Abstract
The structure of cellulolytic enzyme lignin (CEL) prepared from three bamboo species (Neosinocalamus affinis, Bambusa lapidea, and Dendrocalamus brandisii) has been characterized by different analytical methods. The chemical composition analysis revealed a higher lignin content, up to 32.6% of B. lapidea as compared to that of N. affinis (20.7%) and D. brandisii (23.8%). The results indicated that bamboo lignin was a p-hydroxyphenyl-guaiacyl-syringyl (H-G-S) lignin associated with p-coumarates and ferulates. Advanced NMR analyses displayed that the isolated CELs were extensively acylated at the γ-carbon of the lignin side chain (with either acetate and/or p-coumarate groups). Moreover, a predominance of S over G lignin moieties was found in CELs of N. affinis and B. lapidea, with the lowest S/G ratio observed in D. brandisii lignin. Catalytic hydrogenolysis of lignin demonstrated that 4-propyl-substituted syringol/guaiacol and propanol guaiacol/syringol derived from β-O-4′ moieties, and methyl coumarate/ferulate derived from hydroxycinnamic units were identified as the six major monomeric products. We anticipate that the insights of this work could shed light on the sufficient understanding of lignin, which could open a new avenue to facilitate the efficient utilization of bamboo.
1. Introduction
As the cell wall component of terrestrial plants, lignin is widely distributed in nature, and its content is second only to (hemi)cellulose [1,2]. Lignin contains a complex structure of the phenolic polymer, which is especially suitable for the production of aromatic chemicals, and its components vary with different plant materials [3,4,5,6,7,8]. However, most biorefinery schemes focus on the use of easy-to-use compositions, while lignin is relatively underutilized [9,10,11]. For example, about one million t/y of lignosulfonate accounted for only 2% of the total production for commercialization [12,13]. Catalytic conversion of lignin for the production of small molecule chemicals or fuels has received much attention because of its high yield and widespread availability [14]. Nevertheless, it is still a significant challenge for lignin valorization in biorefinery owing to its complexity and heterogeneity of the internal macromolecular structure [15,16]. To solve the above-mentioned challenges, it is necessary to gain a broad understanding of the “main” structural characteristics of lignin to provide a theoretical basis for biomass upgrading, pulping, and biorefinery [14,17].
The content and composition of lignin vary with wood type, cell type and single cell wall layer, and environmental conditions [18]. In recent years, herbaceous plants, such as bamboo and hemp, have gradually attracted extensive attention due to their short growth cycle and mild growth conditions. As typical of fast-growing plants, most of the herbaceous plants contain relatively low lignin contents but with high levels of hydroxycinnamic acid, i.e., p-coumaric acid (pCA) and ferulic acid (FA) [19,20,21,22] Generally, cellulolytic enzyme lignin (CEL), which is obtained by extraction of the residue from cellulase treated ball-milled materials with 96% dioxane solvent, is more representative of the total lignin in lignocellulosic biomass [23,24]. The structural characterization of lignin macromolecules isolated from different hardwood and bamboo species [17,18,25] using advanced nuclear magnetic resonance (NMR) technologies, inducing 13C, 31P, and 2D HSQC, could facilitate the development of efficient utilization strategies to meet current biorefinery toward a circular economy [26].
Over the years, more and more scientific researchers used precious metals (Pd [27,28,29,30,31,32,33], Ru [5,27,34,35,36,37,38], and Pt [39,40,41]) or non-precious metals (Ni [42,43,44,45,46], Fe [47,48], Mo [49,50], and Cu [51,52]) for the catalytic transformation of lignin into chemicals and fuel products. For example, Luterbacher and co-workers [34] reported that adding formaldehyde during biomass pretreatment produced a soluble lignin fraction that could be converted into guaiacyl and syringyl monomers at near theoretical yields (47 mole% of Klason lignin for beech and 78 mole% for a high-syringyl transgenic poplar) during subsequent hydrogenolysis using a Ru/C catalyst. We have recently developed ruthenium nanoparticles (NPs) anchored on defective nitrogen-doped carbon (Ru@NC) via facile pyrolysis of a mixture of ruthenium trichloride and urea with carbon support [53]. Experimental insights indicated that the highly distributed Ru-NPs, constituted by N-enriched graphene shells, have been established as an excellent catalyst for the selective hydrodeoxygenation of lignin and furan derivatives toward biofuel upgrade [53]. As a continuation of our ongoing interest in effectively developing reductive catalytic depolymerization of lignin, we envision that this catalyst exhibits efficient performance for the hydrogenolysis of lignins and β-O-4′ model compounds through the scission of C–O bonds.
In this study, to better unravel the lignin structural variation in three bamboo species (Neosinocalamus affinis, Bambusa lapidea, and Dendrocalamus brandisii), which are widely grown in southwest China, typical CEL preparations were successively isolated from different bamboo species. The composition and structures of the obtained CEL fractions were comprehensively investigated. In order to obtain further insights into their structures, the CEL samples were also analyzed by catalytic hydrogenolysis. The results of this work are important not only for providing new insights into the bamboo lignin characteristics but also for the industrial processing of bamboo for pulp, chemical, or biofuel production.
2. Results and Discussion
2.1. Composition Analysis of Bamboo
The contents of main constituents (i.e., Klason lignin, acid-soluble lignin, glucan, xylan, arabinan, galactan, rhamnan, mannan, glucuronic acid, galacturonic acid, and ash) in the selected three bamboo species (N. affinis, B. lapidea, and D. brandisii) are summarized in Table 1. Quantitative measurement of lignin is an important aspect of the study of lignin structure [54]. It was observed that the total lignin content (Klason lignin plus acid-soluble lignin) of B. lapidea amounted to 32.6%, which was significantly higher than those of N. affinis (20.8%) and D. brandisii (23.8%). This result was consistent with previously reported Phyllostachys pubescens (26.1~28.2%) [12,55,56,57] and Dendrocalamus sinicus (28.6%) [58]. After a general analysis of the chemical composition of the three bamboo species, the variations in the isolated CEL preparations were systematically analyzed, especially with the advanced NMR technologies (13C, 31P, and 2D HSQC).

Table 1.
Abundances (%) of the main constituents of three bamboo species a.
2.2. FT-IR Spectroscopy
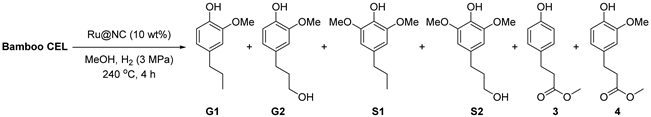
Figure 1 shows the FT-IR spectra of lignin extracted from three species of bamboo, which can identify the characteristic functional groups [33,34,35]. Obviously, the broad absorption band at 3429 cm−1 is associated with the OH stretching vibration. The bands at 1664 and 1655 cm−1 indicate the unconjugated carbonyl of the keto group and the carbonyl stretch of the conjugated carbonate, respectively. The peaks at 1599, 1511, and 1426 cm−1 correspond to aromatic backbone vibrations and C-H deformations, and the methoxy group at 1460 cm−1 is the asymmetric C-H vibration. The analysis of the spectra of the three lignins demonstrated that the aromatic skeleton of the lignin structure was kept well during the isolation process [12]. Syringyl and condensed guaiacyl absorb the band at 1239 cm−1, and the fused G-unit with the C–O stretching at 1253 cm−1 [58,59,60]. The 1125 and 834 cm−1 peaks dominate as unambiguous signals for HGS lignin, which proves that the three bamboo lignins exhibit the pattern of typical grass lignin.
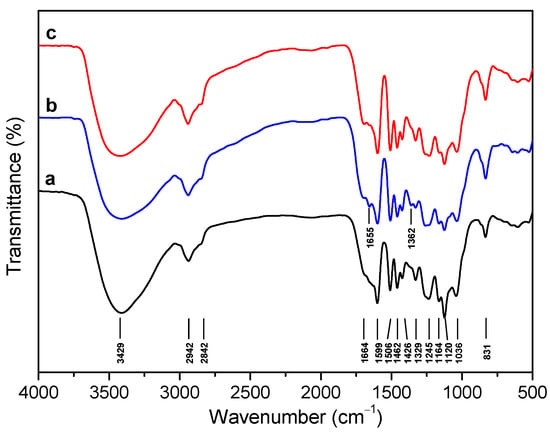
Figure 1.
FT-IR spectra of (a) CELN, (b) CELB, and (c) CELD. CELN, CELB, and CELD were isolated from N. affinis, B. lapidea, and D. brandisii, respectively.
2.3. Molecular Weight Distribution and Thermal Stability
The molecular weight distribution of the CEL preparations after acetylation was determined by gel permeation chromatography (GPC). It was observed that CELN displayed the lowest weight average molecular weight (Mw) of 8.13 kDa in comparison with those of CELB (9.08 kDa) and CELD (9.55 kDa) in Figure 2. However, all these data were comparable with previously reported bamboo CELs [61,62,63]. Moreover, the polydispersity index (Mw/Mn, Ð) of the three CELs was found to be relatively narrow (Ð < 3.0) and with no significant differences (Table 2).
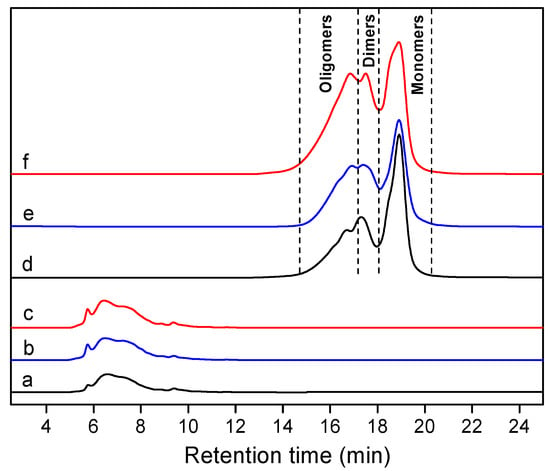
Figure 2.
GPC of (a) CELN, (b) CELB, (c) CELD and lignin oil obtained from the catalytic hydrogenolysis of (d) CELN, (e) CELB, (f) CELD over a Ru@NC catalyst.

Table 2.
Weight-average (Mw), number-average (Mn) molecular weights, and the polydispersity index (Mw/Mn, Ð) of bamboo CELs and the corresponding lignin oil products.
As depicted in Figure 3, the percentages of residues at different stages and the corresponding temperatures (Tm) at the maximum rate of quality loss were obtained from the TG and DTG curves of the three bamboo CELs. Among them, CELN showed the lowest residual amount, whereas CELB and CELD were in an incremental residual amount, indicating that CELN was lower in thermal stability. Moreover, a three-stage weight loss process was observed in the TG curve. The first stage was a slight weight loss due to the volatilization of bound water and loss of the residue extracts at 50~200 °C. The pyrolysis rate was high in the second stage at 200~500 °C, which was due to the pyrolysis of carbohydrates and lignin. The curve in the third stage gradually flattened, which was mainly attributed to the degradation of tar and coke. As discussed above, lignin was easier to convert into char due to its highly agglomerated properties [64,65]. The cleavage of the β-O-4′ bonds mainly occurred at 250~350 °C [66]. By analyzing the DTG curves, CELN exhibited the representative lowest decomposition peak, which might be related to the content of β-O-4′ linkages in it. Therefore, it could be concluded that the thermal stabilities of CELB and CELD were relatively higher than that of CELN.
Figure 3.
TG and DTG curves of (a) CELN, (b) CELB, and (c) CELD.
2.4. NMR Characterization
The 13C NMR spectroscopy provides valid evidence for the analysis of the chemical structure of bamboo lignin [41,42,43]. As shown in Figure 4, the peaks of S-type were identified by signals at 151.1–155.1 ppm (C-3/C-5 etherified and condensed), 146.6–148.7 ppm (C-3/C-5 non-etherified), 138.1 ppm (C-4 etherified), 133.0–136.6 ppm (C-1), and 102.5–106.5 ppm (C-2/C-6). The G-units give signals at 148.7–151.0 ppm (C-3 etherified and condensed), 146.6–148.7 ppm (C-4 etherified), 144.0–146.6 ppm (C-3/C-4 non-etherified), 133.0–136.6 ppm (C-1), 119.5 ppm (C-6), 113.1–118.0 ppm (C-5), and 110.0–113.1 ppm (C-2). The H units were detected at 128.1 ppm (C-2/C-6), and 113.1–118.0 ppm (C-3/C-5). Moreover, the pCA was evidenced by five signals at 166.7, 160.1, 130.5, 125.3, and 113.1–118 ppm, which originated from C-9, C-4, C-2/C-6, C-3/C-5, and C-β in the structures, respectively. Additionally, we assigned the above spectral regions to functional groups and quantified them by integration (Table S1). The analysis results of quantitative 13C NMR spectroscopy further revealed that the bamboo CEL was a typical HGS-type lignin, which was in good accordance with the aforementioned results of FT-IR.
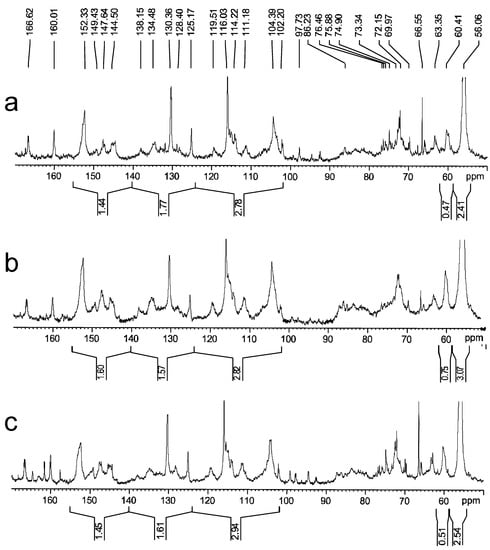
Figure 4.
Quantitative 13C NMR spectra of (a) CELN, (b) CELB, and (c) CELD.
2D HSQC NMR spectrum has been reported to be capable of providing important structural information about the complex polymer substructures bonded between lignin units and S/G ratio. The aliphatic (δC/δH 10–45/0–3.5 ppm), aliphatic-oxygenated (δC/δH 49–90/2.4–5.8 ppm), and aromatic (δC/δH 90–150/5.7–8.0 ppm) regions of the 2D HSQC NMR of CELN are illustrated in Figure 5. The assignments of main lignin cross-signals in the HSQC spectra were assigned according to previously reported literature [12,58,62,67,68,69,70,71], which are listed in Table S2, and the main substructures are depicted in Figure 5.
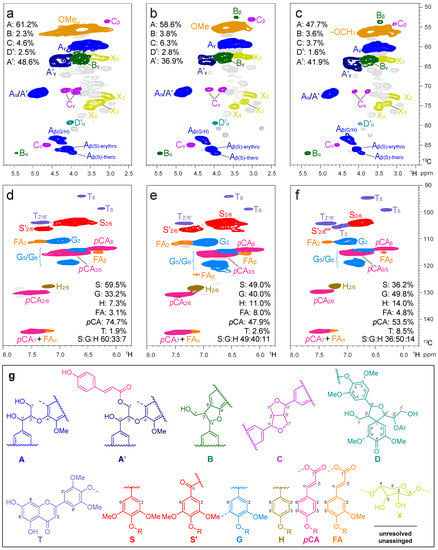
Figure 5.
2D HSQC NMR spectra of the prepared (a,d) CELN, (b,e) CELB, and (c,f) CELD isolated from ball-milled bamboo. The colors of the contours correspond to the structures drawn. (g) The main structures and lignin-derived monomers found are as follows: (A) β-O-4′ alkyl-aryl ether; (A′) γ-OH with p-coumaroylated β-O-4′ alkyl-aryl ethers; (B) phenylcoumarans; (C) resinols; (D) spirodienones; (T) tricin; (S) syringyl units; (S′) oxidized syringyl units bearing a carbonyl at Cα; (G) guaiacyl units; (H) p-hydroxyphenyl unites; (pCA) p-coumarates; and (FA) ferulates.
The aliphatic-oxygenated region of the spectra (Figure 5b) provided information about the different interunit linkages present in the lignin. In this region, correlation peaks from methoxyls and side chains in β-O-4′ substructures (A, 61.2%) were the most prominent in the HSQC spectra of the isolated CELN. Other substructures were also visible in the HSQC spectrum of the CELN, including signals for phenylcoumarans (B, 2.3%) and resinols (C, 4.6%). Notably, a large predominance of β-O-4′ linkages (up to 48.6% of all linkages) was observed at δC/δH 63.5/4.21, which was assigned to the γ-acylated lignin units A′. This indicated that the structure of native lignin was highly remarkable, being extensively acylated (acetylated and/or p-coumaroylated) in bamboo [69]. The main cross-signals from S, G, and H units are visible in the aromatic region of the HSQC spectra (Figure 5c), which correspond to the benzenic rings of lignin units. The prominent signal at δC/δH 103.9/6.58 ppm was associated with C2,6-H2,6 in S-type units. The peaks at δC/δH 110.9/6.93, 114.4/6.69, and 118.9/6.80 ppm were assigned to C2-H2, C5-H5, and C6-H6 of the G-type unit, respectively. The C2,6-H2,6 of the H-type hydroxyphenyl building block appeared at δC/δH 127.8/7.28 ppm. In addition, the pCA and FA units were detected in the HSQC spectra. The series of signals at δC/δH 130.0/7.35, 115.8/6.81, and 113.8/6.20 ppm were associated with C2,6-H2,6, C3,5-H3,5, and Cβ-Hβ in pCA, respectively. The crossover signals of C2-H2 and Cβ-Hβ in FA appeared at δC/δH 110.9/7.25 and 115.3/6.29 ppm. In addition, the HSQC spectra showed signals of C8-H8 and C6-H6 at δC/δH 94.3/6.62 and 98.9/6.15 ppm, respectively, which were attributed to the grass-specific tricin (T) lignin units [72,73].
To further investigate the differences in the chemical structures of the three bamboo lignin species, quantitative 31P NMR technique was employed (Figure S4), and the quantitative data on the distribution of different OH groups in the CELs are listed in Table S3 [57]. The results of phenolic OH content revealed no significant differences among the three CELs. However, the highest content of aliphatic hydroxyl groups was detected in CELB, and the lowest content of total hydroxyl groups in CELN.
2.5. Catalytic Hydrogenolysis of Lignin
Catalytic hydrogenolysis was developed to produce aromatic products and phenolic moieties from lignin. Typically, alkyl aryl ether linkages in the lignin biomacromolecules are cleaved during this process. Moreover, secondary (benzylic) alcohols are removed, and aliphatic double bonds are reduced, which provides more additional information regarding the characteristics of the lignin side chain. On treatment of bamboo CELN with 10 wt% of Ru@NC at 240 °C and 3 MPa of H2 in MeOH for 4 h in a Parr autoclave, a brown soluble oily product was obtained after extraction with CH2Cl2 (Figure S1). This catalytic hydrogenolysis process afforded a monophenol yield of 26.6 wt% (Table 1), and the detailed monomer distribution is depicted in Figure S5. Both syringyl- and guaiacyl-derived phenols were detected with an S/G ratio of 2.2, slightly higher than that of S/G monomer composition in the original lignin (1.8). Among the monomers, 4-propyl-substituted syringol (S1, 12.2 wt%) and guaiacol (G1, 4.1 wt%) were identified as the two major products, corresponding to 61.9 mol% selectivity of total monomers. Small quantities of 4-n-propanol guaiacol/syringol (G2/S2, 5.1 wt%) were also detected (Table 3). As a typical herbaceous species, bamboo lignin features hydroxycinnamic acid, which is bonded with α-OH or γ-OH of β-O-4′ moieties through ester or ether linkages [74,75]. Accordingly, two specific phenolic monomers (3, 4.1 wt%; 4, 1.1 wt%) were also generated from the pCA and FA units, which amounted to 20.9 mol% selectivity of total monomers. Moreover, the analysis of the oily product (CELON) and by GPC revealed a significant decrease in molecular weight (Mw 0.66 kDa) relative to the initial CELN (Mw 8.13 kDa) (Table 2). These results demonstrated the successful scission of most C–O bonds under such a catalytic condition.

Table 3.
Catalytic hydrogenolysis of bamboo CEL over a Ru@NC catalyst a.
The as-obtained oily product was further characterized by 2D HSQC NMR spectroscopy (Figure 6). All signals of monomeric phenols were assigned based on the comparison with authentic samples. Notably, no detectable signals for lignin linkages remained in Figure 6b, such as β-aryl ether (β-O-4′, A), phenylcoumaran (β-5, B), and resinol (β–β, C), indicating efficient depolymerization of lignin over the Ru@NC catalyst. The cross peaks at δC/δH 13.5/0.85, 24.5/1.53, and 36.6/2.48 ppm ascribed to the propyl end chains in G1/S1 could be easily observed (Figure 6c). A family of cross peaks located at δC/δH 31.8/2.51, 34.6/1.71, and 61.3/3.25 emerged, relating to the propyl end chain in G2/S2. In addition, signal at δC/δH 51.0/3.58 was attributed to the ester group (CO2Me) in the product (Figure 6b). In the aliphatic region, the signal peaks of the C7, C8, and C9 belonging to G- and S-products were found, while the signal peaks of 3 and 4 were mainly found in the aromatic region (Figure 6a,c). The characteristic of the monomers was further verified by the addition of the 3D version in Figure 6d.
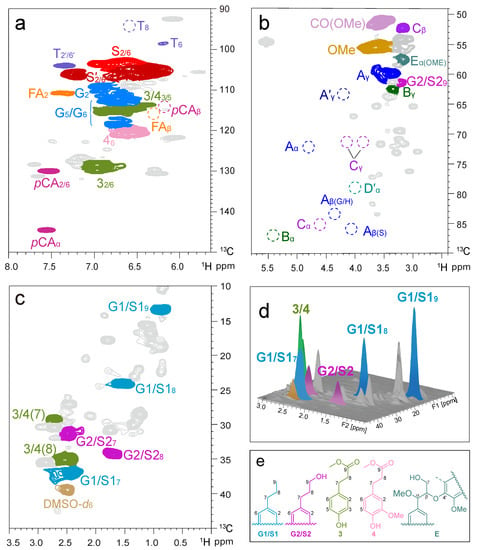
Figure 6.
2D HSQC NMR spectra (in DMSO-d6) of the lignin oily product (a–c) obtained from the Ru@NC-catalytic hydrogenolysis of CELN (in DMSO-d6); (d) three-dimensional version of the 2D HSQC end-chain region; (e) detected lignin monomers. Reaction conditions: CELN (50 mg), Ru@NC catalyst (5 mg, 10 wt%), MeOH (10 mL), 240 °C, H2 (3 MPa at 25 °C, 12 MPa at 240 °C), and 4 h.
We further evaluated the catalytic hydrogenolysis performance of the other two bamboo lignin species over the Ru@NC catalyst (Figures S2 and S3). Notably, CELB afforded a relatively higher yield of phenolic monomer (26.5 wt%) than that of CELD (16.5 wt%), which was due to the high proportion of β-O-4′ linkages in CELB [15,71]. As expected, both CELB and CELD gave G1 and S1 as the dominant products (60.5~64.6% selectivity). Moreover, the 2D HSQC NMR spectroscopies of lignin oils from those two lignin preparations indicated that there were no β-O-4′ structures existing, illustrating the fullest cleavage of C–O linkages, which was in good accordance with the GPC analysis in Table 2 and Figure 2.
2.6. Catalytic Hydrogenolysis of Lignin β-O-4′ Model Compounds
To further understand the pathway of lignin during the Ru@NC-catalyzed hydrogenolysis reactions, the typical lignin β-O-4′ model compounds were tested (Figure 7) [76,77]. Catalytic hydrogenolysis of phenolic β-O-4′ dimer 1 offered G1 (13.6%) and G2 (16.8%) as the major phenolic monomer products (reaction a, Figure S6). Nonphenolic β-O-4′ dimer 2 could efficiently afford 4-propylveratrole (D1) and benzenepropanol (D2) in 62.6% and 24.5% yields, respectively (reaction b, Figure S7). All the product distributions were similar to those of the Ru@NC-catalyzed hydrogenolysis of CEL samples. These results further confirmed that Ru@NC displayed excellent activity for the hydrogenation of C=C/C=O bonds and hydrogenolysis of C–O bonds in β-O-4′ moieties.
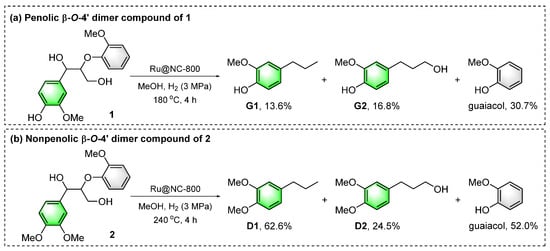
Figure 7.
Catalytic hydrogenolysis of the lignin β-O-4′ model compounds over a Ru@NC catalyst.
2.7. Elemental Composition of Bamboo, Lignin, and Lignin Oil
The elemental compositions of the three bamboo species, CELs, and the corresponding lignin oily products were investigated, which is summarized in Table S4 and depicted in Figure 8 as the van Krevelen diagram [32,78]. The solid, dashed, and dotted lines in the diagram represent the processes of demethanation, decarboxylation, and dehydration reactions, respectively. Notably, the H/C ratio of BambooB was slightly lower than that of the other two bamboo samples (BambooN and BambooD) because of the lower content of carbohydrates, which was consistent with the compositional analysis results in Table 1. It was found that the average H/C molar ratios of lignin oil were significantly higher than those of CELs. This was reasonable as (hemi)celluloses were removed in the preparation of CEL samples, which was further verified by the O/C ratios between bamboo and CEL samples. Moreover, it was found that the average O/C molar ratio of lignin oil products (0.26~0.30) was close to those of CEL samples (0.33~0.35) but much lower than those of bamboo materials (0.57~0.61). The results of reduced oxygen content but increased hydrogen content further indicated the efficient C–O bond scission in the catalytic hydrogenolysis of lignin, which led to deoxygenation and decarboxylation process.
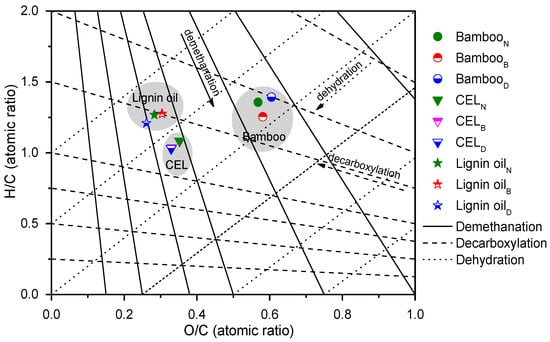
Figure 8.
Atomic H/C versus O/C ratios (van Krevelen diagram) of bamboo, bamboo CELs, and lignin oil obtained from the Ru@NC-catalytic hydrogenolysis of CELs. The lines represent demethanation, dehydration, and decarboxylation pathways. BambooN, BambooB, and BambooD refer to N. affinis, B. lapidea, and D. brandisii, respectively.
3. Materials and Methods
3.1. Materials
Three bamboo species (N. affinis, B. lapidea, and D. brandisii) were harvested from Yunnan Province, China. Methanol (MeOH), dichloromethane (DCM), and tetrahydrofuran (THF) were purchased from Energy Chemical (Shanghai, China). The commercial cellulase was kindly provided by Novozymes (Beijing, China) Biotechnology Co., Ltd. Lignin model samples for catalytic degradation were synthesized independently. Dimeric lignin model compounds were prepared following previously reported procedures with modifications [49,52,79]. The Ru@NC catalyst used in this work was prepared and described in detail as previously described [53].
3.2. Preparation of Cellulolytic Enzyme Lignin
The bamboo raw materials were smashed into sawdust (40~60 mesh), dried in an oven at 60 °C, and then extracted with ethanol/toluene (1:2, v/v) using a Soxhlet extractor for 10 h. The preground and extracted bamboo samples were then planetary ball milled (Fritsch GmbH, Idar-Oberstein, Germany) at 400 rpm for 4 h with zirconium dioxide (ZrO2) vessels containing ZrO2 ball bearings (10 mm × 30). One cycle of the ball-milling condition consists of a 10 min milling and a 10 min cooling cycle. Subsequently, the ball-milled samples were subjected to digestion (72 h × 2) to obtain enzyme lignin samples by cellulose at 50 °C in NaOAc buffer (pH 4.8). After that, the solid residue was obtained after centrifugation (5000 rpm for 5 min), washing three times with deionized water, lyophilization, and finally ladled as CEL.
3.3. Chemical Components Analysis
The structural carbohydrates and lignin, as well as ash in the dewaxed bamboo sawdust, were determined according to the standard analytical procedures (NREL/TP-510-42618 and NREL/TP-510-42622) [80,81].
3.4. Catalytic Hydrogenolysis of Lignin or Lignin Model Compounds
Typically, CEL (50 mg) or lignin model compounds (15 mg), Ru@NC (5 mg), and MeOH (10 mL) were charged into an autoclave (50 mL, Parr Instrument Company, Moline, IL, USA), which was then flushed with N2 for three times and pressurized with 3 MPa H2 at room temperature. Afterwards, the mixture was stirred at 800 rpm and heated to the desired temperature. After the reaction, the autoclave was cooled and depressurized carefully. The reaction mixture was filtered through a nylon 66 membrane filter (0.22 μm), and the insoluble fraction was washed with DCM. Lignin oily product was obtained after removing DCM under a vacuum condition. An external standard (1,3,5-trimethoxybenzene) was added to the lignin oily solution in DCM.
3.5. Characterizations
Gas chromatography-mass spectrometry (GC-MS) and GC analysis were performed for qualitative and quantitative analysis of the aromatic monomers, respectively, as described previously [15,49,52,71].Briefly, GC-MC analyses of the lignin oily product were carried out on a Shimadzu GCMS-QP2010SE equipped with an HP-5 MS (30 m × 250 μm × 0.25 μm, Agilent, Santa Clara, CA, USA) capillary column and an MS detector. GC analyses were conducted with a Shimadzu GC-2010 equipped with an HP-5 column (30 m × 250 μm × 0.25 μm, Agilent) and a flame ionization detector. The monomeric yield obtained from lignins and β-O-4′ model compounds were calculated using Equations (1) and (2), respectively:
Advanced NMR technologies, including 13C, 31P, and 2D HSQC, were used, and analysis of lignin or lignin oily products was performed on a Bruker Ascend-400 MHz spectrometer instrument (Bruker, Hanau, Germany) [52,71]. The molecular weights of lignin and lignin oil were determined by GPC as described previously [52,71].
4. Conclusions
In summary, the structure characterization of CEL isolated from three bamboo species (N. affinis, B. lapidea, and D. brandisii) was investigated. The chemical composition analysis revealed a higher lignin content, up to 32.6% of B. lapidea in comparison with that of N. affinis (20.7%) and D. brandisii (23.8%). The results showed that bamboo lignin was an H-G-S lignin associated with p-coumarates and ferulates, indicating typical characteristics of herbaceous lignin. Moreover, advanced NMR analyses displayed that CEL was extensively acylated at the γ-carbon of the lignin side chain (with either acetate and/or p-coumarate groups). A predominance of S over G lignin moieties was found in CELs of N. affinis and B. lapidea, with the lowest S/G ratio observed in D. brandisii lignin. The catalytic hydrogenolysis of lignin provided deep information on the well-defined low-molecular-weight phenols, giving evidence on the relative abundances of the various C–O bonds and the type of units involved in each of the linkage types. Six major monophenols, e.g., 4-propyl-substituted syringol/guaiacol and propanol guaiacol/syringol derived from β-O-4′moieties, and methyl coumarate/ferulate derived from hydroxycinnamic units, were generated in the range of 16.5~26.6 wt% yields. A sufficient understanding of the structural characteristics of lignin macromolecules in bamboo will facilitate the subsequent utilization of lignocellulosic biomass in an integrated biorefinery.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241210304/s1.
Author Contributions
Conceptualization, L.-P.X., Y.-H.L. and Y.-Q.Y.; methodology, L.-P.X. and Y.-Q.Y.; validation, Y.-H.L., Y.-Q.Y. and Z.-J.S.; investigation, Y.-H.L. and Y.-Q.Y.; formal analysis, Y.-H.L. and Y.-Q.Y.; resources, L.-P.X. and Z.-J.S.; data curation, Y.-H.L. and Y.-Q.Y.; writing—original draft preparation, L.-P.X., Y.-H.L. and Y.-Q.Y.; writing—review and editing, L.-P.X. and R.-C.S.; visualization, S.-L.Z.; supervision, L.-P.X. and R.-C.S.; funding acquisition, L.-P.X. and R.-C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Scientific Research Fund Program for Liaoning Provincial Department of Education (JKZZ20220070), the National Natural Science Foundation of China (22278049 and 51961125207), and the Liaoning Revitalization Talents Program (XLYC2007104).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in the article and its information files.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Wang, T.-P.; Li, H.; Yuan, J.-M.; Li, W.-X.; Li, K.; Huang, Y.-B.; Xiao, L.-P.; Lu, Q. Structures and pyrolytic characteristics of organosolv lignins from typical softwood, hardwood and herbaceous biomass. Ind. Crops Prod. 2021, 171, 113912. [Google Scholar] [CrossRef]
- Li, W.-X.; Xiao, W.-Z.; Yang, Y.-Q.; Wang, Q.; Chen, X.; Xiao, L.-P.; Sun, R.-C. Insights into bamboo delignification with acidic deep eutectic solvents pretreatment for enhanced lignin fractionation and valorization. Ind. Crops Prod. 2021, 170, 113692. [Google Scholar] [CrossRef]
- Das, A.; Rahimi, A.; Ulbrich, A.; Alherech, M.; Motagamwala, A.H.; Bhalla, A.; da Costa Sousa, L.; Balan, V.; Dumesic, J.A.; Hegg, E.L.; et al. Lignin Conversion to Low-Molecular-Weight Aromatics via an Aerobic Oxidation-Hydrolysis Sequence: Comparison of Different Lignin Sources. ACS Sustain. Chem. Eng. 2018, 6, 3367–3374. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Renders, T.; Cooreman, E.; Van den Bosch, S.; Schutyser, W.; Koelewijn, S.F.; Vangeel, T.; Deneyer, A.; Van den Bossche, G.; Courtin, C.M.; Sels, B.F. Catalytic lignocellulose biorefining in n-butanol/water: A one-pot approach toward phenolics, polyols, and cellulose. Green Chem. 2018, 20, 4607–4619. [Google Scholar] [CrossRef]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; da Costa Lopes, A.M.; Łukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Kleinert, M.; Barth, T. Towards a lignincellulosic biorefinery: Direct one-step conversion of lignin to hydrogen-enriched biofuel. Energy Fuels 2008, 22, 1371–1379. [Google Scholar] [CrossRef]
- Ma, Y.; Tan, W.; Wang, J.; Xu, J.; Wang, K.; Jiang, J. Liquefaction of bamboo biomass and production of three fractions containing aromatic compounds. J. Bioresour. Bioprod. 2020, 5, 114–123. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Xue, B.-L.; Sun, R.-C. Quantitative structural characterization of the lignins from the stem and pith of bamboo (Phyllostachys pubescens). Holzforschung 2013, 67, 613–627. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as renewable raw material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; de Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, W.-X.; Yang, Y.-Q.; Chen, X.; Ma, J.; Chen, C.; Xiao, L.-P.; Sun, R.-C. Unlocking Structure-Reactivity Relationships for Catalytic Hydrogenolysis of Lignin into Phenolic Monomers. ChemSusChem 2020, 13, 4548–4556. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Xue, B.-L.; Sun, R.-C. Structural elucidation of inhomogeneous lignins from bamboo. Int. J. Biol. Macromol. 2015, 77, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.B.; Capanema, E.A.; Balakshin, M.Y.; Chang, H.-M.; Jameel, H. Lignin structural variation in hardwood species. J. Agric. Food Chem. 2012, 60, 4923–4930. [Google Scholar] [CrossRef]
- del Rio, J.C.; Rencoret, J.; Gutierrez, A.; Nieto, L.; Jimenez-Barbero, J.; Martinez, A.T. Structural characterization of guaiacyl-rich lignins in flax (Linum usitatissimum) fibers and shives. J. Agric. Food Chem. 2011, 59, 11088–11099. [Google Scholar] [CrossRef]
- Peng, C.; Chen, Q.; Guo, H.; Hu, G.; Li, C.; Wen, J.; Wang, H.; Zhang, T.; Zhao, Z.-K.; Sun, R. Effects of extraction methods on structure and valorization of corn stover lignin by a Pd/C catalyst. ChemCatChem 2017, 9, 1135–1143. [Google Scholar] [CrossRef]
- Shi, Z.-J.; Xiao, L.-P.; Deng, J.; Xu, F.; Sun, R.-C.J.B. Isolation and characterization of soluble polysaccharides of Dendrocalamus brandisii. BioResources 2011, 6, 5151–5166. [Google Scholar]
- Shi, Z.-J.; Xiao, L.-P.; Deng, J.; Sun, R.-C. Isolation and Structural Characterization of Lignin Polymer from Dendrocalamus sinicus. Bioenergy Res. 2013, 6, 1212–1222. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, T.-Q.; Sun, R.-C. Structural elucidation of whole lignin in cell walls of triploid of Populus tomentosa Carr. ACS Sustain. Chem. Eng. 2016, 4, 1006–1015. [Google Scholar] [CrossRef]
- Zhao, B.-C.; Chen, B.-Y.; Yang, S.; Yuan, T.-Q.; Charlton, A.; Sun, R.-C. Structural variation of lignin and lignin–carbohydrate complex in Eucalyptus grandis × E. urophylla during its growth process. ACS Sustain. Chem. Eng. 2017, 5, 1113–1122. [Google Scholar] [CrossRef]
- Wang, H.-M.; Wang, B.; Wen, J.-L.; Yuan, T.-Q.; Sun, R.-C. Structural Characteristics of Lignin Macromolecules from Different Eucalyptus Species. ACS Sustain. Chem. Eng. 2017, 5, 11618–11627. [Google Scholar] [CrossRef]
- Velvizhi, G.; Balakumar, K.; Shetti, N.P.; Ahmad, E.; Kishore Pant, K.; Aminabhavi, T.M. Integrated biorefinery processes for conversion of lignocellulosic biomass to value added materials: Paving a path towards circular economy. Bioresour. Technol. 2022, 343, 126151. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Schutyser, W.; Koelewijn, S.F.; Renders, T.; Courtin, C.M.; Sels, B.F. Tuning the lignin oil OH-content with Ru and Pd catalysts during lignin hydrogenolysis on birch wood. Chem. Commun. 2015, 51, 13158–13161. [Google Scholar] [CrossRef] [PubMed]
- Parsell, T.; Yohe, S.; Degenstein, J.; Jarrell, T.; Klein, I.; Gencer, E.; Hewetson, B.; Hurt, M.; Kim, J.I.; Choudhari, H.; et al. A synergistic biorefinery based on catalytic conversion of lignin prior to cellulose starting from lignocellulosic biomass. Green Chem. 2015, 17, 1492–1499. [Google Scholar] [CrossRef]
- Galkin, M.V.; Smit, A.T.; Subbotina, E.; Artemenko, K.A.; Bergquist, J.; Huijgen, W.J.J.; Samec, J.S.M. Hydrogen-free catalytic fractionation of woody biomass. ChemSusChem 2016, 9, 3280–3287. [Google Scholar] [CrossRef]
- Dou, Z.; Zhang, Z.; Wang, M. Self-hydrogen transfer hydrogenolysis of native lignin over Pd-PdO/TiO2. Appl. Catal. B Environ. 2022, 301, 120767. [Google Scholar] [CrossRef]
- Torr, K.M.; van de Pas, D.J.; Cazeils, E.; Suckling, I.D. Mild hydrogenolysis of in-situ and isolated Pinus radiata lignins. Bioresour. Technol. 2011, 102, 7608–7611. [Google Scholar] [CrossRef]
- Yin, W.-Z.; Xiao, L.-P.; Zou, S.-L.; Li, W.-X.; Wang, H.; Sun, R.-C. Valorization of lignin through reductive catalytic fractionation of fermented corn stover residues. Bioresour. Technol. 2023, 373, 128752. [Google Scholar] [CrossRef]
- Zhang, K.; Li, H.; Xiao, L.-P.; Wang, B.; Sun, R.-C.; Song, G. Sequential utilization of bamboo biomass through reductive catalytic fractionation of lignin. Bioresour. Technol. 2019, 285, 121335. [Google Scholar] [CrossRef]
- Shuai, L.; Amiri, M.T.; Questell-Santiago, Y.M.; Héroguel, F.; Li, Y.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J.S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Odda, A.H.; Liang, K.; Kombo, M.A.; Sahar, S.; Ma, L.-B.; Fang, X.-X.; Xu, A.-W. Metal–acid nanoplate-supported ultrafine Ru nanoclusters for efficient catalytic fractionation of lignin into aromatic alcohols. Green Chem. 2019, 21, 2739–2751. [Google Scholar] [CrossRef]
- Kim, K.H.; Simmons, B.A.; Singh, S. Catalytic transfer hydrogenolysis of ionic liquid processed biorefinery lignin to phenolic compounds. Green Chem. 2017, 19, 215–224. [Google Scholar] [CrossRef]
- Li, T.; Lin, H.; Ouyang, X.; Qiu, X.; Wan, Z. In situ preparation of Ru@N-doped carbon catalyst for the hydrogenolysis of lignin to produce aromatic monomers. ACS Catal. 2019, 9, 5828–5836. [Google Scholar] [CrossRef]
- Zhang, Z.; Lahive, C.W.; Zijlstra, D.S.; Wang, Z.; Deuss, P.J. Sequential Catalytic Modification of the Lignin α-Ethoxylated β-O-4 Motif To Facilitate C–O Bond Cleavage by Ruthenium-Xantphos Catalyzed Hydrogen Transfer. ACS Sustain. Chem. Eng. 2019, 7, 12105–12116. [Google Scholar] [CrossRef]
- Bouxin, F.P.; McVeigh, A.; Tran, F.; Westwood, N.J.; Jarvis, M.C.; Jackson, S.D. Catalytic depolymerisation of isolated lignins to fine chemicals using a Pt/alumina catalyst: Part 1—Impact of the lignin structure. Green Chem. 2015, 17, 1235–1242. [Google Scholar] [CrossRef]
- Yan, N.; Zhao, C.; Dyson, P.J.; Wang, C.; Liu, L.-T.; Kou, Y. Selective degradation of wood lignin over noble-metal catalysts in a two-step process. ChemSusChem 2008, 1, 626–629. [Google Scholar] [CrossRef]
- Ouyang, X.; Huang, X.; Zhu, J.; Boot, M.D.; Hensen, E.J.M. Catalytic conversion of lignin in woody biomass into phenolic monomers in methanol/water mixtures without external hydrogen. ACS Sustain. Chem. Eng. 2019, 7, 13764–13773. [Google Scholar] [CrossRef]
- Luo, H.; Klein, I.M.; Jiang, Y.; Zhu, H.; Liu, B.; Kenttämaa, H.I.; Abu-Omar, M.M. Total utilization of miscanthus biomass, lignin and carbohydrates, using earth abundant nickel catalyst. ACS Sustain. Chem. Eng. 2016, 4, 2316–2322. [Google Scholar] [CrossRef]
- Cheng, C.; Truong, J.; Barrett, J.A.; Shen, D.; Abu-Omar, M.M.; Ford, P.C. Hydrogenolysis of Organosolv Lignin in Ethanol/Isopropanol Media without Added Transition-Metal Catalyst. ACS Sustain. Chem. Eng. 2020, 8, 1023–1030. [Google Scholar] [CrossRef]
- Anderson, E.M.; Katahira, R.; Reed, M.; Resch, M.G.; Karp, E.M.; Beckham, G.T.; Román-Leshkov, Y. Reductive catalytic fractionation of corn stover lignin. ACS Sustain. Chem. Eng. 2016, 4, 6940–6950. [Google Scholar] [CrossRef]
- Wang, S.; Gao, W.; Xiao, L.-P.; Shi, J.; Sun, R.-C.; Song, G. Hydrogenolysis of biorefinery corncob lignin into aromatic phenols over activated carbon-supported nickel. Sustain. Energy Fuels 2019, 3, 401–408. [Google Scholar] [CrossRef]
- Chen, J.; Lu, F.; Si, X.; Nie, X.; Chen, J.; Lu, R.; Xu, J. High Yield Production of Natural Phenolic Alcohols from Woody Biomass Using a Nickel-Based Catalyst. ChemSusChem 2016, 9, 3353–3360. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bouxin, F.P.; Fan, J.; Budarin, V.L.; Hu, C.; Clark, J.H. Microwave-assisted catalytic depolymerization of lignin from birch sawdust to produce phenolic monomers utilizing a hydrogen-free strategy. J. Hazard. Mater. 2021, 402, 123490. [Google Scholar] [CrossRef]
- Agarwal, S.; Chowdari, R.K.; Hita, I.; Heeres, H.J. Experimental Studies on the Hydrotreatment of Kraft Lignin to Aromatics and Alkylphenolics Using Economically Viable Fe-Based Catalysts. ACS Sustain. Chem. Eng. 2017, 5, 2668–2678. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Wang, S.; Li, H.; Li, Z.; Shi, Z.-J.; Xiao, L.; Sun, R.-C.; Fang, Y.; Song, G. Catalytic hydrogenolysis of lignins into phenolic compounds over carbon nanotube supported molybdenum oxide. ACS Catal. 2017, 7, 7535–7542. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Xiao, L.-P.; Guo, X.; Fang, Y.; Sun, R.-C.; Song, G. Fragmentation of Woody Lignocellulose into Primary Monolignols and Their Derivatives. ACS Sustain. Chem. Eng. 2019, 7, 4666–4674. [Google Scholar] [CrossRef]
- Sun, Z.; Bottari, G.; Afanasenko, A.; Stuart, M.C.A.; Deuss, P.J.; Fridrich, B.; Barta, K. Complete lignocellulose conversion with integrated catalyst recycling yielding valuable aromatics and fuels. Nat. Catal. 2018, 1, 82–92. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, L.-P.; Lv, Y.-H.; Yin, W.-Z.; Hou, C.-J.; Sun, R.-C. Metal–Organic-Framework-Derived Copper Catalysts for the Hydrogenolysis of Lignin into Monomeric Phenols. ACS Catal. 2022, 12, 11899–11909. [Google Scholar] [CrossRef]
- Yang, Y.-Q.; Xiao, L.-P.; Xiao, W.-Z.; Li, X.-Y.; Wang, Q.; Sun, R.-C. Nitrogen-doped carbon anchored ruthenium nanoparticles for biofuel upgrade. Fuel 2022, 314, 123100. [Google Scholar] [CrossRef]
- Ghaffar, S.H.; Fan, M. Structural analysis for lignin characteristics in biomass straw. Biomass Bioenergy 2013, 57, 264–279. [Google Scholar] [CrossRef]
- Huang, C.; He, J.; Du, L.; Min, D.; Yong, Q. Structural Characterization of the Lignins from the Green and Yellow Bamboo of Bamboo Culm (Phyllostachys pubescens). J. Wood Chem. Technol. 2016, 36, 157–172. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, T.; Fu, Q.-J.; Bai, Y.-Y.; Peng, F.; Yao, C.-L. Choline chloride-lactic acid deep eutectic solvent for delignification and nanocellulose production of moso bamboo. Cellulose 2019, 26, 9447–9462. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-N.; Yuan, T.-Q.; Xu, F.; Sun, R.-C. Fractionation of bamboo culms by autohydrolysis, organosolv delignification and extended delignification: Understanding the fundamental chemistry of the lignin during the integrated process. Bioresour. Technol. 2013, 150, 278–286. [Google Scholar] [CrossRef]
- Xu, G.; Shi, Z.; Zhao, Y.; Deng, J.; Dong, M.; Liu, C.; Murugadoss, V.; Mai, X.; Guo, Z. Structural characterization of lignin and its carbohydrate complexes isolated from bamboo (Dendrocalamus sinicus). Int. J. Biol. Macromol. 2019, 126, 376–384. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H.; et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl- propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Sun, S.-L.; Wen, J.-L.; Ma, M.-G.; Sun, R.-C.; Jones, G.L. Structural features and antioxidant activities of degraded lignins from steam exploded bamboo stem. Ind. Crops Prod. 2014, 56, 128–136. [Google Scholar] [CrossRef]
- Huang, C.; Zhan, Y.; Wang, J.; Cheng, J.; Meng, X.; Liang, L.; Liang, F.; Deng, Y.; Fang, G.; Ragauskas, A.J. Valorization of bamboo biomass using combinatorial pretreatments. Green Chem. 2022, 24, 3736–3749. [Google Scholar] [CrossRef]
- Bai, Y.-Y.; Xiao, L.-P.; Shi, Z.-J.; Sun, R.-C. Structural Variation of Bamboo Lignin before and after Ethanol Organosolv Pretreatment. Int. J. Mol. Sci. 2013, 14, 21394–21413. [Google Scholar] [CrossRef]
- Huang, C.; Fang, G.; Zhou, Y.; Du, X.; Yu, L.; Meng, X.; Li, M.; Yoo, C.G.; Chen, B.; Zhai, S.; et al. Increasing the Carbohydrate Output of Bamboo Using a Combinatorial Pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 7380–7393. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Oh, S.; Hwang, H.; Kim, U.-J.; Choi, J.W. Structural features and thermal degradation properties of various lignin macromolecules obtained from poplar wood (Populus albaglandulosa). Polym. Degrad. Stab. 2013, 98, 1671–1678. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.Y.; De Hoop, C.F.; Hu, T.; Qi, J.; Shupe, T.F. Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef]
- Kawamoto, H. Lignin pyrolysis reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef]
- Wen, J.-L.; Xue, B.-L.; Xu, F.; Sun, R.-C.; Pinkert, A. Unmasking the structural features and property of lignin from bamboo. Ind. Crops Prod. 2013, 42, 332–343. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Kim, H.; Lu, F.; Ralph, J. Whole plant cell wall characterization using solution-state 2D NMR. Nat. Protoc. 2012, 7, 1579–1589. [Google Scholar] [CrossRef]
- del Río, J.C.; Rencoret, J.; Marques, G.; Gutiérrez, A.; Ibarra, D.; Santos, J.I.; Jiménez-Barbero, J.; Zhang, L.; Martínez, Á.T. Highly Acylated (Acetylated and/or p-Coumaroylated) Native Lignins from Diverse Herbaceous Plants. J. Agric. Food Chem. 2008, 56, 9525–9534. [Google Scholar] [CrossRef]
- del Río, J.C.; Rencoret, J.; Prinsen, P.; Martínez, Á.T.; Ralph, J.; Gutiérrez, A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Xiao, L.-P.; Chen, X.; Wang, S.; Chen, X.-H.; Guo, Y.; Zhai, S.-R. Lignin-First Depolymerization of Lignocellulose into Monophenols over Carbon Nanotube-Supported Ruthenium: Impact of Lignin Sources. ChemSusChem 2022, 15, e202200365. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Rencoret, J.; Lu, F.; Karlen, S.D.; Smith, B.G.; Harris, P.J.; del Río, J.C.; Ralph, J. Tricin-lignins: Occurrence and quantitation of tricin in relation to phylogeny. Plant J. 2016, 88, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Takada, R.; Tobimatsu, Y.; Takeda, Y.; Suzuki, S.; Yamamura, M.; Osakabe, K.; Osakabe, Y.; Sakamoto, M.; Umezawa, T. OsMYB108 loss-of-function enriches p-coumaroylated and tricin lignin units in rice cell walls. Plant J. 2019, 98, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, S.; Ohkubo, K.; Towers, G.H.N. Cinnamic acid derivatives in cell walls of bamboo and bamboo grass. Phytochemistry 1992, 31, 3207–3209. [Google Scholar] [CrossRef]
- del Río, J.C.; Rencoret, J.; Gutiérrez, A.; Kim, H.; Ralph, J. Chapter One—Unconventional lignin monomers—Extension of the lignin paradigm. In Advances in Botanical Research; Sibout, R., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 104, pp. 1–39. [Google Scholar]
- Liu, X.; Li, H.; Xiao, L.-P.; Sun, R.-C.; Song, G. Chemodivergent hydrogenolysis of eucalyptus lignin with Ni@ZIF-8 catalyst. Green Chem. 2019, 21, 1498–1504. [Google Scholar] [CrossRef]
- Strassberger, Z.; Alberts, A.H.; Louwerse, M.J.; Tanase, S.; Rothenberg, G. Catalytic cleavage of lignin β-O-4 link mimics using copper on alumina and magnesia–alumina. Green Chem. 2013, 15, 768–774. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Shi, Z.-J.; Xu, F.; Sun, R.-C. Hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H.; Gao, X.; Guo, X.; Wang, S.; Fang, Y.; Song, G. Rational highly dispersed ruthenium for reductive catalytic fractionation of lignocellulose. Nat. Commun. 2022, 13, 4716. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. In Laboratory Analytical Procedure (NREL/TP-510-42618); NREL: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass. In Laboratory Analytical Procedure (NREL TP-510-42619); NREL: Golden, CO, USA, 2008. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).