Prohibitin Links Cell Cycle, Motility and Invasion in Prostate Cancer Cells
Abstract
1. Introduction
2. Results
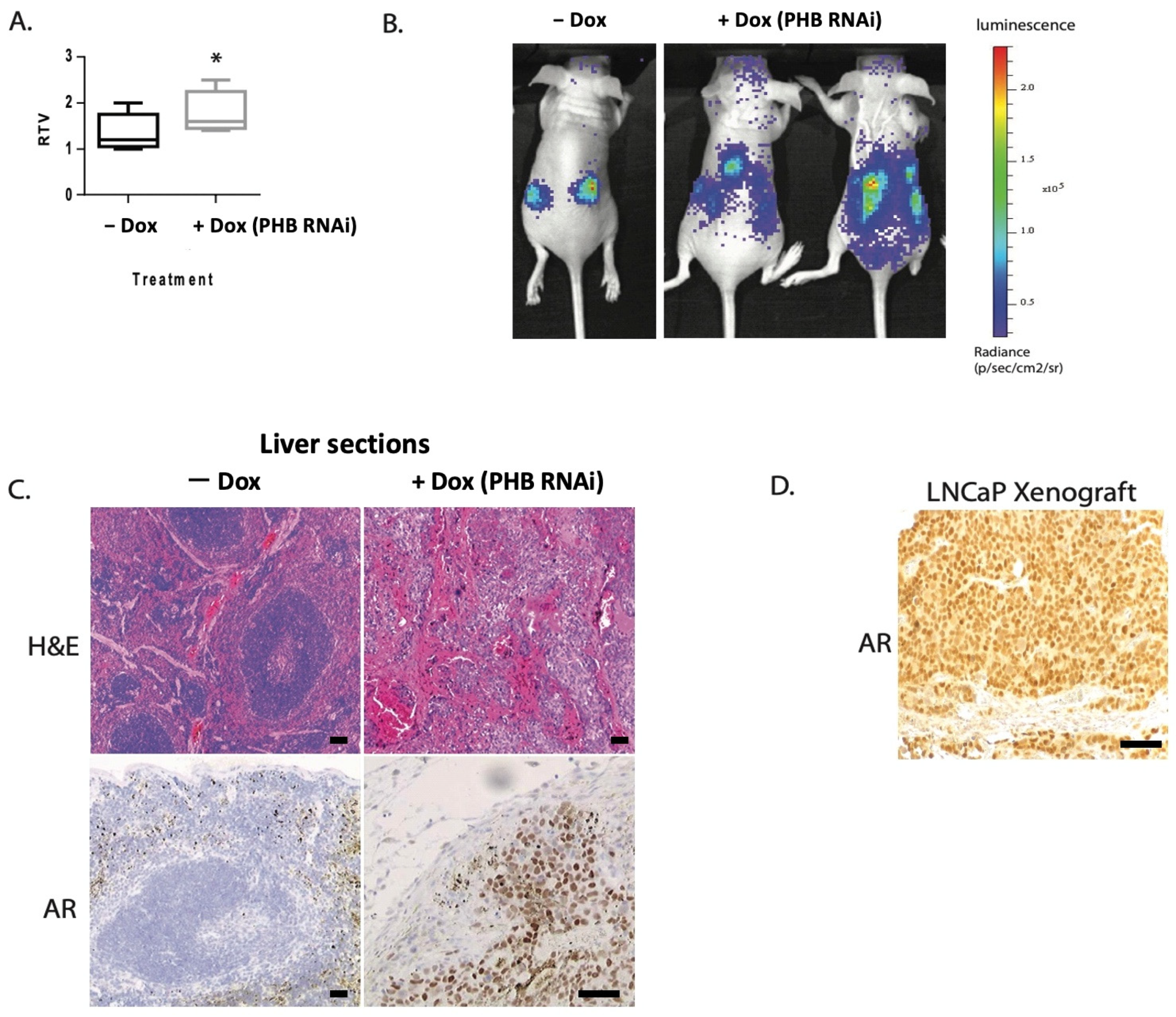
2.1. Doxycycline-Induced PHB siRNA Increases Tumour Growth and Increases Metastatic Potential
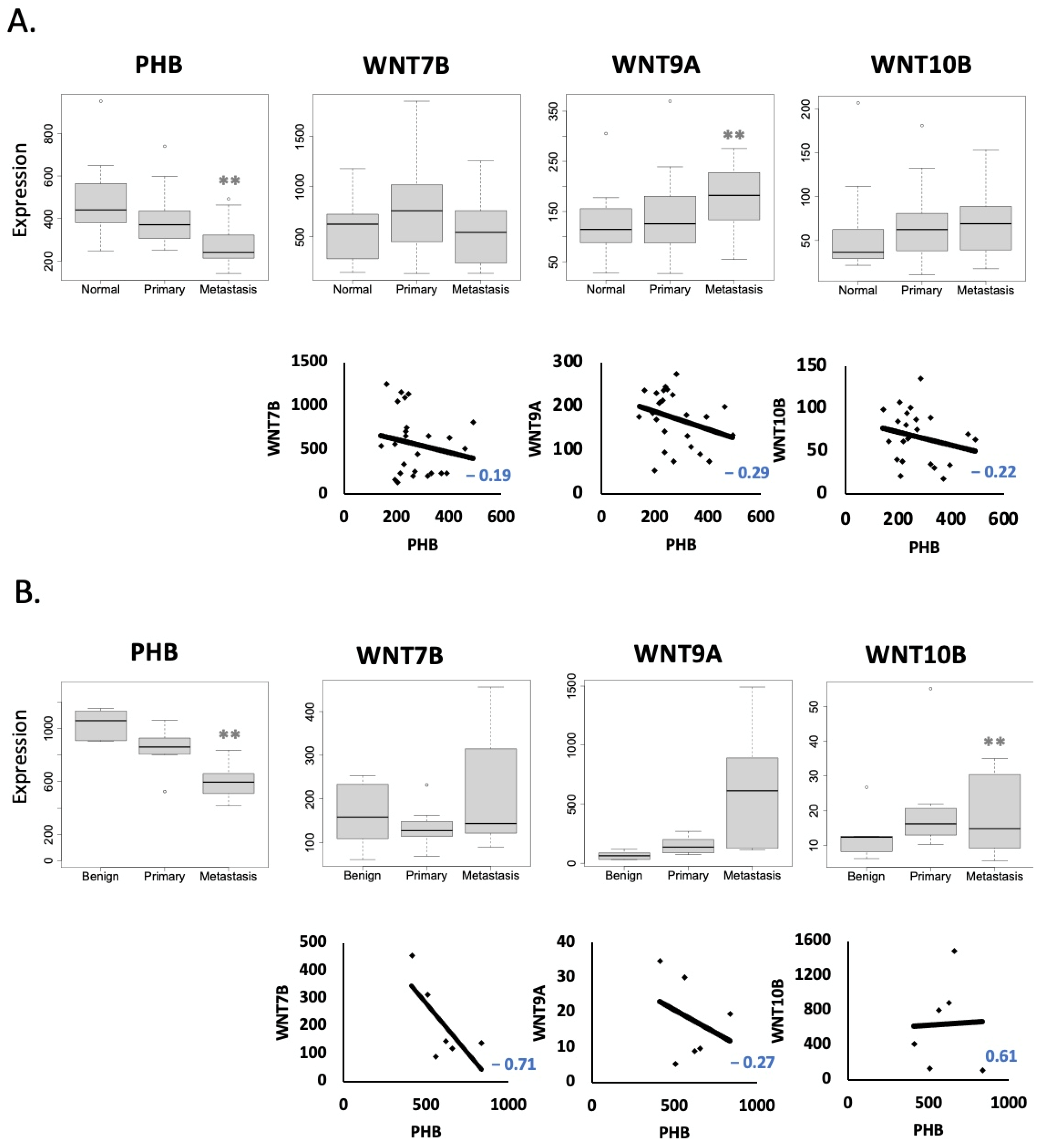
2.2. PHB Expression Is Reduced in Advanced Metastatic PC
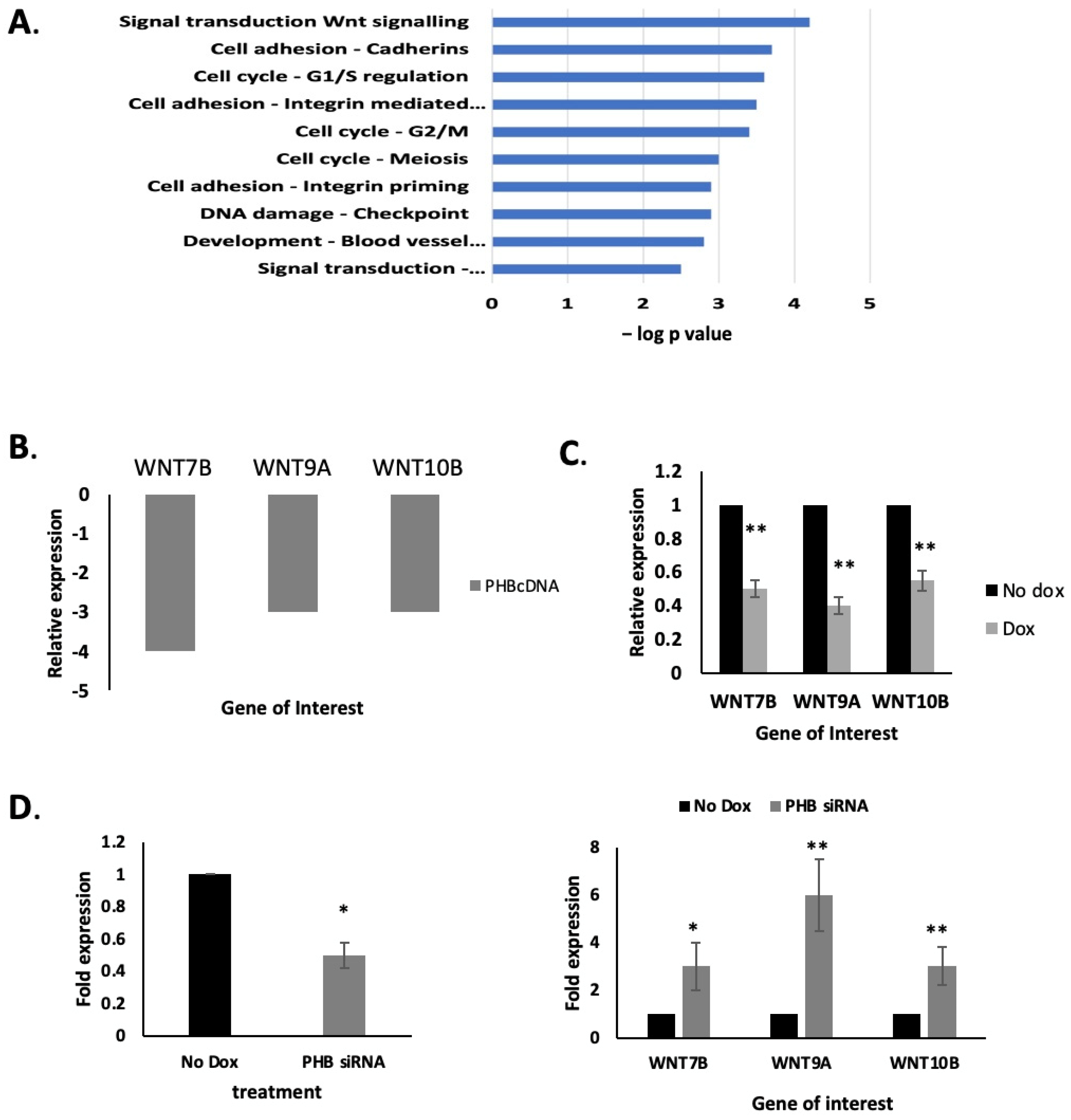
2.3. PHB Manipulation Affects WNT Gene Expression
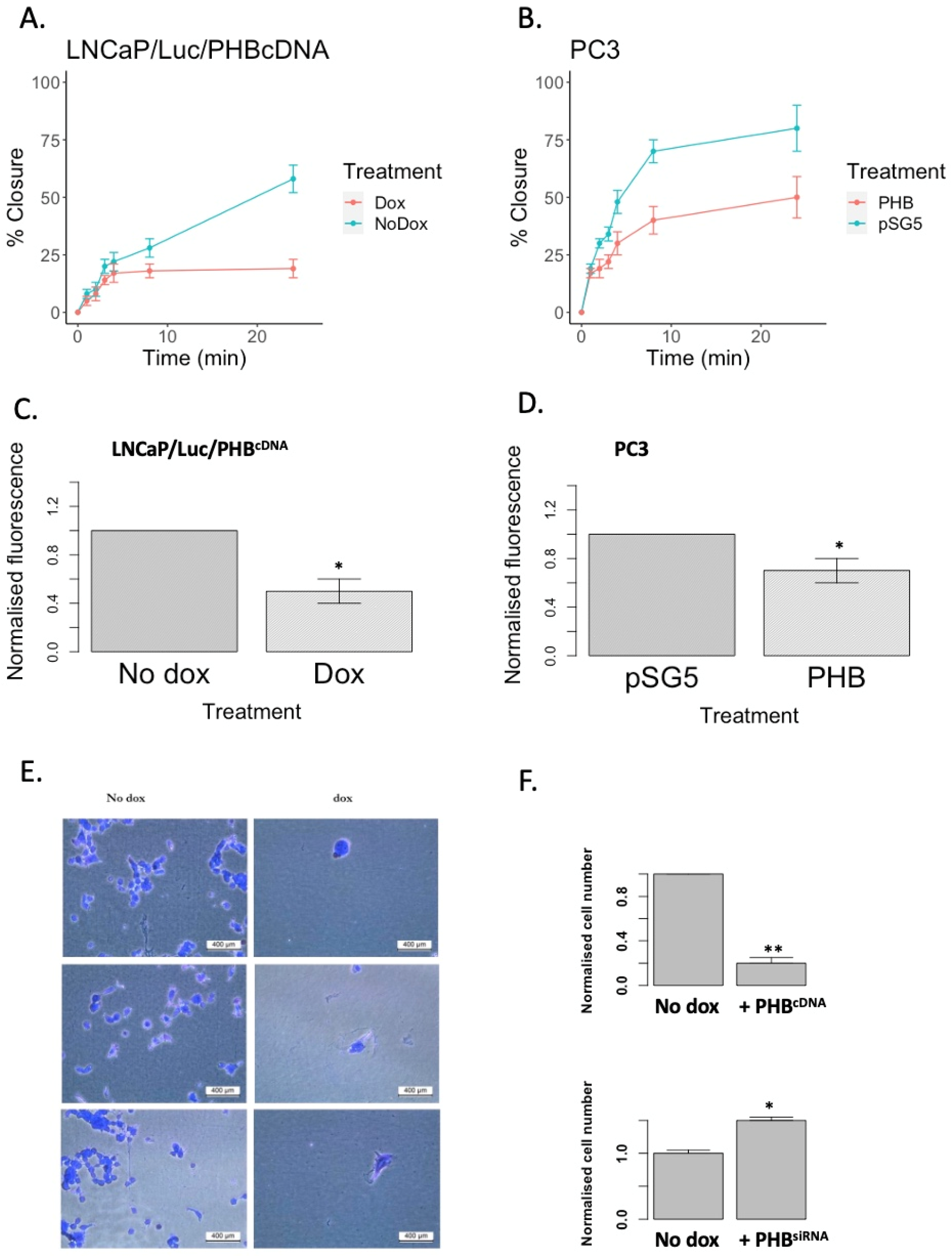
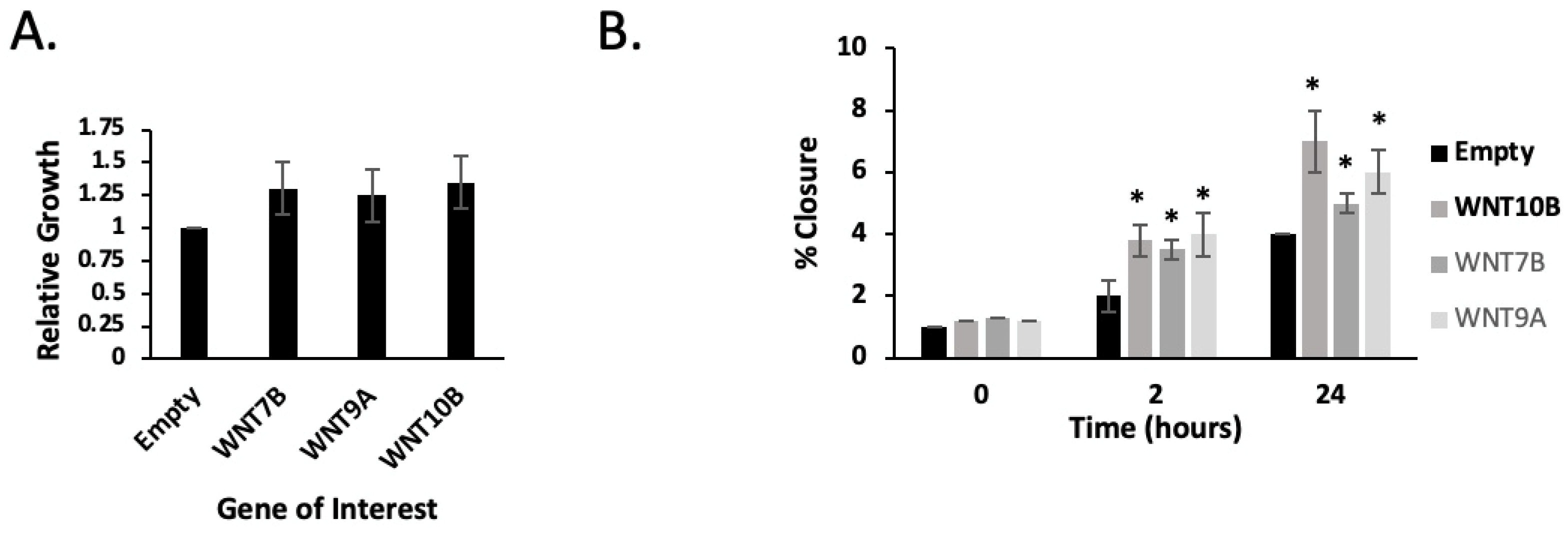
2.4. PHB Overexpression Inhibits the Migration and Adhesion of Both Androgen-Dependent and Androgen-Independent PC Cells
2.5. WNT7B, 9B and WNT10B Are AR- and E2F1-Responsive
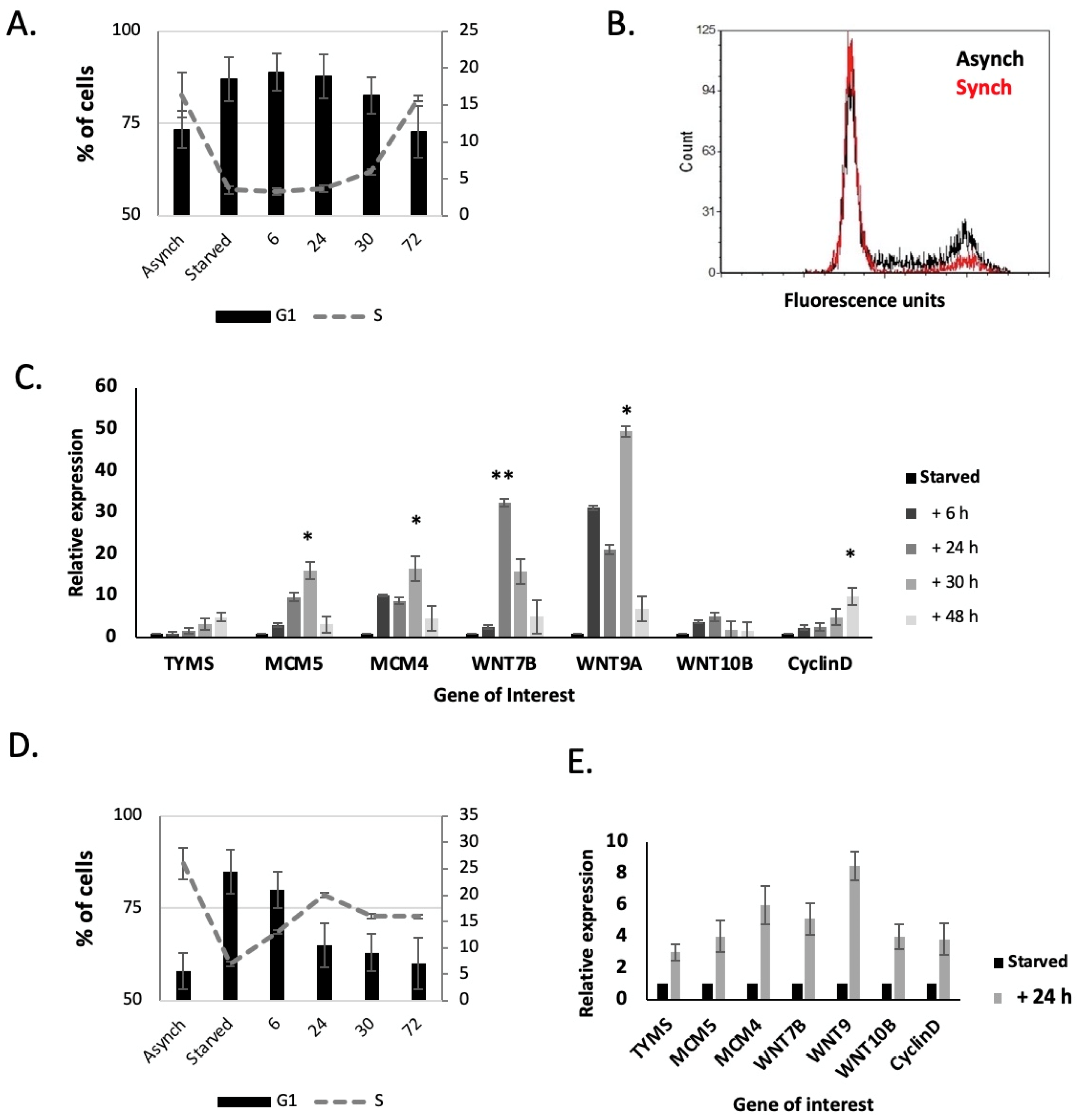
2.6. WNT7B, WNT9A and 10B Expression Are Linked to the Cell Cycle
3. Discussion
4. Materials and Methods
4.1. Cell Line Maintenance
4.2. Transfection and Plasmids
4.3. Cell Synchronization
4.3.1. Isoleucine Synchronization
4.3.2. HeLa Cell Synchronization
4.4. Wound-Healing/Scratch Assay
4.5. Transwell Filter Migration Assay
4.6. Cell to Basement Membrane Adhesion Assay
4.7. RNA Extraction and Quantitative SYBR® Green PCR (Q-PCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gregory, C.W.; He, B.; Johnson, R.T.; Ford, O.H.; Mohler, J.L.; French, F.S.; Wilson, E.M. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001, 61, 4315–4319. [Google Scholar] [PubMed]
- Pisano, C.; Tucci, M.; Di Stefano, R.F.; Turco, F.; Scagliotti, G.V.; Di Maio, M.; Buttigliero, C. Interactions between androgen receptor signaling and other molecular pathways in prostate cancer progression: Current and future clinical implications. Crit. Rev. Oncol. Hematol. 2021, 157, 103185. [Google Scholar] [CrossRef] [PubMed]
- Msaouel, P.; Pissimissis, N.; Halapas, A.; Koutsilieris, M. Mechanisms of bone metastasis in prostate cancer: Clinical implications. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-K.; Dayyani, F.; Gallick, G.E. Steps in prostate cancer progression that lead to bone metastasis. Int. J. Cancer 2011, 128, 2545–2561. [Google Scholar] [CrossRef]
- Dai, C.; Heemers, H.; Sharifi, N. Androgen Signaling in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030452. [Google Scholar] [CrossRef]
- Nuell, M.J.; Stewart, D.A.; Walker, L.; Friedman, V.; Wood, C.M.; Owens, G.A.; Smith, J.R.; Schneider, E.L.; Dell’Orco, R.; Lumpkin, C.K. Prohibitin, an evolutionarily conserved intracellular protein that blocks DNA synthesis in normal fibroblasts and HeLa cells. Mol. Cell. Biol. 1991, 11, 1372–1381. [Google Scholar]
- Nijtmans, L.G.; Artal, S.M.; Grivell, L.A.; Coates, P.J. The mitochondrial PHB complex: Roles in mitochondrial respiratory complex assembly, ageing and degenerative disease. Cell. Mol. Life Sci. 2002, 59, 143–155. [Google Scholar] [CrossRef]
- Wang, S.; Nath, N.; Adlam, M.; Chellappan, S. Prohibitin, a potential tumor suppressor, interacts with RB and regulates E2F function. Oncogene 1999, 18, 3501–3510. [Google Scholar] [CrossRef]
- Koushyar, S.; Economides, G.; Zaat, S.; Jiang, W.; Bevan, C.L.; A Dart, D. The prohibitin-repressive interaction with E2F1 is rapidly inhibited by androgen signalling in prostate cancer cells. Oncogenesis 2017, 6, e333. [Google Scholar] [CrossRef]
- He, B.; Feng, Q.; Mukherjee, A.; Lonard, D.M.; DeMayo, F.J.; Katzenellenbogen, B.S.; Lydon, J.P.; O’Malley, B.W. A repressive role for prohibitin in estrogen signaling. Mol. Endocrinol. 2008, 22, 344–360. [Google Scholar] [CrossRef]
- Gamble, S.C.; Chotai, D.; Odontiadis, M.; A Dart, D.; Brooke, G.N.; Powell, S.M.; Reebye, V.; Varela-Carver, A.; Kawano, Y.; Waxman, J.; et al. Prohibitin, a protein downregulated by androgens, represses androgen receptor activity. Oncogene 2007, 26, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Dart, D.A.; Spencer-Dene, B.; Gamble, S.C.; Waxman, J.; Bevan, C.L. Manipulating prohibitin levels provides evidence for an in vivo role in androgen regulation of prostate tumours. Endocr. Relat. Cancer 2009, 16, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Dart, D.A.; Brooke, G.N.; Sita-Lumsden, A.; Waxman, J.; Bevan, C.L. Reducing prohibitin increases histone acetylation, and promotes androgen independence in prostate tumours by increasing androgen receptor activation by adrenal androgens. Oncogene 2011, 31, 4588–4598. [Google Scholar] [CrossRef]
- Fletcher, C.E.; Dart, D.A.; Sita-Lumsden, A.; Cheng, H.; Rennie, P.S.; Bevan, C.L. Androgen-regulated processing of the oncomir miR-27a, which targets Prohibitin in prostate cancer. Hum. Mol. Genet. 2012, 21, 3112–3127. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gong, S.; Roy-Burman, P.; Lee, P.; Culig, Z. Current mouse and cell models in prostate cancer research. Endocr. Relat. Cancer 2013, 20, R155–R170. [Google Scholar] [CrossRef]
- Byrne, N.M.; Nesbitt, H.; Ming, L.; McKeown, S.R.; Worthington, J.; McKenna, D.J. Androgen deprivation in LNCaP prostate tumour xenografts induces vascular changes and hypoxic stress, resulting in promotion of epithelial-to-mesenchymal transition. Br. J. Cancer 2016, 114, 659–668. [Google Scholar] [CrossRef]
- Chandran, U.R.; Maureen, L.-W.; Dhir, R.; Bisceglia, M.; Lyons-Weiler, M.; Liang, W.; Michalopoulos, G.; Becich, M.; A Monzon, F. Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer 2007, 7, 64. [Google Scholar] [CrossRef]
- Varambally, S.; Yu, J.; Laxman, B.; Rhodes, D.R.; Mehra, R.; Tomlins, S.A.; Shah, R.B.; Chandran, U.; Monzon, F.A.; Becich, M.J.; et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell 2005, 8, 393–406. [Google Scholar] [CrossRef]
- Marín-Aguilera, M.; Codony-Servat, J.; Kalko, S.G.; Fernández, P.L.; Bermudo, R.; Buxo, E.; Ribal, M.J.; Gascón, P.; Mellado, B. Identification of docetaxel resistance genes in castration-resistant prostate cancer. Mol. Cancer Ther. 2012, 11, 329–339. [Google Scholar] [CrossRef]
- Wu, Y.; Grabsch, H.; Ivanova, T.; Tan, I.B.; Murray, J.; Ooi, C.-H.; Wright, A.I.; West, N.P.; Hutchins, G.G.A.; Wu, J.; et al. Comprehensive genomic meta-analysis identifies intra-tumoural stroma as a predictor of survival in patients with gastric cancer. Gut 2013, 62, 1100–1111. [Google Scholar] [CrossRef]
- D’Antonio, J.M.; Griend, D.V.; Isaacs, J.T. DNA licensing as a novel androgen receptor mediated therapeutic target for prostate cancer. Endocr. Relat. Cancer 2009, 16, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Balk, S.P.; Knudsen, K.E. AR, the cell cycle, and prostate cancer. Nucl. Recept. Signal. 2008, 6, e001. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.E.; D’souza-Schorey, C. WNT Signaling in Cell Motility and Invasion: Drawing Parallels between Development and Cancer. Cancers 2016, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Vincan, E. The WNT signaling pathways and cell adhesion. Front. Biosci. (Landmark Ed.) 2012, 17, 784–804. [Google Scholar] [CrossRef]
- Koushyar, S.; Meniel, V.S.; Phesse, T.J.; Pearson, H.B. Exploring the WNT Pathway as a Therapeutic Target for Prostate Cancer. Biomolecules 2022, 12, 309. [Google Scholar] [CrossRef]
- Zheng, D.; Decker, K.F.; Zhou, T.; Chen, J.; Qi, Z.; Jacobs, K.; Weilbaecher, K.N.; Corey, E.; Long, F.; Jia, L. Role of WNT7B-induced noncanonical pathway in advanced prostate cancer. Mol. Cancer Res. 2013, 11, 482–493. [Google Scholar] [CrossRef]
- Hu, M.; Xie, J.; Liu, Z.; Wang, X.; Liu, M.; Wang, J. Comprehensive Analysis Identifying WNT Ligands Gene Family for Biochemical Recurrence in Prostate Adenocarcinoma and Construction of a Nomogram. J. Comput. Biol. 2020, 27, 1656–1667. [Google Scholar] [CrossRef]
- Madueke, I.; Hu, W.-Y.; Hu, D.; Swanson, S.M.; Griend, D.V.; Abern, M.; Prins, G.S. The role of WNT10B in normal prostate gland development and prostate cancer. Prostate 2019, 79, 1692–1704. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koushyar, S.; Uysal-Onganer, P.; Jiang, W.G.; Dart, D.A. Prohibitin Links Cell Cycle, Motility and Invasion in Prostate Cancer Cells. Int. J. Mol. Sci. 2023, 24, 9919. https://doi.org/10.3390/ijms24129919
Koushyar S, Uysal-Onganer P, Jiang WG, Dart DA. Prohibitin Links Cell Cycle, Motility and Invasion in Prostate Cancer Cells. International Journal of Molecular Sciences. 2023; 24(12):9919. https://doi.org/10.3390/ijms24129919
Chicago/Turabian StyleKoushyar, Sarah, Pinar Uysal-Onganer, Wen Guo Jiang, and Dafydd Alwyn Dart. 2023. "Prohibitin Links Cell Cycle, Motility and Invasion in Prostate Cancer Cells" International Journal of Molecular Sciences 24, no. 12: 9919. https://doi.org/10.3390/ijms24129919
APA StyleKoushyar, S., Uysal-Onganer, P., Jiang, W. G., & Dart, D. A. (2023). Prohibitin Links Cell Cycle, Motility and Invasion in Prostate Cancer Cells. International Journal of Molecular Sciences, 24(12), 9919. https://doi.org/10.3390/ijms24129919








