Unravelling the Interplay between Cardiac Metabolism and Heart Regeneration
Abstract
1. Introduction
2. The Role of Metabolic Substrates in Heart Regeneration
2.1. Glucose Metabolism
2.2. Fatty Acid Metabolism
2.3. Ketone Body Metabolism
2.4. Amino Acid Metabolism
3. The Role of Mitochondria in Heart Regeneration
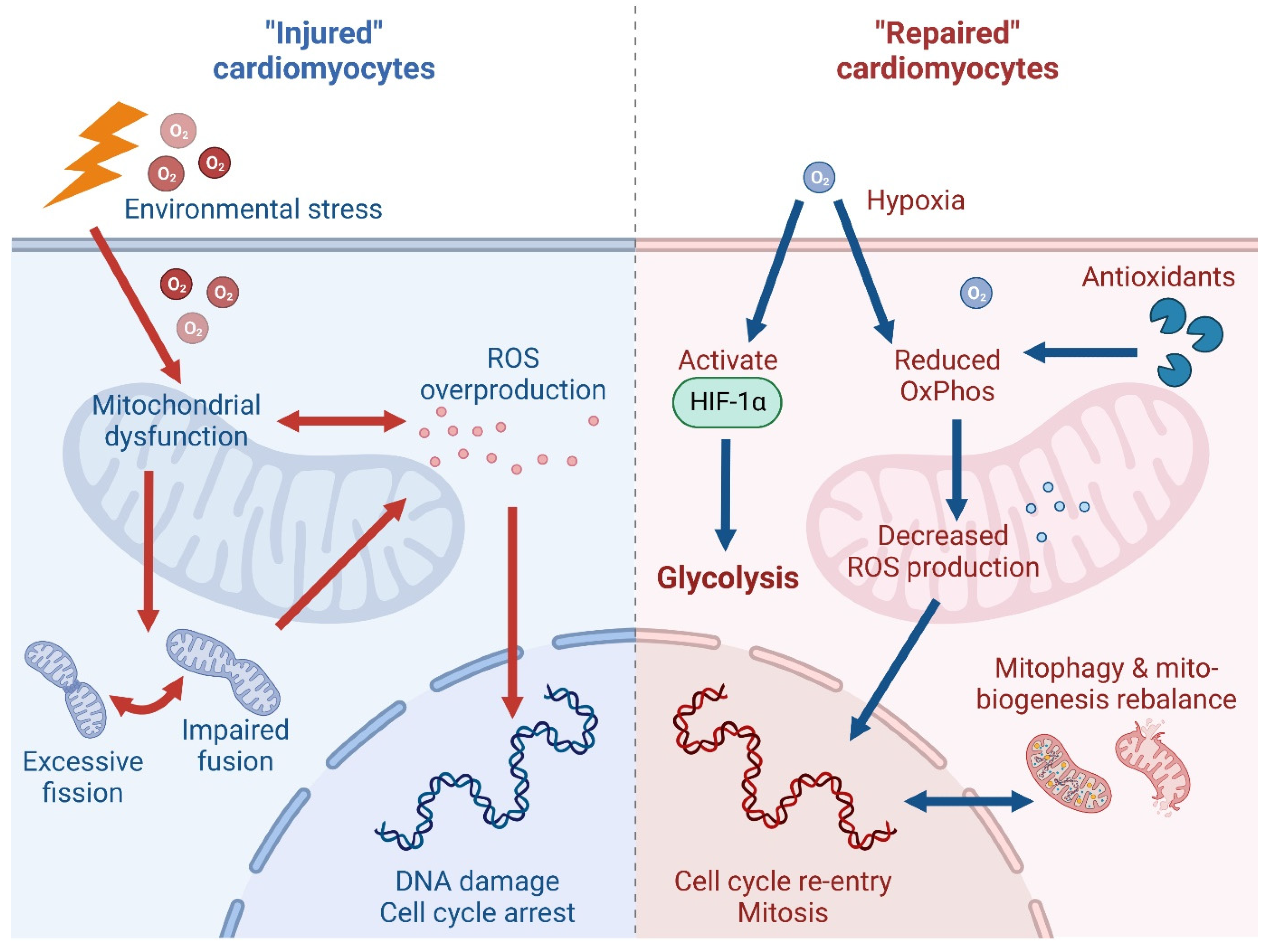
3.1. Oxidative Phosphorylation and Reactive Oxygen Species
3.2. Hypoxia Conditioning
3.3. Mitochondrial Quality Control
4. Potential Strategies to Promote Heart Regeneration through Metabolic Modulation
4.1. Long Non-Coding RNAs
4.2. Hormones
5. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Cleland, J.G.; McGowan, J. Heart failure due to ischaemic heart disease: Epidemiology, pathophysiology and progression. J. Cardiovasc. Pharmacol. 1999, 33 (Suppl. 3), S17–S29. [Google Scholar] [CrossRef]
- Vedin, O.; Lam, C.S.P.; Koh, A.S.; Benson, L.; Teng, T.H.K.; Tay, W.T.; Braun, O.O.; Savarese, G.; Dahlstrom, U.; Lund, L.H. Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: A nationwide cohort study. Circ. Heart Fail. 2017, 10, e003875. [Google Scholar] [CrossRef] [PubMed]
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Xanthopoulos, A.; Butler, J. Cardiovascular aging and heart failure: JACC review topic of the week. J. Am. Coll. Cardiol. 2019, 74, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Orogo, A.M.; Gustafsson, A.B. Cell death in the myocardium: My heart won’t go on. IUBMB Life 2013, 65, 651–656. [Google Scholar] [CrossRef]
- Yehualashet, A.S.; Belachew, T.F.; Kifle, Z.D.; Abebe, A.M. Targeting cardiac metabolic pathways: A role in ischemic management. Vasc. Health Risk Manag. 2020, 16, 353–365. [Google Scholar] [CrossRef]
- van Empel, V.P.; Bertrand, A.T.; Hofstra, L.; Crijns, H.J.; Doevendans, P.A.; De Windt, L.J. Myocyte apoptosis in heart failure. Cardiovasc. Res. 2005, 67, 21–29. [Google Scholar] [CrossRef]
- Saraste, A.; Pulkki, K.; Kallajoki, M.; Heikkila, P.; Laine, P.; Mattila, S.; Nieminen, M.S.; Parvinen, M.; Voipio-Pulkki, L.M. Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur. J. Clin. Investig. 1999, 29, 380–386. [Google Scholar] [CrossRef]
- Crow, M.T.; Mani, K.; Nam, Y.J.; Kitsis, R.N. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ. Res. 2004, 95, 957–970. [Google Scholar] [CrossRef]
- Narula, J.; Haider, N.; Virmani, R.; DiSalvo, T.G.; Kolodgie, F.D.; Hajjar, R.J.; Schmidt, U.; Semigran, M.J.; Dec, G.W.; Khaw, B.A. Apoptosis in myocytes in end-stage heart failure. N. Engl. J. Med. 1996, 335, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, M.A.; Murry, C.E. Heart regeneration. Nature 2011, 473, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Chiong, M.; Wang, Z.V.; Pedrozo, Z.; Cao, D.J.; Troncoso, R.; Ibacache, M.; Criollo, A.; Nemchenko, A.; Hill, J.A.; Lavandero, S. Cardiomyocyte death: Mechanisms and translational implications. Cell Death Dis. 2011, 2, e244. [Google Scholar] [CrossRef]
- Neubauer, S. The failing heart--an engine out of fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef]
- Lam, N.T.; Sadek, H.A. Neonatal heart regeneration: Comprehensive literature review. Circulation 2018, 138, 412–423. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, E.; Zhao, M.; Chong, Z.; Fan, C.; Tang, Y.; Hunter, J.D.; Borovjagin, A.V.; Walcott, G.P.; Chen, J.Y.; et al. Regenerative potential of neonatal porcine hearts. Circulation 2018, 138, 2809–2816. [Google Scholar] [CrossRef]
- Ye, L.; D’Agostino, G.; Loo, S.J.; Wang, C.X.; Su, L.P.; Tan, S.H.; Tee, G.Z.; Pua, C.J.; Pena, E.M.; Cheng, R.B.; et al. Early regenerative capacity in the porcine heart. Circulation 2018, 138, 2798–2808. [Google Scholar] [CrossRef]
- Haubner, B.J.; Schneider, J.; Schweigmann, U.; Schuetz, T.; Dichtl, W.; Velik-Salchner, C.; Stein, J.I.; Penninger, J.M. Functional recovery of a human neonatal heart after severe myocardial infarction. Circ. Res. 2016, 118, 216–221. [Google Scholar] [CrossRef]
- Yap, E.P.; Chan, X.; Yu, F.; Cong, S.; Ramachandra, C.J.A. Mending a broken heart: Can gene modulation bolster therapeutic performance of adult stem cells? Cond. Med. 2021, 4, 71–87. [Google Scholar]
- Lopaschuk, G.D.; Jaswal, J.S. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J. Cardiovasc. Pharmacol. 2010, 56, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Collins-Nakai, R.L.; Itoi, T. Developmental changes in energy substrate use by the heart. Cardiovasc. Res. 1992, 26, 1172–1180. [Google Scholar] [CrossRef]
- Puente, B.N.; Kimura, W.; Muralidhar, S.A.; Moon, J.; Amatruda, J.F.; Phelps, K.L.; Grinsfelder, D.; Rothermel, B.A.; Chen, R.; Garcia, J.A.; et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 2014, 157, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Karwi, Q.G.; Uddin, G.M.; Ho, K.L.; Lopaschuk, G.D. Loss of metabolic flexibility in the failing heart. Front. Cardiovasc. Med. 2018, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Spelat, R.; Ferro, F.; Contessotto, P.; Aljaabary, A.; Martin-Saldana, S.; Jin, C.; Karlsson, N.G.; Grealy, M.; Hilscher, M.M.; Magni, F.; et al. Metabolic reprogramming and membrane glycan remodeling as potential drivers of zebrafish heart regeneration. Commun. Biol. 2022, 5, 1365. [Google Scholar] [CrossRef]
- Ordono, J.; Perez-Amodio, S.; Ball, K.; Aguirre, A.; Engel, E. The generation of a lactate-rich environment stimulates cell cycle progression and modulates gene expression on neonatal and hiPSC-derived cardiomyocytes. Biomater. Adv. 2022, 139, 213035. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Spafford, M.A.; Marsh, D.R. Glycolysis is predominant source of myocardial ATP production immediately after birth. Am. J. Physiol. 1991, 261, H1698–H1705. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac energy metabolism in heart failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Wood, I.S.; Trayhurn, P. Glucose transporters (GLUT and SGLT): Expanded families of sugar transport proteins. Br. J. Nutr. 2003, 89, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.D. Glucose transport in the heart. Front. Biosci. 2004, 9, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Smoak, I.W.; Branch, S. Glut-1 expression and its response to hypoglycemia in the embryonic mouse heart. Anat. Embryol. 2000, 201, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Morissette, M.R.; Howes, A.L.; Zhang, T.; Heller Brown, J. Upregulation of GLUT1 expression is necessary for hypertrophy and survival of neonatal rat cardiomyocytes. J. Mol. Cell Cardiol. 2003, 35, 1217–1227. [Google Scholar] [CrossRef]
- Fajardo, V.M.; Feng, I.; Chen, B.Y.; Perez-Ramirez, C.A.; Shi, B.; Clark, P.; Tian, R.; Lien, C.L.; Pellegrini, M.; Christofk, H.; et al. GLUT1 overexpression enhances glucose metabolism and promotes neonatal heart regeneration. Sci. Rep. 2021, 11, 8669. [Google Scholar] [CrossRef]
- Soto-Heredero, G.; Gomez de Las Heras, M.M.; Gabande-Rodriguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef]
- Chen, Z.; Dudek, J.; Maack, C.; Hofmann, U. Pharmacological inhibition of GLUT1 as a new immunotherapeutic approach after myocardial infarction. Biochem. Pharmacol. 2021, 190, 114597. [Google Scholar] [CrossRef]
- Israelsen, W.J.; Vander Heiden, M.G. Pyruvate kinase: Function, regulation and role in cancer. Semin. Cell Dev. Biol. 2015, 43, 43–51. [Google Scholar] [CrossRef]
- Magadum, A.; Singh, N.; Kurian, A.A.; Munir, I.; Mehmood, T.; Brown, K.; Sharkar, M.T.K.; Chepurko, E.; Sassi, Y.; Oh, J.G.; et al. Pkm2 regulates cardiomyocyte cell cycle and promotes cardiac regeneration. Circulation 2020, 141, 1249–1265. [Google Scholar] [CrossRef]
- Rees, M.L.; Subramaniam, J.; Li, Y.; Hamilton, D.J.; Frazier, O.H.; Taegtmeyer, H. A PKM2 signature in the failing heart. Biochem. Biophys. Res. Commun. 2015, 459, 430–436. [Google Scholar] [CrossRef]
- Hauck, L.; Dadson, K.; Chauhan, S.; Grothe, D.; Billia, F. Inhibiting the Pkm2/b-catenin axis drives in vivo replication of adult cardiomyocytes following experimental MI. Cell Death Differ. 2021, 28, 1398–1417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hulver, M.W.; McMillan, R.P.; Cline, M.A.; Gilbert, E.R. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr. Metab. 2014, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, R.; Marin-Juez, R.; El-Sammak, H.; Beisaw, A.; Ramadass, R.; Kuenne, C.; Guenther, S.; Konzer, A.; Bhagwat, A.M.; Graumann, J.; et al. Stimulation of glycolysis promotes cardiomyocyte proliferation after injury in adult zebrafish. EMBO Rep. 2020, 21, e49752. [Google Scholar] [CrossRef]
- Ramachandra, C.J.; Mehta, A.; Wong, P.; Shim, W. ErbB4 Activated p38gamma MAPK isoform mediates early cardiogenesis through NKx2.5 in human pluripotent stem cells. Stem Cells 2016, 34, 288–298. [Google Scholar] [CrossRef]
- Ramachandra, C.J.; Mehta, A.; Lua, C.H.; Chitre, A.; Ja, K.P.; Shim, W. ErbB receptor tyrosine kinase: A molecular switch between cardiac and neuroectoderm specification in human pluripotent stem cells. Stem Cells 2016, 34, 2461–2470. [Google Scholar] [CrossRef]
- Honkoop, H.; de Bakker, D.E.; Aharonov, A.; Kruse, F.; Shakked, A.; Nguyen, P.D.; de Heus, C.; Garric, L.; Muraro, M.J.; Shoffner, A.; et al. Single-cell analysis uncovers that metabolic reprogramming by ErbB2 signaling is essential for cardiomyocyte proliferation in the regenerating heart. eLife 2019, 8, e50163. [Google Scholar] [CrossRef]
- Aharonov, A.; Shakked, A.; Umansky, K.B.; Savidor, A.; Genzelinakh, A.; Kain, D.; Lendengolts, D.; Revach, O.Y.; Morikawa, Y.; Dong, J.; et al. ERBB2 drives YAP activation and EMT-like processes during cardiac regeneration. Nat. Cell Biol. 2020, 22, 1346–1356. [Google Scholar] [CrossRef]
- Zhou, L.; Jiang, S.; Fu, Q.; Smith, K.; Tu, K.; Li, H.; Zhao, Y. FASN, ErbB2-mediated glycolysis is required for breast cancer cell migration. Oncol. Rep. 2016, 35, 2715–2722. [Google Scholar] [CrossRef]
- Li, J.; Dong, L.; Wei, D.; Wang, X.; Zhang, S.; Li, H. Fatty acid synthase mediates the epithelial-mesenchymal transition of breast cancer cells. Int. J. Biol. Sci. 2014, 10, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, T.; Mukai, R.; Oka, S.I.; Zhai, P.; Nakada, Y.; Yang, Z.; Mizushima, W.; Nakahara, T.; Warren, J.S.; Abdellatif, M.; et al. YAP mediates compensatory cardiac hypertrophy through aerobic glycolysis in response to pressure overload. J. Clin. Investig. 2022, 132, e150595. [Google Scholar] [CrossRef]
- Ancey, P.B.; Contat, C.; Boivin, G.; Sabatino, S.; Pascual, J.; Zangger, N.; Perentes, J.Y.; Peters, S.; Abel, E.D.; Kirsch, D.G.; et al. GLUT1 expression in tumor-associated neutrophils promotes lung cancer growth and resistance to radiotherapy. Cancer Res. 2021, 81, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Onay-Besikci, A. Regulation of cardiac energy metabolism in newborn. Mol. Cell Biochem. 2006, 287, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Iruretagoyena, J.I.; Davis, W.; Bird, C.; Olsen, J.; Radue, R.; Teo Broman, A.; Kendziorski, C.; Splinter BonDurant, S.; Golos, T.; Bird, I.; et al. Metabolic gene profile in early human fetal heart development. Mol. Hum. Reprod. 2014, 20, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Bou Khzam, L.; Son, N.H.; Mullick, A.E.; Abumrad, N.A.; Goldberg, I.J. Endothelial cell CD36 deficiency prevents normal angiogenesis and vascular repair. Am. J. Transl. Res. 2020, 12, 7737–7761. [Google Scholar]
- Mathison, M.; Rosengart, T.K. Heart regeneration: The endothelial cell comes first. J. Thorac. Cardiovasc. Surg. 2018, 155, 1128–1129. [Google Scholar] [CrossRef]
- Singh, S.; Prakash, S.; Gupta, S.K. Angiogenesis: A critical determinant for cardiac regeneration. Mol. Ther. Nucleic Acids 2022, 29, 88–89. [Google Scholar] [CrossRef]
- Ja, K.P.; Miao, Q.; Zhen Tee, N.G.; Lim, S.Y.; Nandihalli, M.; Ramachandra, C.J.A.; Mehta, A.; Shim, W. iPSC-derived human cardiac progenitor cells improve ventricular remodelling via angiogenesis and interstitial networking of infarcted myocardium. J. Cell Mol. Med. 2016, 20, 323–332. [Google Scholar] [CrossRef]
- Myu Mai Ja, K.P.; Lim, K.P.; Chen, A.; Ting, S.; Li, S.Q.; Tee, N.; Ramachandra, C.; Mehta, A.; Wong, P.; Oh, S.; et al. Construction of a vascularized hydrogel for cardiac tissue formation in a porcine model. J. Tissue Eng. Regen. Med. 2018, 12, e2029–e2038. [Google Scholar] [CrossRef]
- Jabs, M.; Rose, A.J.; Lehmann, L.H.; Taylor, J.; Moll, I.; Sijmonsma, T.P.; Herberich, S.E.; Sauer, S.W.; Poschet, G.; Federico, G.; et al. Inhibition of endothelial notch signaling impairs fatty acid transport and leads to metabolic and vascular remodeling of the adult heart. Circulation 2018, 137, 2592–2608. [Google Scholar] [CrossRef]
- Zhao, L.; Ben-Yair, R.; Burns, C.E.; Burns, C.G. Endocardial Notch signaling promotes cardiomyocyte proliferation in the regenerating zebrafish heart through Wnt pathway antagonism. Cell Rep. 2019, 26, 546–554.e545. [Google Scholar] [CrossRef]
- Wang, W.; Hu, Y.F.; Pang, M.; Chang, N.; Yu, C.; Li, Q.; Xiong, J.W.; Peng, Y.; Zhang, R. BMP and Notch signaling pathways differentially regulate cardiomyocyte proliferation during ventricle regeneration. Int. J. Biol. Sci. 2021, 17, 2157–2166. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Staudacher, S.; Poppelreuther, M.; Stremmel, W.; Ehehalt, R.; Fullekrug, J. Protein mediated fatty acid uptake: Synergy between CD36/FAT-facilitated transport and acyl-CoA synthetase-driven metabolism. Arch. Biochem. Biophys. 2014, 546, 8–18. [Google Scholar] [CrossRef]
- Goldenberg, J.R.; Wang, X.; Lewandowski, E.D. Acyl CoA synthetase-1 links facilitated long chain fatty acid uptake to intracellular metabolic trafficking differently in hearts of male versus female mice. J. Mol. Cell Cardiol. 2016, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, M.; Tan, J.; Shen, C.; Deng, S.; Fu, X.; Gao, S.; Li, H.; Zhang, X.; Cai, W. Targeting ACSL1 promotes cardiomyocyte proliferation and cardiac regeneration. Life Sci. 2022, 294, 120371. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yan, J.; Li, Z.Y.; Fan, Y.J.; Jiang, Z.L.; Shyy, J.Y.; Chien, S. Cyclic stretch promotes vascular homing of endothelial progenitor cells via Acsl1 regulation of mitochondrial fatty acid oxidation. Proc. Natl. Acad. Sci. USA 2023, 120, e2219630120. [Google Scholar] [CrossRef] [PubMed]
- Gilde, A.J.; van der Lee, K.A.; Willemsen, P.H.; Chinetti, G.; van der Leij, F.R.; van der Vusse, G.J.; Staels, B.; van Bilsen, M. Peroxisome proliferator-activated receptor (PPAR) alpha and PPARbeta/delta, but not PPARgamma, modulate the expression of genes involved in cardiac lipid metabolism. Circ. Res. 2003, 92, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Djouadi, F.; Brandt, J.M.; Weinheimer, C.J.; Leone, T.C.; Gonzalez, F.J.; Kelly, D.P. The role of the peroxisome proliferator-activated receptor alpha (PPAR alpha) in the control of cardiac lipid metabolism. Prostaglandins Leukot. Essent. Fatty Acids 1999, 60, 339–343. [Google Scholar] [CrossRef]
- Cao, T.; Liccardo, D.; LaCanna, R.; Zhang, X.; Lu, R.; Finck, B.N.; Leigh, T.; Chen, X.; Drosatos, K.; Tian, Y. Fatty acid oxidation promotes cardiomyocyte proliferation rate but does not change cardiomyocyte number in infant mice. Front. Cell Dev. Biol. 2019, 7, 42. [Google Scholar] [CrossRef]
- Ding, L.; Liang, X.; Zhu, D.; Lou, Y. Peroxisome proliferator-activated receptor alpha is involved in cardiomyocyte differentiation of murine embryonic stem cells in vitro. Cell Biol. Int. 2007, 31, 1002–1009. [Google Scholar] [CrossRef]
- Lim, G.B. Inhibiting fatty acid oxidation promotes cardiomyocyte proliferation. Nat. Rev. Cardiol. 2020, 17, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, C.J.A.; Mehta, A.; Wong, P.; Ja, K.; Fritsche-Danielson, R.; Bhat, R.V.; Hausenloy, D.J.; Kovalik, J.P.; Shim, W. Fatty acid metabolism driven mitochondrial bioenergetics promotes advanced developmental phenotypes in human induced pluripotent stem cell derived cardiomyocytes. Int. J. Cardiol. 2018, 272, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Czarnowska, E.; Domal-Kwiatkowska, D.; Reichman-Warmusz, E.; Bierla, J.B.; Sowinska, A.; Ratajska, A.; Goral-Radziszewska, K.; Wojnicz, R. The correlation of PPARalpha activity and cardiomyocyte metabolism and structure in idiopathic dilated cardiomyopathy during heart failure progression. PPAR Res. 2016, 2016, 7508026. [Google Scholar] [CrossRef]
- Roy, R.L.T.; Gao, E.; Zhang, X.Y.; Tian, Y. Activation or inhibition of PPARα-mediated fatty acid β-oxidation does not active cardiomyocyte proliferation in normal or infarcted adult mice. bioRxiv 2019. preprint. [Google Scholar] [CrossRef]
- Balasse, E.O.; Fery, F. Ketone body production and disposal: Effects of fasting, diabetes, and exercise. Diabetes Metab. Rev. 1989, 5, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Wentz, A.E.; d’Avignon, D.A.; Weber, M.L.; Cotter, D.G.; Doherty, J.M.; Kerns, R.; Nagarajan, R.; Reddy, N.; Sambandam, N.; Crawford, P.A. Adaptation of myocardial substrate metabolism to a ketogenic nutrient environment. J. Biol. Chem. 2010, 285, 24447–24456. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Yurista, S.R.; Nguyen, C.T.; Rosenzweig, A.; de Boer, R.A.; Westenbrink, B.D. Ketone bodies for the failing heart: Fuels that can fix the engine? Trends Endocrinol. Metab. 2021, 32, 814–826. [Google Scholar] [CrossRef]
- Kim, S.; Jeon, J.M.; Kwon, O.K.; Choe, M.S.; Yeo, H.C.; Peng, X.; Cheng, Z.; Lee, M.Y.; Lee, S. Comparative proteomic analysis reveals the upregulation of ketogenesis in cardiomyocytes differentiated from induced pluripotent stem cells. Proteomics 2019, 19, e1800284. [Google Scholar] [CrossRef]
- Chong, D.; Gu, Y.; Zhang, T.; Xu, Y.; Bu, D.; Chen, Z.; Xu, N.; Li, L.; Zhu, X.; Wang, H.; et al. Neonatal ketone body elevation regulates postnatal heart development by promoting cardiomyocyte mitochondrial maturation and metabolic reprogramming. Cell Discov. 2022, 8, 106. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Gregorich, Z.; Prajnamitra, R.P.; Lundy, D.J.; Ma, T.Y.; Huang, Y.H.; Lee, Y.C.; Ruan, S.C.; Lin, J.H.; Lin, P.J.; et al. Metabolic changes associated with cardiomyocyte dedifferentiation enable adult mammalian cardiac regeneration. Circulation 2022, 146, 1950–1967. [Google Scholar] [CrossRef]
- Weis, E.M.; Puchalska, P.; Nelson, A.B.; Taylor, J.; Moll, I.; Hasan, S.S.; Dewenter, M.; Hagenmuller, M.; Fleming, T.; Poschet, G.; et al. Ketone body oxidation increases cardiac endothelial cell proliferation. EMBO Mol. Med. 2022, 14, e14753. [Google Scholar] [CrossRef]
- Cong, S.C., X.; Yap, E.P.; Ramachandra, C.J.A.; Hausenloy, D.J. Insights into the potential cardioprotective mechanisms of SGLT2 inhibitors. Cond. Med. 2022, 5, 1–10. [Google Scholar]
- Murashige, D.; Jang, C.; Neinast, M.; Edwards, J.J.; Cowan, A.; Hyman, M.C.; Rabinowitz, J.D.; Frankel, D.S.; Arany, Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 2020, 370, 364–368. [Google Scholar] [CrossRef] [PubMed]
- McGarrah, R.W.; White, P.J. Branched-chain amino acids in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, P.; Capparelli, R.; Iannelli, A.; Iannelli, D. Role of branched-chain amino acid metabolism in type 2 diabetes, obesity, cardiovascular disease and non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2022, 23, 4325. [Google Scholar] [CrossRef]
- Beppu, T.; Nitta, H.; Hayashi, H.; Imai, K.; Okabe, H.; Nakagawa, S.; Hashimoto, D.; Chikamoto, A.; Ishiko, T.; Yoshida, M.; et al. Effect of branched-chain amino acid supplementation on functional liver regeneration in patients undergoing portal vein embolization and sequential hepatectomy: A randomized controlled trial. J. Gastroenterol. 2015, 50, 1197–1205. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Zhu, F.; Duan, Q. The role of branched chain amino acids metabolic disorders in tumorigenesis and progression. Biomed. Pharmacother. 2022, 153, 113390. [Google Scholar] [CrossRef]
- Vellai, T. How the amino acid leucine activates the key cell-growth regulator mTOR. Nature 2021, 596, 192–194. [Google Scholar] [CrossRef]
- Davogustto, G.E.; Salazar, R.L.; Vasquez, H.G.; Karlstaedt, A.; Dillon, W.P.; Guthrie, P.H.; Martin, J.R.; Vitrac, H.; De La Guardia, G.; Vela, D.; et al. Metabolic remodeling precedes mTORC1-mediated cardiac hypertrophy. J. Mol. Cell Cardiol. 2021, 158, 115–127. [Google Scholar] [CrossRef]
- Miklas, J.W.; Levy, S.; Hofsteen, P.; Mex, D.I.; Clark, E.; Muster, J.; Robitaille, A.M.; Sivaram, G.; Abell, L.; Goodson, J.M.; et al. Amino acid primed mTOR activity is essential for heart regeneration. iScience 2022, 25, 103574. [Google Scholar] [CrossRef]
- Dowling, R.J.; Topisirovic, I.; Alain, T.; Bidinosti, M.; Fonseca, B.D.; Petroulakis, E.; Wang, X.; Larsson, O.; Selvaraj, A.; Liu, Y.; et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 2010, 328, 1172–1176. [Google Scholar] [CrossRef]
- Csibi, A.; Fendt, S.M.; Li, C.; Poulogiannis, G.; Choo, A.Y.; Chapski, D.J.; Jeong, S.M.; Dempsey, J.M.; Parkhitko, A.; Morrison, T.; et al. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell 2013, 153, 840–854. [Google Scholar] [CrossRef]
- Mehta, A.; Ramachandra, C.J.; Sequiera, G.L.; Sudibyo, Y.; Nandihalli, M.; Yong, P.J.; Koh, C.H.; Shim, W. Phasic modulation of Wnt signaling enhances cardiac differentiation in human pluripotent stem cells by recapitulating developmental ontogeny. Biochim. Biophys. Acta 2014, 1843, 2394–2402. [Google Scholar] [CrossRef]
- Balatskyi, V.V.; Sowka, A.; Dobrzyn, P.; Piven, O.O. WNT/beta-catenin pathway is a key regulator of cardiac function and energetic metabolism. Acta Physiol. 2023, 237, e13912. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.Y.; Ruiz-Velasco, A.; Bui, T.; Collins, L.; Wang, X.; Liu, W. Mitochondrial function in the heart: The insight into mechanisms and therapeutic potentials. Br. J. Pharmacol. 2019, 176, 4302–4318. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Shirihai, O.S. Mitochondrial signal transduction. Cell Metab. 2022, 34, 1620–1653. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Pizzo, P.; Filadi, R. Calcium, mitochondria and cell metabolism: A functional triangle in bioenergetics. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1068–1078. [Google Scholar] [CrossRef]
- Bauer, T.M.; Murphy, E. Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ. Res. 2020, 126, 280–293. [Google Scholar] [CrossRef]
- Ramachandra, C.J.A.; Chua, J.; Cong, S.; Kp, M.M.J.; Shim, W.; Wu, J.C.; Hausenloy, D.J. Human-induced pluripotent stem cells for modelling metabolic perturbations and impaired bioenergetics underlying cardiomyopathies. Cardiovasc. Res. 2021, 117, 694–711. [Google Scholar] [CrossRef]
- Lazou, A.; Ramachandra, C.J. Protecting the mitochondria in cardiac disease. Int. J. Mol. Sci. 2022, 23, 8115. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Ramachandra, C.J.A.; Mai Ja, K.M.; Yap, J.; Shim, W.; Wei, L.; Hausenloy, D.J. Mechanisms underlying diabetic cardiomyopathy: From pathophysiology to novel therapeutic targets. Cond. Med. 2020, 3, 82–97. [Google Scholar]
- Lemieux, H.; Semsroth, S.; Antretter, H.; Hofer, D.; Gnaiger, E. Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. Int. J. Biochem. Cell Biol. 2011, 43, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Carley, A.N.; Taegtmeyer, H.; Lewandowski, E.D. Matrix revisited: Mechanisms linking energy substrate metabolism to the function of the heart. Circ. Res. 2014, 114, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, C.J.A.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Lin, Y.H.; Hausenloy, D.J. Mitochondria in acute myocardial infarction and cardioprotection. eBioMedicine 2020, 57, 102884. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Duan, S.; Yi, F.; Ocampo, A.; Liu, G.H.; Izpisua Belmonte, J.C. Mitochondrial regulation in pluripotent stem cells. Cell Metab. 2013, 18, 325–332. [Google Scholar] [CrossRef]
- Khacho, M.; Harris, R.; Slack, R.S. Mitochondria as central regulators of neural stem cell fate and cognitive function. Nat. Rev. Neurosci. 2019, 20, 34–48. [Google Scholar] [CrossRef]
- Chakrabarty, R.P.; Chandel, N.S. Mitochondria as signaling organelles control mammalian stem cell fate. Cell Stem Cell 2021, 28, 394–408. [Google Scholar] [CrossRef]
- Ramachandra, C.J.A.; Mai Ja, K.P.M.; Lin, Y.H.; Shim, W.; Boisvert, W.A.; Hausenloy, D.J. Induced pluripotent stem cells for modelling energetic alterations in hypertrophic cardiomyopathy. Cond. Med. 2019, 2, 142–151. [Google Scholar]
- Vujic, A.; Koo, A.N.M.; Prag, H.A.; Krieg, T. Mitochondrial redox and TCA cycle metabolite signaling in the heart. Free. Radic. Biol. Med. 2021, 166, 287–296. [Google Scholar] [CrossRef]
- Chen, Y.R.; Zweier, J.L. Cardiac mitochondria and reactive oxygen species generation. Circ. Res. 2014, 114, 524–537. [Google Scholar] [CrossRef]
- Sakaguchi, A.; Nishiyama, C.; Kimura, W. Cardiac regeneration as an environmental adaptation. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118623. [Google Scholar] [CrossRef]
- Sakaguchi, A.; Kimura, W. Metabolic regulation of cardiac regeneration: Roles of hypoxia, energy homeostasis, and mitochondrial dynamics. Curr. Opin. Genet. Dev. 2021, 70, 54–60. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Heims-Waldron, D.; Bezzerides, V.; Guatimosim, S.; Guo, Y.; Gu, F.; Zhou, P.; Lin, Z.; Ma, Q.; et al. Mitochondrial cardiomyopathy caused by elevated reactive oxygen species and impaired cardiomyocyte proliferation. Circ. Res. 2018, 122, 74–87. [Google Scholar] [CrossRef]
- Ramachandra, C.J.A.; Cong, S.; Chan, X.; Yap, E.P.; Yu, F.; Hausenloy, D.J. Oxidative stress in cardiac hypertrophy: From molecular mechanisms to novel therapeutic targets. Free Radic. Biol. Med. 2021, 166, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, G.; Li, M.; Hesse, M.; Ma, Y.; Chen, W.; Huang, H.; Liu, Y.; Xu, W.; Tang, Y.; et al. LDHA-mediated metabolic reprogramming promoted cardiomyocyte proliferation by alleviating ROS and inducing M2 macrophage polarization. Redox Biol. 2022, 56, 102446. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Velasquez, S.; Durrani, S.; Jiang, M.; Neiman, M.; Crocker, J.S.; Benoit, J.B.; Rubinstein, J.; Paul, A.; Ahmed, R.P. MicroRNA-1825 induces proliferation of adult cardiomyocytes and promotes cardiac regeneration post ischemic injury. Am. J. Transl. Res. 2017, 9, 3120–3137. [Google Scholar] [PubMed]
- Dambrova, M.; Zuurbier, C.J.; Borutaite, V.; Liepinsh, E.; Makrecka-Kuka, M. Energy substrate metabolism and mitochondrial oxidative stress in cardiac ischemia/reperfusion injury. Free Radic. Biol. Med. 2021, 165, 24–37. [Google Scholar] [CrossRef]
- Kimura, W.; Muralidhar, S.; Canseco, D.C.; Puente, B.; Zhang, C.C.; Xiao, F.; Abderrahman, Y.H.; Sadek, H.A. Redox signaling in cardiac renewal. Antioxid. Redox Signal. 2014, 21, 1660–1673. [Google Scholar] [CrossRef]
- Baharlooie, M.; Peymani, M.; Nasr-Esfahani, M.H.; Ghaedi, K. Pioglitazone mediates cardiac progenitor formation through increasing ROS levels. BioMed Res. Int. 2022, 2022, 1480345. [Google Scholar] [CrossRef]
- Liang, J.; Wu, M.; Chen, C.; Mai, M.; Huang, J.; Zhu, P. Roles of reactive oxygen species in cardiac differentiation, reprogramming, and regenerative therapies. Oxid. Med. Cell Longev. 2020, 2020, 2102841. [Google Scholar] [CrossRef]
- Ghadge, S.K.; Muhlstedt, S.; Ozcelik, C.; Bader, M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol. Ther. 2011, 129, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Herrero, D.; Albericio, G.; Higuera, M.; Herranz-Lopez, M.; Garcia-Brenes, M.A.; Cordero, A.; Roche, E.; Sepulveda, P.; Mora, C.; Bernad, A. The vascular niche for adult cardiac progenitor cells. Antioxidants 2022, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Bou-Teen, D.; Kaludercic, N.; Weissman, D.; Turan, B.; Maack, C.; Di Lisa, F.; Ruiz-Meana, M. Mitochondrial ROS and mitochondria-targeted antioxidants in the aged heart. Free Radic. Biol. Med. 2021, 167, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Lazar, E.; Sadek, H.A.; Bergmann, O. Cardiomyocyte renewal in the human heart: Insights from the fall-out. Eur. Heart J. 2017, 38, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.D.; Guerquin-Kern, J.L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2013, 493, 433–436. [Google Scholar] [CrossRef]
- Neidig, L.E.; Weinberger, F.; Palpant, N.J.; Mignone, J.; Martinson, A.M.; Sorensen, D.W.; Bender, I.; Nemoto, N.; Reinecke, H.; Pabon, L.; et al. Evidence for minimal cardiogenic potential of stem cell antigen 1-positive cells in the adult mouse heart. Circulation 2018, 138, 2960–2962. [Google Scholar] [CrossRef]
- Ferreira-Martins, J.; Ogorek, B.; Cappetta, D.; Matsuda, A.; Signore, S.; D’Amario, D.; Kostyla, J.; Steadman, E.; Ide-Iwata, N.; Sanada, F.; et al. Cardiomyogenesis in the developing heart is regulated by c-kit-positive cardiac stem cells. Circ. Res. 2012, 110, 701–715. [Google Scholar] [CrossRef]
- Sandstedt, J.; Jonsson, M.; Kajic, K.; Sandstedt, M.; Lindahl, A.; Dellgren, G.; Jeppsson, A.; Asp, J. Left atrium of the human adult heart contains a population of side population cells. Basic. Res. Cardiol. 2012, 107, 255. [Google Scholar] [CrossRef]
- Hesse, M.; Welz, A.; Fleischmann, B.K. Heart regeneration and the cardiomyocyte cell cycle. Pflugers Arch. 2018, 470, 241–248. [Google Scholar] [CrossRef]
- Rigaud, V.O.; Zarka, C.; Kurian, J.; Harlamova, D.; Elia, A.; Kasatkin, N.; Johnson, J.; Behanan, M.; Kraus, L.; Pepper, H.; et al. UCP2 modulates cardiomyocyte cell cycle activity, acetyl-CoA, and histone acetylation in response to moderate hypoxia. JCI Insight 2022, 7, e155475. [Google Scholar] [CrossRef]
- Knox, C.; Camberos, V.; Ceja, L.; Monteon, A.; Hughes, L.; Longo, L.; Kearns-Jonker, M. Long-term hypoxia maintains a state of dedifferentiation and enhanced stemness in fetal cardiovascular progenitor cells. Int. J. Mol. Sci. 2021, 22, 9382. [Google Scholar] [CrossRef] [PubMed]
- Bo, B.; Li, S.; Zhou, K.; Wei, J. The regulatory role of oxygen metabolism in exercise-induced cardiomyocyte regeneration. Front. Cell Dev. Biol. 2021, 9, 664527. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Qiu, L.; Feng, B.; Jiang, C.; Huang, Y.; Zhang, H.; Zhang, H.; Hong, H.; Liu, J. Role of blood oxygen saturation during post-natal human cardiomyocyte cell cycle activities. JACC Basic. Transl. Sci. 2020, 5, 447–460. [Google Scholar] [CrossRef]
- Korski, K.I.; Kubli, D.A.; Wang, B.J.; Khalafalla, F.G.; Monsanto, M.M.; Firouzi, F.; Echeagaray, O.H.; Kim, T.; Adamson, R.M.; Dembitsky, W.P.; et al. Hypoxia prevents mitochondrial dysfunction and senescence in human c-Kit(+) cardiac progenitor cells. Stem Cells 2019, 37, 555–567. [Google Scholar] [CrossRef]
- Payan, S.M.; Hubert, F.; Rochais, F. Cardiomyocyte proliferation, a target for cardiac regeneration. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118461. [Google Scholar] [CrossRef]
- Nakada, Y.; Canseco, D.C.; Thet, S.; Abdisalaam, S.; Asaithamby, A.; Santos, C.X.; Shah, A.M.; Zhang, H.; Faber, J.E.; Kinter, M.T.; et al. Hypoxia induces heart regeneration in adult mice. Nature 2017, 541, 222–227. [Google Scholar] [CrossRef]
- Mohamed, T.M.A.; Abouleisa, R.; Hill, B.G. Metabolic determinants of cardiomyocyte proliferation. Stem Cells 2022, 40, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, M.; Weng, H. Induction of the mitochondrial NDUFA4L2 protein by HIF-1a regulates heart regeneration by promoting the survival of cardiac stem cell. Biochem. Biophys. Res. Commun. 2018, 503, 2226–2233. [Google Scholar] [CrossRef]
- Feyen, D.A.M.; Gaetani, R.; Doevendans, P.A.; Sluijter, J.P.G. Stem cell-based therapy: Improving myocardial cell delivery. Adv. Drug. Deliv. Rev. 2016, 106, 104–115. [Google Scholar] [CrossRef]
- Barbash, I.M.; Chouraqui, P.; Baron, J.; Feinberg, M.S.; Etzion, S.; Tessone, A.; Miller, L.; Guetta, E.; Zipori, D.; Kedes, L.H.; et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation 2003, 108, 863–868. [Google Scholar] [CrossRef]
- Jang, W.B.; Park, J.H.; Ji, S.T.; Lee, N.K.; Kim, D.Y.; Kim, Y.J.; Jung, S.Y.; Kang, S.; Lamichane, S.; Lamichane, B.D.; et al. Cytoprotective roles of a novel compound, MHY-1684, against hyperglycemia-induced oxidative stress and mitochondrial dysfunction in human cardiac progenitor cells. Oxid. Med. Cell Longev. 2018, 2018, 4528184. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, P.; Wang, X.L.; Zhang, S.; Devejian, N.; Bennett, E.; Cai, C. Sulfiredoxin-1 enhances cardiac progenitor cell survival against oxidative stress via the upregulation of the ERK/NRF2 signal pathway. Free Radic. Biol. Med. 2018, 123, 8–19. [Google Scholar] [CrossRef]
- Fan, H.; He, Z.; Huang, H.; Zhuang, H.; Liu, H.; Liu, X.; Yang, S.; He, P.; Yang, H.; Feng, D. Mitochondrial quality control in cardiomyocytes: A critical role in the progression of cardiovascular diseases. Front. Physiol. 2020, 11, 252. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.B.; Kwek, X.Y.; Katwadi, K.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Ismail, N.I.; Lin, Y.H.; Yap, E.P.; Lim, S.Y.; Ja, K.; et al. Targeting mitochondrial fission using mdivi-1 in a clinically relevant large animal model of acute myocardial infarction: A pilot study. Int. J. Mol. Sci. 2019, 20, 3972. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W., 2nd; Vega, R.B.; Kelly, D.P. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes. Dev. 2015, 29, 1981–1991. [Google Scholar] [CrossRef]
- Li, L.; Tao, G.; Hill, M.C.; Zhang, M.; Morikawa, Y.; Martin, J.F. Pitx2 maintains mitochondrial function during regeneration to prevent myocardial fat deposition. Development 2018, 145, dev168609. [Google Scholar] [CrossRef]
- Picca, A.; Mankowski, R.T.; Burman, J.L.; Donisi, L.; Kim, J.S.; Marzetti, E.; Leeuwenburgh, C. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat. Rev. Cardiol. 2018, 15, 543–554. [Google Scholar] [CrossRef]
- Kalkhoran, S.B.; Hernandez-Resendiz, S.; Ong, S.G.; Ramachandra, C.J.A.; Hausenloy, D.J. Mitochondrial shaping proteins as novel treatment targets for cardiomyopathies. Cond. Med. 2020, 3, 216–226. [Google Scholar]
- Rahman, A.; Li, Y.; Ismail, N.I.; Chan, T.K.; Li, Y.; Xu, D.; Zhou, H.; Ong, S.B. The calcineurin-Drp1-mediated mitochondrial fragmentation is aligned with the differentiation of c-Kit cardiac progenitor cells. Int. J. Stem Cells 2023, 16, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Park, J.H.; Jang, W.B.; Ji, S.T.; Jung, S.Y.; Kim da, Y.; Kang, S.; Kim, Y.J.; Yun, J.; Kim, J.H.; et al. High glucose causes human cardiac progenitor cell dysfunction by promoting mitochondrial fission: Role of a GLUT1 blocker. Biomol. Ther. 2016, 24, 363–370. [Google Scholar] [CrossRef]
- Rosdah, A.A.; Bond, S.T.; Sivakumaran, P.; Hoque, A.; Oakhill, J.S.; Drew, B.G.; Delbridge, L.M.D.; Lim, S.Y. Mdivi-1 protects human W8B2(+) cardiac stem cells from oxidative stress and simulated ischemia-reperfusion injury. Stem Cells Dev. 2017, 26, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Lampert, M.A.; Gustafsson, A.B. Mitochondria and autophagy in adult stem cells: Proliferate or differentiate. J. Muscle Res. Cell Motil. 2020, 41, 355–362. [Google Scholar] [CrossRef]
- Lampert, M.A.; Orogo, A.M.; Najor, R.H.; Hammerling, B.C.; Leon, L.J.; Wang, B.J.; Kim, T.; Sussman, M.A.; Gustafsson, A.B. BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation. Autophagy 2019, 15, 1182–1198. [Google Scholar] [CrossRef] [PubMed]
- Mu, N.; Zhang, T.; Zhu, Y.; Lu, B.; Zheng, Q.; Duan, J. The mechanism by which miR-494-3p regulates PGC1-alpha-mediated inhibition of mitophagy in cardiomyocytes and alleviation of myocardial ischemia-reperfusion injury. BMC Cardiovasc. Disord. 2023, 23, 204. [Google Scholar] [CrossRef]
- Guo, Q.Y.; Yang, J.Q.; Feng, X.X.; Zhou, Y.J. Regeneration of the heart: From molecular mechanisms to clinical therapeutics. Mil. Med. Res. 2023, 10, 18. [Google Scholar] [CrossRef]
- Doppler, S.A.; Deutsch, M.A.; Lange, R.; Krane, M. Cardiac regeneration: Current therapies-future concepts. J. Thorac. Dis. 2013, 5, 683–697. [Google Scholar]
- Bolli, R.; Solankhi, M.; Tang, X.L.; Kahlon, A. Cell therapy in patients with heart failure: A comprehensive review and emerging concepts. Cardiovasc. Res. 2022, 118, 951–976. [Google Scholar] [CrossRef]
- Pianca, N.; Sacchi, F.; Umansky, K.B.; Chirivì, M.; Iommarini, L.; al, e. Glucocorticoid receptor antagonization propels endogenous cardiomyocyte proliferation and cardiac regeneration. Nat. Cardiovasc. Res. 2022, 1, 617–633. [Google Scholar] [CrossRef]
- Ivy, J.R.; Carter, R.N.; Zhao, J.F.; Buckley, C.; Urquijo, H.; Rog-Zielinska, E.A.; Panting, E.; Hrabalkova, L.; Nicholson, C.; Agnew, E.J.; et al. Glucocorticoids regulate mitochondrial fatty acid oxidation in fetal cardiomyocytes. J. Physiol. 2021, 599, 4901–4924. [Google Scholar] [CrossRef]
- Gay, M.S.; Dasgupta, C.; Li, Y.; Kanna, A.; Zhang, L. Dexamethasone induces cardiomyocyte terminal differentiation via epigenetic repression of cyclin D2 gene. J. Pharmacol. Exp. Ther. 2016, 358, 190–198. [Google Scholar] [CrossRef]
- Chattergoon, N.N.; Giraud, G.D.; Thornburg, K.L. Thyroid hormone inhibits proliferation of fetal cardiac myocytes in vitro. J. Endocrinol. 2007, 192, R1–R8. [Google Scholar] [CrossRef]
- Tan, L.; Bogush, N.; Naib, H.; Perry, J.; Calvert, J.W.; Martin, D.I.K.; Graham, R.M.; Naqvi, N.; Husain, A. Redox activation of JNK2alpha2 mediates thyroid hormone-stimulated proliferation of neonatal murine cardiomyocytes. Sci. Rep. 2019, 9, 17731. [Google Scholar] [CrossRef]
- Bogush, N.; Tan, L.; Naib, H.; Faizullabhoy, E.; Calvert, J.W.; Iismaa, S.E.; Gupta, A.; Ramchandran, R.; Martin, D.I.K.; Graham, R.M.; et al. DUSP5 expression in left ventricular cardiomyocytes of young hearts regulates thyroid hormone (T3)-induced proliferative ERK1/2 signaling. Sci. Rep. 2020, 10, 21918. [Google Scholar] [CrossRef] [PubMed]
- McClure, T.D.; Young, M.E.; Taegtmeyer, H.; Ning, X.H.; Buroker, N.E.; Lopez-Guisa, J.; Portman, M.A. Thyroid hormone interacts with PPARalpha and PGC-1 during mitochondrial maturation in sheep heart. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2258–H2264. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Payumo, A.Y.; Cutie, S.; Hoang, A.; Zhang, H.; Guyot, R.; Lunn, D.; Bigley, R.B.; Yu, H.; Wang, J.; et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science 2019, 364, 184–188. [Google Scholar] [CrossRef]
- Anderson, K.M.; Anderson, D.M. LncRNAs at the heart of development and disease. Mamm. Genome 2022, 33, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Mongelli, A.; Martelli, F.; Farsetti, A.; Gaetano, C. The dark that matters: Long non-coding RNAs as master regulators of cellular metabolism in non-communicable diseases. Front. Physiol. 2019, 10, 369. [Google Scholar] [CrossRef]
- Frank, S.; Aguirre, A.; Hescheler, J.; Kurian, L. A lncRNA perspective into (re)building the heart. Front. Cell Dev. Biol. 2016, 4, 128. [Google Scholar] [CrossRef]
- Liu, N.; Kataoka, M.; Wang, Y.; Pu, L.; Dong, X.; Fu, X.; Zhang, F.; Gao, F.; Liang, T.; Pei, J.; et al. LncRNA LncHrt preserves cardiac metabolic homeostasis and heart function by modulating the LKB1-AMPK signaling pathway. Basic. Res. Cardiol. 2021, 116, 48. [Google Scholar] [CrossRef]
- Yuan, T.; Krishnan, J. Non-coding RNAs in cardiac regeneration. Front. Physiol. 2021, 12, 650566. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Li, H.; Fan, Y.; Zhang, Q.J.; Li, X.; Wu, L.J.; Chen, Z.J.; Zhu, C.; Qian, L.M. Identification of differentially expressed lncRNAs involved in transient regeneration of the neonatal C57BL/6J mouse heart by next-generation high-throughput RNA sequencing. Oncotarget 2017, 8, 28052–28062. [Google Scholar] [CrossRef]
- Li, B.; Hu, Y.; Li, X.; Jin, G.; Chen, X.; Chen, G.; Chen, Y.; Huang, S.; Liao, W.; Liao, Y.; et al. Sirt1 antisense long noncoding RNA promotes cardiomyocyte proliferation by enhancing the stability of Sirt1. J. Am. Heart Assoc. 2018, 7, e009700. [Google Scholar] [CrossRef]
- Planavila, A.; Iglesias, R.; Giralt, M.; Villarroya, F. Sirt1 acts in association with PPARalpha to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc. Res. 2011, 90, 276–284. [Google Scholar] [CrossRef]
- Walker, B.R. Glucocorticoids and cardiovascular disease. Eur. J. Endocrinol. 2007, 157, 545–559. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Jellyman, J.K.; Fletcher, A.J.W.; Fowden, A.L.; Giussani, D.A. Glucocorticoid maturation of fetal cardiovascular function. Trends Mol. Med. 2020, 26, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Oakley, R.H.; Cruz-Topete, D.; Cidlowski, J.A. Dual role for glucocorticoids in cardiomyocyte hypertrophy and apoptosis. Endocrinology 2012, 153, 5346–5360. [Google Scholar] [CrossRef]
- Grais, I.M.; Sowers, J.R. Thyroid and the heart. Am. J. Med. 2014, 127, 691–698. [Google Scholar] [CrossRef]
- Janssen, R.; Muller, A.; Simonides, W.S. Cardiac thyroid hormone metabolism and heart failure. Eur. Thyroid. J. 2017, 6, 130–137. [Google Scholar] [CrossRef]
- Ross, I.; Omengan, D.B.; Huang, G.N.; Payumo, A.Y. Thyroid hormone-dependent regulation of metabolism and heart regeneration. J. Endocrinol. 2022, 252, R71–R82. [Google Scholar] [CrossRef]
- Payumo, A.Y.; Chen, X.; Hirose, K.; Chen, X.; Hoang, A.; Khyeam, S.; Yu, H.; Wang, J.; Chen, Q.; Powers, N.; et al. Adrenergic-thyroid hormone interactions drive postnatal thermogenesis and loss of mammalian heart regenerative capacity. Circulation 2021, 144, 1000–1003. [Google Scholar] [CrossRef]
- Gerdes, A.M.; Kriseman, J.; Bishop, S.P. Changes in myocardial cell size and number during the development and reversal of hyperthyroidism in neonatal rats. Lab. Investig. 1983, 48, 598–602. [Google Scholar] [PubMed]
- Parizadeh, S.M.; Jafarzadeh-Esfehani, R.; Ghandehari, M.; Parizadeh, M.R.; Ferns, G.A.; Avan, A.; Hassanian, S.M. Stem cell therapy: A novel approach for myocardial infarction. J. Cell Physiol. 2019, 234, 16904–16912. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.K.; Rhee, J.W.; Wu, J.C. Adult stem cell therapy and heart failure, 2000 to 2016: A systematic review. JAMA Cardiol. 2016, 1, 831–841. [Google Scholar] [CrossRef]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef]
- Hokimoto, S.; Yasue, H.; Fujimoto, K.; Yamamoto, H.; Nakao, K.; Kaikita, K.; Sakata, R.; Miyamoto, E. Expression of angiotensin-converting enzyme in remaining viable myocytes of human ventricles after myocardial infarction. Circulation 1996, 94, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- El-Sabban, M.E.; Hassan, K.A.; Birbari, A.E.; Bitar, K.M.; Bikhazi, A.B. Angiotensin II binding and extracellular matrix remodelling in a rat model of myocardial infarction. J. Renin Angiotensin Aldosterone Syst. 2000, 1, 369–378. [Google Scholar] [CrossRef]
- Stuck, B.J.; Lenski, M.; Bohm, M.; Laufs, U. Metabolic switch and hypertrophy of cardiomyocytes following treatment with angiotensin II are prevented by AMP-activated protein kinase. J. Biol. Chem. 2008, 283, 32562–32569. [Google Scholar] [CrossRef] [PubMed]
- Pellieux, C.; Montessuit, C.; Papageorgiou, I.; Lerch, R. Angiotensin II downregulates the fatty acid oxidation pathway in adult rat cardiomyocytes via release of tumour necrosis factor-alpha. Cardiovasc. Res. 2009, 82, 341–350. [Google Scholar] [CrossRef]
- Segersvard, H.; Lakkisto, P.; Forsten, H.; Immonen, K.; Kosonen, R.; Palojoki, E.; Kankuri, E.; Harjula, A.; Laine, M.; Tikkanen, I. Effects of angiotensin II blockade on cardiomyocyte regeneration after myocardial infarction in rats. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 92–102. [Google Scholar] [CrossRef] [PubMed]
- de Boer, R.A.; Pinto, Y.M.; Suurmeijer, A.J.; Pokharel, S.; Scholtens, E.; Humler, M.; Saavedra, J.M.; Boomsma, F.; van Gilst, W.H.; van Veldhuisen, D.J. Increased expression of cardiac angiotensin II type 1 (AT1) receptors decreases myocardial microvessel density after experimental myocardial infarction. Cardiovasc. Res. 2003, 57, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Sladek, T.; Sladkova, J.; Kolar, F.; Papousek, F.; Cicutti, N.; Korecky, B.; Rakusan, K. The effect of AT1 receptor antagonist on chronic cardiac response to coronary artery ligation in rats. Cardiovasc. Res. 1996, 31, 568–576. [Google Scholar] [CrossRef]
- Liu, C.; Fan, Y.; Zhou, L.; Zhu, H.Y.; Song, Y.C.; Hu, L.; Wang, Y.; Li, Q.P. Pretreatment of mesenchymal stem cells with angiotensin II enhances paracrine effects, angiogenesis, gap junction formation and therapeutic efficacy for myocardial infarction. Int. J. Cardiol. 2015, 188, 22–32. [Google Scholar] [CrossRef]

| Hormone | Treatment | Animal/Cellular Model | Effects on Cardiomyocyte Proliferation | Proliferation Markers Evaluated | Effects on Cardiac Metabolism | References |
|---|---|---|---|---|---|---|
| Glucocorticoid | Cardiomyocyte-specific knockout of glucocorticoid receptors | Neonatal C57BL/6 mice (P1) | Promoted | Ki67; BrdU; Aurora B; Nucleation | Decreased fatty acid oxidation; increased glycolysis | [160] |
| Dexamethasone | Pregnant C57BL/6J mice (E13.5 or E16.5) | Failed to induce fatty acid oxidation | [161] | |||
| Pregnant C57BL/6J mice (E17.5) | Decreased fatty acid oxidation | |||||
| Neonatal Sprague Dawley rats (P2) | Inhibited | Ki67; Nucleation | [162] | |||
| Thyroid hormones | T3 | Ovine (Ovis aries) foetal cardiomyocytes (~135 days gestation) | Inhibited | BrdU | [163] | |
| Neonatal cardiomyocytes from C57BL/6 mice (P2-P4) | Increased | PHH3; EdU | Increased ROS production | [164] | ||
| Neonatal C57BL/6 mice (P6) | Increased | PHH3; EdU | [165] | |||
| Thyroidectomy | Neonatal sheep (P30) | Reduced mitochondrial maturation and biogenesis | [166] | |||
| Myh6-Cre;ThraDN/+ | Neonatal C57BL/6 mice (P14) | Increased | Ki67; EdU; Aurora B; Nucleation | Downregulation of mitochondrial genes | [167] | |
| Myh6-Cre;ThraDN/+ | Adult C57BL/6 mice post-IRI injury | Increased | Ki67; EdU; Aurora B; Nucleation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, F.; Cong, S.; Yap, E.P.; Hausenloy, D.J.; Ramachandra, C.J. Unravelling the Interplay between Cardiac Metabolism and Heart Regeneration. Int. J. Mol. Sci. 2023, 24, 10300. https://doi.org/10.3390/ijms241210300
Yu F, Cong S, Yap EP, Hausenloy DJ, Ramachandra CJ. Unravelling the Interplay between Cardiac Metabolism and Heart Regeneration. International Journal of Molecular Sciences. 2023; 24(12):10300. https://doi.org/10.3390/ijms241210300
Chicago/Turabian StyleYu, Fan, Shuo Cong, En Ping Yap, Derek J. Hausenloy, and Chrishan J. Ramachandra. 2023. "Unravelling the Interplay between Cardiac Metabolism and Heart Regeneration" International Journal of Molecular Sciences 24, no. 12: 10300. https://doi.org/10.3390/ijms241210300
APA StyleYu, F., Cong, S., Yap, E. P., Hausenloy, D. J., & Ramachandra, C. J. (2023). Unravelling the Interplay between Cardiac Metabolism and Heart Regeneration. International Journal of Molecular Sciences, 24(12), 10300. https://doi.org/10.3390/ijms241210300






