Significance of Type II Collagen Posttranslational Modifications: From Autoantigenesis to Improved Diagnosis and Treatment of Rheumatoid Arthritis
Abstract
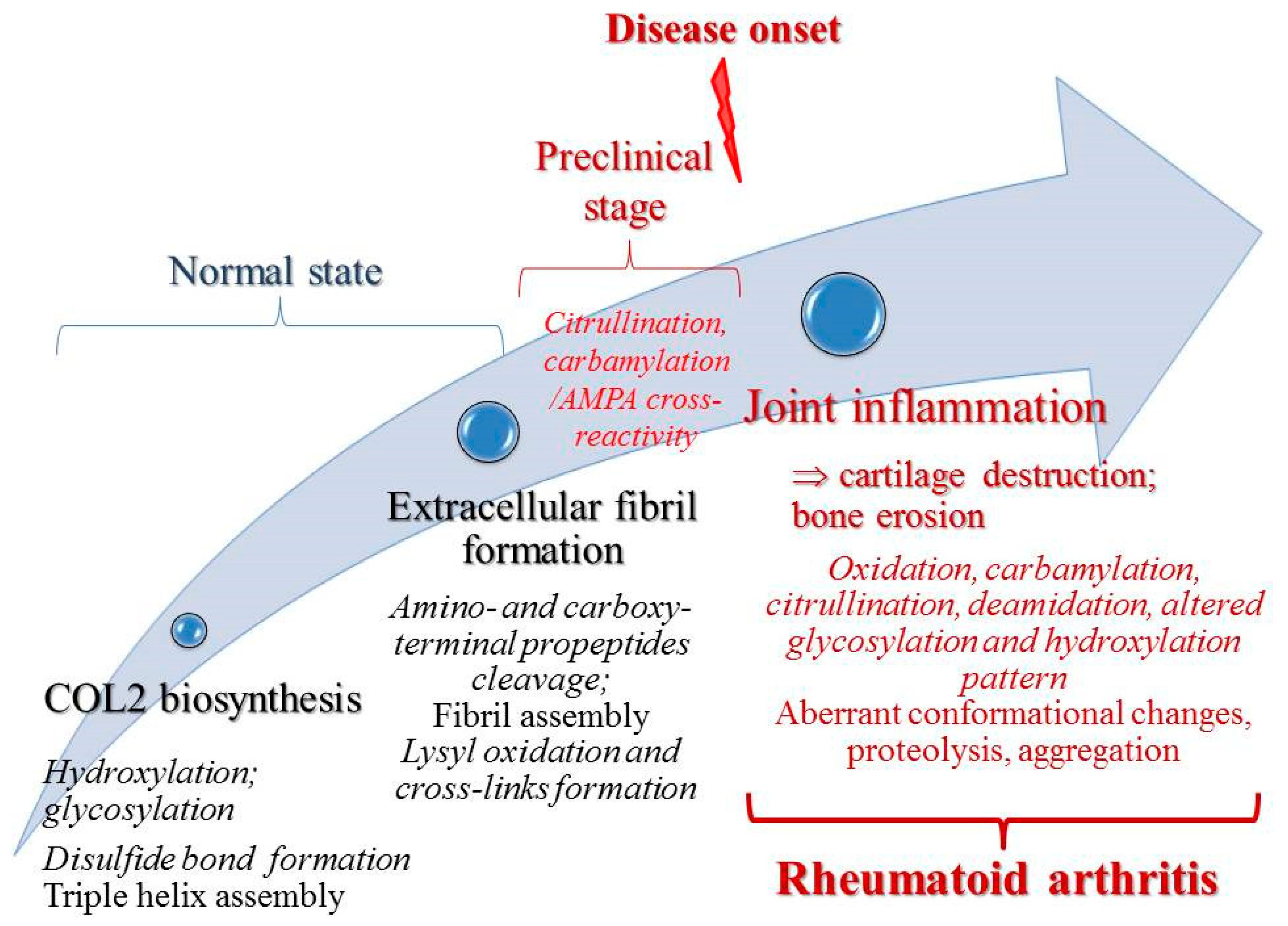
1. Introduction
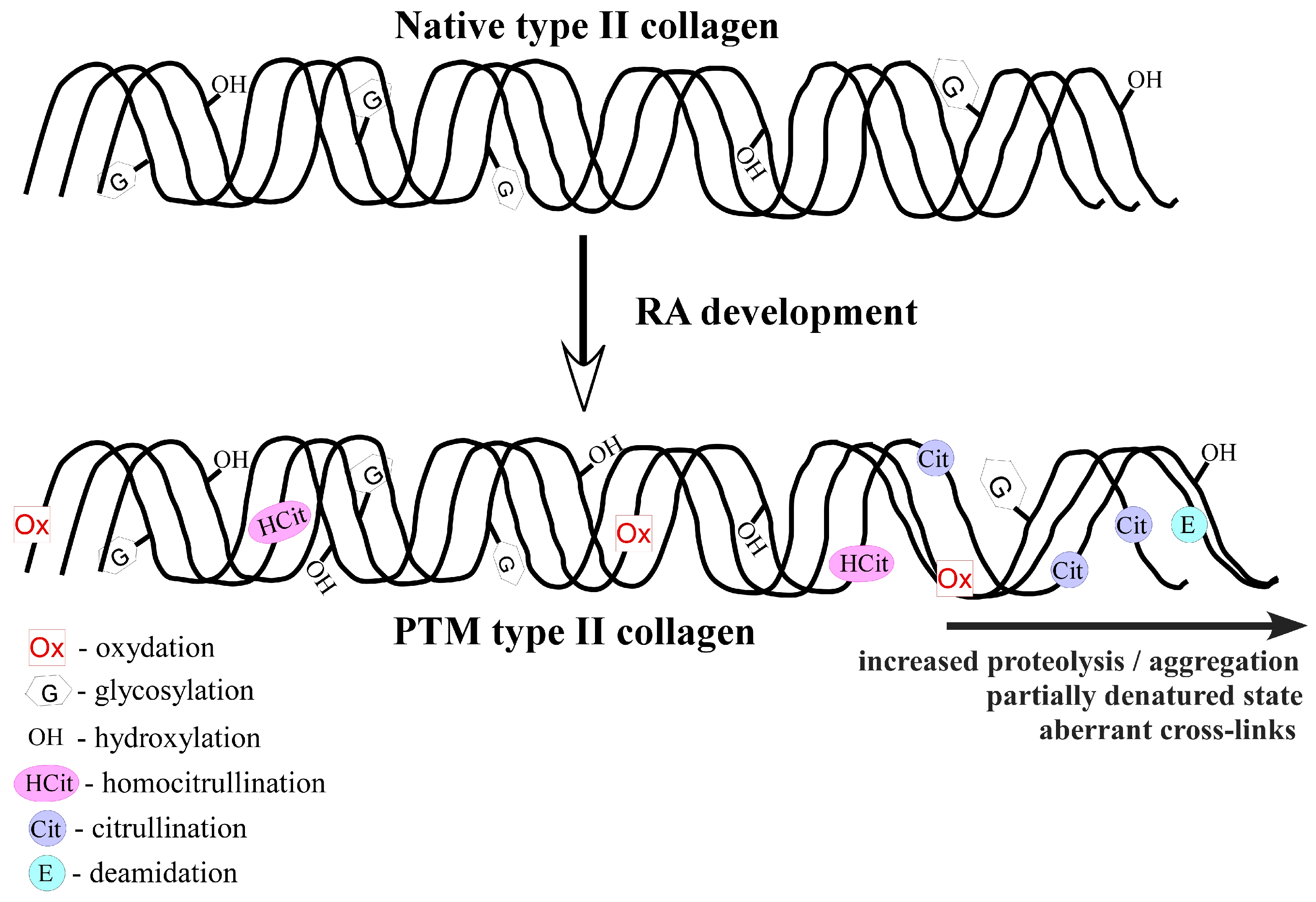
2. Collagen Type II (COL2) Posttranslational Modifications in Rheumatoid Arthritis
2.1. Hydroxylation
2.2. Glycosylation
2.3. Citrullination
2.4. Carbamylation
2.5. Oxidative Modifications
Glycation
2.6. Deamidation
2.7. Candidate Modifications/Other Modifications
3. COL2 PTMs Contribution to RA Diagnosis and Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scherer, H.U.; Haupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef]
- Figus, F.A.; Piga, M.; Azzolin, I.; McConnell, R.; Iagnocco, A. Rheumatoid arthritis: Extra-articular manifestations and comorbidities. Autoimmun. Rev. 2021, 20, 102776. [Google Scholar] [CrossRef]
- Deane, K.D.; Holers, V.M. The Natural History of Rheumatoid Arthritis. Clin. Ther. 2019, 41, 1256–1269. [Google Scholar] [CrossRef]
- Debreova, M.; Culenova, M.; Smolinska, V.; Nicodemou, A.; Csobonyeiova, M.; Danisovic, L. Rheumatoid arthritis: From synovium biology to cell-based therapy. Cytotherapy 2022, 24, 365–375. [Google Scholar] [CrossRef]
- Cheng, C.F.; Liao, H.J.; Wu, C.S. Tissue microenvironment dictates inflammation and disease activity in rheumatoid arthritis. J. Formos. Med. Assoc. Taiwan Yi Zhi 2022, 121, 1027–1033. [Google Scholar] [CrossRef]
- Mueller, A.L.; Payandeh, Z.; Mohammadkhani, N.; Mubarak, S.M.H.; Zakeri, A.; Alagheband Bahrami, A.; Brockmueller, A.; Shakibaei, M. Recent Advances in Understanding the Pathogenesis of Rheumatoid Arthritis: New Treatment Strategies. Cells 2021, 10, 3017. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. The immunology of rheumatoid arthritis. Nat. Immunol. 2021, 22, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Karami, J.; Aslani, S.; Jamshidi, A.; Garshasbi, M.; Mahmoudi, M. Genetic implications in the pathogenesis of rheumatoid arthritis; an updated review. Gene 2019, 702, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Prim. 2018, 4, 18001. [Google Scholar] [CrossRef]
- Shams, S.; Martinez, J.M.; Dawson, J.R.D.; Flores, J.; Gabriel, M.; Garcia, G.; Guevara, A.; Murray, K.; Pacifici, N.; Vargas, M.V.; et al. The Therapeutic Landscape of Rheumatoid Arthritis: Current State and Future Directions. Front. Pharmacol. 2021, 12, 680043. [Google Scholar] [CrossRef]
- Sandhu, G.; Thelma, B.K. New Druggable Targets for Rheumatoid Arthritis Based on Insights from Synovial Biology. Front. Immunol. 2022, 13, 834247. [Google Scholar] [CrossRef]
- Nel, H.J.; Malmstrom, V.; Wraith, D.; Thomas, R. Autoantigens in rheumatoid arthritis and the potential for antigen-specific tolerising immunotherapy. Lancet Rheumatol. 2020, 2, E712–E723. [Google Scholar] [CrossRef]
- Poulsen, T.B.G.; Damgaard, D.; Jorgensen, M.M.; Senolt, L.; Blackburn, J.M.; Nielsen, C.H.; Stensballe, A. Identification of Novel Native Autoantigens in Rheumatoid Arthritis. Biomedicines 2020, 8, 141. [Google Scholar] [CrossRef]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, M.V.; Schett, G.; Steffen, U. Autoantibodies in Rheumatoid Arthritis: Historical Background and Novel Findings. Clin. Rev. Allergy Immunol. 2022, 63, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; Stohl, W.; Benoit, V.; Tan, S.M.; Johansen, L.; Ditzel, H.J. Patients with inflammatory arthritic diseases harbor elevated serum and synovial fluid levels of free and immune-complexed glucose-6-phosphate isomerase (G6PI). Biochem. Biophys. Res. Commun. 2006, 349, 838–845. [Google Scholar] [CrossRef]
- Konig, M.F.; Giles, J.T.; Nigrovic, P.A.; Andrade, F. Antibodies to native and citrullinated RA33 (hnRNP A2/B1) challenge citrullination as the inciting principle underlying loss of tolerance in rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 2022–2028. [Google Scholar] [CrossRef]
- Fritsch, R.; Eselbock, D.; Skriner, K.; Jahn-Schmid, B.; Scheinecker, C.; Bohle, B.; Tohidast-Akrad, M.; Hayer, S.; Neumuller, J.; Pinol-Roma, S.; et al. Characterization of autoreactive T cells to the autoantigens heterogeneous nuclear ribonucleoprotein A2 (RA33) and filaggrin in patients with rheumatoid arthritis. J. Immunol. 2002, 169, 1068–1076. [Google Scholar] [CrossRef]
- Onuora, S. Immunology: BiP peptides induce epitope-specific T-cell reactions in RA. Nat. Rev. Rheumatol. 2015, 11, 259. [Google Scholar] [CrossRef]
- Corsiero, E.; Pratesi, F.; Prediletto, E.; Bombardieri, M.; Migliorini, P. NETosis as Source of Autoantigens in Rheumatoid Arthritis. Front. Immunol. 2016, 7, 485. [Google Scholar] [CrossRef] [PubMed]
- van Venrooij, W.J.; Pruijn, G.J. Citrullination: A small change for a protein with great consequences for rheumatoid arthritis. Arthritis Res. 2000, 2, 249–251. [Google Scholar] [CrossRef]
- Song, J.; Schwenzer, A.; Wong, A.; Turcinov, S.; Rims, C.; Martinez, L.R.; Arribas-Layton, D.; Gerstner, C.; Muir, V.S.; Midwood, K.S.; et al. Shared recognition of citrullinated tenascin-C peptides by T and B cells in rheumatoid arthritis. JCI Insight 2021, 6, 145217. [Google Scholar] [CrossRef]
- Manivel, V.A.; Mullazehi, M.; Padyukov, L.; Westerlind, H.; Klareskog, L.; Alfredsson, L.; Saevarsdottir, S.; Ronnelid, J. Anticollagen type II antibodies are associated with an acute onset rheumatoid arthritis phenotype and prognosticate lower degree of inflammation during 5 years follow-up. Ann. Rheum. Dis. 2017, 76, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, S.F.; Stockman, A.; Rowley, M.J. Collagen Autoantibodies and Their Relationship to CCP Antibodies and Rheumatoid Factor in the Progression of Early Rheumatoid Arthritis. Antibodies 2017, 6, 6. [Google Scholar] [CrossRef]
- Kim, W.U.; Cho, M.L.; Jung, Y.O.; Min, S.Y.; Park, S.W.; Min, D.J.; Yoon, J.H.; Kim, H.Y. Type II collagen autoimmunity in rheumatoid arthritis. Am. J. Med. Sci. 2004, 327, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Hurysz, B.; Bottini, N. Emerging proteoglycans and proteoglycan-targeted therapies in rheumatoid arthritis. Am. J. Physiol. Cell Physiol. 2022, 322, C1061–C1067. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhang, Y.; Thiel, A.; Rudwaleit, M.; Shi, S.L.; Radbruch, A.; Poole, R.; Braun, J.; Sieper, J. Predominant cellular immune response to the cartilage autoantigenic G1 aggrecan in ankylosing spondylitis and rheumatoid arthritis. Rheumatology 2003, 42, 846–855. [Google Scholar] [CrossRef]
- Tizaoui, K.; Yang, J.W.; Lee, K.H.; Kim, J.H.; Kim, M.; Yoon, S.; Jung, Y.; Park, J.B.; An, K.; Choi, H.; et al. The role of YKL-40 in the pathogenesis of autoimmune diseases: A comprehensive review. Int. J. Biol. Sci. 2022, 18, 3731–3746. [Google Scholar] [CrossRef]
- Ge, C.; Tong, D.; Lonnblom, E.; Liang, B.; Cai, W.; Fahlquist-Hagert, C.; Li, T.; Kastbom, A.; Gjertsson, I.; Dobritzsch, D.; et al. Antibodies to Cartilage Oligomeric Matrix Protein Are Pathogenic in Mice and May Be Clinically Relevant in Rheumatoid Arthritis. Arthritis Rheumatol. 2022, 74, 961–971. [Google Scholar] [CrossRef]
- Courtenay, J.S.; Dallman, M.J.; Dayan, A.D.; Martin, A.; Mosedale, B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature 1980, 283, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Brand, D.D.; Kang, A.H.; Rosloniec, E.F. Immunopathogenesis of collagen arthritis. Springer Semin. Immunopathol. 2003, 25, 3–18. [Google Scholar] [CrossRef]
- Kim, W.U.; Yoo, W.H.; Park, W.; Kang, Y.M.; Kim, S.I.; Park, J.H.; Lee, S.S.; Joo, Y.S.; Min, J.K.; Hong, Y.S.; et al. IgG antibodies to type II collagen reflect inflammatory activity in patients with rheumatoid arthritis. J. Rheumatol. 2000, 27, 575–581. [Google Scholar] [PubMed]
- Nandakumar, K.S. Pathogenic antibody recognition of cartilage. Cell Tissue Res. 2010, 339, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Manivel, V.A.; Sohrabian, A.; Wick, M.C.; Mullazehi, M.; Hakansson, L.D.; Ronnelid, J. Anti-type II collagen immune complex-induced granulocyte reactivity is associated with joint erosions in RA patients with anti-collagen antibodies. Arthritis Res. Ther. 2015, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Di Sante, G.; Tolusso, B.; Fedele, A.L.; Gremese, E.; Alivernini, S.; Nicolo, C.; Ria, F.; Ferraccioli, G. Collagen Specific T-Cell Repertoire and HLA-DR Alleles: Biomarkers of Active Refractory Rheumatoid Arthritis. EBioMedicine 2015, 2, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Snir, O.; Backlund, J.; Bostrom, J.; Andersson, I.; Kihlberg, J.; Buckner, J.H.; Klareskog, L.; Holmdahl, R.; Malmstrom, V. Multifunctional T cell reactivity with native and glycosylated type II collagen in rheumatoid arthritis. Arthritis Rheum. 2012, 64, 2482–2488. [Google Scholar] [CrossRef]
- Backlund, J.; Carlsen, S.; Hoger, T.; Holm, B.; Fugger, L.; Kihlberg, J.; Burkhardt, H.; Holmdahl, R. Predominant selection of T cells specific for the glycosylated collagen type II epitope (263–270) in humanized transgenic mice and in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2002, 99, 9960–9965. [Google Scholar] [CrossRef]
- Sekine, T.; Kato, T.; Masuko-Hongo, K.; Nakamura, H.; Yoshino, S.; Nishioka, K.; Yamamoto, K. Type II collagen is a target antigen of clonally expanded T cells in the synovium of patients with rheumatoid arthritis. Ann. Rheum. Dis. 1999, 58, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.U.; Kim, K.J. T cell proliferative response to type II collagen in the inflammatory process and joint damage in patients with rheumatoid arthritis. J. Rheumatol. 2005, 32, 225–230. [Google Scholar]
- Dzhambazov, B.; Nandakumar, K.S.; Kihlberg, J.; Fugger, L.; Holmdahl, R.; Vestberg, M. Therapeutic vaccination of active arthritis with a glycosylated collagen type II peptide in complex with MHC class II molecules. J. Immunol. 2006, 176, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Long, J.; Liang, F.; Liu, N.; Sun, Y.; Xi, Y. Different protective efficacies of a novel antigen-specific DNA vaccine encoding chicken type II collagen via intramuscular, subcutaneous, and intravenous vaccination against experimental rheumatoid arthritis. Biomed. Pharmacother. 2021, 144, 112294. [Google Scholar] [CrossRef]
- Hou, C.; Li, N.; Liu, M.; Chen, J.; Elango, J.; Rahman, S.U.; Bao, B.; Wu, W. Therapeutic Effect of Nile Tilapia Type II Collagen on Rigidity in CD8+ Cells by Alleviating Inflammation and Rheumatoid Arthritis in Rats by Oral Tolerance. Polymers 2022, 14, 1284. [Google Scholar] [CrossRef]
- Clemente-Casares, X.; Blanco, J.; Ambalavanan, P.; Yamanouchi, J.; Singha, S.; Fandos, C.; Tsai, S.; Wang, J.; Garabatos, N.; Izquierdo, C.; et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 2016, 530, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, L.L.; Xu, J.H.; Xiao, F.; Bao, C.D.; Ni, L.Q.; Li, X.F.; Wu, Y.Q.; Sun, L.Y.; Zhang, R.H.; et al. A multicenter, double-blind, randomized, controlled phase III clinical trial of chicken type II collagen in rheumatoid arthritis. Arthritis Res. Ther. 2009, 11, R180. [Google Scholar] [CrossRef] [PubMed]
- Trentham, D.E.; Dynesius-Trentham, R.A.; Orav, E.J.; Combitchi, D.; Lorenzo, C.; Sewell, K.L.; Hafler, D.A.; Weiner, H.L. Effects of oral administration of type II collagen on rheumatoid arthritis. Science 1993, 261, 1727–1730. [Google Scholar] [CrossRef]
- Barnett, M.L.; Kremer, J.M.; St Clair, E.W.; Clegg, D.O.; Furst, D.; Weisman, M.; Fletcher, M.J.; Chasan-Taber, S.; Finger, E.; Morales, A.; et al. Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 1998, 41, 290–297. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958. [Google Scholar] [CrossRef]
- Leitinger, B. Transmembrane collagen receptors. Annu. Rev. Cell Dev. Biol. 2011, 27, 265–290. [Google Scholar] [CrossRef]
- Myllyharju, J.; Kivirikko, K.I. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. TIG 2004, 20, 33–43. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Yamauchi, M.; Sricholpech, M. Lysine post-translational modifications of collagen. Essays Biochem. 2012, 52, 113–133. [Google Scholar] [CrossRef]
- Walsh, C.T.; Garneau-Tsodikova, S.; Gatto, G.J., Jr. Protein posttranslational modifications: The chemistry of proteome diversifications. Angew. Chem. Int. Ed. 2005, 44, 7342–7372. [Google Scholar] [CrossRef] [PubMed]
- Khoury, G.A.; Baliban, R.C.; Floudas, C.A. Proteome-wide post-translational modification statistics: Frequency analysis and curation of the swiss-prot database. Sci. Rep. 2011, 1, 90. [Google Scholar] [CrossRef]
- Craveur, P.; Narwani, T.J.; Rebehmed, J.; de Brevern, A.G. Investigation of the impact of PTMs on the protein backbone conformation. Amino Acids 2019, 51, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Doyle, H.A.; Mamula, M.J. Posttranslational modifications of self-antigens. Ann. N. Y. Acad. Sci. 2005, 1050, 1–9. [Google Scholar] [CrossRef]
- Eggleton, P.; Haigh, R.; Winyard, P.G. Consequence of neo-antigenicity of the ‘altered self’. Rheumatology 2008, 47, 567–571. [Google Scholar] [CrossRef]
- Anderton, S.M. Post-translational modifications of self antigens: Implications for autoimmunity. Curr. Opin. Immunol. 2004, 16, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.J.; Ju, J.H. Impact of Posttranslational Modification in Pathogenesis of Rheumatoid Arthritis: Focusing on Citrullination, Carbamylation, and Acetylation. Int. J. Mol. Sci. 2021, 22, 10576. [Google Scholar] [CrossRef]
- Becart, S.; Whittington, K.B.; Prislovsky, A.; Rao, N.L.; Rosloniec, E.F. The role of posttranslational modifications in generating neo-epitopes that bind to rheumatoid arthritis-associated HLA-DR alleles and promote autoimmune T cell responses. PLoS ONE 2021, 16, e0245541. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Trouw, L.A.; Rispens, T.; Toes, R.E.M. Beyond citrullination: Other post-translational protein modifications in rheumatoid arthritis. Nat. Rev. Rheumatol. 2017, 13, 331–339. [Google Scholar] [CrossRef]
- Steen, J.; Forsstrom, B.; Sahlstrom, P.; Odowd, V.; Israelsson, L.; Krishnamurthy, A.; Badreh, S.; Mathsson Alm, L.; Compson, J.; Ramskold, D.; et al. Recognition of Amino Acid Motifs, Rather Than Specific Proteins, by Human Plasma Cell-Derived Monoclonal Antibodies to Posttranslationally Modified Proteins in Rheumatoid Arthritis. Arthritis Rheumatol. 2019, 71, 196–209. [Google Scholar] [CrossRef]
- Moten, D.; Teneva, I.; Apostolova, D.; Batsalova, T.; Dzhambazov, B. Molecular Mimicry of the Rheumatoid Arthritis-Related Immunodominant T-Cell Epitope within Type II Collagen (CII260-270) by the Bacterial L-Asparaginase. Int. J. Mol. Sci. 2022, 23, 9149. [Google Scholar] [CrossRef] [PubMed]
- Raposo, B.; Merky, P.; Lundqvist, C.; Yamada, H.; Urbonaviciute, V.; Niaudet, C.; Viljanen, J.; Kihlberg, J.; Kyewski, B.; Ekwall, O.; et al. T cells specific for post-translational modifications escape intrathymic tolerance induction. Nat. Commun. 2018, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Corthay, A.; Backlund, J.; Broddefalk, J.; Michaelsson, E.; Goldschmidt, T.J.; Kihlberg, J.; Holmdahl, R. Epitope glycosylation plays a critical role for T cell recognition of type II collagen in collagen-induced arthritis. Eur. J. Immunol. 1998, 28, 2580–2590. [Google Scholar] [CrossRef]
- Schellekens, G.A.; de Jong, B.A.; van den Hoogen, F.H.; van de Putte, L.B.; van Venrooij, W.J. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J. Clin. Investig. 1998, 101, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Cantagrel, A.; Degboe, Y. New autoantibodies associated with rheumatoid arthritis recognize posttranslationally modified self-proteins. Jt. Bone Spine 2016, 83, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Dzhambazov, B.; Holmdahl, M.; Yamada, H.; Lu, S.; Vestberg, M.; Holm, B.; Johnell, O.; Kihlberg, J.; Holmdahl, R. The major T cell epitope on type II collagen is glycosylated in normal cartilage but modified by arthritis in both rats and humans. Eur. J. Immunol. 2005, 35, 357–366. [Google Scholar] [CrossRef]
- Kivirikko, K.I.; Myllyla, R. Posttranslational enzymes in the biosynthesis of collagen: Intracellular enzymes. Methods Enzymol. 1982, 82 Pt A, 245–304. [Google Scholar] [CrossRef]
- Gjaltema, R.A.; Bank, R.A. Molecular insights into prolyl and lysyl hydroxylation of fibrillar collagens in health and disease. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Tenni, R.; Viola, M.; Welser, F.; Sini, P.; Giudici, C.; Rossi, A.; Tira, M.E. Interaction of decorin with CNBr peptides from collagens I and II. Evidence for multiple binding sites and essential lysyl residues in collagen. Eur. J. Biochem. 2002, 269, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ge, Y.; Cheng, Q.; Zhang, Q.; Fang, L.; Zheng, J. Decorin is a pivotal effector in the extracellular matrix and tumour microenvironment. Oncotarget 2018, 9, 5480–5491. [Google Scholar] [CrossRef] [PubMed]
- Notbohm, H.; Nokelainen, M.; Myllyharju, J.; Fietzek, P.P.; Muller, P.K.; Kivirikko, K.I. Recombinant human type II collagens with low and high levels of hydroxylysine and its glycosylated forms show marked differences in fibrillogenesis in vitro. J. Biol. Chem. 1999, 274, 8988–8992. [Google Scholar] [CrossRef]
- Myers, L.K.; Myllyharju, J.; Nokelainen, M.; Brand, D.D.; Cremer, M.A.; Stuart, J.M.; Bodo, M.; Kivirikko, K.I.; Kang, A.H. Relevance of posttranslational modifications for the arthritogenicity of type II collagen. J. Immunol. 2004, 172, 2970–2975. [Google Scholar] [CrossRef]
- Yamada, H.; Dzhambazov, B.; Bockermann, R.; Blom, T.; Holmdahl, R. A transient post-translationally modified form of cartilage type II collagen is ignored by self-reactive T cells. J. Immunol. 2004, 173, 4729–4735. [Google Scholar] [CrossRef]
- Michaelsson, E.; Malmstrom, V.; Reis, S.; Engstrom, A.; Burkhardt, H.; Holmdahl, R. T cell recognition of carbohydrates on type II collagen. J. Exp. Med. 1994, 180, 745–749. [Google Scholar] [CrossRef]
- Michaelsson, E.; Andersson, M.; Engstrom, A.; Holmdahl, R. Identification of an immunodominant type-II collagen peptide recognized by T cells in H-2q mice: Self tolerance at the level of determinant selection. Eur. J. Immunol. 1992, 22, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Rosloniec, E.F.; Whittington, K.B.; Zaller, D.M.; Kang, A.H. HLA-DR1 (DRB1*0101) and DR4 (DRB1*0401) use the same anchor residues for binding an immunodominant peptide derived from human type II collagen. J. Immunol. 2002, 168, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Diab, B.Y.; Lambert, N.C.; L’Faqihi, F.E.; Loubet-Lescoulie, P.; de Preval, C.; Coppin, H. Human collagen II peptide 256-271 preferentially binds to HLA-DR molecules associated with susceptibility to rheumatoid arthritis. Immunogenetics 1999, 49, 36–44. [Google Scholar] [CrossRef]
- Fugger, L.; Rothbard, J.B.; Sonderstrup-McDevitt, G. Specificity of an HLA-DRB1*0401-restricted T cell response to type II collagen. Eur. J. Immunol. 1996, 26, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Andersson, E.C.; Hansen, B.E.; Jacobsen, H.; Madsen, L.S.; Andersen, C.B.; Engberg, J.; Rothbard, J.B.; McDevitt, G.S.; Malmstrom, V.; Holmdahl, R.; et al. Definition of MHC and T cell receptor contacts in the HLA-DR4 restricted immunodominant epitope in type II collagen and characterization of collagen-induced arthritis in HLA-DR4 and human CD4 transgenic mice. Proc. Natl. Acad. Sci. USA 1998, 95, 7574–7579. [Google Scholar] [CrossRef]
- Ohnishi, Y.; Tsutsumi, A.; Sakamaki, T.; Sumida, T. T cell epitopes of type II collagen in HLA-DRB1*0101 or DRB1*0405-positive Japanese patients with rheumatoid arthritis. Int. J. Mol. Med. 2003, 11, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, W.U.; Cho, M.L.; Lee, S.K.; Youn, J.; Kim, S.I.; Yoo, W.H.; Park, J.H.; Min, J.K.; Lee, S.H.; et al. Enhanced T cell proliferative response to type II collagen and synthetic peptide CII (255–274) in patients with rheumatoid arthritis. Arthritis Rheum. 1999, 42, 2085–2093. [Google Scholar] [CrossRef]
- Batsalova, T.; Dzhambazov, B.; Merky, P.; Backlund, A.; Backlund, J. Breaking T cell tolerance against self type II collagen in HLA-DR4-transgenic mice and development of autoimmune arthritis. Arthritis Rheum. 2010, 62, 1911–1920. [Google Scholar] [CrossRef]
- Ge, C.; Weisse, S.; Xu, B.; Dobritzsch, D.; Viljanen, J.; Kihlberg, J.; Do, N.N.; Schneider, N.; Lanig, H.; Holmdahl, R.; et al. Key interactions in the trimolecular complex consisting of the rheumatoid arthritis-associated DRB1*04:01 molecule, the major glycosylated collagen II peptide and the T-cell receptor. Ann. Rheum. Dis. 2022, 81, 480–489. [Google Scholar] [CrossRef]
- Sun, L.; Middleton, D.R.; Wantuch, P.L.; Ozdilek, A.; Avci, F.Y. Carbohydrates as T-cell antigens with implications in health and disease. Glycobiology 2016, 26, 1029–1040. [Google Scholar] [CrossRef]
- Witalison, E.E.; Thompson, P.R.; Hofseth, L.J. Protein Arginine Deiminases and Associated Citrullination: Physiological Functions and Diseases Associated with Dysregulation. Curr. Drug Targets 2015, 16, 700–710. [Google Scholar] [CrossRef]
- Zhu, D.; Song, W.; Jiang, Z.; Zhou, H.; Wang, S. Citrullination: A modification important in the pathogenesis of autoimmune diseases. Clin. Immunol. 2022, 245, 109134. [Google Scholar] [CrossRef] [PubMed]
- Valesini, G.; Gerardi, M.C.; Iannuccelli, C.; Pacucci, V.A.; Pendolino, M.; Shoenfeld, Y. Citrullination and autoimmunity. Autoimmun. Rev. 2015, 14, 490–497. [Google Scholar] [CrossRef]
- Alghamdi, M.; Alasmari, D.; Assiri, A.; Mattar, E.; Aljaddawi, A.A.; Alattas, S.G.; Redwan, E.M. An Overview of the Intrinsic Role of Citrullination in Autoimmune Disorders. J. Immunol. Res. 2019, 2019, 7592851. [Google Scholar] [CrossRef]
- van Delft, M.A.M.; Huizinga, T.W.J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef] [PubMed]
- Willemze, A.; Trouw, L.A.; Toes, R.E.; Huizinga, T.W. The influence of ACPA status and characteristics on the course of RA. Nat. Rev. Rheumatol. 2012, 8, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Shidara, K.; Inoue, E.; Hoshi, D.; Sato, E.; Nakajima, A.; Momohara, S.; Taniguchi, A.; Yamanaka, H. Anti-cyclic citrullinated peptide antibody predicts functional disability in patients with rheumatoid arthritis in a large prospective observational cohort in Japan. Rheumatol. Int. 2012, 32, 361–366. [Google Scholar] [CrossRef]
- Tilvawala, R.; Nguyen, S.H.; Maurais, A.J.; Nemmara, V.V.; Nagar, M.; Salinger, A.J.; Nagpal, S.; Weerapana, E.; Thompson, P.R. The Rheumatoid Arthritis-Associated Citrullinome. Cell Chem. Biol. 2018, 25, 691–704.e6. [Google Scholar] [CrossRef]
- Too, C.L.; Murad, S.; Hansson, M.; Alm, L.M.; Dhaliwal, J.S.; Holmdahl, R.; Jakobsson, P.J.; Alfredsson, L.; Klareskog, L.; Ronnelid, J.; et al. Differences in the Spectrum of Anti-Citrullinated Protein Antibody Fine Specificities between Malaysian and Swedish Patients with Rheumatoid Arthritis: Implications for Disease Pathogenesis. Arthritis Rheumatol. 2017, 69, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Tong, D.; Liang, B.; Lonnblom, E.; Schneider, N.; Hagert, C.; Viljanen, J.; Ayoglu, B.; Stawikowska, R.; Nilsson, P.; et al. Anti-citrullinated protein antibodies cause arthritis by cross-reactivity to joint cartilage. JCI Insight 2017, 2, e93688. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Y.; Liang, B.; Xu, R.; Xu, B.; Lonnblom, E.; Feng, H.; Bai, J.; Stawikowska, R.; Ge, C.; et al. Rheumatoid arthritis sera antibodies to citrullinated collagen type II bind to joint cartilage. Arthritis Res. Ther. 2022, 24, 257. [Google Scholar] [CrossRef]
- Liang, B.; Ge, C.; Lonnblom, E.; Lin, X.; Feng, H.; Xiao, L.; Bai, J.; Ayoglu, B.; Nilsson, P.; Nandakumar, K.S.; et al. The autoantibody response to cyclic citrullinated collagen type II peptides in rheumatoid arthritis. Rheumatology 2019, 58, 1623–1633. [Google Scholar] [CrossRef]
- Carubbi, F.; Alunno, A.; Gerli, R.; Giacomelli, R. Post-Translational Modifications of Proteins: Novel Insights in the Autoimmune Response in Rheumatoid Arthritis. Cells 2019, 8, 657. [Google Scholar] [CrossRef]
- Won, P.; Kim, Y.; Jung, H.; Rim, Y.A.; Sohn, D.H.; Robinson, W.H.; Moon, S.J.; Ju, J.H. Pathogenic Role of Circulating Citrullinated Antigens and Anti-Cyclic Monoclonal Citrullinated Peptide Antibodies in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 692242. [Google Scholar] [CrossRef]
- Uysal, H.; Bockermann, R.; Nandakumar, K.S.; Sehnert, B.; Bajtner, E.; Engstrom, A.; Serre, G.; Burkhardt, H.; Thunnissen, M.M.; Holmdahl, R. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J. Exp. Med. 2009, 206, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Haag, S.; Schneider, N.; Mason, D.E.; Tuncel, J.; Andersson, I.E.; Peters, E.C.; Burkhardt, H.; Holmdahl, R. Identification of new citrulline-specific autoantibodies, which bind to human arthritic cartilage, by mass spectrometric analysis of citrullinated type II collagen. Arthritis Rheumatol. 2014, 66, 1440–1449. [Google Scholar] [CrossRef]
- Dusad, A.; Duryee, M.J.; Shaw, A.T.; Klassen, L.W.; Anderson, D.R.; Wang, D.; Ren, K.; Gravallese, E.M.; O’Dell, J.R.; Mikuls, T.R.; et al. Induction of bone loss in DBA/1J mice immunized with citrullinated autologous mouse type II collagen in the absence of adjuvant. Immunol. Res. 2014, 58, 51–60. [Google Scholar] [CrossRef]
- Thiele, G.M.; Duryee, M.J.; Dusad, A.; Hunter, C.D.; Lacy, J.P.; Anderson, D.R.; Wang, D.; O’Dell, J.R.; Mikuls, T.R.; Klassen, L.W. Citrullinated mouse collagen administered to DBA/1J mice in the absence of adjuvant initiates arthritis. Int. Immunopharmacol. 2012, 13, 424–431. [Google Scholar] [CrossRef]
- Szarka, E.; Babos, F.; Magyar, A.; Huber, K.; Szittner, Z.; Papp, K.; Prechl, J.; Pozsgay, J.; Neer, Z.; Adori, M.; et al. Recognition of new citrulline-containing peptide epitopes by autoantibodies produced in vivo and in vitro by B cells of rheumatoid arthritis patients. Immunology 2014, 141, 181–191. [Google Scholar] [CrossRef]
- Turunen, S.; Hannonen, P.; Koivula, M.K.; Risteli, L.; Risteli, J. Separate and overlapping specificities in rheumatoid arthritis antibodies binding to citrulline- and homocitrulline-containing peptides related to type I and II collagen telopeptides. Arthritis Res. Ther. 2015, 17, 2. [Google Scholar] [CrossRef]
- Snir, O.; Widhe, M.; Hermansson, M.; von Spee, C.; Lindberg, J.; Hensen, S.; Lundberg, K.; Engstrom, A.; Venables, P.J.; Toes, R.E.; et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum. 2010, 62, 44–52. [Google Scholar] [CrossRef]
- Brink, M.; Verheul, M.K.; Ronnelid, J.; Berglin, E.; Holmdahl, R.; Toes, R.E.; Klareskog, L.; Trouw, L.A.; Rantapaa-Dahlqvist, S. Anti-carbamylated protein antibodies in the pre-symptomatic phase of rheumatoid arthritis, their relationship with multiple anti-citrulline peptide antibodies and association with radiological damage. Arthritis Res. Ther. 2015, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Snir, O.; Widhe, M.; von Spee, C.; Lindberg, J.; Padyukov, L.; Lundberg, K.; Engstrom, A.; Venables, P.J.; Lundeberg, J.; Holmdahl, R.; et al. Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: Association with HLA-DRB1 alleles. Ann. Rheum. Dis. 2009, 68, 736–743. [Google Scholar] [CrossRef]
- De Santis, M.; Ceribelli, A.; Cavaciocchi, F.; Generali, E.; Massarotti, M.; Isailovic, N.; Crotti, C.; Scherer, H.U.; Montecucco, C.; Selmi, C. Effects of type II collagen epitope carbamylation and citrullination in human leucocyte antigen (HLA)-DR4+ monozygotic twins discordant for rheumatoid arthritis. Clin. Exp. Immunol. 2016, 185, 309–319. [Google Scholar] [CrossRef]
- Ge, C.; Xu, B.; Liang, B.; Lonnblom, E.; Lundstrom, S.L.; Zubarev, R.A.; Ayoglu, B.; Nilsson, P.; Skogh, T.; Kastbom, A.; et al. Structural Basis of Cross-Reactivity of Anti-Citrullinated Protein Antibodies. Arthritis Rheumatol. 2019, 71, 210–221. [Google Scholar] [CrossRef]
- Sipila, K.; Haag, S.; Denessiouk, K.; Kapyla, J.; Peters, E.C.; Denesyuk, A.; Hansen, U.; Konttinen, Y.; Johnson, M.S.; Holmdahl, R.; et al. Citrullination of collagen II affects integrin-mediated cell adhesion in a receptor-specific manner. FASEB J. 2014, 28, 3758–3768. [Google Scholar] [CrossRef] [PubMed]
- Myers, L.K.; Ouyang, Y.X.; Patel, J.R.; Odens, H.H.; Woo-Rasberry, V.; Park, J.; Yi, A.K.; Rosloniec, E.F.; Brand, D.D.; Stuart, J.M.; et al. Role of Citrullinated Collagen in Autoimmune Arthritis. Int. J. Mol. Sci. 2022, 23, 9833. [Google Scholar] [CrossRef]
- Carvalheiro, T.; Garcia, S.; Pascoal Ramos, M.I.; Giovannone, B.; Radstake, T.; Marut, W.; Meyaard, L. Leukocyte Associated Immunoglobulin Like Receptor 1 Regulation and Function on Monocytes and Dendritic Cells During Inflammation. Front. Immunol. 2020, 11, 1793. [Google Scholar] [CrossRef]
- Foulquier, C.; Sebbag, M.; Clavel, C.; Chapuy-Regaud, S.; Al Badine, R.; Mechin, M.C.; Vincent, C.; Nachat, R.; Yamada, M.; Takahara, H.; et al. Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum. 2007, 56, 3541–3553. [Google Scholar] [CrossRef]
- Ishina, I.A.; Zakharova, M.Y.; Kurbatskaia, I.N.; Mamedov, A.E.; Belogurov, A.A., Jr.; Gabibov, A.G. MHC Class II Presentation in Autoimmunity. Cells 2023, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Sidney, J.; Becart, S.; Zhou, M.; Duffy, K.; Lindvall, M.; Moore, E.C.; Moore, E.L.; Rao, T.; Rao, N.; Nielsen, M.; et al. Citrullination only infrequently impacts peptide binding to HLA class II MHC. PLoS ONE 2017, 12, e0177140. [Google Scholar] [CrossRef]
- Hill, J.A.; Southwood, S.; Sette, A.; Jevnikar, A.M.; Bell, D.A.; Cairns, E. Cutting edge: The conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J. Immunol. 2003, 171, 538–541. [Google Scholar] [CrossRef]
- Chemin, K.; Pollastro, S.; James, E.; Ge, C.; Albrecht, I.; Herrath, J.; Gerstner, C.; Tandre, K.; Sampaio Rizzi, T.; Ronnblom, L.; et al. A Novel HLA-DRB1*10:01-Restricted T Cell Epitope from Citrullinated Type II Collagen Relevant to Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Trier, N.H.; Holm, B.E.; Slot, O.; Locht, H.; Lindegaard, H.; Svendsen, A.; Houen, G. Physical Characteristics of a Citrullinated Pro-Filaggrin Epitope Recognized by Anti-Citrullinated Protein Antibodies in Rheumatoid Arthritis Sera. PLoS ONE 2016, 11, e0168542. [Google Scholar] [CrossRef]
- Sokolove, J.; Bromberg, R.; Deane, K.D.; Lahey, L.J.; Derber, L.A.; Chandra, P.E.; Edison, J.D.; Gilliland, W.R.; Tibshirani, R.J.; Norris, J.M.; et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS ONE 2012, 7, e35296. [Google Scholar] [CrossRef]
- Willemze, A.; Bohringer, S.; Knevel, R.; Levarht, E.W.; Stoeken-Rijsbergen, G.; Houwing-Duistermaat, J.J.; van der Helm-van Mil, A.H.; Huizinga, T.W.; Toes, R.E.; Trouw, L.A. The ACPA recognition profile and subgrouping of ACPA-positive RA patients. Ann. Rheum. Dis. 2012, 71, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Sahlström, P.; Hansson, M.; Steen, J.; Amara, K.; Titcombe, P.J.; Forsstrom, B.; Stalesen, R.; Israelsson, L.; Piccoli, L.; Lundberg, K.; et al. Different Hierarchies of Anti-Modified Protein Autoantibody Reactivities in Rheumatoid Arthritis. Arthritis Rheumatol. 2020, 72, 1643–1657. [Google Scholar] [CrossRef]
- Holers, V.M.; Demoruelle, M.K.; Kuhn, K.A.; Buckner, J.H.; Robinson, W.H.; Okamoto, Y.; Norris, J.M.; Deane, K.D. Rheumatoid arthritis and the mucosal origins hypothesis: Protection turns to destruction. Nat. Rev. Rheumatol. 2018, 14, 542–557. [Google Scholar] [CrossRef]
- Song, W.; Ye, J.; Pan, N.; Tan, C.; Herrmann, M. Neutrophil Extracellular Traps Tied to Rheumatoid Arthritis: Points to Ponder. Front. Immunol. 2020, 11, 578129. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.E.; Causey, C.P.; Knuckley, B.; Slack-Noyes, J.L.; Thompson, P.R. Protein arginine deiminase 4 (PAD4): Current understanding and future therapeutic potential. Curr. Opin. Drug Discov. Dev. 2009, 12, 616–627. [Google Scholar]
- Carmona-Rivera, C.; Carlucci, P.M.; Moore, E.; Lingampalli, N.; Uchtenhagen, H.; James, E.; Liu, Y.; Bicker, K.L.; Wahamaa, H.; Hoffmann, V.; et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci. Immunol. 2017, 2, eaag3358. [Google Scholar] [CrossRef]
- Kongpachith, S.; Lingampalli, N.; Ju, C.H.; Blum, L.K.; Lu, D.R.; Elliott, S.E.; Mao, R.; Robinson, W.H. Affinity Maturation of the Anti-Citrullinated Protein Antibody Paratope Drives Epitope Spreading and Polyreactivity in Rheumatoid Arthritis. Arthritis Rheumatol. 2019, 71, 507–517. [Google Scholar] [CrossRef]
- Kissel, T.; Reijm, S.; Slot, L.M.; Cavallari, M.; Wortel, C.M.; Vergroesen, R.D.; Stoeken-Rijsbergen, G.; Kwekkeboom, J.C.; Kampstra, A.; Levarht, E.; et al. Antibodies and B cells recognising citrullinated proteins display a broad cross-reactivity towards other post-translational modifications. Ann. Rheum. Dis. 2020, 79, 472–480. [Google Scholar] [CrossRef]
- Wu, C.Y.; Yang, H.Y.; Luo, S.F.; Lai, J.H. From Rheumatoid Factor to Anti-Citrullinated Protein Antibodies and Anti-Carbamylated Protein Antibodies for Diagnosis and Prognosis Prediction in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 686. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, J.S.; Verheul, M.K.; Stoop, J.N.; Liu, B.; Ioan-Facsinay, A.; van Veelen, P.A.; de Ru, A.H.; Janssen, G.M.C.; Hegen, M.; Rapecki, S.; et al. Breach of autoreactive B cell tolerance by post-translationally modified proteins. Ann. Rheum. Dis. 2017, 76, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Knevel, R.; Suwannalai, P.; van der Linden, M.P.; Janssen, G.M.; van Veelen, P.A.; Levarht, N.E.; van der Helm-van Mil, A.H.; Cerami, A.; Huizinga, T.W.; et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc. Natl. Acad. Sci. USA 2011, 108, 17372–17377. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; van de Stadt, L.A.; Levarht, E.W.; Huizinga, T.W.; Hamann, D.; van Schaardenburg, D.; Toes, R.E.; Trouw, L.A. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; van de Stadt, L.A.; Levarht, E.W.; Huizinga, T.W.; Toes, R.E.; Trouw, L.A.; van Schaardenburg, D. Anti-carbamylated protein antibodies are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum. 2013, 65, 911–915. [Google Scholar] [CrossRef]
- Jaisson, S.; Pietrement, C.; Gillery, P. Protein Carbamylation: Chemistry, Pathophysiological Involvement, and Biomarkers. Adv. Clin. Chem. 2018, 84, 1–38. [Google Scholar] [CrossRef]
- Sirpal, S. Myeloperoxidase-mediated lipoprotein carbamylation as a mechanistic pathway for atherosclerotic vascular disease. Clin. Sci. 2009, 116, 681–695. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef] [PubMed]
- Turunen, S.; Koivula, M.K.; Melkko, J.; Alasaarela, E.; Lehenkari, P.; Risteli, J. Different amounts of protein-bound citrulline and homocitrulline in foot joint tissues of a patient with anti-citrullinated protein antibody positive erosive rheumatoid arthritis. J. Transl. Med. 2013, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Pruijn, G.J. Citrullination and carbamylation in the pathophysiology of rheumatoid arthritis. Front. Immunol. 2015, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Stoop, J.N.; Liu, B.S.; Shi, J.; Jansen, D.T.; Hegen, M.; Huizinga, T.W.; Trouw, L.A.; Toes, R.E. Antibodies specific for carbamylated proteins precede the onset of clinical symptoms in mice with collagen induced arthritis. PLoS ONE 2014, 9, e102163. [Google Scholar] [CrossRef]
- Kampstra, A.S.B.; Dekkers, J.S.; Volkov, M.; Dorjee, A.L.; Hafkenscheid, L.; Kempers, A.C.; van Delft, M.; Kissel, T.; Reijm, S.; Janssen, G.M.C.; et al. Different classes of anti-modified protein antibodies are induced on exposure to antigens expressing only one type of modification. Ann. Rheum. Dis. 2019, 78, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Turunen, S.; Koivula, M.K.; Risteli, L.; Risteli, J. Anticitrulline antibodies can be caused by homocitrulline-containing proteins in rabbits. Arthritis Rheum. 2010, 62, 3345–3352. [Google Scholar] [CrossRef]
- Shi, J.; Willemze, A.; Janssen, G.M.; van Veelen, P.A.; Drijfhout, J.W.; Cerami, A.; Huizinga, T.W.; Trouw, L.A.; Toes, R.E. Recognition of citrullinated and carbamylated proteins by human antibodies: Specificity, cross-reactivity and the ‘AMC-Senshu’ method. Ann. Rheum. Dis. 2013, 72, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Scinocca, M.; Bell, D.A.; Racape, M.; Joseph, R.; Shaw, G.; McCormick, J.K.; Gladman, D.D.; Pope, J.; Barra, L.; Cairns, E. Antihomocitrullinated fibrinogen antibodies are specific to rheumatoid arthritis and frequently bind citrullinated proteins/peptides. J. Rheumatol. 2014, 41, 270–279. [Google Scholar] [CrossRef]
- Volkov, M.; Kampstra, A.S.B.; van Schie, K.A.; Kawakami, A.; Tamai, M.; Kawashiri, S.; Maeda, T.; Huizinga, T.W.J.; Toes, R.E.M.; van der Woude, D. Evolution of anti-modified protein antibody responses can be driven by consecutive exposure to different post-translational modifications. Arthritis Res. Ther. 2021, 23, 298. [Google Scholar] [CrossRef]
- Gorisse, L.; Pietrement, C.; Vuiblet, V.; Schmelzer, C.E.; Kohler, M.; Duca, L.; Debelle, L.; Fornes, P.; Jaisson, S.; Gillery, P. Protein carbamylation is a hallmark of aging. Proc. Natl. Acad. Sci. USA 2016, 113, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Eekhoff, J.D.; Fang, F.; Lake, S.P. Multiscale mechanical effects of native collagen cross-linking in tendon. Connect. Tissue Res. 2018, 59, 410–422. [Google Scholar] [CrossRef]
- Goldberga, I.; Li, R.; Duer, M.J. Collagen Structure-Function Relationships from Solid-State NMR Spectroscopy. Acc. Chem. Res. 2018, 51, 1621–1629. [Google Scholar] [CrossRef]
- Jaisson, S.; Lorimier, S.; Ricard-Blum, S.; Sockalingum, G.D.; Delevallee-Forte, C.; Kegelaer, G.; Manfait, M.; Garnotel, R.; Gillery, P. Impact of carbamylation on type I collagen conformational structure and its ability to activate human polymorphonuclear neutrophils. Chem. Biol. 2006, 13, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Panwar, P.; Butler, G.S.; Jamroz, A.; Azizi, P.; Overall, C.M.; Bromme, D. Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 2018, 65, 30–44. [Google Scholar] [CrossRef]
- Jaisson, S.; Larreta-Garde, V.; Bellon, G.; Hornebeck, W.; Garnotel, R.; Gillery, P. Carbamylation differentially alters type I collagen sensitivity to various collagenases. Matrix Biol. 2007, 26, 190–196. [Google Scholar] [CrossRef]
- Zeltz, C.; Gullberg, D. Post-translational modifications of integrin ligands as pathogenic mechanisms in disease. Matrix Biol. 2014, 40, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Eggleton, P.; Nissim, A.; Ryan, B.J.; Whiteman, M.; Winyard, P.G. Detection and isolation of human serum autoantibodies that recognize oxidatively modified autoantigens. Free Radic. Biol. Med. 2013, 57, 79–91. [Google Scholar] [CrossRef]
- Ryan, B.J.; Nissim, A.; Winyard, P.G. Oxidative post-translational modifications and their involvement in the pathogenesis of autoimmune diseases. Redox Biol. 2014, 2, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Nissim, A.; Winyard, P.G.; Corrigall, V.; Fatah, R.; Perrett, D.; Panayi, G.; Chernajovsky, Y. Generation of neoantigenic epitopes after posttranslational modification of type II collagen by factors present within the inflamed joint. Arthritis Rheum. 2005, 52, 3829–3838. [Google Scholar] [CrossRef] [PubMed]
- Shahab, U.; Ahmad, S.; Moinuddin; Dixit, K.; Habib, S.; Alam, K.; Ali, A. Hydroxyl radical modification of collagen type II increases its arthritogenicity and immunogenicity. PLoS ONE 2012, 7, e31199. [Google Scholar] [CrossRef] [PubMed]
- Strollo, R.; Ponchel, F.; Malmstrom, V.; Rizzo, P.; Bombardieri, M.; Wenham, C.Y.; Landy, R.; Perret, D.; Watt, F.; Corrigall, V.M.; et al. Autoantibodies to posttranslationally modified type II collagen as potential biomarkers for rheumatoid arthritis. Arthritis Rheum. 2013, 65, 1702–1712. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, L.; Regueiro, C.; Amhaz-Escanlar, S.; Pena, C.; Herbello-Hermelo, P.; Moreda-Pineiro, A.; Rodriguez-Garcia, J.; Mera-Varela, A.; Perez-Pampin, E.; Gonzalez, A. Antibodies against 4 Atypical Post-Translational Protein Modifications in Patients with Rheumatoid Arthritis. Diagnostics 2022, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, C.; Jaisson, S.; Gorisse, L.; Tessier, F.J.; Niquet-Leridon, C.; Jacolot, P.; Pietrement, C.; Gillery, P. Carbamylation and glycation compete for collagen molecular aging in vivo. Sci. Rep. 2019, 9, 18291. [Google Scholar] [CrossRef]
- Jost, T.; Zipprich, A.; Glomb, M.A. Analysis of Advanced Glycation Endproducts in Rat Tail Collagen and Correlation to Tendon Stiffening. J. Agric. Food Chem. 2018, 66, 3957–3965. [Google Scholar] [CrossRef] [PubMed]
- Gautieri, A.; Passini, F.S.; Silvan, U.; Guizar-Sicairos, M.; Carimati, G.; Volpi, P.; Moretti, M.; Schoenhuber, H.; Redaelli, A.; Berli, M.; et al. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biol. 2017, 59, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Newkirk, M.M.; Goldbach-Mansky, R.; Lee, J.; Hoxworth, J.; McCoy, A.; Yarboro, C.; Klippel, J.; El-Gabalawy, H.S. Advanced glycation end-product (AGE)-damaged IgG and IgM autoantibodies to IgG-AGE in patients with early synovitis. Arthritis Res. Ther. 2003, 5, R82–R90. [Google Scholar] [CrossRef]
- Drinda, S.; Franke, S.; Canet, C.C.; Petrow, P.; Brauer, R.; Huttich, C.; Stein, G.; Hein, G. Identification of the advanced glycation end products Nε-carboxymethyllysine in the synovial tissue of patients with rheumatoid arthritis. Ann. Rheum. Dis. 2002, 61, 488–492. [Google Scholar] [CrossRef]
- Chen, J.R.; Takahashi, M.; Suzuki, M.; Kushida, K.; Miyamoto, S.; Inoue, T. Comparison of the concentrations of pentosidine in the synovial fluid, serum and urine of patients with rheumatoid arthritis and osteoarthritis. Rheumatology 1999, 38, 1275–1278. [Google Scholar] [CrossRef]
- Kim, S.Y. Transglutaminase 2 in inflammation. Front. Biosci. (Landmark Ed.) 2006, 11, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- Dzhambazov, B.; Lindh, I.; Engstrom, A.; Holmdahl, R. Tissue transglutaminase enhances collagen type II-induced arthritis and modifies the immunodominant T-cell epitope CII260-270. Eur. J. Immunol. 2009, 39, 2412–2423. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, L.; Bang, H.; Regueiro, C.; Nuno, L.; Triguero-Martinez, A.; Peiteado, D.; Ortiz, A.M.; Villalba, A.; Martinez-Feito, A.; Balsa, A.; et al. Improved classification of rheumatoid arthritis with a score including anti-acetylated ornithine antibodies. Sci. Rep. 2020, 10, 19263. [Google Scholar] [CrossRef]
- Colasanti, T.; Sabatinelli, D.; Mancone, C.; Giorgi, A.; Pecani, A.; Spinelli, F.R.; Di Giamberardino, A.; Navarini, L.; Speziali, M.; Vomero, M.; et al. Homocysteinylated alpha 1 antitrypsin as an antigenic target of autoantibodies in seronegative rheumatoid arthritis patients. J. Autoimmun. 2020, 113, 102470. [Google Scholar] [CrossRef] [PubMed]
- Geborek, P.; Saxne, T.; Pettersson, H.; Wollheim, F.A. Synovial fluid acidosis correlates with radiological joint destruction in rheumatoid arthritis knee joints. J. Rheumatol. 1989, 16, 468–472. [Google Scholar] [PubMed]
- Lardner, A. The effects of extracellular pH on immune function. J. Leukoc. Biol. 2001, 69, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, K.; Ottosson, L.; Westman, E.; Sunnerhagen, M.; Hultenby, K.; Harris, H.E. A pH-induced modification of CII increases its arthritogenic properties. J. Autoimmun. 2004, 23, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef] [PubMed]
- Trentham, D.E. Evidence that type II collagen feeding can induce a durable therapeutic response in some patients with rheumatoid arthritis. Ann. N. Y. Acad. Sci. 1996, 778, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Page, A.; Fusil, F.; Cosset, F.L. Antigen-specific tolerance approach for rheumatoid arthritis: Past, present and future. Jt. Bone Spine 2021, 88, 105164. [Google Scholar] [CrossRef]
- Barnett, M.L.; Combitchi, D.; Trentham, D.E. A pilot trial of oral type II collagen in the treatment of juvenile rheumatoid arthritis. Arthritis Rheum. 1996, 39, 623–628. [Google Scholar] [CrossRef]
- Zhu, P.; Li, X.Y.; Wang, H.K.; Jia, J.F.; Zheng, Z.H.; Ding, J.; Fan, C.M. Oral administration of type-II collagen peptide 250–270 suppresses specific cellular and humoral immune response in collagen-induced arthritis. Clin. Immunol. 2007, 122, 75–84. [Google Scholar] [CrossRef]
- Wenhart, C.; Holthoff, H.P.; Reimann, A.; Li, Z.; Fassbender, J.; Ungerer, M. A fructosylated peptide derived from a collagen II T cell epitope for long-term treatment of arthritis (FIA-CIA) in mice. Sci. Rep. 2021, 11, 17345. [Google Scholar] [CrossRef] [PubMed]
- Gertel, S.; Serre, G.; Shoenfeld, Y.; Amital, H. Immune tolerance induction with multiepitope peptide derived from citrullinated autoantigens attenuates arthritis manifestations in adjuvant arthritis rats. J. Immunol. 2015, 194, 5674–5680. [Google Scholar] [CrossRef] [PubMed]
- Gertel, S.; Karmon, G.; Vainer, S.; Shovman, O.; Cornillet, M.; Serre, G.; Shoenfeld, Y.; Amital, H. Immunomodulation of RA Patients’ PBMC with a Multiepitope Peptide Derived from Citrullinated Autoantigens. Mediat. Inflamm. 2017, 2017, 3916519. [Google Scholar] [CrossRef] [PubMed]
- Hansson, M.; Mathsson, L.; Schlederer, T.; Israelsson, L.; Matsson, P.; Nogueira, L.; Jakobsson, P.J.; Lundberg, K.; Malmstrom, V.; Serre, G.; et al. Validation of a multiplex chip-based assay for the detection of autoantibodies against citrullinated peptides. Arthritis Res. Ther. 2012, 14, R201. [Google Scholar] [CrossRef] [PubMed]
- Koivula, M.K.; Heliovaara, M.; Rissanen, H.; Palosuo, T.; Knekt, P.; Immonen, H.; Risteli, J. Antibodies binding to citrullinated telopeptides of type I and type II collagens and to mutated citrullinated vimentin synergistically predict the development of seropositive rheumatoid arthritis. Ann. Rheum. Dis. 2012, 71, 1666–1670. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Yoshida, T.; Sudo, A. Tenascin-C in Osteoarthritis and Rheumatoid Arthritis. Front. Immunol. 2020, 11, 577015. [Google Scholar] [CrossRef] [PubMed]
| PTM Type | Test Objects | Effect |
|---|---|---|
| Glycosylated COL2259–273 | B10.Q mice/ (BALB/c × B10.Q) F2 CIA model | Inhibition of CIA incidence and severity; reduced anti-CII antibody levels Reduction in arthritis progression in chronic stage |
| Fructosylated COL2259–273 | DBA/1 mice FIA-CIA model * | Amelioration of disease severity No effect on antibody levels |
| Citrullinated multiepitope peptide containing COL2 sequence | Lewis rats AIA model * | Ameliorated disease Increased regulatory T-cell populations Reduced Th17 populations |
| PBMC from RA patients | Increased regulatory T-cell populations and TGF-β expression levels Reduced Th17 populations; downregulation of IL-1β and TNF-α expression |
| Detected Antibody Specificity | Results/Benefits |
|---|---|
| Citrullinated carboxy-terminal COL1 and COL2 telopeptides (TELO-I and TELO-II, respectively); Mutated citrullinated vimentin (MCV) | Prediction of seropositive RA |
| Citrullinated C1 epitopes of COL2 and multiepitope peptides; 11 other citrullinated peptides containing epitopes from α-enolase, vimentin, fibrinogen, filaggrin | Improved diagnostic value Potential guidance for personalized treatment |
| Cyclic citrullinated COL2 peptides | Identification of new epitopes Improved specificity for RA diagnosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batsalova, T.; Dzhambazov, B. Significance of Type II Collagen Posttranslational Modifications: From Autoantigenesis to Improved Diagnosis and Treatment of Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 9884. https://doi.org/10.3390/ijms24129884
Batsalova T, Dzhambazov B. Significance of Type II Collagen Posttranslational Modifications: From Autoantigenesis to Improved Diagnosis and Treatment of Rheumatoid Arthritis. International Journal of Molecular Sciences. 2023; 24(12):9884. https://doi.org/10.3390/ijms24129884
Chicago/Turabian StyleBatsalova, Tsvetelina, and Balik Dzhambazov. 2023. "Significance of Type II Collagen Posttranslational Modifications: From Autoantigenesis to Improved Diagnosis and Treatment of Rheumatoid Arthritis" International Journal of Molecular Sciences 24, no. 12: 9884. https://doi.org/10.3390/ijms24129884
APA StyleBatsalova, T., & Dzhambazov, B. (2023). Significance of Type II Collagen Posttranslational Modifications: From Autoantigenesis to Improved Diagnosis and Treatment of Rheumatoid Arthritis. International Journal of Molecular Sciences, 24(12), 9884. https://doi.org/10.3390/ijms24129884








