High-Strength, High-Water-Retention Hemicellulose-Based Hydrogel and Its Application in Urea Slow Release
Abstract
1. Introduction
2. Results and Discussion
2.1. Microstructure Analysis of HC-CSN-Fe3+ Hydrogels
2.2. Functional Group Analysis of HC-CSN-Fe3+ Hydrogels
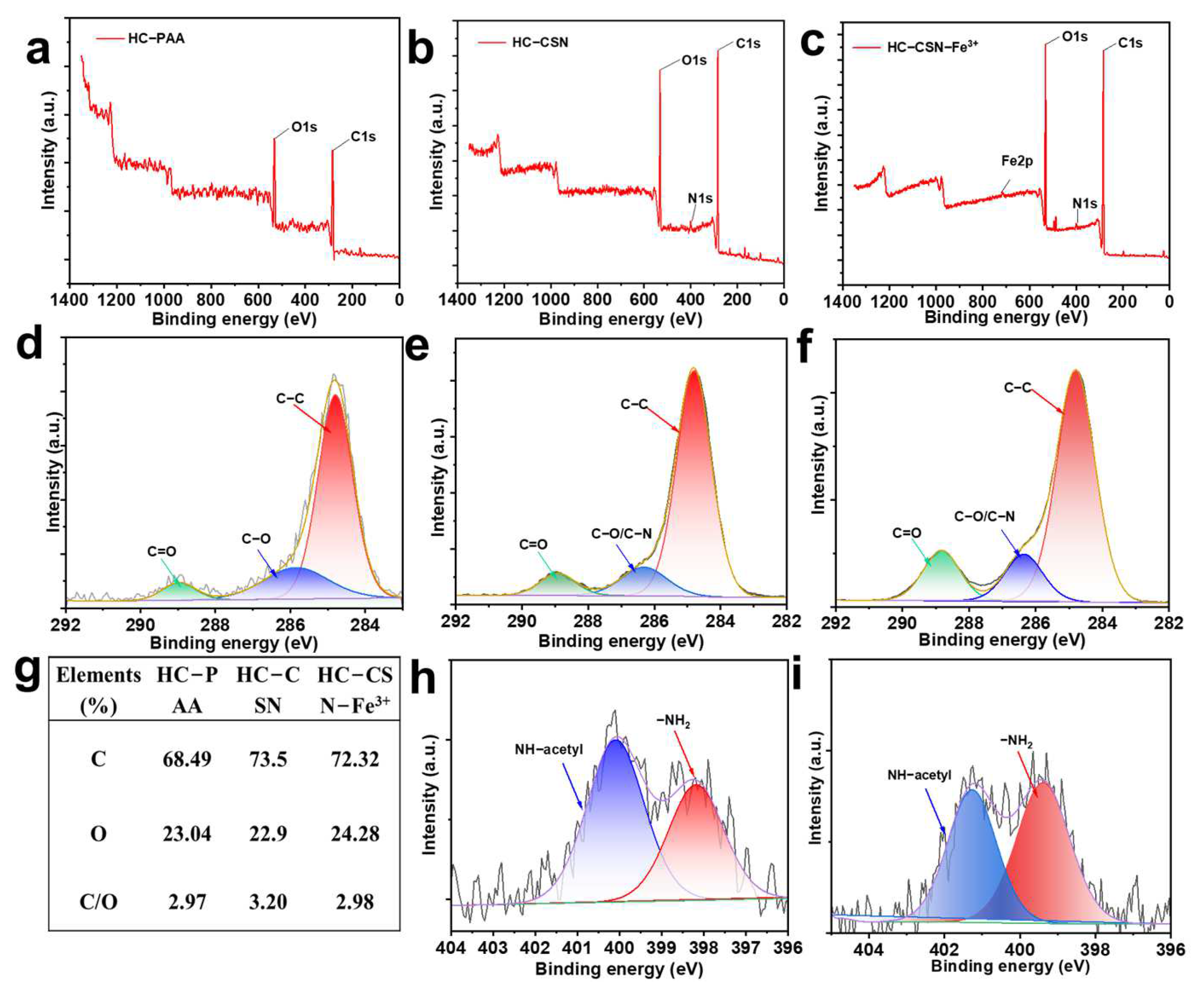
2.3. Surface Elemental Analysis of HC-CSN-Fe3+ Hydrogels
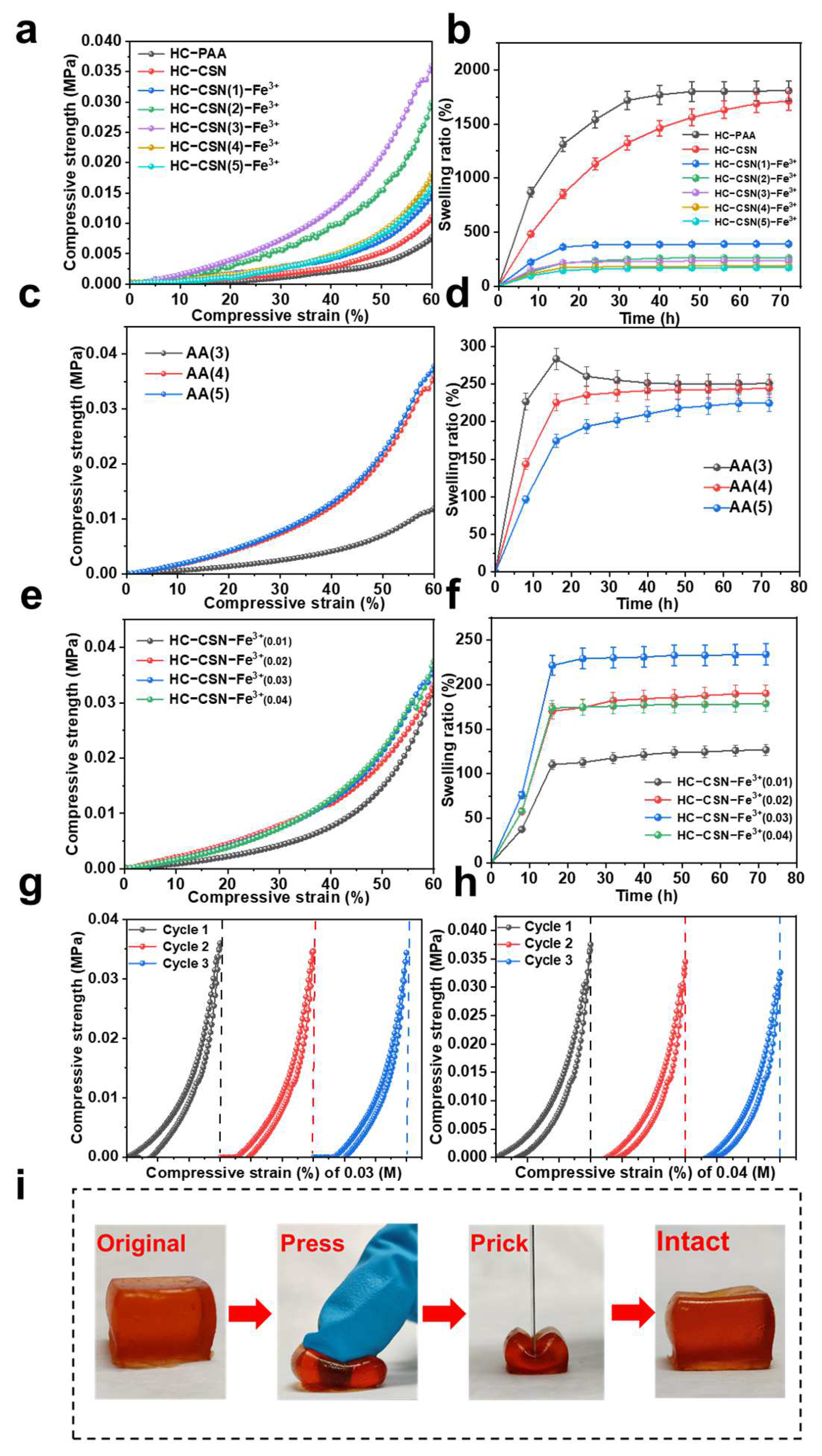
2.4. Effects of Hemicellulose and Chitosan Content on HC-CSN-Fe3+ Hydrogel Properties
2.5. Effect of AA Addition on HC-CSN-Fe3+ Hydrogel Properties
2.6. Effect of Iron Ion Concentration on HC-CSN-Fe3+ Hydrogel Properties
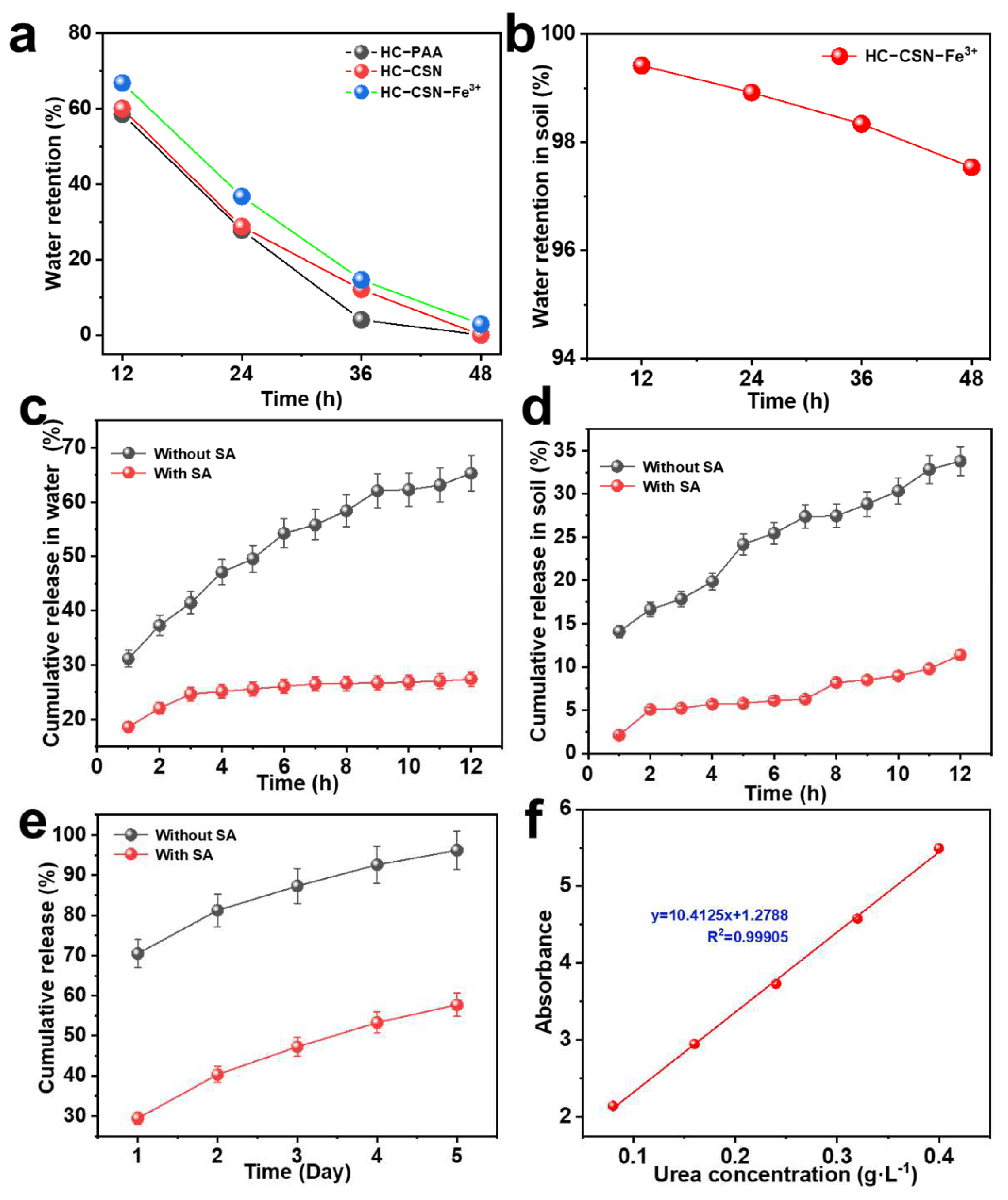
2.7. Analysis of Water Retention Properties of HC-CSN-Fe3+ Hydrogels
2.8. Rheological Analysis of HC-CSN-Fe3+ Hydrogels
2.9. Analysis of Antioxidant Properties of HC-CSN-Fe3+ Hydrogels
2.10. Analysis of UV Resistance of HC-CSN-Fe3+ Hydrogels
2.11. Analysis of Urea Release Performance of HC-CSN-Fe3+/SA Core-Shell Hydrogels
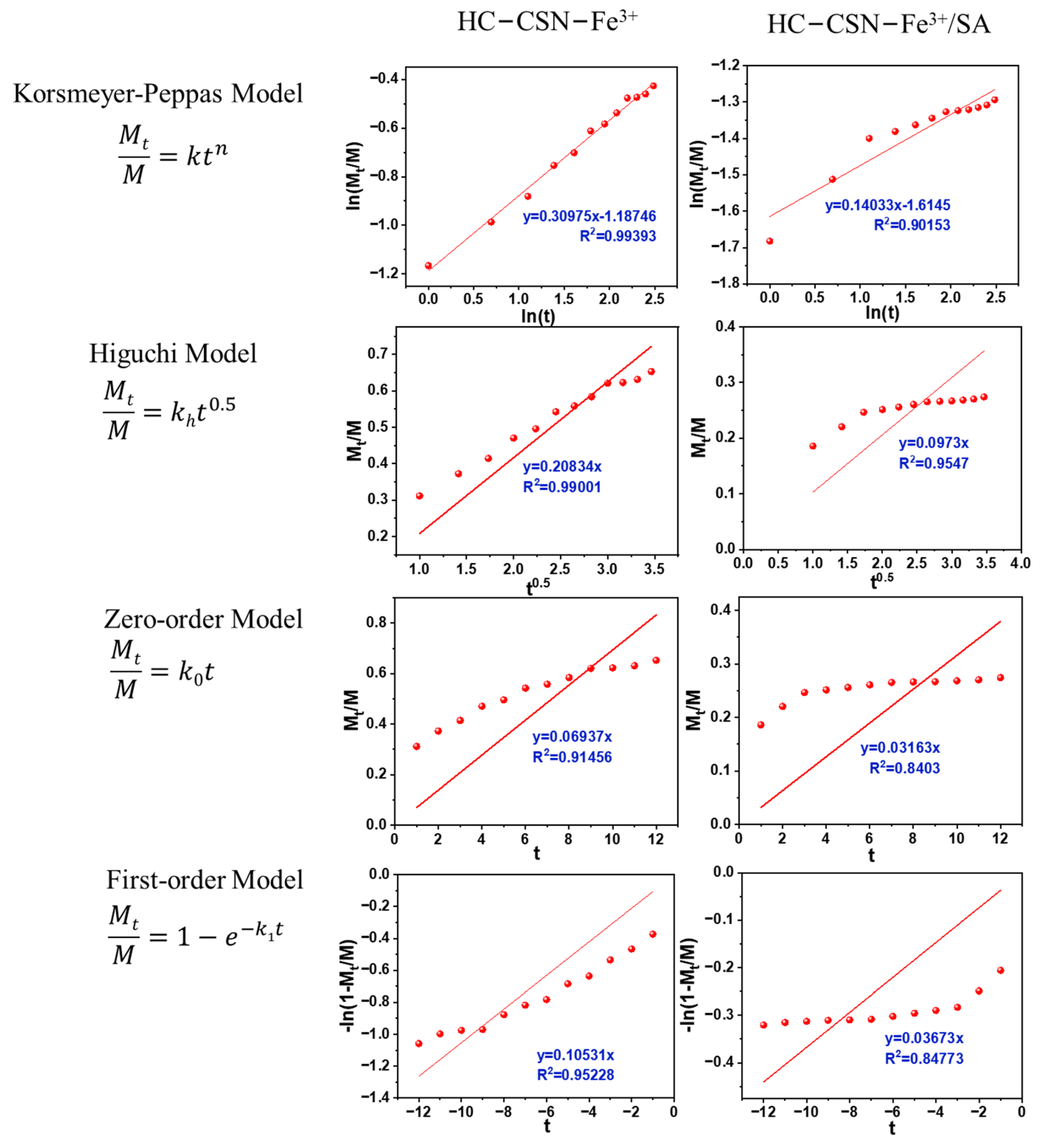
2.12. Analysis of Urea Release Kinetics by HC-CSN-Fe3+/SA Core-Shell Hydrogel in Water
2.13. Analysis of Urea Release Kinetics by HC-CSN-Fe3+/SA Core-Shell Hydrogels in Soil
3. Materials and Methods
3.1. Materials
3.2. Preparation of Hemicellulose-Based Hydrogels and Preparation of Sustained-Release Urea Systems
3.3. Characterization Analysis of HC-CSN-Fe3+ Hydrogels
3.4. Analysis of Compression Properties of HC-CSN-Fe3+ Hydrogels
3.5. Analysis of Swelling Ratio of HC-CSN-Fe3+ Hydrogels
3.6. Analysis of Water Retention Properties of HC-CSN-Fe3+ Hydrogels
3.7. Rheological Analysis of HC-CSN-Fe3+ Hydrogels
3.8. Analysis of Antioxidant Properties of HC-CSN-Fe3+ Hydrogels
3.9. Analysis of UV Resistance of HC-CSN-Fe3+ Hydrogels
3.10. Analysis of Urea Release Performance of HC-CSN-Fe3+/SA Core-Shell Hydrogels
3.11. Release Kinetics Analysis of Urea using HC-CSN-Fe3+/SA Core-Shell Hydrogels
- (1)
- Korsmeyer–Peppas model
- (2)
- Higuchi model
- (3)
- Zero-order model
- (4)
- First-order model
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Irfan, S.A.; Razali, R.; KuShaari, K.; Mansor, N.; Azeem, B.; Versypt, A.N.F. A review of mathematical modeling and simulation of controlled-release fertilizers. J. Control. Release 2018, 271, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Yan, X. How to feed the world while reducing nitrogen pollution. Nature 2023, 613, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhang, H.; Chen, X.; Zhang, C.; Ma, W.; Huang, C.; Zhang, W.; Mi, G.; Miao, Y.; Li, X.; et al. Pursuing sustainable productivity with millions of smallholder farmers. Nature 2018, 555, 363. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Seggiani, M.; Cinelli, P.; Elnaby, H.M.H.; Azaam, M.M. Swelling capacity of sugarcane bagasse-g-poly(acrylamide)/attapulgite superabsorbent composites and their application as slow release fertilizer. Eur. Polym. J. 2020, 133, 109769. [Google Scholar] [CrossRef]
- Pereira, E.I.; Nogueira, A.R.A.; Cruz, C.C.T.; Guimarães, G.G.F.; Foschini, M.M.; Bernardi, A.C.C.; Ribeiro, C. Controlled urea release employing nanocomposites increases the efficiency of nitrogen use by forage. ACS Sustain. Chem. Eng. 2017, 5, 9993–10001. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, C.; Li, G.; Jiang, Y.; Hou, P.; Xue, L.; Yang, L.; Ding, Y. Lower dose of controlled/slow release fertilizer with higher rice yield and N utilization in paddies: Evidence from a meta-analysis. Field Crop. Res. 2023, 294, 108879. [Google Scholar] [CrossRef]
- Wei, H.; Wang, H.; Chu, H.; Li, J. Preparation and characterization of slow-release and water-retention fertilizer based on starch and halloysite. Int. J. Biol. Macromol. 2019, 133, 1210–1218. [Google Scholar] [CrossRef]
- Li, L.; Zhao, J.; Sun, Y.; Yu, F.; Ma, J. Ionically cross-linked sodium alginate/kappa-carrageenan double-network gel beads with low-swelling, enhanced mechanical properties, and excellent adsorption performance. Chem. Eng. J. 2019, 372, 1091–1103. [Google Scholar] [CrossRef]
- Yang, J.; Lin, Y. Spatiotemporal evolution and driving factors of fertilizer reduction control in Zhejiang Province. Sci. Total. Environ. 2019, 660, 650–659. [Google Scholar] [CrossRef]
- Kaur, J.; Sharma, K.; Kaushik, A. Waste hemp-stalk derived nutrient encapsulated aerogels for slow release of fertilizers: A step towards sustainable agriculture. J. Environ. Chem. Eng. 2023, 11, 109582. [Google Scholar] [CrossRef]
- Rodrigues Sousa, H.; Sá Lima, I.; Matheus Lima Neris, L.; Santos Silva, A.; Maria Silva Santos Nascimento, A.; Pereira de Araújo, F.; Felippe Ratke, R.; Anteveli Osajima, J.; Loiola Edvan, R.; Kauany da Silva Azevedo, C.; et al. Innovative hydrogels made from babassu mesocarp for technological application in agriculture. J. Mol. Liq. 2023, 376, 121463. [Google Scholar] [CrossRef]
- Wu, C.; Dan, Y.; Tian, D.; Zheng, Y.; Wei, S.; Xiang, D. Facile fabrication of MOF(Fe)@alginate aerogel and its application for a high-performance slow-release N-fertilizer. Int. J. Biol. Macromol. 2020, 145, 1073–1079. [Google Scholar] [CrossRef]
- Mathew, A.P.; Uthaman, S.; Cho, K.-H.; Cho, C.-S.; Park, I.-K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef]
- Rabat, N.E.; Hashim, S.; Majid, R.A. Effect of different monomers on water retention properties of slow release fertilizer hydrogel. In Proceedings of the 4th International Conference on Process Engineering and Advanced Materials (ICPEAM), Kuala Lumpur, Malaysia, 15–17 August 2016; pp. 201–207. [Google Scholar]
- Li, J.; Liu, B.J.; Liu, L.; Luo, Y.D.; Zeng, F.Y.; Qin, C.R.; Liang, C.; Huang, C.X.; Yao, S.Q. Pretreatment of poplar with eco-friendly levulinic acid to achieve efficient utilization of biomass. Bioresour. Technol. 2023, 376, 128855. [Google Scholar] [CrossRef]
- Liu, B.J.; Liu, L.; Deng, B.J.; Huang, C.X.; Zhu, J.T.; Liang, L.L.; He, X.L.; Wei, Y.X.; Qin, C.R.; Liang, C.; et al. Application and prospect of organic acid pretreatment in lignocellulosic biomass separation: A review. Int. J. Biol. Macromol. 2022, 222, 1400–1413. [Google Scholar] [CrossRef]
- Liu, L.; Liu, B.; Li, X.; Wang, Z.; Mu, L.; Qin, C.; Liang, C.; Huang, C.; Yao, S. Mannitol assisted oxalic acid pretreatment of poplar for the deconstruction and separation of hemicellulose. Ind. Crop. Prod. 2023, 200, 116811. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Liang, J.; Wang, F.; Bao, Y.; Qin, C.; Liang, C.; Huang, C.; Yao, S. Rapid and mild fractionation of hemicellulose through recyclable mandelic acid pretreatment. Bioresour. Technol. 2023, 382, 129154. [Google Scholar] [CrossRef]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.M.; Feng, C.Q.; Liu, X.Y.; Qin, F.Y.; Liang, C.; Bian, H.Y.; Qin, C.R.; Yao, S.Q. Green, efficient extraction of bamboo hemicellulose using freeze-thaw assisted alkali treatment. Bioresour. Technol. 2021, 333, 125107. [Google Scholar] [CrossRef]
- Deng, B.; Hou, Y.; Wang, F.; Bao, Y.; Zeng, F.; Qin, C.; Liang, C.; Huang, C.; Ma, J.; Yao, S. Highly selective separation of eucalyptus hemicellulose by salicylic acid treatment with both aromatic and hydroxy acids. Bioresour. Technol. 2022, 355, 127304. [Google Scholar] [CrossRef]
- Li, Z.; Pan, X. Strategies to modify physicochemical properties of hemicelluloses from biorefinery and paper industry for packaging material. Rev. Environ. Sci. Bio/Technol. 2018, 17, 47–69. [Google Scholar] [CrossRef]
- Peng, X.; Ren, J.; Sun, R. An efficient method for the synthesis of hemicellulosic derivatives with bifunctional groups in butanol/water medium and their rheological properties. Carbohydr. Polym. 2011, 83, 1922–1928. [Google Scholar] [CrossRef]
- Agustin, M.B.; Nakatsubo, F.; Yano, H. Improving the thermal stability of wood-based cellulose by esterification. Carbohydr. Polym. 2018, 192, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Farhat, W.; Venditti, R.; Ayoub, A.; Prochazka, F.; Fernández-de-Alba, C.; Mignard, N.; Taha, M.; Becquart, F. Towards thermoplastic hemicellulose: Chemistry and characteristics of poly-(ε-caprolactone) grafting onto hemicellulose backbones. Mater. Des. 2018, 153, 298–307. [Google Scholar] [CrossRef]
- Liu, X.; Luan, S.; Li, W. Utilization of waste hemicelluloses lye for superabsorbent hydrogel synthesis. Int. J. Biol. Macromol. 2019, 132, 954–962. [Google Scholar] [CrossRef]
- Maleki, L.; Edlund, U.; Albertsson, A.-C. Synthesis of full interpenetrating hemicellulose hydrogel networks. Carbohydr. Polym. 2017, 170, 254–263. [Google Scholar] [CrossRef]
- Gao, C.D.; Ren, J.L.; Zhao, C.; Kong, W.Q.; Dai, Q.Q.; Chen, Q.F.; Liu, C.F.; Sun, R.C. Xylan-based temperature/pH sensitive hydrogels for drug controlled release. Carbohydr. Polym. 2016, 151, 189–197. [Google Scholar] [CrossRef]
- Gong, X.Q.; Fu, C.L.; Alam, N.; Ni, Y.H.; Chen, L.H.; Huang, L.L.; Hu, H.C. Tannic acid modified hemicellulose nanoparticle reinforced ionic hydrogels with multi-functions for human motion strain sensor applications. Ind. Crop. Prod. 2022, 176, 114412. [Google Scholar] [CrossRef]
- Lian, Y.X.; Zhang, J.; Li, N.; Ping, Q.W. Preparation of hemicellulose-based hydrogel and its application as an adsorbent towards heavy metal ions. Bioresources 2018, 13, 3208–3218. [Google Scholar] [CrossRef]
- Zhang, X.N.; Wang, Y.J.; Sun, S.; Hou, L.; Wu, P.; Wu, Z.L.; Zheng, Q. A tough and stiff hydrogel with tunable water content and mechanical properties based on the synergistic effect of hydrogen bonding and hydrophobic interaction. Macromolecules 2018, 51, 8136–8146. [Google Scholar] [CrossRef]
- Kong, W.Q.; Huang, D.Y.; Xu, G.B.; Ren, J.L.; Liu, C.F.; Zhao, L.H.; Sun, R.C. Graphene oxide/polyacrylamide/aluminum ion cross-linked carboxymethyl hemicellulose nanocomposite hydrogels with very tough and elastic properties. Chem.-Asian J. 2016, 11, 1697–1704. [Google Scholar] [CrossRef]
- Essawy, H.A.; Ghazy, M.B.M.; Abd El-Hai, F.; Mohamed, M.F. Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef]
- Wang, Y.F.; Liu, M.Z.; Ni, B.L.; Xie, L.H. Kappa-carrageenan-sodium alginate beads and superabsorbent coated nitrogen fertilizer with slow-release, water-retention, and anticompaction properties. Ind. Eng. Chem. Res. 2012, 51, 1413–1422. [Google Scholar] [CrossRef]
- Cui, F.H.; Zi, H.; Liu, H.S.; Zhang, S.B.; Yuan, B. A study of starch-urea-water mixtures with a combination of molecular Chock tor dynamics simulation and traditional characterization methods. Int. J. Biol. Macromol. 2020, 148, 121–128. [Google Scholar] [CrossRef]
- Li, Y.; Yao, M.Z.; Luo, Y.D.; Li, J.; Wang, Z.L.; Liang, C.; Qin, C.R.; Huang, C.X.; Yao, S.Q. Polydopamine-reinforced hemicellulose-based multifunctional flexible hydrogels for human movement sensing and self-powered transdermal drug delivery. ACS Appl. Mater. Interfaces 2023, 15, 5883–5896. [Google Scholar] [CrossRef]
- Luo, Y.D.; Li, Y.; Cao, L.M.; Zhu, J.T.; Deng, B.J.; Hou, Y.J.; Liang, C.; Huang, C.X.; Qin, C.R.; Yao, S.Q. High efficiency and clean separation of eucalyptus components by glycolic acid pretreatment. Bioresour. Technol. 2021, 341, 125757. [Google Scholar] [CrossRef]
- Shang, J.; Shao, Z.Z.; Chen, X. Chitosan-based electroactive hydrogel. Polymer 2008, 49, 5520–5525. [Google Scholar] [CrossRef]
- Peng, X.W.; Zhong, L.X.; Ren, J.L.; Sun, R.C. Highly effective adsorption of heavy metal ions from aqueous solutions by macroporous xylan-rich hemicelluloses-based hydrogel. J. Agric. Food Chem. 2012, 60, 3909–3916. [Google Scholar] [CrossRef]
- Wang, H.X.; Qan, J.; Ding, F.Y. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Feng, C.Q.; Zhu, J.T.; Hou, Y.J.; Qin, C.R.; Chen, W.Q.; Nong, Y.H.; Liao, Z.P.; Liang, C.; Bian, H.Y.; Yao, S.Q. Effect of temperature on simultaneous separation and extraction of hemicellulose using p-toluenesulfonic acid treatment at atmospheric pressure. Bioresour. Technol. 2022, 348, 126793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Wu, X.J.; Qin, Z.H.; Sun, X.; Zhang, H.; Yu, Q.Y.; Yao, M.M.; He, S.S.; Dong, X.R.; Yao, F.L.; et al. Dual physically cross-linked carboxymethyl cellulose-based hydrogel with high stretchability and toughness as sensitive strain sensors. Cellulose 2020, 27, 9975–9989. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca stress simulation of metal-on-metal total hip arthroplasty during normal walking Activity. Materials 2021, 14, 7554. [Google Scholar] [CrossRef] [PubMed]
- Ammarullah, M.I.; Hartono, R.; Supriyono, T.; Santoso, G.; Sugiharto, S.; Permana, M.S. Polycrystalline diamond as a potential material for the hard-on-hard bearing of total hip prosthesis: Von Mises Stress Analysis. Biomedicines 2023, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Wibowo, D.B.; Kurdi, O.; Tauviqirrahman, M.; Jamari, J. Minimizing risk of failure from ceramic-on-ceramic total hip prosthesis by selecting ceramic materials based on tresca Stress. Sustainability 2022, 14, 13413. [Google Scholar] [CrossRef]
- Hamilton, A.; Breslin, C.B. The development of a highly sensitive urea sensor due to the formation of an inclusion complex between urea and sulfonated-beta-cyclodextrin. Electrochim. Acta 2014, 125, 250–257. [Google Scholar] [CrossRef]
- Santoso, B.; Ammarullah, M.I.; Haryati, S.; Sofijan, A.; Bustan, M.D. Power and energy optimization of carbon based lithium-ion battery from water spinach. J. Ecol. Eng. 2023, 24, 213–223. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Permana, M.S.; Winarni, T.I.; van der Heide, E. Adopted walking condition for computational simulation approach on bearing of hip joint prosthesis: Review over the past 30 years. Heliyon 2022, 8, e12050. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In silico contact pressure of metal-on-metal total hip implant with different materials subjected to gait loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Tauviqirrahman, M.; Ammarullah, M.I.; Jamari, J.; Saputra, E.; Winarni, T.I.; Kurniawan, F.D.; Shiddiq, S.A.; van der Heide, E. Analysis of contact pressure in a 3D model of dual-mobility hip joint prosthesis under a gait cycle. Sci. Rep. 2023, 13, 3564. [Google Scholar] [CrossRef]
- Salaha, Z.F.M.; Ammarullah, M.I.; Abdullah, N.N.A.A.; Aziz, A.U.A.; Gan, H.-S.; Abdullah, A.H.; Abdul Kadir, M.R.; Ramlee, M.H. Biomechanical effects of the porous structure of gyroid and voronoi hip implants: A finite element analysis using an experimentally validated model. Materials 2023, 16, 3298. [Google Scholar] [CrossRef]
- Tauviqirrahman, M.; Jamari, J.; Susilowati, S.; Pujiastuti, C.; Setiyana, B.; Pasaribu, A.H.; Ammarullah, M.I. Performance Comparison of Newtonian and Non-Newtonian Fluid on a Heterogeneous Slip/No-Slip Journal Bearing System Based on CFD-FSI Method. Fluids 2022, 7, 225. [Google Scholar] [CrossRef]


| Samples | HC/CSN Ratio (g·.g−1) | Fe3+ (mol·L−1) | AA (mL) |
|---|---|---|---|
| HC-CSN-Fe3+ | 5:1 | 0.03 | 4 |
| HC-CSN-Fe3+ | 5:2 | 0.03 | 4 |
| HC-CSN-Fe3+ | 5:3 | 0.03 | 4 |
| HC-CSN-Fe3+ | 5:4 | 0.03 | 4 |
| HC-CSN-Fe3+ | 5:5 | 0.03 | 4 |
| HC-CSN-Fe3+ | 5:3 | 0.03 | 3 |
| HC-CSN-Fe3+ | 5:3 | 0.03 | 4 |
| HC-CSN-Fe3+ | 5:3 | 0.03 | 5 |
| HC-CSN-Fe3+ | 5:3 | 0.01 | 4 |
| HC-CSN-Fe3+ | 5:3 | 0.02 | 4 |
| HC-CSN-Fe3+ | 5:3 | 0.03 | 4 |
| HC-CSN-Fe3+ | 5:3 | 0.04 | 4 |
| Kinetic Models | Parameter | Gel | Gel-SA |
|---|---|---|---|
| Korsmeyer-Peppas | R2 | 0.9939 | 0.9015 |
| n | 0.3098 | 0.1403 | |
| k | 0.3050 | 0.1990 | |
| Higuchi | R2 | 0.9900 | 0.9547 |
| kh | 0.2083 | 0.0973 | |
| Zero-order | R2 | 0.9146 | 0.8403 |
| k0 | 0.0694 | 0.0316 | |
| First-order | R2 | 0.9523 | 0.8477 |
| k1 | 0.1053 | 0.0367 |
| Kinetic Models | Parameter | Gel | Gel-SA |
|---|---|---|---|
| Korsmeyer-Peppas | R2 | 0.9690 | 0.8794 |
| n | 0.3688 | 0.4033 | |
| k | 0.1301 | 0.0343 | |
| Higuchi | R2 | 0.9958 | 0.9894 |
| kh | 0.1004 | 0.0288 | |
| Zero-order | R2 | 0.9385 | 0.9563 |
| k0 | 0.0338 | 0.0098 | |
| First-order | R2 | 0.9528 | 0.9595 |
| k1 | 0.3992 | 0.0103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Deng, B.; Wang, S.; Ma, Y.; Long, X.; Wang, F.; Qin, C.; Liang, C.; Yao, S. High-Strength, High-Water-Retention Hemicellulose-Based Hydrogel and Its Application in Urea Slow Release. Int. J. Mol. Sci. 2023, 24, 9208. https://doi.org/10.3390/ijms24119208
Hou Y, Deng B, Wang S, Ma Y, Long X, Wang F, Qin C, Liang C, Yao S. High-Strength, High-Water-Retention Hemicellulose-Based Hydrogel and Its Application in Urea Slow Release. International Journal of Molecular Sciences. 2023; 24(11):9208. https://doi.org/10.3390/ijms24119208
Chicago/Turabian StyleHou, Yajun, Baojuan Deng, Shanshan Wang, Yun Ma, Xing Long, Fei Wang, Chengrong Qin, Chen Liang, and Shuangquan Yao. 2023. "High-Strength, High-Water-Retention Hemicellulose-Based Hydrogel and Its Application in Urea Slow Release" International Journal of Molecular Sciences 24, no. 11: 9208. https://doi.org/10.3390/ijms24119208
APA StyleHou, Y., Deng, B., Wang, S., Ma, Y., Long, X., Wang, F., Qin, C., Liang, C., & Yao, S. (2023). High-Strength, High-Water-Retention Hemicellulose-Based Hydrogel and Its Application in Urea Slow Release. International Journal of Molecular Sciences, 24(11), 9208. https://doi.org/10.3390/ijms24119208








