pyridylmethylamino group. J. Pestic. Sci. 1993, 18, 31–40. [Google Scholar] [CrossRef]
- Liang, P.; Tian, Y.A.; Biondi, A.; Desneux, N.; Gao, X.W. Short-term and transgenerational effects of the neonicotinoid nitenpyram on susceptibility to insecticides in two whitefly species. Ecotoxicology 2012, 21, 1889–1898. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, X.; Ali, E.; Liao, X.; Jin, R.; Ren, Z.; Wan, H.; Li, J. Characterization of nitenpyram resistance in Nilaparvata lugens (Stål). Pestic. Biochem. Physiol. 2019, 157, 26–32. [Google Scholar] [CrossRef]
- Khoa, D.B.; Thang, B.X.; Liem, N.V.; Holst, N.; Kristensen, M. Variation in susceptibility of eight insecticides in the brown planthopper Nilaparvata lugens in three regions of Vietnam 2015-2017. PLoS ONE 2018, 13, e0204962. [Google Scholar] [CrossRef]
- Datta, J.; Wei, Q.; Yang, Q.; Wan, P.-J.; He, J.-C.; Wang, W.-X.; Lai, F.-X.; Ali, M.P.; Fu, Q. Current resistance status of the brown planthopper Nilaparvata lugens (Stål) to commonly used insecticides in China and Bangladesh. Crop. Prot. 2021, 150, 105789. [Google Scholar] [CrossRef]
- Sun, J.; Fang, J.; Xia, L.; Yang, J.; Shen, X. Studies on the insecticidal activity of imidacloprid and its application in paddy fields against the brown planthopper, Nilapavarta lugens) (Homoptera: Delphacidae). Kun Chong Xue Bao Acta Entomol. Sin. 1996, 39, 37–45. [Google Scholar]
- He, J.; Li, B.; Xie, M.; Lai, F.; Hu, G.; Fu, Q. Laboratory Bioactivity Study on Neonicotinoid and Other Rice Paddy Used Insecticides Against the Brown Planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Chin. J. Rice Sci. 2019, 33, 467–478. (In Chinese) [Google Scholar]
- Xing, P.; Duan, M.; Liu, Y.; Mo, Z.; Lai, R.; Tang, X. Enhancement of Yield, Grain Quality Characters, 2-Acetyl-1-Pyrroline Content, and Photosynthesis of Fragrant Rice Cultivars by Foliar Application of Paclobutrazol. J. Plant Growth Regul. 2022, 42, 748–758. [Google Scholar]
- Wu, H.; Chen, H.; Zhang, Y.; Zhang, Y.; Zhu, D.; Xiang, J. Effects of 1-aminocyclopropane-1-carboxylate and paclobutrazol on the endogenous hormones of two contrasting rice varieties under submergence stress. Plant Growth Regul. 2018, 87, 109–121. [Google Scholar] [CrossRef]
- Khunpon, B.; Cha-um, S.; Faiyue, B.; Uthaibutra, J.; Saengnil, K. Paclobutrazol mitigates salt stress in indica rice seedlings by enhancing glutathione metabolism and glyoxalase system. Biologia 2018, 73, 1267–1276. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Kao, C.H. Abscisic acid accumulation and cadmium tolerance in rice seedlings. Physiol. Plant 2005, 124, 71–80. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, P.; Mo, X.; Hu, L.; Jin, N.; Chen, X.; Yu, Z.; Meng, J.; Erb, M.; Shang, Z.; et al. Induction of defense in cereals by 4-fluorophenoxyacetic acid suppresses insect pest populations and increases crop yields in the field. Proc. Natl. Acad. Sci. USA 2020, 117, 12017–12028. [Google Scholar] [CrossRef]
- Wang, W.; Jin, N.; Mo, X.; Wu, J.; Lu, J.; Lou, Y. Exogenous Gibberellin GA3 Enhances Defense Responses in Rice to the Brown Planthopper Nilaparvata lugens (Stål). J. Plant Biol. 2020, 64, 379–387. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Kamran, M.; Cui, W.; Ahmad, I.; Meng, X.; Zhang, X.; Su, W.; Chen, J.; Ahmad, S.; Fahad, S.; Han, Q.; et al. Effect of paclobutrazol, a potential growth regulator on stalk mechanical strength, lignin accumulation and its relation with lodging resistance of maize. Plant Growth Regul. 2017, 84, 317–332. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Chai, M.; Ren, M.; Yuan, J.; Yang, W.; Dong, Y.; Liu, B.; Jian, Q.; Wang, S.; et al. Gibberellins modulate local auxin biosynthesis and polar auxin transport by negatively affecting flavonoid biosynthesis in the root tips of rice. Plant Sci. 2020, 298, 110545. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Feng, L.; Liang, X.; Li, J.; Liao, G.; Zhu, L.; Fu, K.; Fan, W.; Wang, S.; Liu, J. Characterizing the Mechanism of Serotonin Alleviates Rice Resistance to Brown Planthopper Nilaparvata lugens (Homoptera: Delphacidae) Nymphs. Agronomy 2022, 12, 3191. [Google Scholar] [CrossRef]
- Yang, Z.; Li, N.; Kitano, T.; Li, P.; Spindel, J.E.; Wang, L.; Bai, G.; Xiao, Y.; McCouch, S.R.; Ishihara, A.; et al. Genetic mapping identifies a rice naringenin O-glucosyltransferase that influences insect resistance. Plant J. 2021, 106, 1401–1413. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, T.; Wang, W.; Cao, T.; Li, R.; Lou, Y. Silencing OsSLR1 enhances the resistance of rice to the brown planthopper Nilaparvata lugens. Plant Cell Environ. 2017, 40, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Shao, X.; Zhang, Y.; Deng, Y.; Xu, X.; Liu, Z.; Li, Z. Specific Synergist for Neonicotinoid Insecticides: IPPA08, a cis-Neonicotinoid Compound with a Unique Oxabridged Substructure. J. Agric. Food Chem. 2016, 64, 5148–5155. [Google Scholar] [CrossRef] [PubMed]
- Young, S.L. A systematic review of the literature reveals trends and gaps in integrated pest management studies conducted in the United States. Pestic. Manag. Sci. 2017, 73, 1553–1558. [Google Scholar] [CrossRef]
- Huang, S.; Luo, H.; Ashraf, U.; Abrar, M.; He, L.; Zheng, A.; Wang, Z.; Zhang, T.; Tang, X. Seed Treatment with Paclobutrazol Affects Early Growth, Photosynthesis, Chlorophyll Fluorescence and Physiology of Rice. Appl. Ecol. Environ. Res. 2019, 17, 999–1012. [Google Scholar] [CrossRef]
- Ramoutar, D.; Cowles, R.S.; Requintina, E., Jr.; Alm, S.R. Synergism between demethylation inhibitor fungicides or gibberellin inhibitor plant growth regulators and bifenthrin in a pyrethroid-resistant population of Listronotus maculicollis (Coleoptera: Curculionidae). J. Econ. Entomol. 2010, 103, 1810–1814. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef]
- Sasi, M.; Awana, M.; Samota, M.K.; Tyagi, A.; Kumar, S.; Sathee, L.; Krishnan, V.; Praveen, S.; Singh, A. Plant growth regulator induced mitigation of oxidative burst helps in the management of drought stress in rice (Oryza sativa L.). Environ. Exp. Bot. 2021, 185, 104413. [Google Scholar] [CrossRef]
- Hao, P.Y.; Feng, Y.L.; Zhou, Y.S.; Song, X.M.; Li, H.L.; Ma, Y.; Ye, C.L.; Yu, X.P. Schaftoside Interacts With NlCDK1 Protein: A Mechanism of Rice Resistance to Brown Planthopper, Nilaparvata lugens. Front. Plant Sci. 2018, 9, 710. [Google Scholar] [PubMed]
- Araujo, F.F.; Santos, M.N.; Costa, L.C.; Moreira, K.F.; Araujo, M.N.; Martinez, P.A.H.; Finger, F.L. Changes on Potato Leaf Metabolism and Anatomy Induced by Plant Growth Regulators. J. Agric. Sci. 2019, 11, 139–147. [Google Scholar] [CrossRef]
- Dinler, B.S.; Cetinkaya, H.; Sergiev, I.; Shopova, E.; Todorova, D. Paclobutrazol induced non-enzymatic antioxidants and polyamine levels in soybean plants grown under salinity stress. Botanica 2021, 27, 149–159. [Google Scholar] [CrossRef]
- Qi, J.; Li, J.; Han, X.; Li, R.; Wu, J.; Yu, H.; Hu, L.; Xiao, Y.; Lu, J.; Lou, Y. Jasmonic acid carboxyl methyltransferase regulates development and herbivory-induced defense response in rice. J. Integr. Plant Biol. 2016, 58, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Furstenberg-Hagg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef]
- Orozco-Cárdenas, M.L.; Narváez-Vásquez, J.; Ryan, C.A. Hydrogen Peroxide Acts as a Second Messenger for the Induction of Defense Genes in Tomato Plants in Response to Wounding, Systemin, and Methyl Jasmonate. Plant Cell 2001, 13, 179–191. [Google Scholar] [CrossRef]
- Hu, L.; Ye, M.; Li, R.; Lou, Y. OsWRKY53, a versatile switch in regulating herbivore-induced defense responses in rice. Plant Signal. Behav. 2016, 11, e1169357. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Qi, J.; Ren, N.; Cheng, J.; Erb, M.; Mao, B.; Lou, Y. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J. 2009, 60, 638–648. [Google Scholar] [CrossRef]
- Lu, J.; Ju, H.; Zhou, G.; Zhu, C.; Erb, M.; Wang, X.; Wang, P.; Lou, Y. An EAR-motif-containing ERF transcription factor affects herbivore-induced signaling, defense and resistance in rice. Plant J. 2011, 68, 583–596. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Jasmonic Acid-Mediated-Induced Resistance in Groundnut (Arachis hypogaea L.) Against Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J. Plant Growth Regul. 2011, 30, 512–523. [Google Scholar] [CrossRef]
- Singh, I.; Sarao, P.S.; Sharma, N. Antibiosis components and antioxidant defense of rice as mechanism of resistance to brown planthopper, Nilaparvata lugens (Stål). Cereal Res. Commun. 2017, 45, 284–295. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Salem, M.A.; Hasanuzzaman, M.; Nahar, K. Plant growth regulator interactions results enhancement of antioxidant enzymes inCatharanthus roseus. J. Plant Interact. 2010, 5, 135–145. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Zu, H.; Zeng, X.; Baldwin, I.T.; Lou, Y.; Li, R. Molecular dissection of rice phytohormone signaling involved in resistance to a piercing-sucking herbivore. New. Phytol. 2021, 230, 1639–1652. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Yao, J.; Mei, C.S.; Tong, X.H.; Zeng, L.J.; Li, Q.; Xiao, L.T.; Sun, T.P.; Li, J.; Deng, X.W.; et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 2012, 109, E1192–E1200. [Google Scholar] [CrossRef] [PubMed]
- Desta, B.; Amare, G. Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric. 2021, 8, 1. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Bao, Q.; Chu, Y.; Sun, H.; Huang, Y. Jasmonic acid alleviates cadmium toxicity through regulating the antioxidant response and enhancing the chelation of cadmium in rice (Oryza sativa L.). Environ. Pollut. 2022, 304, 119178. [Google Scholar] [CrossRef]
- Wang, W.X.; Lai, F.X.; Wan, P.J.; Fu, Q.; Zhu, T.H. Molecular Characterization of Ca(2+)/Calmodulin-Dependent Protein Kinase II Isoforms in Three Rice Planthoppers-Nilaparvata lugens, Laodelphax striatellus, and Sogatella furcifera. Int. J. Mol. Sci. 2019, 20, 3014. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Kefayati, Z.; Motamed, S.M.; Shojaii, A.; Noori, M.; Ghods, R. Antioxidant Activity and Phenolic and Flavonoid Contents of the Extract and Subfractions of Euphorbia splendida Mobayen. Pharmacogn. Res. 2017, 9, 362–365. [Google Scholar]
- Gu, Z.; Wang, D.; Gong, Q.; You, J.; Ren, Q.; An, H.; Zhou, Y.; Jiang, H. Metabolomic analysis of rapeseed priming with H2O2 in response to germination under chilling stress. Plant Growth Regul. 2022, 99, 477–491. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hong, H.; Chen, L.; Li, J.; Sheng, J.; Shen, L. LeMAPK1, LeMAPK2, and LeMAPK3 are associated with nitric oxide-induced defense response against Botrytis cinerea in the Lycopersicon esculentum fruit. J. Agric. Food Chem. 2014, 62, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Dou, T.; Gao, F.; Wang, G.; Dong, Y.; Song, N.; An, S.; Yin, X.; Liu, X.; Ren, Y. Sublethal Effects of Thiamethoxam on Biological Traits and Detoxification Enzyme Activities in the Small Brown Planthopper, Laodelphax striatellus (Fallen). J. Econ. Entomol. 2022, 115, 2051–2060. [Google Scholar] [CrossRef] [PubMed]

| Day after Treatment | Treatment | Slope ± SE | LC50 (mg/L) 95% C.L. a | χ2 (df) | SR b |
|---|---|---|---|---|---|
| 0 | NIT | 2.01 ± 0.29 | 7.55 (6.50–8.69) | 5.46 (4) | 15.1 |
| PZ + NIT | 2.39 ± 0.41 | 0.50 (0.42–0.58) | 0.66 (7) | ||
| 12 | NIT | 1.21 ± 0.19 | 525.37 (326.95–1076.06) | 15.51 (5) | 4.94 |
| PZ + NIT | 1.46 ± 0.20 | 106.30 (68.89–157.17) | 16.08 (5) |
| Treatment | Phenolic Content/μmol·g−1 FW | Flavonoids Content/mg·g−1 FW | MDA Content/mg·g−1 FW |
|---|---|---|---|
| BPH | 0.000 *** | 0.000 *** | 0.000 *** |
| NIT | 0.002 ** | 0.000 *** | 0.62 |
| PZ | 0.000 *** | 0.000 *** | 0.000 *** |
| BPH×NIT | 0.899 | 0.611 | 0.102 |
| BPH×PZ | 0.000 *** | 0.76 | 0.022 * |
| NIT×PZ | 0.419 | 0.812 | 0.748 |
| BPH×NIT×PZ | 0.592 | 0.296 | 0.984 |
| BPH Treatment | H2O2 6 h after BPH Infestion/μmol g−1 FW | H2O2 12 h after BPH Infestion/μmol g−1 FW | POD Activity/U | PPO Activity/U |
|---|---|---|---|---|
| BPH | 0.000 *** | 0.063 | 0.000 *** | 0.556 |
| NIT | 0.054 | 0.126 | 0.001 ** | 0.010 * |
| PZ | 0.000 *** | 0.000 *** | 0.102 | 0.000 *** |
| BPH × NIT | 0.416 | 0.485 | 0.443 | 0.62 |
| BPH × PZ | 0.138 | 0.805 | 0.198 | 0.109 |
| NIT × PZ | 0.449 | 0.638 | 0.000 *** | 0.042 * |
| BPH × NIT × PZ | 0.701 | 0.221 | 0.940 | 0.188 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Wei, Q.; Wan, P.; Wang, W.; Lai, F.; He, J.; Fu, Q. Effect of Paclobutrazol Application on Enhancing the Efficacy of Nitenpyram against the Brown Planthopper, Nilaparvata lugens. Int. J. Mol. Sci. 2023, 24, 10490. https://doi.org/10.3390/ijms241310490
Zhu X, Wei Q, Wan P, Wang W, Lai F, He J, Fu Q. Effect of Paclobutrazol Application on Enhancing the Efficacy of Nitenpyram against the Brown Planthopper, Nilaparvata lugens. International Journal of Molecular Sciences. 2023; 24(13):10490. https://doi.org/10.3390/ijms241310490
Chicago/Turabian StyleZhu, Xuhui, Qi Wei, Pinjun Wan, Weixia Wang, Fengxiang Lai, Jiachun He, and Qiang Fu. 2023. "Effect of Paclobutrazol Application on Enhancing the Efficacy of Nitenpyram against the Brown Planthopper, Nilaparvata lugens" International Journal of Molecular Sciences 24, no. 13: 10490. https://doi.org/10.3390/ijms241310490
APA StyleZhu, X., Wei, Q., Wan, P., Wang, W., Lai, F., He, J., & Fu, Q. (2023). Effect of Paclobutrazol Application on Enhancing the Efficacy of Nitenpyram against the Brown Planthopper, Nilaparvata lugens. International Journal of Molecular Sciences, 24(13), 10490. https://doi.org/10.3390/ijms241310490







